Introduction
Diabetic nephropathy (DN) is one of the most common
microvascular complications of diabetes mellitus (DM) and a major
cause of the end-stage renal disease (ESRD) worldwide (1). DN is characterized by persistent
proteinuria, glomerulosclerosis and progressive decline in renal
function. Approximately 30-40% of patients with DM also have DN,
which seriously affects their quality of life and causes a
substantial economic burden (2).
Conventional treatments with strict control of hyperglycemia and
hypertension may only delay the progression of DN to a certain
extent, but cannot stop or reverse it (3). Therefore, it is crucial to
investigate DN pathogenesis and actively search for specific and
effective therapeutic targets.
Transfer RNA (tRNA)-derived small RNAs (tsRNAs) are
a class of non-coding RNA derived from mature tRNA or tRNA
precursors. Depending on their cleavage site and length, tsRNAs are
classified into tRNA halves (tiRNAs) and tRNA-derived fragments
(tRFs). tRFs, approximately 16-40 nucleotides (nt) in length, are
further classified into 5'-tRF, 3'-tRF and tRF-1 series, according
to their origin (4). tRFs exist in
all types of tissues and cells and are highly conserved, with a
stable structure and tissue-specific expression (5). tRFs are involved in regulating
processes such as cell proliferation, priming of viral reverse
transcriptases, regulation of gene expression, RNA processing,
modulation of the DNA damage response, tumor suppression and
neurodegeneration (6,7), and are closely associated with
various human diseases (8). In
particular, tRFs are aberrantly expressed in tumor cells, including
those of ovarian (9), non-small
cell lung (10), breast (11) and gastric cancer (12), and affect tumor development. For
instance, Han et al (13)
found that tRF-3008A inhibited the proliferation and migration of
colorectal cancer in vivo and in vitro by
destabilizing FOXK1 in an argonaute RISC component 1-dependent
manner. Furthermore, circulating tRF-7816 may be a novel potential
biomarker for the diagnosis of patients with early-stage
non-triple-negative breast cancer (14). However, studies of the potential
functions of tRFs in DN are limited. In previous studies by our
group, several differentially expressed (DE) tRFs were identified
in podocytes using high-throughput sequencing and were confirmed to
potentially have a significant regulatory role in the
differentiation and damage processes of podocytes (15,16).
In the current study, the DE profiles of serum tRFs
in patients with DN and DM were analysed using high-throughput
sequencing. The reliability of the sequencing results was verified
using reverse transcription-quantitative PCR (RT-qPCR). MiRanda was
used to predict the target genes of the DE tRFs. Gene Ontology (GO)
and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses were
performed to predict the potential functions of the DE tRFs. The
present study aimed to determine the DE profiles and potential
functions of tRFs in the development of DN, thus providing new
therapeutic targets for DN.
Materials and methods
Patients and samples
In total, three patients with type 2 DN and three
patients with type 2 diabetes mellitus (DM), who visited The Second
Affiliated Hospital of Nanjing Medical University (Nanjing, China)
between March 2021 and October 2021, were enrolled in the present
study. The inclusion criteria were as follows: i) Both groups met
the World Health Organization diagnostic criteria for DM, i.e.
fasting postprandial glucose ≥7.0 mmol/l, 2-h glucose ≥11.1 mmol/l
based on an oral glucose tolerance test, random glucose ≥11.1
mmol/l in patients with typical symptoms or glycosylated hemoglobin
type a1c ≥6.5%; ii) the DN group met the criteria of at least two
urine albumin to urine creatinine ratio results, i.e. ≥30 mg/g from
three measurements performed within 3-6 months. Patients with type
1 DM, malignancy and other comorbid renal diseases, such as chronic
nephritis and IgA nephropathy, cardiovascular disease, liver
disease or a history of kidney-damaging drug administration, were
excluded. A 5-ml peripheral blood sample from each participant was
collected and centrifuged to obtain the serum sample, which was
stored at -80˚C for subsequent experiments. This study was approved
by the Ethics Committee of The Second Affiliated Hospital of
Nanjing Medical University (Nanjing, China) and all participants
provided written informed consent. The baseline clinical
characteristics of the study participants are presented in Table I.
 | Table IClinical characteristics of the
subjects. |
Table I
Clinical characteristics of the
subjects.
| Characteristic | DM (n=3) | DN (n=3) | P-value |
|---|
| Sex | | | |
|
Male | 1 | 1 | - |
|
Female | 2 | 2 | - |
| Age, years | 74.0±4.583 | 70.0±11.27 | 0.5995 |
| Fasting blood
glucose, mmol/l | 9.383±3.636 | 8.217±3.004 | 0.6904 |
| Systolic blood
pressure, mmHg | 139.3±4.619 | 140.3±1.528 | 0.7398 |
| Diastolic blood
pressure, mmHg | 80.00±2.00 | 79.33±10.07 | 0.9158 |
| Glycosylated
hemoglobin type a1c, % | 9.233±2.515 | 8.067±1.955 | 0.5603 |
| Blood urea
nitrogen, mmol/l | 5.50±2.485 | 7.51±2.982 | 0.4205 |
| Serum creatinine
µmol/l | 60.27±2.053 | 120.1±33.51 | 0.0366a |
| Uric acid
µmol/l | 236.7±27.21 | 360.0±49.69 | 0.0196a |
| Urine albumin to
urine creatinine ratio, mg/g | <30 | >30 | - |
Total RNA extraction and RT-qPCR
Total RNA from the serum of patients with DN and DM
was prepared for RT-qPCR. Total RNA was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. The concentration
and purity of the RNA were determined using a NanoDrop-1000
spectrophotometer (Thermo Fisher Scientific, Inc.). According to
the instructions of the riboSCRIPT™ Reverse Transcription Kit (cat.
no. MR101; Guangzhou RiboBio Co., Ltd.), 10 µl RT reaction system
was used to reverse-transcribe the extracted RNA into cDNA.
Subsequently, qPCR was performed using 5 µl of 2X SYBR, 0.4 µl of
forward and 0.4 µl of reverse primers on the StepOne Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
Bulge-Loop™ micro (mi)RNA qRT-PCR Starter Kit (cat. no. C10211-2;
Guangzhou RiboBio Co., Ltd.) was used to quantify tRFs expression
using specific stem-loop primers synthesized by Guangzhou RiboBio
Co., Ltd. The following thermocycling conditions were used for
qPCR: Initial denaturation at 95˚C for 5 min, followed by 95˚C for
2 sec, 60˚C for 20 sec and 70˚C for 10 sec for 40 cycles. MiR-39-3p
(17) served as the external
reference and target gene expression was calculated using the
2-ΔΔCq method (18).
Each set of experiments was performed in triplicate. Details of the
primers are presented in Table
II.
 | Table IIPrimers used in the present study and
their catalogue numbers. |
Table II
Primers used in the present study and
their catalogue numbers.
| Primer | Cat. no. |
|---|
| Bulge-Loop
tRF5-GlyCCC Primer Set, 200T |
MQPS0004671-1-200 |
| Bulge-Loop
tRF3-GlyGCC Primer Set, 200T |
MQPS0004672-1-200 |
| Bulge-Loop
tRF3-IleAAT Primer Set, 200T |
MQPS0004673-1-200 |
| Bulge-Loop
tRF5-GluCTC Primer Set, 200T |
MQPS0004674-1-200 |
| Bulge-Loop
tRF5-AlaCGC Primer Set, 200T |
MQPS0004675-1-200 |
| Bulge-Loop
tRF5-ValCAC Primer Set, 200T |
MQPS0004676-1-200 |
| Cel-miR-39-3p
Standard RNA | MiRB0000010 |
High-throughput sequencing
Total RNA from the serum of patients with DN and DM
was treated using the rtStar™ tRF&tiRNA Pretreatment Kit (cat.
no. AS-FS-005; Arraystar, Inc.) to remove excess modifications and
ensure efficient RT. The 3' and 5' miRNA adapters were then ligated
to the pretreated RNA. cDNA was synthesized and amplified using
proprietary reverse transcription and amplification primers
(NEBNext® Multiplex Small RNA Library Prep Set for
Illumina; cat. no. E7330l; Illumina, Inc.). Subsequently, 134-160
bp PCR fragments, equivalent to 14-40 nt small RNA, were extracted
and purified using an automatic gel cutter. The identification and
quantitative analysis of the library were performed using an
Agilent 2100 BioAnalyzer (Agilent Technologies, Inc.). Thereafter,
the qualified library was diluted to a 1.3 ml final volume and 1.8
pM final concentration. Finally, libraries with the same
concentration and volume were loaded into the NextSeq 500/550V2 kit
(cat. no. FC-404-2005; Illumina, Inc.) and sequenced on an Illumina
NextSeq 500 system (Illumina, Inc.), according to the
manufacturer's instructions. Correlation analysis, hierarchical
clustering, scatter plots and principal component analysis (PCA)
were performed using R (v.4.1.3) software (https://www.r-project.org/). P<0.05 and |log2 fold
change (FC)| ≥1 were considered to indicate a statistically
significant difference. The sequences of DE tRFs are listed in
Table III.
 | Table IIIDifferentially expressed tRFs in
diabetic nephropathy. |
Table III
Differentially expressed tRFs in
diabetic nephropathy.
| tRF identifier | tRF sequence |
Log2(fold change) | P-value | Regulation |
|---|
| tRF5-GluCTC |
5'-TCCCTGGTGGTCTAGTGGTTAGGATTCGGCG-3' | 2.09 | 0.003 | Up |
| tRF5-AlaCGC |
5'-GGGGGTGTAGCTCAGTGGTAGAGCGCGTGC-3' | 2.09 | 0.012 | Up |
| tRF5-ValCAC |
5'-GTTTCCGTAGTGTAGTGGTTATCACGTTCGC-3' | 1.80 | 0.032 | Up |
| tRF5-GlyCCC |
5'-GCGCCGCTGGTGTAGTGGTATCATGCAAGA-3' | -1.21 | <0.001 | Down |
| tRF3-GlyGCC |
5'-TCGATTCCCGGCCCATGCACCA-3' | -1.64 | 0.040 | Down |
| tRF3-IleAAT |
5'TCGATCCCCGTACGGGCCACCA-3' | -3.36 | 0.035 | Down |
Target gene prediction and GO and KEGG
analyses
MiRanda (http://www.microrna.org/microrna/home.do) was used to
predict the target genes of DE tRFs. DAVID (https://david.ncifcrf.gov/) was used to explore the
potential biological functions and molecular mechanisms of these
target genes via GO and KEGG enrichment analyses and GO functional
classification and KEGG pathway classification maps were generated.
Cytoscape (3.9.0; https://cytoscape.org/) was used to construct the
signaling pathway regulation network for predicted target
genes.
Statistical analysis
Statistical analyses were conducted with GraphPad
Prism 8 software (GraphPad Software; Dotmatics). The measurement
data are expressed as the mean ± standard deviation. The unpaired
t-test was used for statistical comparison between two groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
DE profiles of tRFs between DN and
DM
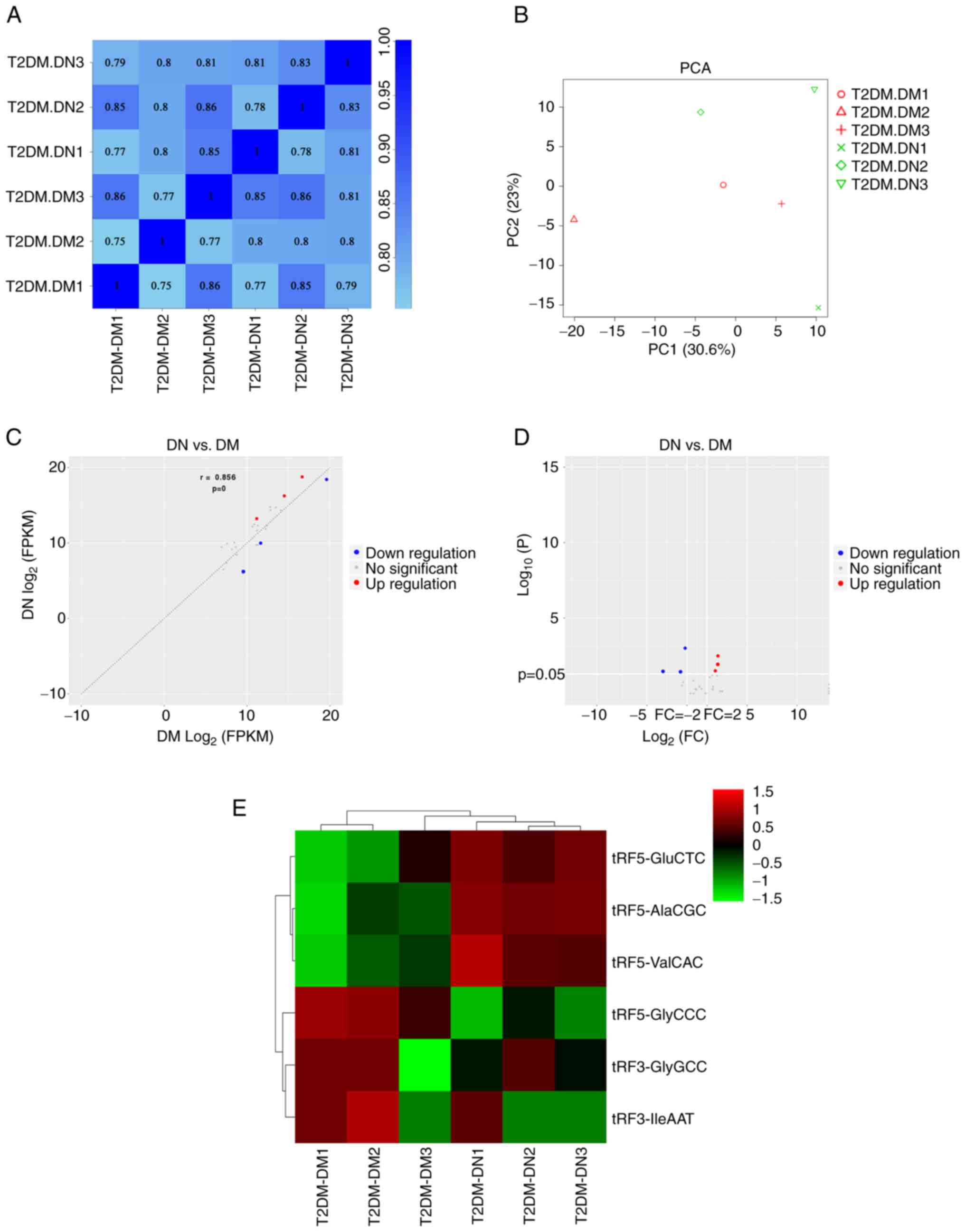
The correlation of expression levels between samples
is an important indicator for testing the reliability of the
experiment and validating the sample selection. The correlation
coefficient for any two samples exhibited a high degree of
similarity (Fig. 1A). In addition,
PCA was used to reduce the complexity of the data and analyze the
variation between samples. The results suggested a distinguishable
tRF expression profile among the six samples (Fig. 1B). In total, 30 tRFs were
identified as dysregulated in the DN group. Based on the screening
conditions of P<0.05 and |log2FC| ≥1, six tRFs were identified
as DE, of which three were upregulated and three were downregulated
(Fig. 1C and D). The cluster analysis indicated that
the samples in the same group had similar clustering patterns and
expression profiles, thereby suggesting that the differences
between the samples were small. The expression of tRF5-GluCTC,
tRF5-AlaCGC and tRF5-ValCAC was upregulated, whereas that of
tRF5-GlyCCC, tRF3-GlyGCC and tRF3-IleAAT was downregulated
(Fig. 1E).
Validation of DE tRFs
To verify the reliability of the sequencing results,
RT-qPCR was used to detect the expression levels of the DE tRFs in
the sera of patients with DN and DM. Compared with that in the DM
group, the expression of tRF5-GluCTC, tRF5-AlaCGC and tRF5-ValCAC
was upregulated, while that of tRF5-GlyCCC, tRF3-GlyGCC and
tRF3-IleAAT was downregulated in the DN group (Fig. 2). The RT-qPCR results were
consistent with the sequencing data.
Prediction of target genes
MiRanda was used to predict the target genes of DE
tRFs. The upregulated tRFs, tRF5-GluCTC, tRF5-AlaCGC and
tRF5-ValCAC, corresponded with 4,671, 3,813 and 5,234 target genes,
respectively. The downregulated tRFs, tRF5-GlyCCC, tRF3-GlyGCC and
tRF3-IleAAT, corresponded with 677, 3,448 and 856 target genes,
respectively. The top 20 target genes with the target scores for
each tRF are listed in Table
IV.
 | Table IVTarget genes with target scores in
the top 20. |
Table IV
Target genes with target scores in
the top 20.
| tRF | Targets |
Log2(fold change) | P-value |
|---|
| tRF5-GluCTC | C22orf46, ADAM11,
KCNC4, TTC34, HEMK1, C1orf95, CD276, POTEC, TAB1, ATP8B3, FAM126B,
ZNF395, CWC25, ADAM11, MMP24 | 2.09 | 0.003 |
| tRF5-AlaCGC | TTC34, FCER2,
ADGRA1, UNC5A, TRIOBP, RP1-37E16.12, SOX12, PSMC1, UBE2L3, MPRIP,
UBE2L3, EHD2, C1orf95 | 2.09 | 0.012 |
| tRF5-ValCAC | CLSTN2, TAB3,
NBPF19, NBPF12, NCBP3, SHPRH, ZFAT, HEATR6, NBPF11, SEMA5A,
KIAA1958, PPP1R21, MMACHC, ZNF891, MPV17L, DMTF1, DMTF1, DMTF1 | 1.80 | 0.032 |
| tRF5-GlyCCC | SLC25A10, NCK2,
KLC2, HNF1A, ESPL1, PPARD, EIF4G1, KIAA0930, CCDC151, STARD3,
TSEN54, PCSK6, MDH2, INO80B, CDHR5, CDK18, GTPBP3, SDF4, XPC | -1.21 | <0.001 |
| tRF3-GlyGCC | METTL21A, SMAD2,
ORAI2, CFLAR, CYP20A1, NFIX, NAT8L, TMOD3, RP11-5A19.5, KLC1,
TMEM184A, KLC1 | -1.21 | <0.001 |
| tRF3-IleAAT | MAFF, ARL2-SNX15,
TRPV4, SNRNP70, IQSEC3, FAM57A, YIPF4, DCAF11, ACIN1, MAP3K9,
HCLS1, DLG3 | -3.36 | 0.035 |
GO and KEGG enrichment analysis
To further investigate the potential functions of
these target genes, GO analyses, including the categories
biological process, cellular component and molecular function, and
KEGG pathway enrichment analyses were performed. The top 30
enriched GO terms and top 30 enriched KEGG pathways are presented
in Fig. 3. The significantly
enriched terms in the category cellular component were
micro-ribonucleoprotein complex, the Rad51 paralog
(Rad51)B-Rad51C-Rad51D-X-Ray repair cross complementing 2 (XRCC2)
complex and ribbon synapse, and the enriched primary molecular
function terms were nuclear factor of activated T-cells (NFAT)
protein binding and fibroblast growth factor-activated receptor
activity (Fig. 3A and B). The KEGG enrichment analysis indicated
that the target genes were mainly enriched in axon guidance and
neurotrophin, AMPK, mTOR and ErbB signaling pathways (Fig. 3C and D). The target genes associated with the
enriched signaling pathways are presented in Fig. 3E.
Discussion
DN pathogenesis is complex and involves various
biomolecules and signaling pathways, including advanced glycation
end products (19), the
renin-angiotensin-aldosterone system (20), oxidative stress and inflammation
(21). Traditional treatments such
as strict control of hyperglycemia and hypertension cannot
effectively delay the progression of DM to ESRD (3). Hence, researchers have been
increasingly committed to identifying specific and effective
therapeutic targets for DN.
tRNA, an essential molecule responsible for
transporting amino acids to the ribosome for protein synthesis, has
been neglected in the past. A previous study indicated that tRNAs
serve as major sources of small non-coding RNAs (sncRNAs) with
unique and diverse functions (22). These tRNA-derived sncRNAs are not
the result of random degradation but are produced by precise
biogenic processes. Under various stress conditions, tRNA
precursors and mature tRNAs are sheared by specific nucleic acid
endonucleases to produce tRFs and tiRNAs. tRFs have attracted much
attention in recent years because of their extensive biological
functions. tRFs are abundantly expressed in various body fluids and
are second only to miRNAs in abundance (23,24).
Studies indicate that tRFs may have an miRNA-like regulatory
effect, regulating gene expression by affecting mRNA stability. For
instance, Goodarzi et al (25) found that tRFs from tRNAGlu,
tRNAAsp, tRNAGly and tRNATyr suppress the stability of multiple
oncogenic transcripts in breast cancer cells by competitively
binding the RNA-binding protein YBX1. In addition, tRFs may
regulate the translation process by affecting ribosome biogenesis,
binding ribosomes and influencing the translation initiation
process, and may interact with cytochrome C to regulate apoptosis
and regulate gene expression as new epigenetic regulators (26,27).
tRFs may also be involved in a variety of human diseases, such as
cancer (28), kidney injury
(29), viral infectious (30) and neurodegenerative diseases
(31). In particular, tRFs have
been confirmed to be dysregulated in a variety of cancer tissues,
including ovarian, gastric, pancreatic and colon cancers, and are
associated with the proliferation, invasion and migration of tumor
cells (32). In the current study,
high-throughput sequencing was used to identify six DE tRFs between
DN and DM. The expression of tRF5-GluCTC, tRF5-AlaCGC and
tRF5-ValCAC was significantly upregulated, whereas that of
tRF5-GlyCCC, tRF3-GlyGCC and tRF3-IleAAT was significantly
downregulated in patients with DN. RT-qPCR was performed to
validate the expression levels and confirmed the results of the DE
analysis. It may be hypothesized that the DE tRFs are associated
with the development of DN.
GO analysis was performed to explore the potential
biological functions of the DE tRFs. In the biological process
category, anterior/posterior axon guidance had a high enrichment
ratio. Axon guidance is the process by which axons originating from
neurons form accurate synapses and is essential for the development
of the nervous system. Semaphorin is an important axon guidance
factor that contains the semiotic structural domain and semaphoring
3A (Sema3A) is currently the most widely studied semaphorin
(33). Aggarwal et al
(34) found that Sema3A expression
was significantly upregulated in podocytes from patients with
advanced DN and it was able to disrupt the glomerular filtration
barrier, leading to massive proteinuria and renal failure. Further
mechanistic studies revealed that Sema3A induced laminin and
collagen IV accumulation in the glomerulus via nephrin, αvβ3
integrin and microtubule-associated monooxygenase calponin and LIM
domain containing 1 interaction with plexin-A1, thereby resulting
in diffuse podocyte peduncle loss and F-actin collapse. In
addition, the axon guidance factor Netrin-1 and its receptor unc-5
netrin receptor B are involved in early DN angiogenesis (35). In the cellular component category,
the most enriched terms were the micro-ribonucleoprotein complex,
ribbon synapse and Rad51B-Rad51C-Rad51D-XRCC2 complex. Oxidative
stress is an independent risk factor for DN development. In
patients with DN stimulated by persistent hyperglycemia, the level
of oxidative stress is elevated and free radical production
increases, leading to increased DNA damage (36). Rad51 protein is the core protein
involved in DNA damage repair. The Rad51B - Rad51C - Rad51D - XRCC2
complex is involved in the entire process of DNA repair and is
essential for maintaining genome integrity (37). In the molecular function category,
NFAT protein binding and fibroblast growth factor (FGFs)-activated
receptor activity were the most enriched terms. NFAT is a
transcription factor with pleiotropic regulatory functions and is
expressed in a variety of immune cells (38). NFAT is also widely expressed in
other cells. NFAT is activated in response to high glucose
stimulation, which exacerbates podocyte damage (39). FGFs are a group of growth factors
with multiple isoforms that participate in angiogenesis, wound
healing, embryonic development and various endocrine signaling
pathways (40,41). FGF1 and FGF21 are promising
therapeutic targets for DN. They delay DN-related renal injury by
regulating glucolipid metabolism, inhibiting inflammation, reducing
oxidative stress and improving insulin resistance. Conversely, FGF2
and FGF23 have been associated with worsening renal function in DN
(42). In conclusion, GO analysis
suggested that the DE tRFs may be associated with DN pathology.
KEGG pathway analysis demonstrated that the target
genes were enriched in several pathways, including axon guidance,
neurotrophin, AMPK, mTOR and ErbB signaling pathways. Neurotrophin
signaling pathways are correlated with axon guidance. Each of these
neurotrophic factors [nerve growth factor, brain-derived
neurotrophic factor (BDNF), neurotrophin (NT)-3-3 and NT-4] may
interact with tropomyosin receptor kinase (Trk) or p75NT receptors
to activate Ras, PI3K, NF-κB and Jun kinase (43). miR-365 regulates high-fat
diet/streptozotocin-induced DN fibrosis by targeting the BDNF-TrkB
signaling axis (44). The mTOR and
AMPK signaling pathways are the most important nutrient-sensing
signaling pathways and are the most common pathways regulating
cellular autophagy and disruption of autophagic homeostasis in
renal cells affects DN progression (45). A study on the effect of butyrate on
DN-induced muscle atrophy implied that butyrate was able to
activate free fatty acid receptor 2-mediated PI3K/Akt/mTOR
signaling to inhibit autophagy and exert a protective effect on
DN-induced muscle atrophy (46).
In addition, Li et al (47)
investigated the role of the vitamin D receptor (VDR) in autophagy
in DN and found that VDR deficiency led to a more severe autophagy
defect in diabetic mice. However, paricalcitol or VDR
overexpression restored autophagy defects. Furthermore, this study
reported for the first time that paricalcitol ameliorates autophagy
defects in high glucose-induced HK-2 cells, partially through the
Ca2+-calcium/calmodulin-dependent protein kinase kinase
2-AMPK pathway. In summary, the present KEGG analysis suggested
that these DE tRFs may be involved in the regulation of DN via
multiple signaling pathways.
Despite these findings, the current study still
faced certain limitations. First, the relatively small sample size
may have led to a large statistical difference between the samples;
thus, further investigations based on a large sample size are
needed. Furthermore, the present results were only based on tRF
sequencing analysis and bioinformatics predictions. Thus, further
functional validation studies using in vitro and in
vivo models should be performed. Hence, in the future, a more
in-depth study will be performed to confirm and further explore the
present findings.
In conclusion, the current study examined the
expression of serum tRFs in DN and identified six DE tRFs.
Bioinformatics analysis indicated that these DE tRFs may be
associated with the pathological processes of DN. The present
findings provide a new perspective and theoretical basis for the
study of therapeutic targets for DN.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Natural Science
Foundation of China (grant no. 81970664), the Natural Science
Foundation of Jiangsu Province (grant nos. BK20191082 and
BK20211385), 789 Outstanding Talent Program of SAHNMU (grant nos.
789ZYRC202080119 and 789ZYRC202090251), the National Innovation and
Entrepreneurship Training Program for College Students (grant no.
202210312038Z) and the Science and Technology Development
Foundation of Nanjing Medical University (grant no.
NMUB2020049).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the Sequence Read Archive database
under accession no. PRJNA916973 (https://www.ncbi.nim.nih.gov/sra/PRJNA916973).
Authors' contributions
CH and LD wrote the manuscript, prepared the figures
and tables and confirm the authenticity of all the raw data. JJ and
YQ collected the samples and performed the sequencing. ZX, HS and
SZ were responsible for collating and analyzing the data. WG and AZ
designed the study and revised the manuscript. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Second Affiliated Hospital of Nanjing Medical University
[approval no. (2022)-KY-162.01] and all participants provided
written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sagoo MK and Gnudi L: Diabetic
nephropathy: An overview. Methods Mol Biol. 2067:3–7.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Umanath K and Lewis JB: Update on diabetic
nephropathy: Core curriculum 2018. Am J Kidney Dis. 71:884–895.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Samsu N: Diabetic nephropathy: Challenges
in pathogenesis, diagnosis, and treatment. Biomed Res Int.
2021(1497449)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Krishna S, Raghavan S, DasGupta R and
Palakodeti D: tRNA-derived fragments (tRFs): Establishing their
turf in post-transcriptional gene regulation. Cell Mol Life Sci.
78:2607–2619. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhu P, Yu J and Zhou P: Role of
tRNA-derived fragments in cancer: Novel diagnostic and therapeutic
targets tRFs in cancer. Am J Cancer Res. 10:393–402.
2020.PubMed/NCBI
|
|
6
|
Kumar P, Kuscu C and Dutta A: Biogenesis
and function of transfer RNA-related fragments (tRFs). Trends
Biochem Sci. 41:679–689. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Winek K, Lobentanzer S, Nadorp B, Dubnov
S, Dames C, Jagdmann S, Moshitzky G, Hotter B, Meisel C, Greenberg
DS, et al: Transfer RNA fragments replace microRNA regulators of
the cholinergic poststroke immune blockade. Proc Natl Acad Sci USA.
117:32606–32616. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pandey KK, Madhry D, Ravi Kumar YS,
Malvankar S, Sapra L, Srivastava RK, Bhattacharyya S and Verma B:
Regulatory roles of tRNA-derived RNA fragments in human
pathophysiology. Mol Ther Nucleic Acids. 26:161–173.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Panoutsopoulou K, Dreyer T, Dorn J,
Obermayr E, Mahner S, Gorp TV, Braicu I, Zeillinger R, Magdolen V,
Avgeris M and Scorilas A: tRNAGlyGCC-derived internal
fragment (i-tRF-GlyGCC) in ovarian cancer treatment outcome and
progression. Cancers (Basel). 14(24)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yang W, Gao K, Qian Y, Huang Y, Xiang Q,
Chen C, Chen Q, Wang Y, Fang F, He Q, et al: A novel tRNA-derived
fragment AS-tDR-007333 promotes the malignancy of NSCLC via the
HSPB1/MED29 and ELK4/MED29 axes. J Hematol Oncol.
15(53)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kwon NH, Lee JY and Kim S: Role of tRNAs
in breast cancer regulation. Adv Exp Med Biol. 1187:121–145.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Cui H, Li H, Wu H, Du F, Xie X, Zeng S,
Zhang Z, Dong K, Shang L, Jing C and Li L: A novel 3'tRNA-derived
fragment tRF-Val promotes proliferation and inhibits apoptosis by
targeting EEF1A1 in gastric cancer. Cell Death Dis.
13(471)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Han Y, Peng Y, Liu S, Wang X, Cai C, Guo
C, Chen Y, Gao L, Huang Q, He M, et al: tRF3008A suppresses the
progression and metastasis of colorectal cancer by destabilizing
FOXK1 in an AGO-dependent manner. J Exp Clin Cancer Res.
41(32)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huang Y, Ge H, Zheng M, Cui Y, Fu Z, Wu X,
Xia Y, Chen L, Wang Z, Wang S and Xie H: Serum tRNA-derived
fragments (tRFs) as potential candidates for diagnosis of nontriple
negative breast cancer. J Cell Physiol. 235:2809–2824.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Shi H, Yu M, Wu Y, Cao Y, Li S, Qu G, Gong
J, Gan W and Zhang A: tRNA-derived fragments (tRFs) contribute to
podocyte differentiation. Biochem Biophys Res Commun. 521:1–8.
2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li S, Liu Y, He X, Luo X, Shi H, Qu G, Wen
X, Gan W, Wang J and Zhang A: tRNA-derived fragments in podocytes
with adriamycin-induced injury reveal the potential mechanism of
idiopathic nephrotic syndrome. Biomed Res Int.
2020(7826763)2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Poel D, Buffart TE, Oosterling-Jansen J,
Verheul HM and Voortman J: Evaluation of several methodological
challenges in circulating miRNA qPCR studies in patients with head
and neck cancer. Exp Mol Med. 50(e454)2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mengstie MA, Chekol Abebe E, Behaile
Teklemariam A, Tilahun Mulu A, Agidew MM, Teshome Azezew M, Zewde
EA and Agegnehu Teshome A: Endogenous advanced glycation end
products in the pathogenesis of chronic diabetic complications.
Front Mol Biosci. 9(1002710)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rubin MF and Townsend RR: Aldosterone
blockade in diabetic nephropathy: Relative risks and potential
promise. J Am Soc Nephrol. 20:2487–2489. 2009.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Winiarska A, Knysak M, Nabrdalik K,
Gumprecht J and Stompór T: Inflammation and oxidative stress in
diabetic kidney disease: Inflammation and oxidative stress in
diabetic kidney disease: The targets for SGLT2 inhibitors and GLP-1
receptor agonists. Int J Mol Sci. 22(10822)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Tosar JP and Cayota A: Extracellular tRNAs
and tRNA-derived fragments. RNA Biol. 17:1149–1167. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yu X, Xie Y, Zhang S, Song X, Xiao B and
Yan Z: tRNA-derived fragments: Mechanisms underlying their
regulation of gene expression and potential applications as
therapeutic targets in cancers and virus infections. Theranostics.
11:461–469. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lee YS, Shibata Y, Malhotra A and Dutta A:
A novel class of small RNAs: tRNA-derived RNA fragments (tRFs).
Genes Dev. 23:2639–2649. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Goodarzi H, Liu X, Nguyen HC, Zhang S,
Fish L and Tavazoie SF: Endogenous tRNA-derived fragments suppress
breast cancer progression via YBX1 displacement. Cell. 161:790–802.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chen Q, Yan M, Cao Z, Li X, Zhang Y, Shi
J, Feng GH, Peng H, Zhang X, Zhang Y, et al: Sperm tsRNAs
contribute to intergenerational inheritance of an acquired
metabolic disorder. Science. 351:397–400. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Fu Y, Lee I, Lee YS and Bao X: Small
non-coding transfer RNA-derived RNA fragments (tRFs): Their
biogenesis, function and implication in human diseases. Genomics
Inform. 13:94–101. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Gupta T, Malkin MG and Huang S: tRNA
function and dysregulation in cancer. Front Cell Dev Biol.
10(886642)2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li D, Zhang H, Wu X, Dai Q, Tang S, Liu Y,
Yang S and Zhang W: Role of tRNA derived fragments in renal
ischemia-reperfusion injury. Ren Fail. 44:815–825. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Deng J, Ptashkin RN, Chen Y, Cheng Z, Liu
G, Phan T, Deng X, Zhou J, Lee I, Lee YS and Bao X: Respiratory
syncytial virus utilizes a tRNA fragment to suppress antiviral
responses through a novel targeting mechanism. Mol Ther.
23:1622–1629. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Tian H, Hu Z and Wang C: The therapeutic
potential of tRNA-derived small RNAs in neurodegenerative
disorders. Aging Dis. 13:389–401. 2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yu M, Lu B, Zhang J, Ding J, Liu P and Lu
Y: tRNA-derived RNA fragments in cancer: current status and future
perspectives. J Hematol Oncol. 13(121)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Nakanishi Y, Kang S and Kumanogoh A: Axon
guidance molecules in immunometabolic diseases. Inflamm Regen.
42(5)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Aggarwal PK, Veron D, Thomas DB, Siegel D,
Moeckel G, Kashgarian M and Tufro A: Semaphorin3a promotes advanced
diabetic nephropathy. Diabetes. 64:1743–1759. 2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jiao X, Zhang D, Hong Q, Yan L, Han Q,
Shao F, Cai G, Chen X and Zhu H: Netrin-1 works with UNC5B to
regulate angiogenesis in diabetic kidney disease. Front Med.
14:293–304. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zou YL, Luo WB, Xie L, Mao XB, Wu C and
You ZP: Targeting human 8-oxoguanine DNA glycosylase to
mitochondria protects cells from high glucose-induced apoptosis.
Endocrine. 60:445–457. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu Y, Tarsounas M, O'Regan P and West SC:
Role of RAD51C and XRCC3 in genetic recombination and DNA repair. J
Biol Chem. 282:1973–1979. 2007.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Macian F: NFAT proteins: Key regulators of
T-cell development and function. Nat Rev Immunol. 5:472–484.
2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nijenhuis T, Sloan AJ, Hoenderop JG,
Flesche J, van Goor H, Kistler AD, Bakker M, Bindels RJ, de Boer
RA, Möller CC, et al: Angiotensin II contributes to podocyte injury
by increasing TRPC6 expression via an NFAT-mediated positive
feedback signaling pathway. Am J Pathol. 179:1719–1732.
2011.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Itoh N, Ohta H and Konishi M: Endocrine
FGFs: Evolution, physiology, pathophysiology, and pharmacotherapy.
Front Endocrinol (Lausanne). 6(154)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Zhang J and Li Y: . Therapeutic uses of
FGFs. Semin Cell Dev Biol. 53:144–154. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Deng J, Liu Y, Liu Y, Li W and Nie X: The
multiple roles of fibroblast growth factor in diabetic nephropathy.
J Inflamm Res. 14:5273–5290. 2021.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Skaper SD: Neurotrophic factors: An
overview. Methods Mol Biol. 1727:1–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Zhao P, Li X, Li Y, Zhu J, Sun Y and Hong
J: Mechanism of miR-365 in regulating BDNF-TrkB signal axis of
HFD/STZ induced diabetic nephropathy fibrosis and renal function.
Int Urol Nephrol. 53:2177–2187. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ding Y and Choi ME: Autophagy in diabetic
nephropathy. J Endocrinol. 224:R15–R30. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Tang G, Du Y, Guan H, Jia J, Zhu N, Shi Y,
Rong S and Yuan W: Butyrate ameliorates skeletal muscle atrophy in
diabetic nephropathy by enhancing gut barrier function and
FFA2-mediated PI3K/Akt/mTOR signals. Br J Pharmacol. 179:159–178.
2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li A, Yi B, Han H, Yang S, Hu Z, Zheng L,
Wang J, Liao Q and Zhang H: Vitamin D-VDR (vitamin D receptor)
regulates defective autophagy in renal tubular epithelial cell in
streptozotocin-induced diabetic mice via the AMPK pathway.
Autophagy. 18:877–890. 2022.PubMed/NCBI View Article : Google Scholar
|