Introduction
Pruritis is an unpleasant sensation that causes an
intense desire to rub or scratch (1). Pruritus is the primary cause of poor
quality of life in patients with hypersensitive skin disease
(2). The desire to scratch in
hypersensitive skin disease is not resolved by scratching and it
becomes stronger as the patient scratches (3,4).
Repeated scratching weakens the skin barrier and exacerbates skin
lesions (5). Therefore, pruritus
control may be key to improving the quality of life of patients
with hypersensitive skin disease.
Astrocytes are a type of glial cell involved in
regulating neuronal excitability in the central nervous system
(CNS) (6,7). Astrocytes mediate itching by
releasing signaling molecules, such as ATP, that activate C-fibers
(8). However, a recent study
showed that inositol 1,4,5-trisphosphate receptor type 1 (IP3R1) is
important for both the IL-6-induced sustained activation of signal
transducer and activator of transcription 3 (STAT3) and expression
of genes associated with reactive astrocytes (9). STAT3 serves an important role in the
transformation of reactive astrocytes (9). Reactive astrocytes intensify the
itching sensation by sensitizing gastrin releasing peptide receptor
(GRPR)-positive neurons to GRP itch neuropeptides via lipocalin-2
(LCN2) secretion in hypersensitive skin disease models such as
atopic dermatitis (10). Previous
studies have showed that pharmacological inhibition of STAT3
decreases reactivity of astrocytes (11,12).
Therefore, modulating STAT3 in reactive astrocytes is a useful
strategy to control the itch.
Diospyros lotus, also called date plum or
Caucasian persimmon, is a deciduous plant native to Asian
countries, including Korea, Japan and China, and Southeast Europe.
It can be eaten directly or by processing the leaves into herbal
tea (13,14). Diospyros lotus leaf extract
(DLE) has been used in traditional medicine as an antitumor,
antidiabetic, sedative, astringent, antipyretic or laxative agent
(13). Our previous studies showed
that DLE contains flavonoids such as myricitrin (MC), gallic acid,
astragalin, myricetin-3-O-galactoside and myricetin with
anti-atopic dermatitis, anti-inflammatory, antioxidant and
anti-obesity effects (15-17).
However, to the best of our knowledge, effects of DLE on
histamine-independent pruritus and its mechanism of action in
activated astrocytes have not been reported yet.
Therefore, the present study aimed to investigate
the effect of DLE and MC on histamine-independent pruritus in
IL-6-stimulated astrocytes and chloroquine-injected mouse
models.
Materials and methods
Plant materials
Diospyros lotus leaves were collected in June
2020 from Cheonjam mountain, Jeonju-si, Jeollabuk-do, Republic of
Korea. The leaves were washed five times with water and dried in a
windy area with shade. Dried leaves (100 g) were mixed with 70%
(v/v) ethanol (2 l) at room temperature for 48 h. The extracted
sample was filtered using 0.5 µm filter paper (ADVANTEC), Thee
extract was concentrated at 45˚C using a vacuum concentrator (EYELA
Rotary evaporator N-1100, EYELA) to obtain DLE in powder form.
Materials
MC was purchased from Tokyo Chemical Industry Co.,
Ltd. Quanti-MAX™ WST-8 Cell Viability Assay kit (cat. no. QM1000)
and WestGlow™ FEMTO Chemiluminescent substrate (BWF0100) were
obtained from Biomax Co. Ltd. ReadyShield® Protease and Phosphatase
Inhibitor Cocktail, cat. no. PPC2020) and Chloroquine diphosphate
salt were purchased from Sigma-Aldrich (Merck KGaA).
Radioimmunoprecipitation assay (RIPA) buffer and phosphorylated
(p)-STAT3 (44-384G), STAT3 (MA1-13042) glial fibrillary acidic
protein (GFAP) (14-9892-82), LCN2 (PA5-79590), IP3R1 (PA1-901) and
goat anti-mouse IgG Alexa Fluor 488 (A-11001) antibodies were
purchased from Thermo Fisher Scientific, Inc. Actin (sc-8432),
m-IgGκ BP-HRP (sc-516102) and mouse anti-rabbit IgG-HRP (cat. no.
sc-2357) were purchased from Santa Cruz Biotechnology, Inc.
Hematoxylin and eosin (H&E) stain kit (ab245880) was purchased
from Abcam. ProLong® gold antifade reagent with DAPI mounting
solution (8961) was came from Cell signaling (Danvers, MA,
USA).
Cell culture
Mouse-origin astrocytes (CRL-2535™) and DMEM (cat.
no. 30-2002) were purchased from American Type Culture Collection.
Fetal bovine serum (cat. no. 16000044) and
Penicillin-Streptomycin-Glutamine (100X; cat. no. 10378016) were
purchased from Thermo Fisher Scientific, Inc. Astrocytes were
cultured and maintained in DMEM supplemented with 10% fetal bovine
serum, 100 U/ml penicillin and 100 µg/ml streptomycin in a 5%
CO2 incubator at 37˚C.
Cell viability
Cell viability was analyzed using Quanti-MAX™ WST-8.
Astrocytes (3x105 cells/ml) were seeded into 96-well
plates and cultured at 37˚C for 24 h. Then, DLE (0.0, 12.5, 25.0,
50.0, 100.0 and 200.0 µg/ml) or MC (0, 10, 20, 30, 50, 75 and 100
µM) were treated and further incubated at 37˚C for 20 h.
Subsequently, 10 µl Quanti-MAX™ WST-8 reagent was added to each
well followed by incubation at 37˚C for 4 h. The absorbance of each
well was measured at 450 nm using a spectrophotometer (Tecan Group,
Ltd.). The cell viability was calculated based on the absorbance
compared with the control group without the sample added.
Protein extraction and western
blotting
Astrocytes (3x105 cells/ml) were cultured
in 60-mm cell culture dishes at 37˚C for 24 h, treated with DLE (50
and 100 µg/ml) or MC (10 and 20 µM), and incubated at 37˚C for 1 h.
These cells were stimulated with IL-6 (10 ng/ml) at 37˚C for 30 min
or 24 h. Total protein was extracted from each sample using
protease inhibitor-treated RIPA buffer. Following quantification by
Bradford assay, 50 µg protein was loaded in each lane and separated
by SDS-PAGE on 10 or 12% gel. Separated proteins were transferred
onto a polyvinylidene fluoride membrane, blocked with 5% bovine
serum albumin (BSA) (A0100-010; GenDEPOT) at room temperature for 1
h and washed three times with Tris-buffered saline with 1% Tween-20
(TBST) solution (10 min each wash). These membranes were incubated
with antibodies against STAT3 (1:1,000), p-STAT3 (1:1,000), IP3R
(1:1000), LCN2 (1:2000), GFAP (1:1000) and β-actin (1:500) at 4˚C
overnight. After washing five times with TBST, membranes were
incubated with anti-mouse (1:5,000; cat. no. sc-516102, Santa Cruz
Biotechnology, Inc.) or rabbit (1:5,000) (sc-2357, Santa Cruz
Biotechnology, Inc.) horseradish peroxidase-conjugated secondary
antibodies containing BSA for 2 h at room temperature.
Subsequently, membranes were washed three times with TBST solution
(10 min each) and visualized with an imaging system (ALLIANCE LD4;
Uvitec, Cambridge, UK) using EZ-Western Lumi Pico Alpha
chemiluminescent reagent (DG-WP250; DoGenBio, Seoul, South Korea).
Band densities were analyzed using ImageJ 1.53e (National
Institutes of Health) with β-actin as the loading control.
Reverse transcription-quantitative
(RT-q)PCR
Astrocytes (3x105 cells/ml) were cultured
in 60-mm cell culture dishes at 37˚C for 24 h, treated and
incubated at 37˚C for 1 h with DLE (0, 50 and 100 µg/ml) or MC (0
10, and 20 µg/ml) and stimulated with IL-6 (10 ng/ml) at 37˚C for 4
h. Total RNA was extracted and purified using a GeneAll®
Ribospin™ II extraction kit (GeneAll Biotechnology Co., Ltd.),
according to the manufacturer's instructions. The concentration of
total RNA isolated and purified was determined by spectrophotometry
(Optizen NanoQ plus; KLAB). The ReverTra Ace™ qPCR RT Master Mix
cDNA synthesis kit (Toyobo Life Science) was used to synthesize
cDNA using 5 µg each RNA sample. qPCR was performed using the Power
SYBR® Green Master Mix (Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions. The following
thermocycling conditions were used for qPCR: Initial denaturation
at 95˚C for 3 min, followed by 40 cycles of denaturation at 95˚C
for 30 sec, annealing at 60˚C for 10 sec and extension at 72˚C for
30 sec. The following primer pairs were used for qPCR: LCN2
forward, 5'-CCAGTTCGCCATGGTATTTT-3' and reverse,
5'-GGTGGGGACAGAGAAGATGA-3' and GAPDH forward,
5'-GGCTACACTGAGGACCAGGT-3' GAPDH reverse,
5'-TCCACCACCCTGTTGCTGTA-3'.
Animals and experimental design
Specific-pathogen-free male ICR mice (25 4 weeks-old
21 g) were obtained from Orient Bio, Inc. Mice were housed in a
room with standard environmental conditions (temperature of 22±2˚C,
50-60% humidity and a 12/12-h light/dark cycle) and were provided
free access to a commercial standard laboratory diet and water.
Experimental procedures were performed according to Jeonju
University Institutional Animal Care and Use Committee guidelines
(18). During the experimental
period, the health of animals was monitored daily, and no mice met
the humane endpoints specified, such as a weight loss of over 20%,
appetite loss for more than 2 days, dyspnea, increased heart rate,
self-harm, jaundice, persistent diarrhea/vomiting, and decreased
response to external stimuli. After a 1-week acclimation period,
mice were shaved on their back using an electric shaver and then
randomly assigned to four groups (n=5/group): 1, Normal control
(200 µl of saline); 2, chloroquine (50 µg/site); 3, chloroquine +
200 mg/kg DLE and 4, chloroquine + 20 mg/kg MC. Normal saline, DLE,
and MC were administered orally, while chloroquine was administered
via subcutaneous injection. After the experiment, mice were
anesthetized with a 2-6% isoflurane for induction and maintained at
a 1-3% concentration. Skin thickness was measured with vernier
calipers and blood samples (600-800 µl) were collected from the
orbital venous plexus. Subsequently, mice were euthanized through
cervical dislocation and, after confirming the absence of
respiratory and heartbeat, the dorsal skin samples were
collected.
Scratching behavior analysis
The hair on the back was shaved with a hair clipper
1 day before the start of the experiment. At 1 h before chloroquine
injection, mice in groups 1 and 2 were orally administered with
saline, whereas those in groups 3 and 4 were orally administered
with DLE or MC. After 30 min CCTV recording immediately after
chloroquine injection, five researchers evaluated scratching
behavior in a double-blind manner. The back skin was collected for
histological analysis and the vertebrae were collected for
immunofluorescence staining.
Histopathological examination
Tissues were fixed in 4% paraformaldehyde at 4˚C for
24 h and washed five times with phosphate-buffered saline (PBS) at
room temperature for a total of 24 h. Samples were dehydrated in
ascending ethanol series (60-100%) at room temperature for 30 min
at each concentration. Samples were washed twice in xylene (2 h
each at room temperature), embedded three times in paraffin (1 h
each at 60˚C). Finally, new paraffin was poured into the tissues at
60˚C and solidified at 4˚C for 1 h to form a block.
Paraffin-embedded tissue samples were cut into 5-µm-thick sections
using a microtome (Leica Microsystems GmbH). Samples were stained
using the H&E staining kit (cat. no. ab245880, Abcam) according
to the manufacturer's protocol.
Immunofluorescence staining
Astrocytes (3x105 cells/ml) were cultured
in 4-well cell culture slides at 37˚C for 24 h, treated at 37˚C for
1 h with DLE (100 µg/ml) or MC (20 µM) and then stimulated with
IL-6 (10 ng/ml) at 37˚C for 24 h. The cells were fixed with ice
cold methanol (99.9%) for 20 min, blocked with PBS containing 1%
BSA at room temperature for 1 h and then incubated at 4˚C for 16 h
after injection with GFAP primary antibody (1:100). After washing
three times for 10 min with PBS with 0.1% Tween-20, the cells were
incubated in the dark at room temperature for 2 h with goat
anti-mouse IgG Alexa Fluor 488 secondary antibody (1:1,000),
followed by mounting with a mounting solution containing DAPI.
After drying overnight, images were captured at 400x using a
fluorescence microscope (Carl Zeiss AG).
Segments of the fifth lumbar vertebra were fixed in
4% paraformaldehyde at 37˚C for 4 h and incubated with PBS
containing 30% sucrose at 4˚C for 24 h. Tissues were cut into 30-µm
sections using a cryotome (Amos Scientific Pty, Ltd.). Samples were
washed three times in PBS (10 min each) and incubated at room
temperature for 1 h in PBS containing 0.3% Triton X-100 and 2% BSA.
Samples were incubated overnight at 4˚C with GFAP antibody. After
washing five times with PBS, sections were washed with PBS and
incubated with the goat anti-mouse IgG Alexa Fluor 488 secondary
antibody (1:1000) at room temperature for 2 h. After washing five
times with PBS, samples were mounted with DAPI-containing mounting
medium. After drying overnight, images were captured at 400x using
a fluorescence microscope (Carl Zeiss AG).
Statistical analysis
All statistical analysis was performed using SPSS
version 26.0 (IBM, Armonk, NY, USA). Data are presented as the mean
± standard deviation (n=3). Statistical analysis was performed
using one-way ANOVA followed by Tukey's post hoc test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Effects of DLE and MC on astrocyte
survival
Cell viability assay was performed to investigate
the effect of DLE and MC on astrocytes. Results showed that DLE was
not cytotoxic at ≤100 µg/ml. However, DLE was cytotoxic at
concentrations >200 µg/ml (Fig.
1A). MC was not toxic at concentrations ≤30 µM; however, it was
cytotoxic at concentrations >50 µM (Fig. 1B). Therefore, in the subsequent
experiments, cells were treated with ≤100 µg/ml DLE and ≤30 µM
MC.
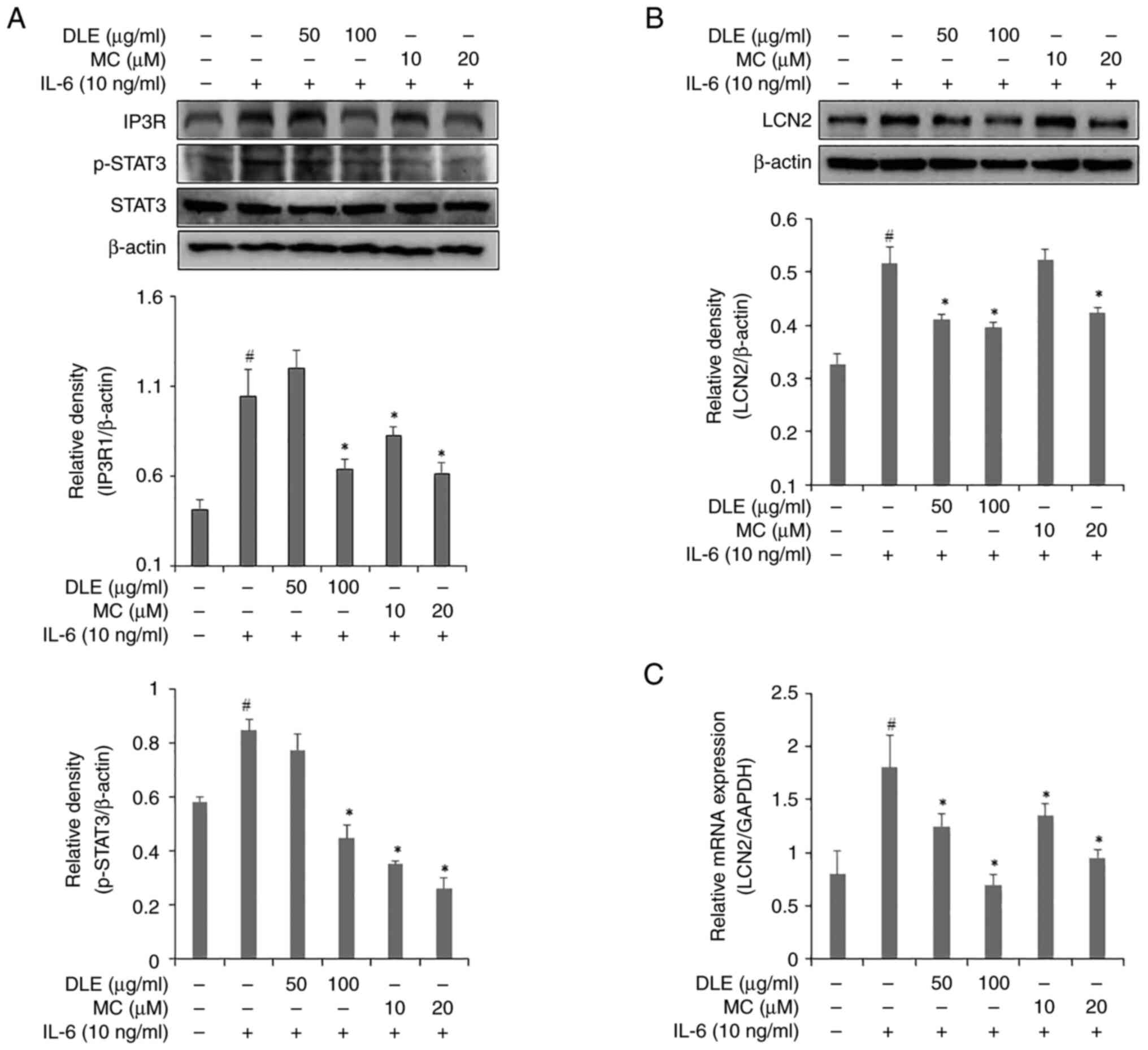
Effects of DLE and MC on STAT3 and
IP3R1 activation and LCN2 production
To investigate the effects of DLE and MC on STAT3
and IP3R expression, western blotting was performed (Fig. 2A). The expression of IP3R showed a
significant decrease compared with the IL-6 alone treated group at
all DLE concentrations except 50 µg/ml. Similarly, 50 µg/ml DLE did
not inhibit STAT3 expression. However, 100 µg/ml DLE significantly
decreased compared to the IL-6 alone treated group expression of
STAT3. Expression of STAT3 was decreased in a
concentration-dependent manner starting from 10 µM MC. The effects
of DLE and MC on LCN2 production were assessed using western
blotting (Fig. 2B) and RT-qPCR
(Fig. 2C). DLE treatment similarly
inhibited LCN2 expression at both 50 and 100 µg/ml concentrations,
and the inhibitory effect showed a significant decrease compared to
the IL-6 alone treated group. However, MC treatment significantly
decreased LCN2 expression only at 20µM compared to the control
group. RT-qPCR results showed that both DLE and MC decreased LCN2
mRNA expression in a dose-dependent manner compared to the control
group.
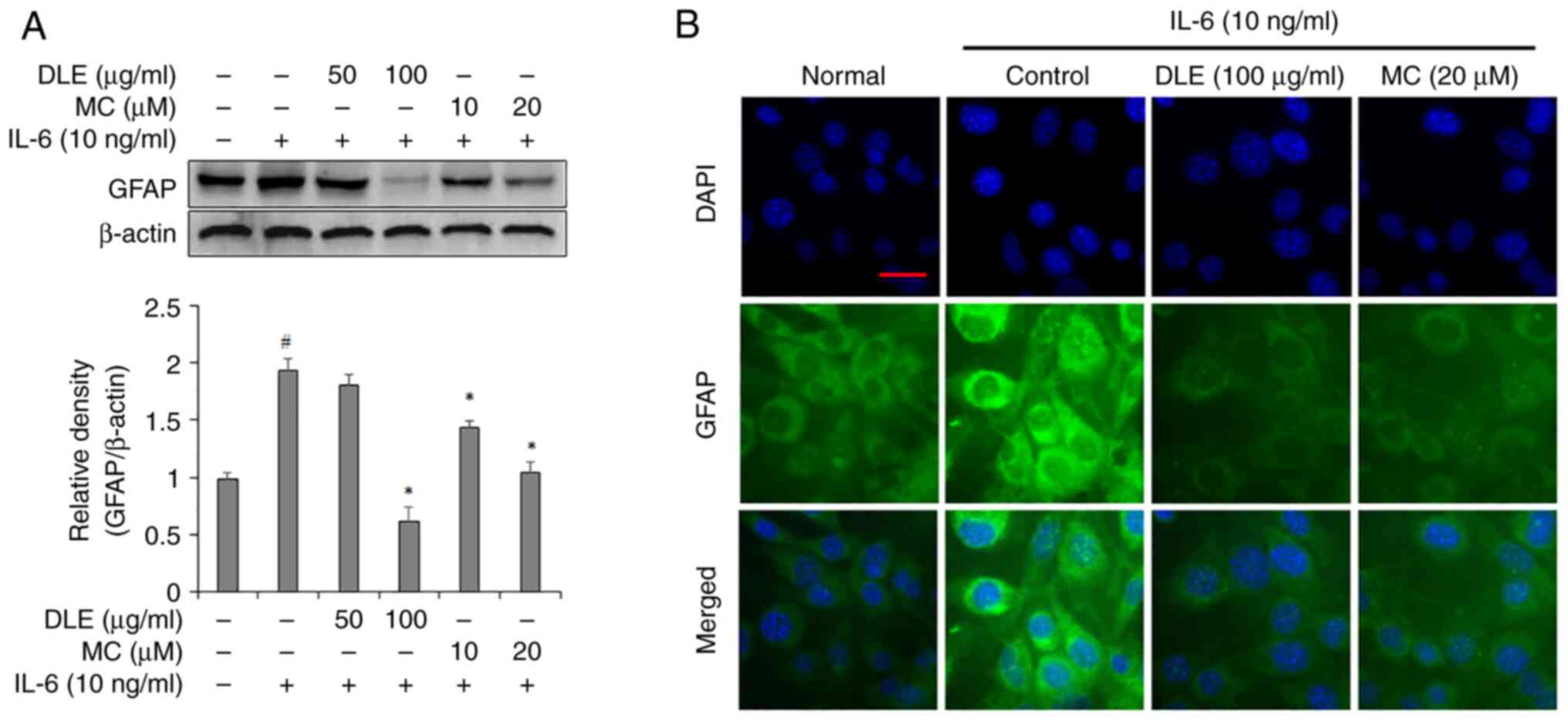
Effects of DLE and MC on GFAP
expression in IL-6-treated astrocytes
GFAP expression was assessed using western blotting
and immunofluorescence staining. Western blotting analysis
indicated that 50 µg/ml DLE did not significant decrease in GFAP
expression compared with the IL-6 alone treated group but 100 µg/ml
DLE significantly suppressed GFAP expression. The treatment with MC
resulted in a dose-dependent decrease in GFAP expression in
astrocytes and showed a significant reduction compared to the group
treated with IL-6 alone (Fig. 3A).
Immunofluorescent staining assay showed that the expression of GFAP
was decreased by treatment with DLE and MC, which was consistent
with the results of the western blotting assay (Fig. 3B).
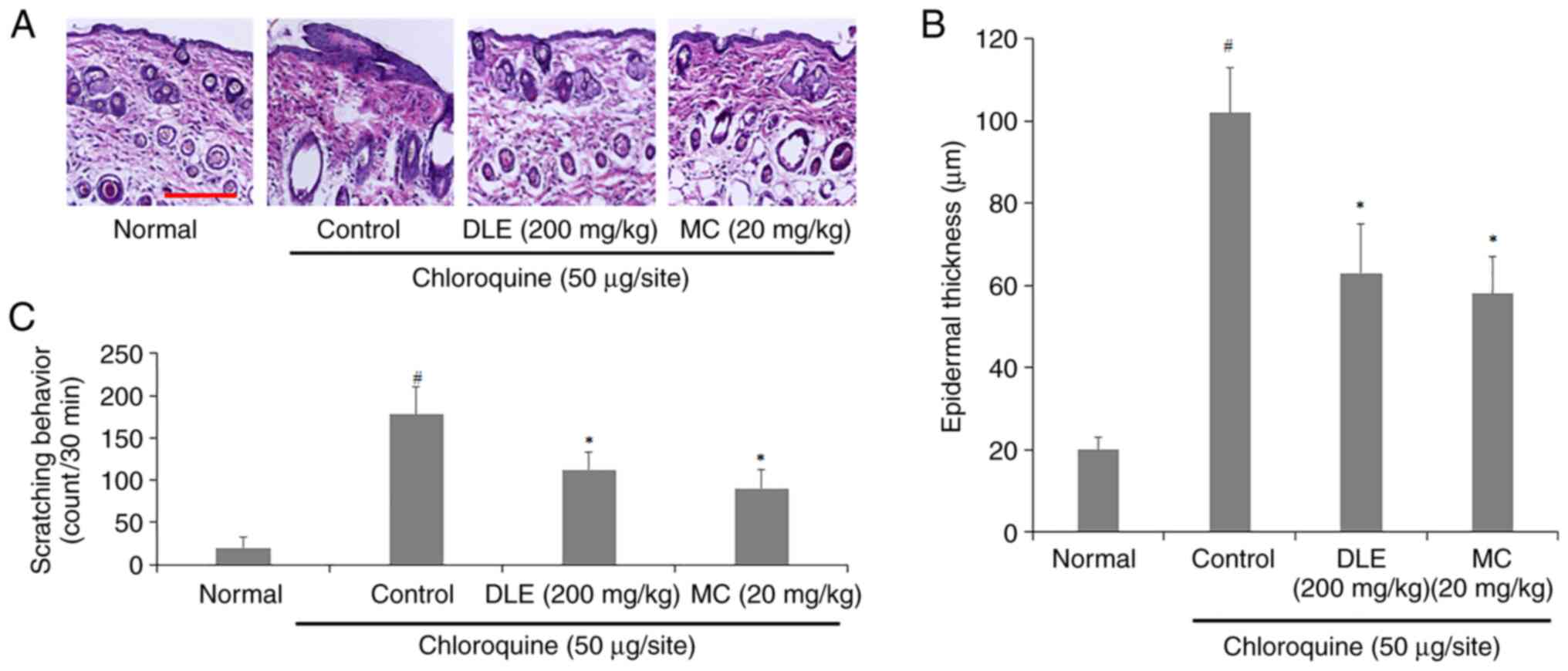
Effect of DLE and MC on histological
changes in the skin and scratching behavior of chloroquine-injected
mice
To investigate the effects of DLE and MC on
histological changes of chloroquine-injected mice, the back skin
was observed through H&E staining. Severe edema was observed in
the back skin of the control group, whereas this was decreased in
the DLE and MC-administered groups (Fig. 4A). Additionally, DLE and MC
administration resulted in a decrease in epidermal thickness
compared to the control group (Fig.
4B). The frequency of scratching behavior also significantly
decreased in DLE and MC-administered groups (Fig. 4C).
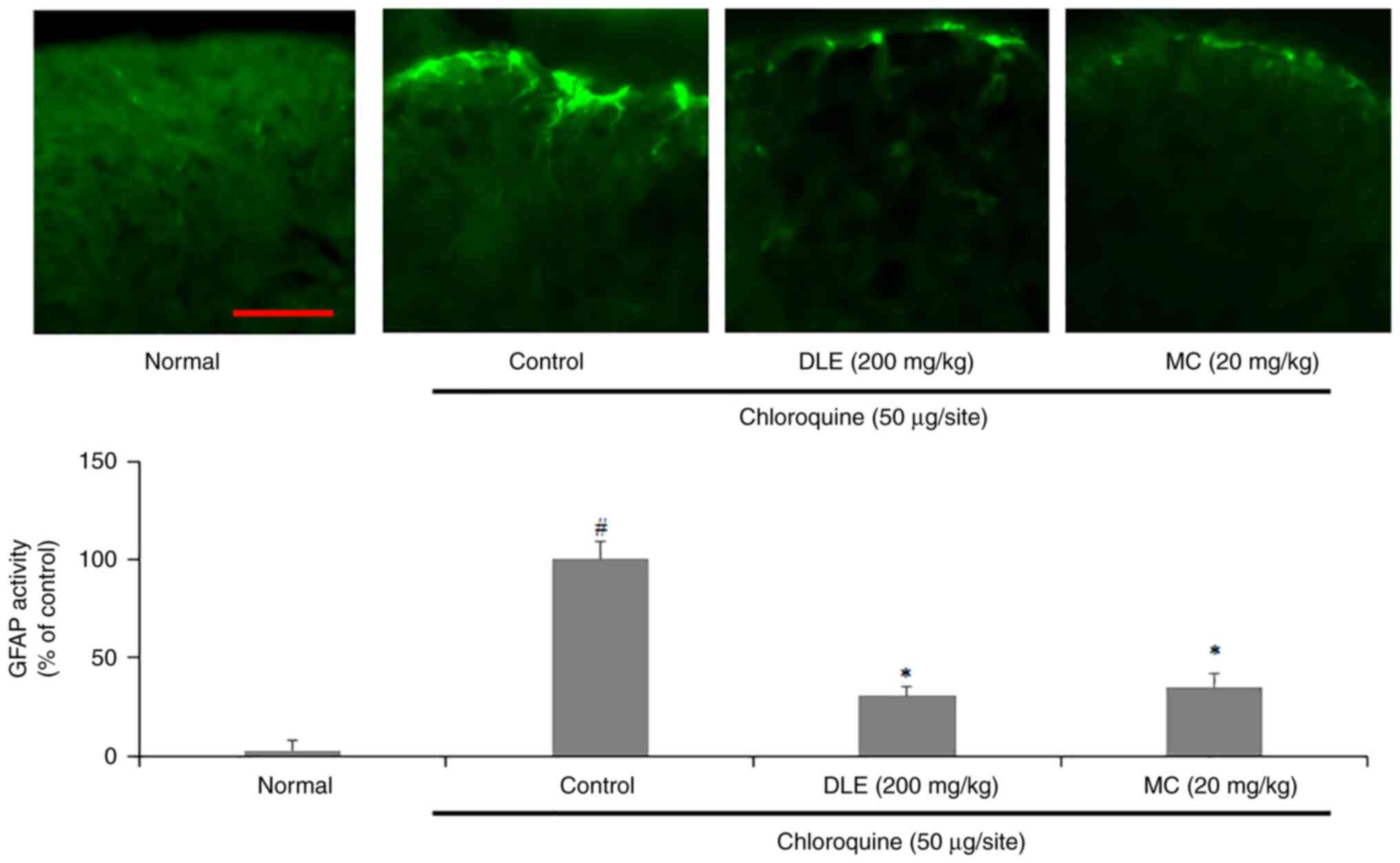
Effect of DLE and MC on mouse spinal
dorsal horn (SDH) astrocyte activity
To investigate effects of DLE and MC on SDH
astrocyte activity, a GFAP-specific fluorescence staining assay of
spinal sections collected from the mice was performed. The
expression of GFAP was significantly increased in the control group
compared with that in the normal group; however, administration of
DLE or MC significantly decreased the expression of GFAP (Fig. 5).
Discussion
Our previous studies demonstrated that
administration of DLE could inhibit biomarkers such as
pro-inflammatory cytokines, immune cell infiltration, IgE and
clinical score in atopic dermatitis animal models (16,19).
However, to the best of our knowledge, it is unclear whether DLE
could control the pruritus. Therefore, the present study
investigated the antipruritic effects of DLE and its primary
component MC in reactive astrocytes and an animal model of
histamine-independent itch.
STAT3 is activated by phosphorylation of JAK after
activation of the IL-6 family cytokine receptors (20). However, a recent study reported
that IL-6 induces sustained STAT3 activation in astrocytes via
IP3R1/transient receptor potential cation channel (TRPC)-mediated
Ca2+ signaling (9).
Activation of STAT3 in astrocytes increases expression of LCN2 and
enhances pruritus via activation of GRP/GRPR signaling (21). The present study showed that DLE
and MC treatment decreased expression of STAT3, IP3R1 and LCN2 in
IL-6-stimulated astrocytes. The present results indicated that DLE
and MC suppressed pruritus by suppressing the expression of LCN2,
which suggested that the antipruritic effect of DLE and MC may be
associated with IP3R1/TRPC channel inhibition. Furthermore, based
on previous reports of symptom control through the inhibition of
receptors by natural substances in allergic disease (22,23),
it is hypothesized that DLE and MC could serve as effective itch
relievers.
The present study used a mouse model of
chloroquine-induced pruritus to investigate the in vivo
antipruritic effect of DLE and MC. Chloroquine induces itch through
the GRP/GRPR signaling pathway and this is not dependent on the
histamine release (24,25). Therefore, chloroquine is a suitable
tool to induce histamine-independent pruritus. The present results
showed a decrease in scratching behavior in mice with
chloroquine-induced pruritus following treatment with DLE or MC.
Considering that pruritus occurred through GRP/GRPR signaling due
to subcutaneous injection of chloroquine, the inhibitory effect of
DLE and MC in mice was hypothesized to be related to LCN2
inhibitory effect that was observed in vitro.
GFAP is a target gene for STAT3 and is often
investigated to determine the reactive state of astrocytes
(12,26). Reactive astrocytes have been
reported in chronic pain caused by nerve damage; studies reporting
an increased expression of GFAP in SDH in pruritus-induced mice
demonstrated that this is also an important indicator for pruritus
(9,21,27).
Thus, decreasing or knocking down expression of GFAP might be
beneficial in ameliorating pruritus sensation. In the present
study, DLE and MC inhibited the expression of GFAP both in
vivo and in vitro. The inhibitory effect of GFAP
expression in cells was hypothesized to be due to the inhibition of
STAT3 expression. However, the present study did not find
conclusive evidence that the GFAP inhibitory effect of DLE and MC
in vivo was the result of STAT3 inhibition. Therefore, it is
necessary to clarify if DLE and MC inhibit STAT3 expression in a
chronic itch model in a future study.
Our previous study confirmed that MC, myricetin,
myricetin-3-O-galactoside, gallic acid and astragalin are present
in DLE; of these, the dominant component is MC and its content is
45 mg/g (28). On this basis, it
was estimated that 100 µg/ml DLE used in the present study
contained ~4.5 µg MC. This concentration was lower than the 10 and
20 µg/ml MC used in the study. Despite this, the results suggested
that the antipruritic effect of DLE was not solely attributed to MC
as a concentration of 100 µg/ml DLE showed improved performance
compared with that 20 µg/ml MC. Further studies are necessary to
investigate the components of DLE and their mechanisms of
action.
In conclusion, the present results demonstrated that
DLE and its primary component, MC, suppressed expression of LCN2
through inhibition of IP3R1 and STAT3 activation in astrocytes.
Furthermore, in chloroquine-injected mice, it was confirmed that
administration of DLE and MC ameliorated scratching behavior and
inhibited SDH astrocyte activity. The results of this study suggest
that DLE and MC may be suitable candidates as oral therapeutic
agents for the treatment and management of pruritus.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National
Research Foundation of Korea Grant funded by the Korean Government
(approval nos. NRF-2019R1F1A1060332 and NRF-2022R1F1A1064419).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JS made major contributions to the study design and
manuscript writing. BC was involved in the study conceptualization,
manuscript review and editing. JP and EK conducted formal analysis
and investigation. YK and SJ contributed to the study
conceptualization, data curation, manuscript review and editing,
and project management. All authors have read and approved the
final manuscript. BC and SJ confirm the authenticity of all the raw
data
Ethics approval and consent to
participate
The present study was approved by Jeonju University
Institutional Animal Care and Use Committee (approval no.
JJU-IACUC-2018-3).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Savin JA: How should we define itching? J
Am Acad Dermatol. 39:268–269. 1998.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Erturk IE, Arican O, Omurlu IK and Sut N:
Effect of the pruritus on the quality of life: A preliminary study.
Ann Dermatol. 24:406–412. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ikoma A, Steinhoff M, Ständer S,
Yosipovitch G and Schmelz M: The neurobiology of itch. Nat Rev
Neurosci. 7:535–547. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
4
|
Dhand A and Aminoff MJ: The neurology of
itch. Brain. 137:313–322. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ikoma A: Updated neurophysiology of itch.
Biol Pharm Bull. 36:1235–1240. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Jensen CJ, Massie A and De Keyser J:
Immune players in the CNS: The astrocyte. J Neuroimmune Pharmacol.
8:824–839. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tsuda M: Spinal dorsal horn astrocytes:
New players in chronic itch. Allergol Int. 66:31–35.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shiratori-Hayashi M and Tsuda M: Spinal
glial cells in itch modulation. Pharmacol Res Perspect.
9(e00754)2021.PubMed/NCBI View
Article : Google Scholar
|
|
9
|
Shiratori-Hayashi M, Yamaguchi C, Eguchi
K, Shiraishi Y, Kohno K, Mikoshiba K, Inoue K, Nishida M and Tsuda
M: Astrocytic STAT3 activation and chronic itch require
IP3R1/TRPC-dependent Ca2+ signals in mice. J
Allergy Clin Immunol. 147:1341–1353. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Koga K, Yamagata R, Kohno K, Yamane T,
Shiratori-Hayashi M, Kohro Y, Tozaki-Saitoh H and Tsuda M:
Sensitization of spinal itch transmission neurons in a mouse model
of chronic itch requires an astrocytic factor. J Allergy Clin
Immunol. 145:183–191.e10. 2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Sriram K, Benkovic SA, Hebert MA, Miller
DB and O'Callaghan JP: Induction of gp130-related cytokines and
activation of JAK2/STAT3 pathway in astrocytes precedes
up-regulation of glial fibrillary acidic protein in the
1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine model of
neurodegeneration: Key signaling pathway for astrogliosis in vivo?
J Biol Chem. 279:19936–19947. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ceyzériat K, Abjean L, Carrillo-de Sauvage
MA, Ben Haim L and Escartin C: The complex STATes of astrocyte
reactivity: How are they controlled by the JAK-STAT3 pathway?
Neuroscience. 330:205–218. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Moghaddam AH, Nabavi SM, Nabavi SF,
Bigdellou R, Mohammadzadeh S and Ebrahimzadeh MA: Antioxidant,
antihemolytic and nephroprotective activity of aqueous extract of
Diospyros lotus seeds. Acta Pol Pharm. 69:687–692. 2012.PubMed/NCBI
|
|
14
|
Rauf A, Abu-Izneid T, Alhumaydhi FA,
Muhammad N, Aljohani ASM, Naz S, Bawazeer S, Wadood A and Mubarak
MS: In vivo analgesic, anti-inflammatory, and sedative activity and
a molecular docking study of dinaphthodiospyrol G isolated from
Diospyros lotus. BMC Complement Med Ther. 20(237)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cho BO, Yin HH, Park SH, Byun EB, Ha HY
and Jang SI: Anti-inflammatory activity of myricetin from Diospyros
lotus through suppression of NF-κB and STAT1 activation and
Nrf2-mediated HO-1 induction in lipopolysaccharide-stimulated
RAW264.7 macrophages. Biosci Biotechnol Biochem. 80:1520–1530.
2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cho BO, Che DN, Yin HH, Shin JY and Jang
SI: Diospyros lotus leaf and grapefruit stem extract
synergistically ameliorate atopic dermatitis-like skin lesion in
mice by suppressing infiltration of mast cells in skin lesions.
Biomed Pharmacother. 89:819–826. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kim BM, Cho BO and Jang SI: Anti-obesity
effects of Diospyros lotus leaf extract in mice with high-fat
diet-induced obesity. Int J Mol Med. 43:603–613. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jeonju University Institutional Animal
Care and Use Committee: Animal Testing Ethics. https://www.jj.ac.kr/sanhak/ethics/iacuc.jsp. Accessed
May 3, 2023.
|
|
19
|
Cho BO, Shin JY, Kim JS, Che DN, Kang HJ,
Kang HJ, Oh H and Kim YS: Enzyme-treated date plum leave extract
ameliorates atopic dermatitis-like skin lesion in hairless mice.
Asian Pac J Trop Biomed. 10(239)2020.
|
|
20
|
Ivashkiv LB and Hu X: Signaling by stats.
Arthritis Res Ther. 6:1–10. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
21
|
Shiratori-Hayashi M, Koga K, Tozaki-Saitoh
H, Kohro Y, Toyonaga H, Yamaguchi C, Hasegawa A, Nakahara T,
Hachisuka J, Akira S, et al: STAT3-dependent reactive astrogliosis
in the spinal dorsal horn underlies chronic itch. Nat Med.
21:927–931. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Lundstrom K, Pham HT and Dinh LD:
Interaction of plant extracts with central nervous system
receptors. Medicines. 4(12)2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Roschek B Jr, Fink RC, McMichael M and
Alberte RS: Nettle extract (Urtica dioica) affects key receptors
and enzymes associated with allergic rhinitis. Phytother Res.
23:920–926. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Sun YG and Chen ZF: A gastrin-releasing
peptide receptor mediates the itch sensation in the spinal cord.
Nature. 448:700–703. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu Q, Tang Z, Surdenikova L, Kim S, Patel
KN, Kim A, Ru F, Guan Y, Weng HJ, Geng Y, et al: Sensory
neuron-specific GPCR Mrgprs are itch receptors mediating
chloroquine-induced pruritus. Cell. 139:1353–1365. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
O'Callaghan JP, Kelly KA, VanGilder RL,
Sofroniew MV and Miller DB: . Early activation of STAT3 regulates
reactive astrogliosis induced by diverse forms of neurotoxicity.
PLoS One. 9(e102003)2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Green D and Dong X: Supporting itch: A new
role for astrocytes in chronic itch. Nat Med. 21:841–842.
2015.PubMed/NCBI View
Article : Google Scholar
|
|
28
|
Cho BO, Che DN, Shin JY, Kang HJ, Kim JH,
Kim HY, Cho WG and Jang SI: Ameliorative effects of Diospyros lotus
leaf extract against UVB-induced skin damage in BALB/c mice. Biomed
Pharmacother. 95:264–274. 2017.PubMed/NCBI View Article : Google Scholar
|