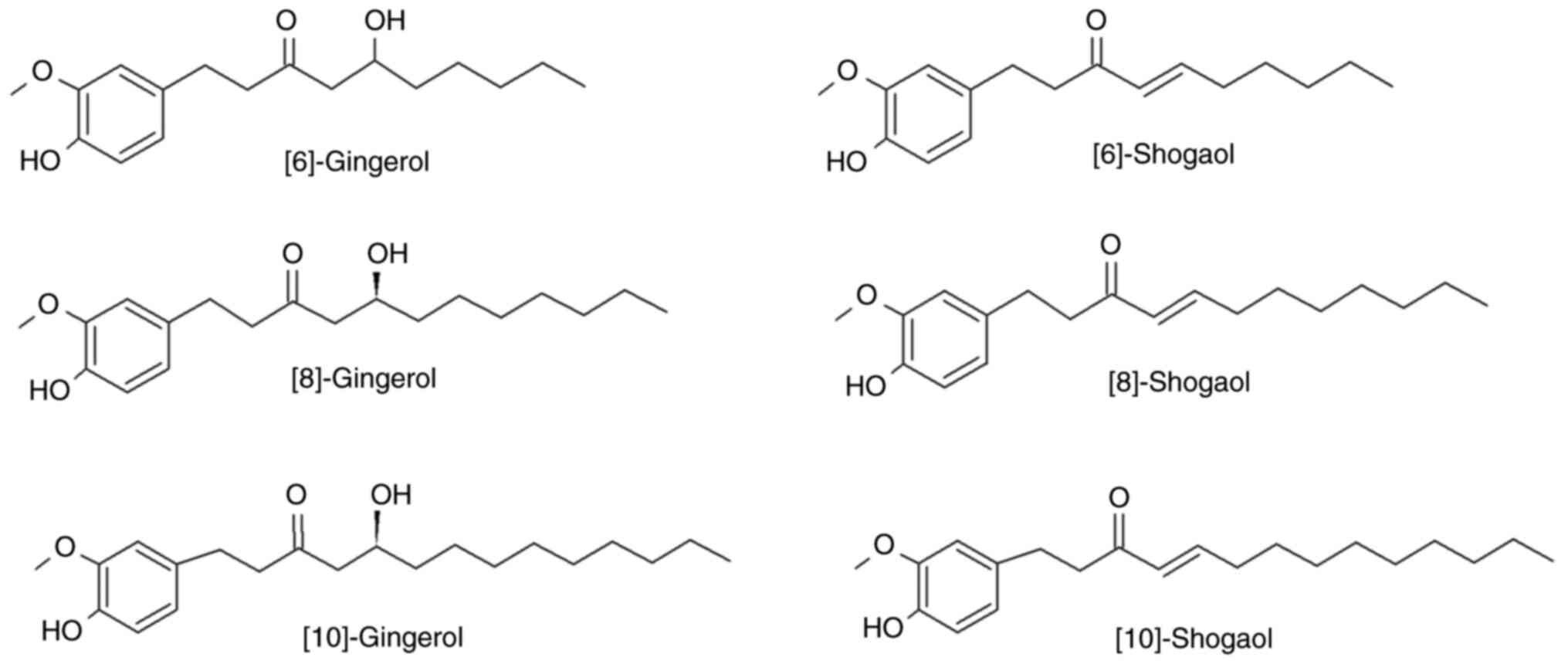
Introduction
The prevalence of obesity has increased rapidly.
According to the World Health Organization (WHO), obesity rates
have increased 3-fold in adults and 5-fold in children and
adolescents from 1975 to 2016(1).
Physiologically, obesity is the result of an excessive increase of
adipose tissue in the body, which is a process related to
hyperplasia and hypertrophy in adipocytes (2). The differentiation from preadipocytes
into adipocytes to store lipid droplets is called adipogenesis, a
process regulated by the activation and expression of different
genes that participate in both the early and late differentiation
stages, such as peroxisome proliferator-activated receptor-γ
(PPAR-γ), CAAT enhancer binding protein α (C/EBPα), acetyl-coenzyme
A carboxylase (ACACA), fatty acid synthase (FASN) and fatty acid
binding protein 4 (FABP4) (3,4).
Fatty tissue and adipocytes are a research focus for treatment of
metabolic diseases (5).
Different treatment options were proposed for the
control and reduction of adiposity. The first-line intervention
includes diet modification, limiting the consumption of total fats
and sugars and increasing the consumption of fruits, vegetables,
legumes, whole grains and nuts, and increased physical activity
(1). However, some patients need
to complement this intervention with pharmacological therapy.
Despite the benefits of the anti-obesity drugs, such as orlistat,
lorcaserin, liraglutide, or phentermine-topiramate, some important
barriers exist to widespread application, including high financial
burden and a wide number of reported side effects like nausea,
vomiting, constipation, hypoglycemia, diarrhea, headache, and
abdominal pain (2). For these
reasons, some bioactive food compounds have attracted attention in
the treatment of obesity, such as capsaicin from chili or caffeine
and ephedrine from guarana due to their properties that activate
vasodilator and endorphin-releasing nerve signals, or thermal
properties, which they increase energy and reduce weight,
respectively (6-9).
Therefore, it is necessary to continue the search and validation of
active compounds that could be used in the treatment of
obesity.
Ginger (Zingiber officinale Roscoe) is a
medicinal plant with various beneficial effects, including
anti-inflammatory, antioxidant, anti-nausea, antiemetic,
lipid-lowering, and recently, the anti-obesogenic effect (10-12).
Ginger contains a variety of phenolic compounds, with the most
notable being gingerols, shogaols and paradols (13). Gingerols constitute the majority of
phenolic compounds in fresh ginger and include 6-, along with 4-,
5-, 8-, 10- and 12-gingerols (14). Gingerols are thermally labile due
to the presence of a β-hydroxy keto group and they are converted
under high temperature to the corresponding shogaols (15). Gingerols (23-25%) and shogaols
(18-25%) are the main bioactive constituents of ginger (14) and potential mediators of its major
therapeutic effects (16).
Some studies demonstrated the anti-adipogenic and
lipolytic effects of these phenols individually. The ability of
6-gingerol to inhibit adipocyte hypertrophy and hyperplasia both
in vitro and in vivo was clearly demonstrated
(17-20).
Suk et al (10,11) showed that treatment with 40 µM of
either 6-shogaol, 8- or 10-gingerol decrease the content of lipids
in 3T3-L1 adipocytes, which is a murine embryonic preadipocyte cell
line widely used for adipogenesis research. Even 6-shogaol was used
in clinical trials where mean body weight, BMI and body fat level
were lower in the group that received capsules of steamed ginger
ethanolic extract rich in 6-shogaol than in the placebo group
(21). However, to the best of our
knowledge, there are no studies that evaluated the anti-obesogenic
effect of the 6-, 8- and 10-gingerol plus 6-, 8- and 10-shogaol on
adipose tissue. Therefore, the current study aimed to evaluate the
lipolytic and anti-adipogenic activity of a mix of the main ginger
phenols in the 3T3-L1 cell line.
Materials and methods
3T3-L1 cell culture and
differentiation
Mouse 3T3-L1 preadipocytes were obtained from the
Immunology Laboratory of the University Center of Health Sciences,
University of Guadalajara, Mexico. Cells were cultured in DMEM
(cat. no. SIG-D6429; Merck KGaA) supplemented with 10% Bovine Calf
Serum (cat. no. 12389812; HyClone; Cytiva) and 1% antibiotics (100
U/ml penicillin and 100 µg/ml streptomycin; cat. no. 15140122;
Thermo Fisher Scientific, Inc.). (maintenance medium) at 37˚C and
5% CO2 in a cell incubator (cat. no. SCO6AD; Sheldon
Manufacturing, Inc.). The cells were seeded in Petri dishes at a
density of 1x105 cells/dish and the maintenance medium
was replaced every 2-3 days until the cells reached 100%
confluence. At 1 day post-confluence (day 0), medium was
substituted with DMEM/F-12 (cat. no. 21041025; Thermo Fisher
Scientific, Inc.) supplemented with 10% FBS (cat. no. 16000044;
Thermo Fisher Scientific, Inc.), 1% antibiotics and an adipogenic
cocktail including 500 µM 3-isobutyl-1-methylxanthine (IBMX), 1 µM
dexamethasone, 1.5 µg/ml insulin and 1 µM rosiglitazone (3T3-L1
Differentiation Kit; cat. no. SIG-DIF001-1KT; Merck KGaA) for 3
days, according to the manufacturer's instructions. On day 3, the
medium was replaced with DMEM/F-12 supplemented (cat. no. 21041025;
Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (cat. no.
16000044; Thermo Fisher Scientific, Inc.), 1% antibiotics and an
adipogenic cocktail including 500 µM IBMX, 1 µM dexamethasone, 1.5
µg/ml insulin and 1 µM rosiglitazone (3T3-L1 Differentiation Kit;
cat. no. SIG-DIF001-1KT; Merck KGaA) for 3 days. On day 3, the
medium was replaced with DMEM supplemented with 10% FBS, 1%
antibiotics and 5 µg/ml insulin, and the cells were cultured for 5
days. The total duration of adipogenesis was 8 days from the
induction of differentiation with adipogenic cocktail and the cells
were incubated at 37˚C and 5% CO2.
Mix of the main ginger phenols
A solution from ginger gingerols and shogaols mix
was acquired from Merck KGaA (cat. no. SIG-G-027-1ML). The solution
contained the main phenols of ginger (6-, 8- and 10-gingerol and
6-, 8- and 10-shogaol) at a standard concentration of 500 µg/ml for
every component (Fig. 1).
3T3-L1 cell treatment
A dose-response curve was generated using 1, 2, 3, 4
and 5 µg/ml phenol-mix during the adipogenesis process (8 days) and
in mature 3T3-L1 adipocytes (48 h) to determine the dose to be used
in subsequent experiments. A total of four study groups were
formed: i) Negative control (3T3-L1 preadipocytes); ii) positive
control (mature 3T3-L1 adipocytes); iii) phenols-pre; and iv)
phenols-post groups. In the phenols-pre group, confluent
preadipocytes were incubated with phenol-mix until mature
adipocytes were formed (day 8). In the phenols-post group, after 8
days of differentiation, cells were treated for a period of 48 h
with phenol-mix. After the treatment with the phenol-mix,
supernatants were collected in tubes and stored at -80˚C; the
3T3-L1 adipocytes were cryopreserved for 1 month. No prior
treatment was performed on the samples at the time of its use in
the experimental analysis.
MTT assay
3T3-L1 preadipocytes were seeded at 5x103
cells/well in 96-well plates (cat. no. 701001; Nest Scientific USA
Inc.). Cells were differentiated as aforementioned in the presence
or absence of the mix of main ginger phenols. After 8 (phenols-pre
group) and 10 days (phenols-post group), 1 mg/ml of MTT (cat. no.
M6494; Thermo Fisher Scientific, Inc.) solution was added and cells
were incubated for 1 h at 37˚C. The dark blue formazan crystals
were dissolved in an extraction buffer containing 20% SDS and 50%
dimethylformamide. Absorbance at 570 nm was measured using a
microplate reader (Multiskan™ GO; cat. no. 51119300;
Thermo Fisher Scientific, Inc.).
Oil Red O staining
3T3-L1 preadipocytes were differentiated as
aforementioned in the presence or absence of the mix of main ginger
phenols. Fully differentiated cells were fixed with 10% (v/v)
formaldehyde solution for 60 min at room temperature and washed 3
times with distilled water. Lipid droplets in mature adipocytes
were then stained with Oil red O (cat. no. O0625; Merck KGaA)
solution for 15 min and washed 3 times with distilled water. The
stained Oil Red O was then dissolved in 100% isopropanol (cat. no.
IB15730; IBI Scientific) and the absorbance was measured at 515 nm
using a microplate reader (Multiskan GO; cat. no. 51119300; Thermo
Fisher Scientific, Inc.).
Determination of glycerol release
Glycerol quantification in supernatants was
performed using the VITROS 350 Chemistry System (QuidelOrtho
Corporation) with TRIG slides (cat. no. OCD-MS-1336544; QuidelOrtho
Corporation), according to the manufacturer's instructions.
mRNA expression quantification using
reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from the study groups with
the RNeasy Mini Kit (cat. no. 74104; Qiagen, Inc.) following the
manufacturer's instructions. Total RNA (1 µg ) was reverse
transcribed into cDNA using the M-MLV Reverse Transcriptase (cat.
no. 28025013; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. The mRNA expression was quantified using
qPCR (LightCycler 96 thermocycler; Roche Diagnostics) using the
OneTaq® Hot Start Master Mix (cat. no. N01-M0484L; New
England Biolabs, Ltd.) and the following TaqMan®
Real-Time PCR Assays for Rn18s (cat. no. Mm03928990_g1), PPAR-γ
(cat. no. Mm00440940_m1), C/EBPα (cat. no. Mm00514283_s1), ACACA
(cat. no. Mm01304257_m1), FASN (cat. no. Mm00662319_m1) and FABP4
(cat. no. Mm00445878_m1), all from Thermo Fisher Scientific, Inc.
The following thermocycling conditions for qPCR were used: Initial
preincubation at 95˚C for 300 sec; 30 cycles of denaturation at
95˚C for 20 sec and amplification at 60˚C for 60 sec; and 1 cycle
of extension at 68˚C for 300 sec. The relative gene expression was
quantified using the 2-ΔΔCq method (19) and normalized against the expression
level of 18S as an internal reference gene. Each analysis and
quantification were independently repeated 3 times.
Statistical analysis
Experimental analyses were performed in triplicate.
Data are expressed as mean ± SD. Differences among groups were
evaluated using one-way ANOVA followed by Tukey's post hoc test.
Data were analyzed using SPSS (version 25; IBM Corp.).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Cell viability and lipid content
dose-response curves with phenol-mix
A dose-response curve with phenol-mix was performed
in the 3T3-L1 cell line to establish the treatment dose.
Subsequently, cell viability was evaluated with the MTT assay and
lipid content with Oil Red O staining. Treatment of immature 3T3-L1
cells with ≥3 µg/ml phenol-mix during the differentiation process
significantly decreased the percentage of living cells (P<0.01;
Fig. 2A); these concentrations
were excluded from subsequent experiments. However, treatment with
the phenols mix did not significantly decrease the cell viability
of mature 3T3-L1 adipocytes (Fig.
2B). Regarding the lipid content, the 3T3-L1 cells treated with
phenol-mix during the adipogenesis process showed a decreased
percentage of lipids compared with that in the positive control
group at every concentration (P<0.001). Particularly, the 2
µg/ml dose lead to the greatest reduction in lipid content compared
to the positive control group (P<0.001; Fig. 3A). Similarly, the mature 3T3-L1
adipocytes treated with 2, 4 (both P<0.01) and 5 µg/ml
(P<0.001) phenol-mix presented a significantly lower percentage
of lipids than the positive control group (Fig. 3B).
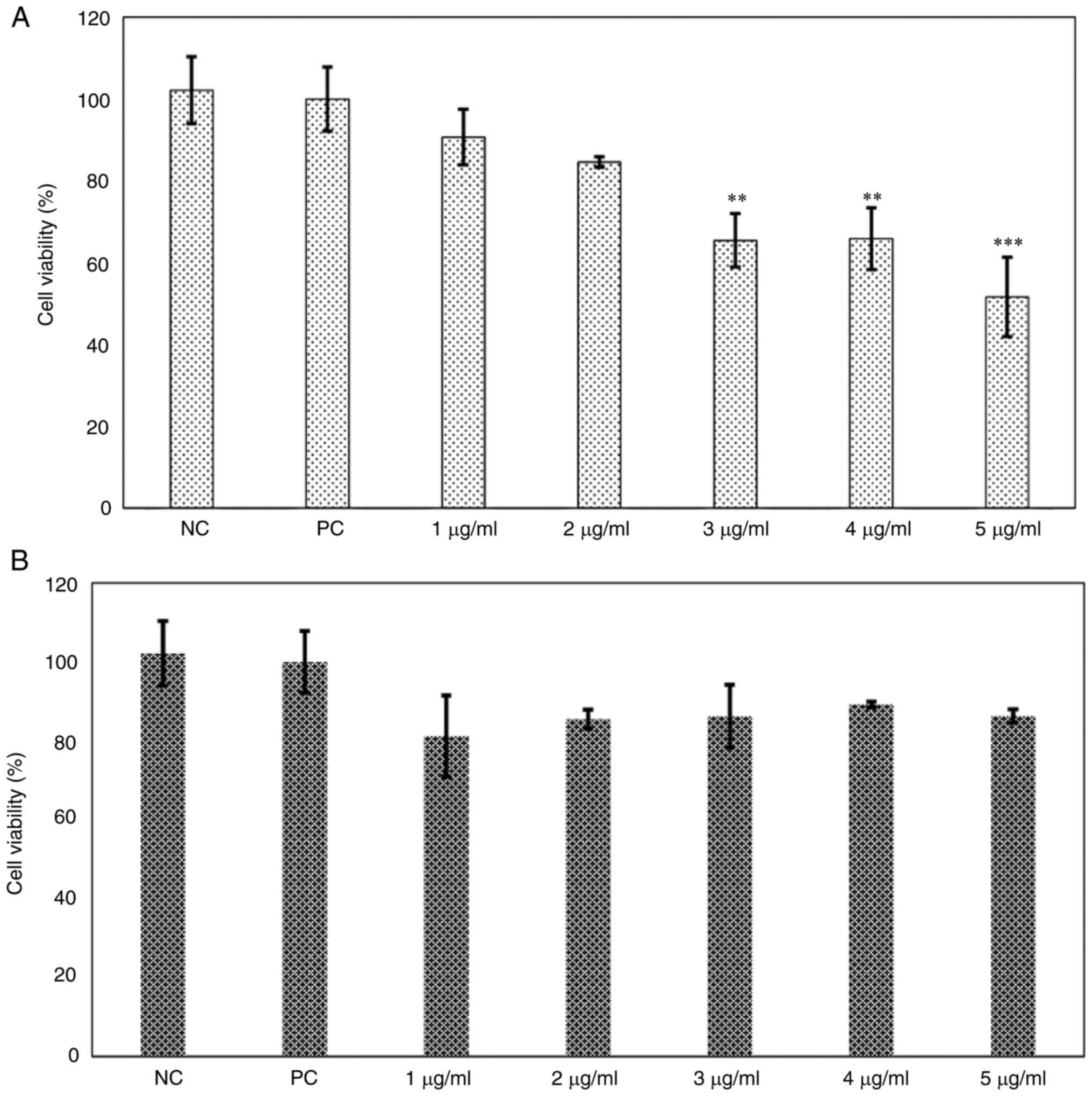 | Figure 2Cell viability dose-response curve of
phenol-mix. (A) 3T3-L1 cells were treated with the phenol-mix at 1,
2, 3, 4 and 5 µg/ml during adipogenic differentiation and cell
viability was determined using MTT assay. (B) Mature 3T3-L1
adipocytes were treated with the phenol-mix at 1,2,3,4 and 5 µg/ml
for 48 h and cell viability was measured using MTT assay. Each bar
represents the mean ± standard deviation of three experiments.
**P<0.01 and ***P<0.001 vs. positive
control. NC, negative control (3T3-L1 cells); PC, positive control
(mature 3T3-L1 adipocytes); phenols-pre, 3T3-L1 cells stimulated
with the phenols mix during adipogenic differentiation;
phenols-post, mature 3T3-L1 adipocytes stimulated with the phenols
mix. |
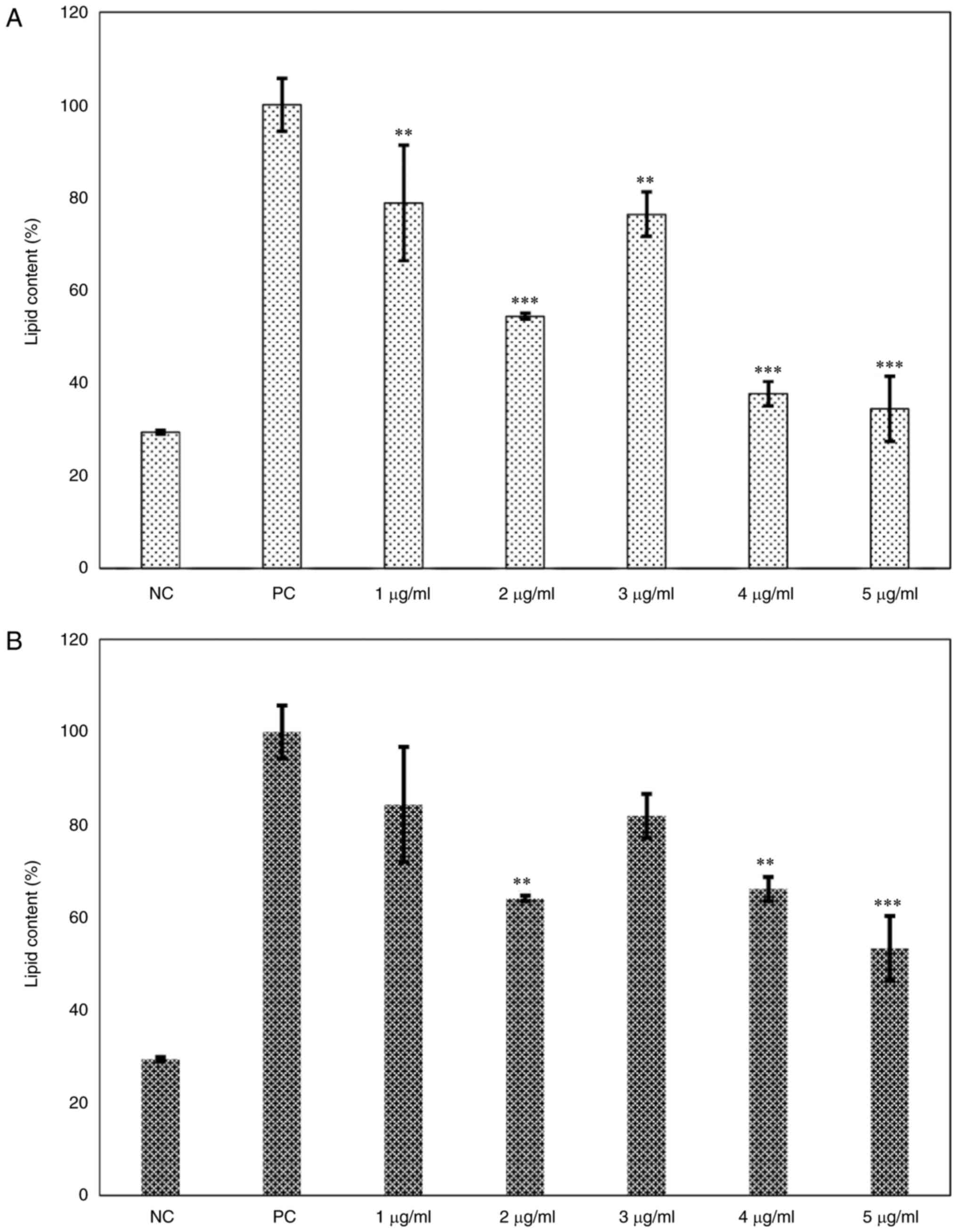 | Figure 3Dose-response curve of the main
ginger phenols mix effects on lipid content. (A) 3T3-L1 cells were
treated with the mix of the main ginger phenols at various
concentrations (1,2,3,4 and 5 µg/ml) during adipogenic
differentiation and lipid content was measured. (B) Mature 3T3-L1
adipocytes were treated with the mix of the main ginger phenols at
various concentrations (1,2,3,4 and 5 µg/ml) for 48 h and lipid
content was measured. Each bar represents the mean ± standard
deviation of three experiments. **P<0.01 and
***P<0.001 vs. positive control. NC, negative control
(3T3-L1 cells); PC, positive control (mature 3T3-L1 adipocytes);
phenols-pre, 3T3-L1 cells stimulated with the phenols mix during
adipogenic differentiation; phenols-post, mature 3T3-L1 adipocytes
stimulated with the phenols mix. |
Treatment with the 2 µg/ml dose both during
adipogenesis and in mature 3T3-L1 adipocytes showed the greatest
reduction of lipid content (P<0.001 and P<0.01, respectively)
without affecting cell viability (P=0.812). For this reason, the 2
µg/ml treatment dose was chosen for the following experiments.
Effects of the phenol-mix on cell
viability and adipocyte differentiation
After determining the optimal treatment dose (2
µg/ml), the negative control, positive control, phenols-pre and
phenols-post study groups were formed to analyze the differences in
cell viability and adipocyte differentiation with or without
treatment with phenol-mix. The percentage of viable cells was not
affected by the treatment with 2 µg/ml in both phenols-pre
(P=0.275) and phenols-post (P=0.710) groups compared with that in
the positive control group (Fig.
4A). Notably, the treatment with 2 µg/ml induced a reduction of
the lipid content by 45.52±7.8 and 35.95±0.76% in the phenols-pre
and -post groups, respectively, compared with that in the positive
control group (P<0.001; Fig.
4B). Oil red O staining revealed lipid content differences
between groups. As expected, negative control 3T3-L1 cells showed
no staining, while high staining was observed in the positive
control (PC; mature 3T3-L1 adipocytes) which is indicative that
adipogenic differentiation has occurred successfully. Finally, in
the groups treated with the phenol-mix during and after
adipogenesis, a qualitative decrease of the dye is observed in
comparison with PC, which is an indirect indicator of a lower
amount of stored lipids (Fig.
4C).
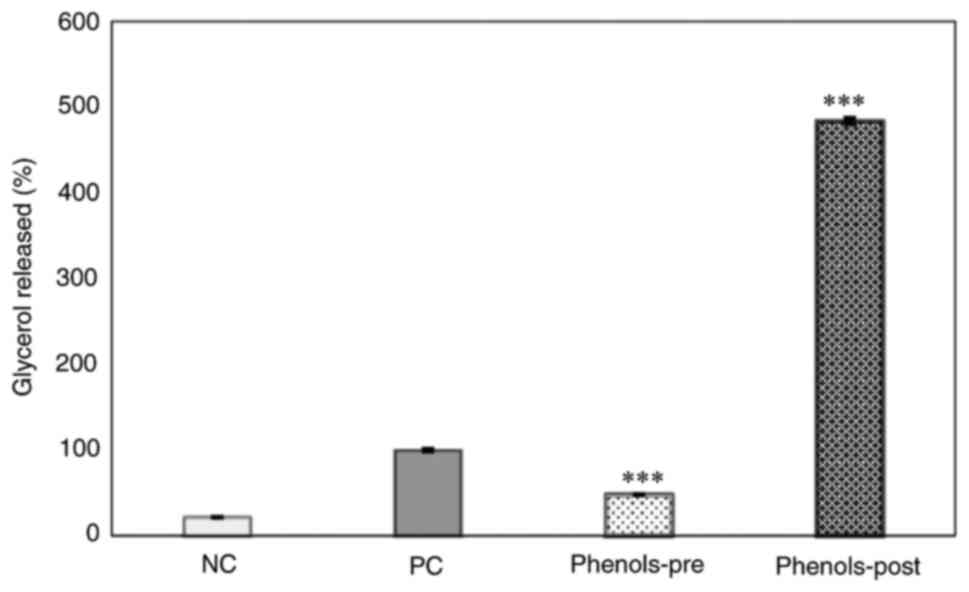
Effects of phenol-mix on glycerol
release from mature 3T3-L1 adipocytes
The glycerol content in the supernatant was measured
as a marker of lipolysis in the four study groups. Results showed
that the phenols-pre group released a lower glycerol amount than
that of the positive control group (P<0.001). Conversely, the
phenols-post group presented a higher glycerol concentration in the
supernatant compared with that in the positive control (P<0.001)
and was numerically increased compared with that in the phenols-pre
group (Fig. 5).
Effects of phenol-mix on proadipogenic
and lipogenic genes in 3T3-L1 adipocytes
Finally, the expression of genes related to the
regulation of adipogenesis and lipogenesis in the study groups was
analyzed. The phenols-pre group showed higher expression at the
mRNA level of C/EBPα, PPAR-γ, FABP4, ACACA and FASN than that of
the positive control group (P<0.05; Fig. 6A-E). By contrast, the phenols-post
group presented a significantly lower expression of C/EBPα, FABP4
and FASN compared with that in the positive control group
(P<0.001; Fig. 6A, C and E).
The expression of PPAR-γ and ACACA was not significantly different
compared with that in the positive control (Fig. 6D).
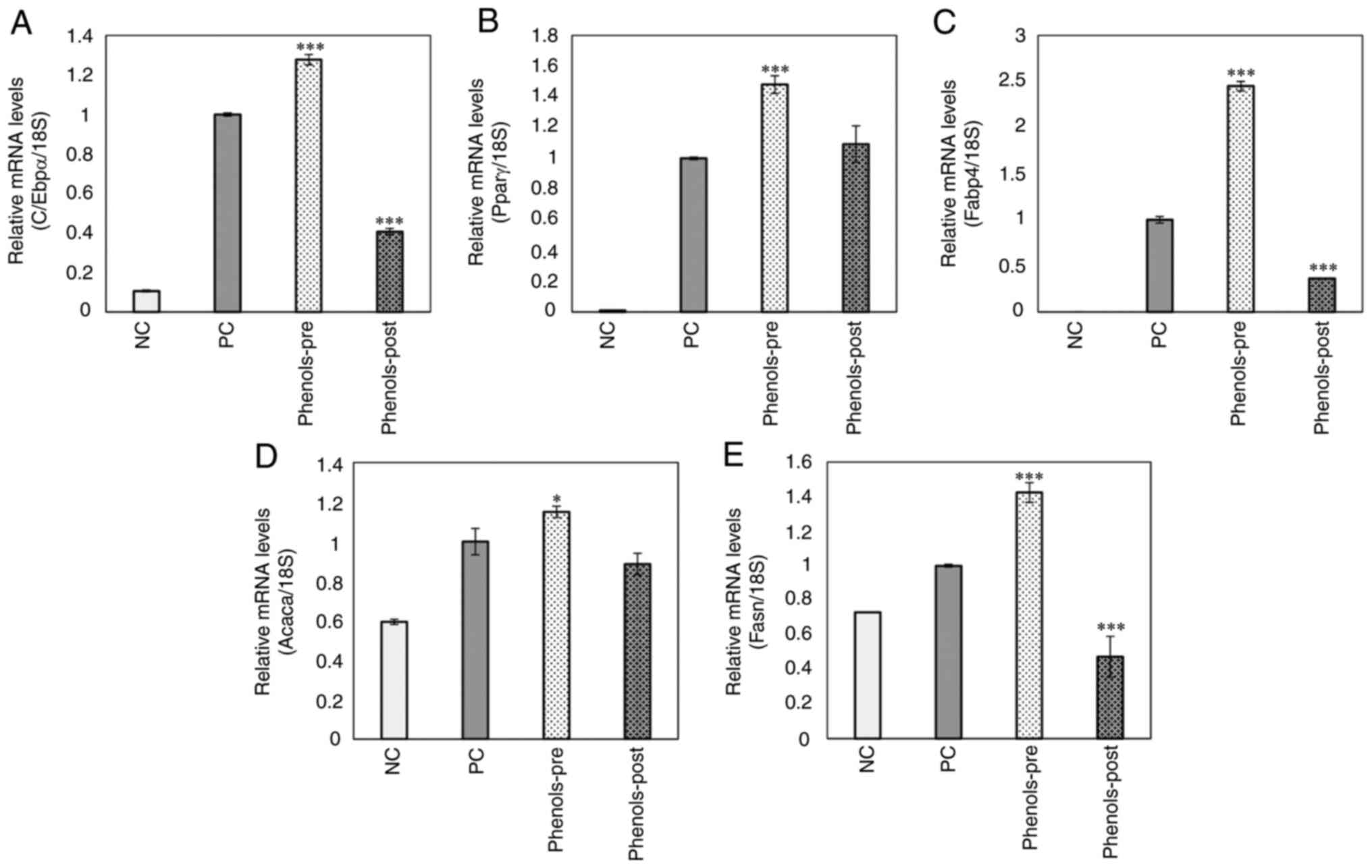 | Figure 6Effects of the main ginger phenols
mix on pro-adipogenic and -lipogenic genes expression. (A) CCAAT
enhancer-binding protein α, (B) peroxisome proliferator-activated
receptor-γ, (C) fatty acid binding protein 4, (D) acetyl-coenzyme A
carboxylase and (E) fatty acid synthase mRNA levels in 3T3-L1
mature adipocytes were examined using reverse
transcription-quantitative PCR. Each bar represents the mean ±
standard deviation of three experiments. *P<0.05 and
***P<0.001 vs positive control. ACACA,
acetyl-coenzyme A carboxylase; C/EBPα, CCAAT enhancer-binding
protein α; FABP4, fatty acid binding protein 4; FASN, fatty acid
synthase; PPAR-γ, peroxisome proliferator-activated receptor-γ; NC,
negative control (3T3-L1 cells); PC, positive control (mature
3T3-L1 adipocytes); phenols-pre, 3T3-L1 cells stimulated with the
phenols mix during adipogenic differentiation; phenols-post, mature
3T3-L1 adipocytes stimulated with the phenols mix. |
Discussion
This study demonstrated that the six main phenols
contained in ginger, i.e. 6-, 8-, 10-gingerol and 6-, 8- and
10-shogaol, could decrease the lipid content in both 3T3-L1 cells
undergoing adipogenic differentiation and in mature adipocytes
derived from this cell line.
Several studies evaluated the ability of
plant-derived extracts to reduce the lipid content in the 3T3-L1
cell line (8,23-26).
These phenols were evaluated individually in different experimental
models that support their antitumor (27,28),
anti-inflammatory (29,30) and anti-obesogenic capacities
(31). Nonetheless, to the best of
our knowledge, there were no previous studies that tested these six
phenols together. Notably, the anti-adipogenic effect of different
gingerols were widely studied in both in vitro and in
vivo models (11,19,20,32-34).
The coordination of the stages of adipocyte differentiation was
shown to be regulated by the sequential activation of
transcriptional factors, including PPAR-γ and various members of
the C/EBP family of transcriptional factors (35). Therefore, the decrease in lipid
content in in vitro model could be attributed to the
suppression of the transcriptional factors that regulate
adipogenesis, i.e. PPAR-γ and C/EBPα (34,36).
However, although the results of the present study in the
phenols-pre group did not reflect the trend observed in other
previous studies, a reduction in the concentration of these
pro-adipogenic markers at the protein level could not be rejected
since studies found discrepancies between the transcriptional and
translational levels of C/EBPα (37,38).
Some studies showed that other natural bioactive compounds could
inhibit the PPAR-γ translocation to the nucleus (23,39,40).
Hence, more studies would be needed to clarify the molecular
mechanism by which gingerols and shogaols decrease the lipid
content during differentiation of 3T3-L1 cells to adipocyte.
Recently, Cheng et al (20) conducted a study using 6-gingerol as
an intervention in a C57BL/6 J high-fat-diet-induced obese mice
that resembled the phenols-post group of the present study. The
authors reported decreases in the expression of PPAR-γ, C/EBPα and
FABP4 from epididymal white adipose tissue both at the
transcriptional and translational levels, consistent with the
results of the current study for the phenols-post group. PPARγ and
C/EBPα were known to form a positive feedback loop with each other
and to promote the expression of genes that encode proteins related
to the adipogenic phenotype (3),
including FABP/aP2 (FABP4 gene), which is induced during adipocyte
differentiation and expressed in adipocytes (41). Therefore, the significant reduction
of these adipogenic markers in the phenol-post group of the present
study indicated that the suppression of adipogenesis could be a key
factor for the anti-obesogenic effect of this group. Noteworthy,
the regulation of the lipid metabolism by these transcriptional
factors could be involved in this process.
The decrease of lipids in mature 3T3-L1 adipocytes
may also be related to the suppression of lipogenesis and lipid
accumulation (31). Some ginger
compounds, specifically 6-shogaol and 6-gingerol, may serve as
PPAR-δ agonists with subsequent effects on energy homeostasis, such
as a reduction of lipid deposition in skeletal muscle and adipose
tissue (42). Moreover, the
mechanisms that mediate the suppression of lipogenesis are
intrinsically linked to the expression levels of lipogenic enzymes
such as acetyl CoA carboxylase (ACC) and fatty acid synthase (FAS)
(43,44). ACC catalyzes the carboxylation of
acetyl-CoA to malonyl-CoA, the rate-limiting step in fatty acid
synthesis; in turn, FAS is involved in energy homeostasis by
converting energy into lipid storage (45). These enzymes are expressed in
adipose tissue at high levels and their expression and activity are
acutely and chronically regulated through transcriptional control
and post-translational modifications are associated with
nutritional status (fasting and feeding) and substrate availability
(46). Results from the present
study revealed significantly decreased expression of FASN in the
phenol-post group, suggesting that the observed decrease in lipid
accumulation in mature adipocytes achieved by these ginger phenols
could be related to the inhibition of the expression of these
lipogenic enzymes. Future in vitro studies should evaluate
the protein expression and activity of FAS and ACC, as well as how
these are regulated by the main bioactive compounds in ginger.
Finally, the current study also showed lipolytic
activity of the mix of phenols derived from ginger on mature 3T3-L1
adipocytes. The lipolytic effect was previously reported for
6-shogaol (11). However, it
should be noted that considerably lower doses were used in the
present study than those reported by previous studies with
individual phenols. For instance, a greater pro-lipolytic effect
was observed in the present study than that achieved by Suk et
al (10,11) who only used 6-shogaol (40 µM), an
observation that also occurred with the anti-obesogenic effect
found in the present study. Therefore, the rest of the phenols
contained in the mix would be responsible for enhancing this
effect. Further studies are required to elucidate the individual
lipolysis-promoting capacities of the rest of the phenols in the
mix.
In conclusion, the present study shows for the first
time the anti-adipogenic and lipolytic effects of a mix of the main
bioactive compounds found in ginger, i.e. 6-, 8-, 10-gingerol and
6-, 8-and 10-shogaol, during the differentiation of 3T3-L1 cells to
adipocytes as well as on mature adipocytes. Future approaches will
need to test the in vivo effects of this mix of ginger
phenols to provide more compressive data that would validate its
use in clinical protocols, as perhaps all or some of them could be
useful for the treatment and prevention of obesity.
Acknowledgements
The authors would like to thank Dr. Trinidad
Garcia-Iglesias (University of Guadalajara, Guadalajara, Mexico)
for having generously donated the 3T3-L1 cell line and the
Biomedical Sciences Research Institute of the University of
Guadalajara for providing equipment used in the present study.
Funding
Funding: This work was partly supported by the University of
Guadalajara (Guadalajara, Mexico; grant no. PIN-2021-II).
Availability of data and materials
The datasets generated and/or analyzed during the
current study are available from the corresponding author upon
reasonable request.
Authors' contributions
JRV and EML contributed to the experimental design.
GGO, MPO and SCRR performed the experiments. WCP and EML performed
the validation of data and statistical analysis. GGO, MPO and JRV
wrote the manuscript. MPO and JRV confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO): Obesity
and Overweight. WHO, Geneva, 2021. https://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight.
Accessed December 12, 2022.
|
|
2
|
Heymsfield SB and Wadden TA: Mechanisms,
pathophysiology, and management of obesity. N Engl J Med.
376:254–266. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ghaben AL and Scherer PE: Adipogenesis and
metabolic health. Nat Rev Mol Cell Biol. 20:242–258.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Farmer SR: Transcriptional control of
adipocyte formation. Cell Metab. 4:263–273. 2006.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bray GA, Kim KK and Wilding JPH: Obesity:
A chronic relapsing progressive disease process. A position
statement of the World Obesity Federation. Obes Rev. 18:715–723.
2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bahmani M, Eftekhari Z, Saki K,
Fazeli-Moghadam E, Jelodari M and Rafieian-Kopaei M: Obesity
phytotherapy: Review of native herbs used in traditional medicine
for obesity. J Evid Based Complementary Altern Med. 21:228–234.
2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yoshioka M, St-Pierre S, Suzuki M and
Tremblay A: Effects of red pepper added to high-fat and
high-carbohydrate meals on energy metabolism and substrate
utilization in Japanese women. Br J Nutr. 80:503–510.
1998.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Oh MJ, Lee HB, Yoo G, Park M, Lee CH, Choi
I and Park HY: Anti-obesity effects of red pepper (Capsicum
annuum L.) leaf extract on 3T3-L1 preadipocytes and high fat
diet-fed mice. Food Funct. 14:292–304. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Boozer CN, Nasser JA, Heymsfield SB, Wang
V, Chen G and Solomon JL: An herbal supplement containing Ma
Huang-Guarana for weight loss: A randomized, double-blind trial.
Int J Obes Relat Metab Disord. 25:316–324. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Suk S, Kwon GT, Lee E, Jang WJ, Yang H,
Kim JH, Thimmegowda NR, Chung MY, Kwon JY, Yang S, et al:
Gingerenone A, a polyphenol present in ginger, suppresses obesity
and adipose tissue inflammation in high-fat diet-fed mice. Mol Nutr
Food Res. 61(1700139)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Suk S, Seo SG, Yu JG and Yang H: A
bioactive constituent of ginger, 6-shogaol, prevents adipogenesis
and stimulates lipolysis in 3T3-L1 adipocytes. J Food Biochem.
40:84–90. 2016.
|
|
12
|
Mao QQ, Xu XY, Cao SY, Gan RY, Corke H,
Beta T and Li HB: Bioactive compounds and bioactivities of ginger
(Zingiber officinale roscoe). Foods. 8:1–21. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Y, Liu J and Zhang Y: Research
progress on chemical constituents of zingiber officinale roscoe.
Biomed Res Int. 2019(5370823)2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yücel Ç, Karatoprak GŞ, Açıkara ÖB, Akkol
EK, Barak TH, Sobarzo-Sánchez E, Aschner M and Shirooie S:
Immunomodulatory and anti-inflammatory therapeutic potential of
gingerols and their nanoformulations. Front Pharmacol.
13(902551)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ali BH, Blunden G, Tanira MO and Nemmar A:
Some phytochemical, pharmacological and toxicological properties of
ginger (Zingiber officinale Roscoe): A review of recent
research. Food Chem Toxicol. 46:409–420. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Baliga MS, Haniadka R, Pereira MM, D'Souza
JJ, Pallaty PL, Bhat HP and Popuri S: Update on the chemopreventive
effects of ginger and its phytochemicals. Crit Rev Food Sci Nutr.
51:499–523. 2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Seo EY: Effects of (6)-gingerol, ginger
component on adipocyte development and differentiation in 3T3-L1. J
Nutr Heal. 48:327–334. 2015.
|
|
18
|
Tzeng TF, Chang CJ and Liu IM: 6-gingerol
inhibits rosiglitazone-induced adipogenesis in 3T3-L1 adipocytes.
Phyther Res. 28:187–192. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Li C and Zhou L: Inhibitory effect
6-gingerol on adipogenesis through activation of the Wnt/β-catenin
signaling pathway in 3T3-L1 adipocytes. Toxicol Vitr. 30:394–401.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Cheng Z, Xiong X, Zhou Y, Wu F, Shao Q,
Dong R, Liu Q and Li L: 6-gingerol ameliorates metabolic disorders
by inhibiting hypertrophy and hyperplasia of adipocytes in
high-fat-diet induced obese mice. Biomed Pharmacother.
146(112491)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Park SH, Jung SJ, Choi EK, Ha KC, Baek HI,
Park YK, Han KH, Jeong SY, Oh JH, Cha YS, et al: The effects of
steamed ginger ethanolic extract on weight and body fat loss: a
randomized, double-blind, placebo-controlled clinical trial. Food
Sci Biotechnol. 29:265–273. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim EJ, Kang MJ, Seo YB, Nam SW and Kim
GD: Acer okamotoanum nakai leaf extract inhibits adipogenesis via
suppressing expression of PPAR γ and C/EBP α in 3T3-L1 cells. J
Microbiol Biotechnol. 28:1645–1653. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Oh MJ, Lee HHL, Lee HB, Do MH, Park M, Lee
CH and Park HY: A water soluble extract of radish greens
ameliorates high fat diet-induced obesity in mice and inhibits
adipogenesis in preadipocytes. Food Funct. 13:7494–7506.
2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Palachai N, Wattanathorn J, Muchimapura S
and Thukham-Mee W: Antimetabolic syndrome effect of phytosome
containing the combined extracts of mulberry and ginger in an
animal model of metabolic syndrome. Oxid Med Cell Longev.
2019(5972575)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pucci M, Mandrone M, Chiocchio I, Sweeney
EM, Tirelli E, Uberti D, Memo M, Poli F, Mastinu A and Abate G:
Different seasonal collections of Ficus carica L. Leaves diversely
modulate lipid metabolism and adipogenesis in 3T3-L1 adipocytes.
Nutrients. 14(2833)2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Pei XD, He ZL, Yao HL, Xiao JS, Li L, Gu
JZ, Shi PZ, Wang JH and Jiang LH: 6-Shogaol from ginger shows
anti-tumor effect in cervical carcinoma via PI3K/Akt/mTOR pathway.
Eur J Nutr. 60:2781–2793. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
de Lima RMT, Dos Reis AC, de Oliveira
Santos JV, de Oliveira Ferreira JR, Lima Braga A, de Oliveira Filho
JWG, de Menezes APM, da Mata AMOF, de Alencar MVOB, do Nascimento
Rodrigues DC, et al: Toxic, cytogenetic and antitumor evaluations
of (6)-gingerol in non-clinical in vitro studies. Biomed
Pharmacother. 115(108873)2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Dugasani S, Pichika MR, Nadarajah VD,
Balijepalli MK, Tandra S and Korlakunta JN: Comparative antioxidant
and anti-inflammatory effects of (6)-gingerol, (8)-gingerol,
(10)-gingerol and (6)-shogaol. J Ethnopharmacol. 127:515–520.
2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Qiu JL, Chai YN, Duan FY, Zhang HJ, Han
XY, Chen LY and Duan F: 6-Shogaol alleviates CCl4-induced liver
fibrosis by attenuating inflammatory response in mice through the
NF-κB pathway. Acta Biochim Pol. 69:363–370. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ebrahimzadeh Attari V, Malek Mahdavi A,
Javadivala Z, Mahluji S, Zununi Vahed S and Ostadrahimi A: A
systematic review of the anti-obesity and weight lowering effect of
ginger (Zingiber officinale Roscoe) and its mechanisms of
action. Phytother Res. 32:577–585. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
32
|
Choi J, Kim KJ, Kim BH, Koh EJ, Seo MJ and
Lee BY: 6-gingerol suppresses adipocyte-derived mediators of
inflammation in vitro and in high-fat diet-induced obese zebra
fish. Planta Med. 83:245–253. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Saravanan G, Ponmurugan P, Deepa MA and
Senthilkumar B: Anti-obesity action of gingerol: effect on lipid
profile, insulin, leptin, amylase and lipase in male obese rats
induced by a high-fat diet. J Sci Food Agric. 94:2972–2977.
2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Jiao W, Mi S, Sang Y, Jin Q, Chitrakar B,
Wang X and Wang S: Integrated network pharmacology and cellular
assay for the investigation of an anti-obesity effect of 6-shogaol.
Food Chem. 374(131755)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Esteve Ràfols M: Adipose tissue: Cell
heterogeneity and functional diversity. Endocrinol Nutr.
61:100–112. 2014.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
36
|
Tzeng TF and Liu IM: 6-Gingerol prevents
adipogenesis and the accumulation of cytoplasmic lipid droplets in
3T3-L1 cells. Phytomedicine. 20:481–487. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Uramaru N, Kawashima A, Osabe M and
Higuchi T: Rhododendrol, a reductive metabolite of raspberry
ketone, suppresses the differentiation of 3T3-L1 cells into
adipocytes. Mol Med Rep. 27:1–9. 2023.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Smith A, Yu X and Yin L: Diazinon exposure
activated transcriptional factors CCAAT-enhancer-binding proteins α
(C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ)
and induced adipogenesis in 3T3-L1 preadipocytes. Pestic Biochem
Physiol. 150(48)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kang MJ, Kim KK, Son BY, Nam SW, Shin PG
and Kim GD: The anti-adipogenic activity of a new cultivar,
pleurotus eryngii var. ferulae ‘beesan no. 2’, through
down-regulation of PPAR γ and C/EBP α in 3T3-L1 cells. J Microbiol
Biotechnol. 26:1836–1844. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zakłos-Szyda M, Pietrzyk N, Szustak M and
Podsędek A: Viburnum opulus L. juice phenolics inhibit mouse 3T3-L1
cells adipogenesis and pancreatic lipase activity. Nutrients.
12(2003)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Furuhashi M, Saitoh S, Shimamoto K and
Miura T: Fatty acid-binding protein 4 (FABP4): Pathophysiological
insights and potent clinical biomarker of metabolic and
cardiovascular diseases. Clin Med Insights Cardiol. 2014:23–33.
2014.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Misawa K, Hashizume K, Yamamoto M,
Minegishi Y, Hase T and Shimotoyodome A: Ginger extract prevents
high-fat diet-induced obesity in mice via activation of the
peroxisome proliferator-activated receptor δ pathway. J Nutr
Biochem. 26:1058–1067. 2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Okamoto M, Irii H, Tahara Y, Ishii H,
Hirao A, Udagawa H, Hiramoto M, Yasuda K, Takanishi A, Shibata S
and Shimizu I: Synthesis of a new (6)-gingerol analogue and its
protective effect with respect to the development of metabolic
syndrome in mice fed a high-fat diet. J Med Chem. 54:6295–6304.
2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Impheng H, Richert L, Pekthong D,
Scholfield CN, Pongcharoen S, Pungpetchara I and Srisawang P:
(6)-Gingerol inhibits de novo fatty acid synthesis and carnitine
palmitoyltransferase-1 activity which triggers apoptosis in HepG2.
Am J Cancer Res. 5(1319)2015.PubMed/NCBI
|
|
45
|
Chirala SS and Wakil SJ: Structure and
function of animal fatty acid synthase. Lipids. 39:1045–1053.
2004.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Batchuluun B, Pinkosky SL and Steinberg
GR: Lipogenesis inhibitors: Therapeutic opportunities and
challenges. Nat Rev Drug Discov. 21:283–305. 2022.PubMed/NCBI View Article : Google Scholar
|