Introduction
Cancer seriously affects health and is responsible
for the death of most people worldwide; there were an estimated
19.3 millon new cases and 10 million cancer deaths worldwide in
2020(1). In recent years,
immunotherapy has emerged as a novel treatment option for advanced
tumors (2). Current research
hotspots include immune checkpoint inhibitors (ICIs) and multiple
ICIs have shown clinical benefits for treatment of certain advanced
cancers. However, factors such as low response rate, disease
pseudoprogression and immune-associated adverse events require the
identification of predictive and prognostic biomarkers to determine
which patients may benefit from cancer immunotherapy (3). Histological markers such as
programmed death-ligand 1 (PD-L1) expression, tumor mutation burden
(TMB), microsatellite instability (MSI) and T cell receptors (TCRs)
are currently well-known predictive biomarkers but they still
present some limitations in clinical application and in predicting
the efficacy of ICIs (4).
There are several cut-off criteria and antibodies
for the PD-L1 expression analysis. For example, in cohort studies
on PD-L1 inhibitors, such as durvalumab (5) and atezolizumab (6) SP163 and SP142 antibodies were used to
evaluate the expression of PD-L1. However, in cohort studies on
PD-1 inhibitors, such as nivolumab (7) and pembrolizumab (8), 28-8 and 22C3 antibodies were used
(9). On the other hand, bias in
defining positivity for PD-L1 expression could be observed among
different cohort studies because of different cut-off values
ranging from 1-50% (10).
Secondly, several limitations exist on the clinical application of
TMB for predicting the efficacy of ICIs. For example, different
cohort studies use different TMB cut-off values (11-14)
Although pembrolizumab was approved by the US Food and Drugs
Administration (FDA) for use in patients with high TMB (TMB-H)
solid tumors (≥10 mutations/megabase), contradictory results are
observed in clinical practice (15). A previous study showed that
patients with TMB-H had a low response rate to ICIs, while patients
with low TMB values benefit from ICI therapies (16). Thirdly, although high MSI (MSI-H)
is approved by FDA as a biomarker for predicting the efficacy of
pembrolizumab in the treatment of solid tumors regardless of tumor
histology, a contradictory phenomenon remains observable in
clinical practice: For example, several studies suggest that
patients with colorectal cancer with microsatellite stability (MSS)
can also have a notable clinical response to PD-1 inhibitors
(17,18).
By contrast, hematological markers are a focus of
clinical research due to their several advantages including
affordability, convenience and non-invasiveness. However,
hematological markers have some limitations. For example, TCR plays
important role in recognizing neoantigens, a prerequisite of T cell
antitumor response. It was demonstrated that increased richness of
TCR clonotypes is correlated with increased overall survival (OS)
time in patients with melanoma treated with ipilimumab (19). This beneficial phenomenon could
also be observed in patients with urothelial carcinoma treated with
atezolizumab, in which long-term clinical benefits were
significantly correlated with expansion of TCR (20). However, contradictory results were
observed with anti-PD-1 therapy (21). Due to the heterogeneity of TCRs and
the need for complicated analytical techniques, such as
high-throughput sequencing and single-cell sequencing techniques,
the clinical application for predicting the efficacy of ICIs is
premature. Therefore, exploring other clinically available and
accessible hematological markers is important. It was reported that
inflammatory responses are associated with apoptosis inhibition,
angiogenesis promotion and DNA damage, which result in tumor
progression (22). Routine
detection of multiple indicators in peripheral blood can reflect
the inflammatory status of patients with cancer. C-reactive protein
(CRP), as a marker of systemic inflammation, can predict the
survival outcomes of patients with cancer treated with ICIs. A
previous report showed that increased CRP was a marker of poor
prognosis in patients with cancer who were treated with ICIs, which
was associated with shortened progression-free survival (PFS) and
OS times (23). Although several
studies investigated the association between CRP levels and ICI
therapy survival outcomes, different conclusions were found in
these studies: For example, among patients with advanced melanoma
who were treated with nivolumab, increased CRP levels were
significantly associated with poor OS and PFS (24). By contrast, in patients with
non-small cell lung cancer (NSCLC) who were treated with nivolumab,
CRP was not correlated with PFS or OS (25). For ICIs other than nivolumab, the
association between CRP and ICI therapy survival outcomes remains
contradictory. For example, Muto et al (26) found that in patients with advanced
melanoma, CRP is not associated with the efficacy of ipilimumab and
OS; however, Shibata et al (27) found that in patients with advanced
NSCLC, increased serum CRP levels at 6 weeks of treatment could
predict longer survival when pembrolizumab was given as first-line
treatment.
Therefore, the present meta-analysis aimed to
explore the association between baseline CRP levels and survival
outcomes of patients with cancer who were treated with ICIs to
provide a basis for improved evaluation of the prognosis.
Materials and methods
Literature search
Electronic databases, including PubMed, EMbase,
Cochrane Library, Web of Science, Chinese National Knowledge
Infrastructure, WanFang, Chinese Literature Biomedical Database and
Weipu Database (28) were searched
to identify cohort studies on the relationship between baseline CRP
levels and ICI survival outcomes from inception to November 2020.
The key words used for the literature search were: ‘C-reactive
protein’, ‘C reactive protein’, ‘CRP’, ‘neoplasms’, ‘tumors’,
‘cancers’, ‘carcinoma’, ‘immunotherapy’, ‘immune checkpoint
inhibitor’, ‘PD-1 inhibitor’, ‘PD-L1 inhibitor’, ‘CTLA-4
inhibitor’, ‘nivolumab’, ‘pembrolizumab’, ‘atezolizumab’,
‘durvalumab’ and ‘ipilimumab’. In addition, the references of
relevant articles were reviewed to identify potentially eligible
studies.
Eligibility criteria
The inclusion criteria were as follows: i) Eligible
patients were pathologically diagnosed with solid tumor and treated
with ICIs alone or ICIs combined with systemic chemotherapy; ii)
reported baseline CRP levels before treatment; iii) provided hazard
ratios (HRs) and 95% CIs for baseline CRP levels and OS or PFS
analysis or the data necessary to calculate them. When duplicated
data were reported in different studies, only the most recent or
highest quality were included.
The exclusion criteria were as follows: i) Reviews,
comment letters, meeting abstracts or case reports; ii) in
vivo or in vitro studies; iii) studies published in a
language other than Chinese or English; iv) immunotherapy regimens
other than ICIs; v) full text was not available or did not provide
all necessary data mentioned in the inclusion criteria above; vi)
provided post-treatment CRP levels or dynamic changes in CRP levels
only.
Data extraction
A total of two authors independently reviewed and
extracted data from the included studies. Any discrepancy was
resolved through discussion with a third author. The data extracted
from the eligible studies included the following items: i) Name of
the first author(s) and year of publication; ii) patient median age
and sex ratio; iii) sample size and types of cancer and ICI drug;
iv) CRP cut-off values; v) HR and 95% CI associated with OS and
PFS. The HRs from multivariate Cox analysis were top-priority for
use when reported.
Quality assessment
The quality assessment of studies was conducted by
two independent researchers according to the Newcastle-Ottawa Scale
(NOS) (29), which assesses the
quality based on three aspects: i) Selection of study subjects; ii)
comparability between groups; and iii) measurement of outcomes. The
maximum score was 9 points and studies scoring ≥6 points were
regarded as high-quality studies.
Statistical analysis
Meta-analysis was conducted using STATA software
(version 14.0; StataCorp,). The HR and corresponding 95% CI were
used to evaluate the association between CRP and ICI survival
outcomes in patients with cancer. Q test was performed to assess
heterogeneity of included studies and the I2 statistic
was calculated to evaluate the total observed variability due to
study heterogeneity. I2>50% and/or P<0.1 was
considered to indicate statistically significant heterogeneity
(30). Subgroup analysis was
performed to identify the source of heterogeneity. A random-effects
model was chosen for the meta-analysis if there was significant
heterogeneity between studies; otherwise, a fixed-effects model was
selected (31). Sensitivity
analysis was performed by excluding each study individually to
assess the stability of the results (32). Publication bias was assessed using
Egger's and Begg's tests, and P<0.05 was considered to indicate
a significant publication bias (33,34).
When significant publication bias was found, Duval and Tweedie's
trim and fill method was used to calculate the effect of potential
data censoring or publication bias on the outcomes of the
meta-analysis (35).
Results
Characteristics of the included
studies
A total of 2,205 relevant studies were identified
through a systematic literature search. Firstly, 390 duplicate
publications were excluded, while 1,766 were excluded after
screening the titles and abstracts, including reviews, meeting
abstracts, laboratory studies and other articles irrelevant to the
present meta-analysis. Among them, 739 articles did not report
survival risks, 954 articles did not involve patients using ICI and
73 articles were case reports that did not have sufficient
data.
After reviewing and screening the full text of the
remaining 49 articles, 36 additional articles were excluded
according to the aforementioned inclusion and exclusion criteria.
Finally, 13 retrospective studies were included in the present
meta-analysis (25,36-47).
The search process is shown in Fig.
1. All included studies were published between 2016 and 2020. A
total of 2,387 patients were included in the present meta-analysis,
while the sample sizes ranged from 36-313 participants. Patients
were primarily diagnosed with NSCLC (6/13; 46.2%), melanoma (2/13;
15.4%), renal cell carcinoma (3/13; 23.1%) and urothelial carcinoma
(2/13; 15.4%). The applications of ICI include anti-CTLA-4,
anti-PD-1 and anti-CTLA-4 combined with anti-PD-1 inhibitors. The
proportion of males was 53-87% in each study, and the mean age was
59-70 years. Regarding prognostic indicators of baseline CRP levels
in patients receiving ICIs, three articles reported OS and PFS,
five reported OS only and five reported PFS only. The CRP cut-off
values were between 3 and 50 mg/l and the value of 10 mg/l was used
frequently. The NOS scores of the included studies ranged from 5-7.
The baseline characteristics of included studies are shown in
Table I; other characteristics,
including study design, country, study period and adjusted
covariates, are listed in Table
SI.
 | Table IBaseline characteristics of included
studies. |
Table I
Baseline characteristics of included
studies.
| First author/s,
year | Median age,
years | Sex,
male/female | Cancer type | Sample size, n | Treatment | CRP cut-off level,
mg/l | Outcome | Newcastle-Ottawa
Scale | (Refs.) |
|---|
| Nakamura et
al, 2016 | 67 | 52/46 | Melanoma | 98 | Nivolumab | 3 | OS | 6 | (36) |
| Oya et al,
2017 | 66 | 87/37 | NSCLC | 124 | Nivolumab | 10 | PFS | 7 | (37) |
| Naqash et
al, 2018 | 64 | 56/31 | NSCLC | 87 | Nivolumab | 50 | OS | 6 | (38) |
| Tanizaki et
al, 2018 | 68 | 90/44 | NSCLC | 134 | Nivolumab | 4.1 | PFS and OS | 6 | (25) |
| Ishihara et
al, 2019 | NA | 45/13 | RCC | 58 | Nivolumab | 10 | PFS and OS | 5 | (39) |
| Yasuoka et
al, 2019 | 69 | 32/8 | UC | 40 | Pembrolizumab | 5 | OS | 7 | (40) |
| Laino et al,
2020 | | | | | | | | | (41) |
|
Cohort
1 | 64 | 121/89 | Melanoma | 206 | Nivolumab | 5.3 | OS | 7 | |
|
Cohort
2 | 59 | 202/114 | Melanoma | 313 | Nivolumab | 5.75 | OS | 7 | |
|
Cohort
3 | 61 | 202/113 | Melanoma | 311 | Ipilimumab | 5.75 | OS | 7 | |
|
Cohort
4 | 59 | 206/108 | Melanoma | 313 | Nivolumab +
ipilimumab | 5.75 | OS | 7 | |
| Tamura et
al, 2020 | 70 | 29/12 | UC | 41 | Pembrolizumab | 10.6 | OS | 6 | (42) |
| Adachi et
al, 2019 | 70 | 206/90 | NSCLC | 296 | Nivolumab | 10 | PFS | 7 | (43) |
| Suzuki et
al, 2019 | 68 | 47/18 | RCC | 65 | Nivolumab | 21 | PFS and OS | 7 | (44) |
| Inomata et
al, 2018 | NA | 54/18 | NSCLC | 36 |
Nivolumab/Pembrolizumab | 10 | PFS | 6 | (45) |
| Noguchi et
al, 2020 | 68.5 | 51/13 | RCC | 64 | Nivolumab | 15 | PFS | 7 | (46) |
| Taniguchi et
al, 2017 | 68 | 135/66 | NSCLC | 201 | Nivolumab | 3 | PFS | 6 | (47) |
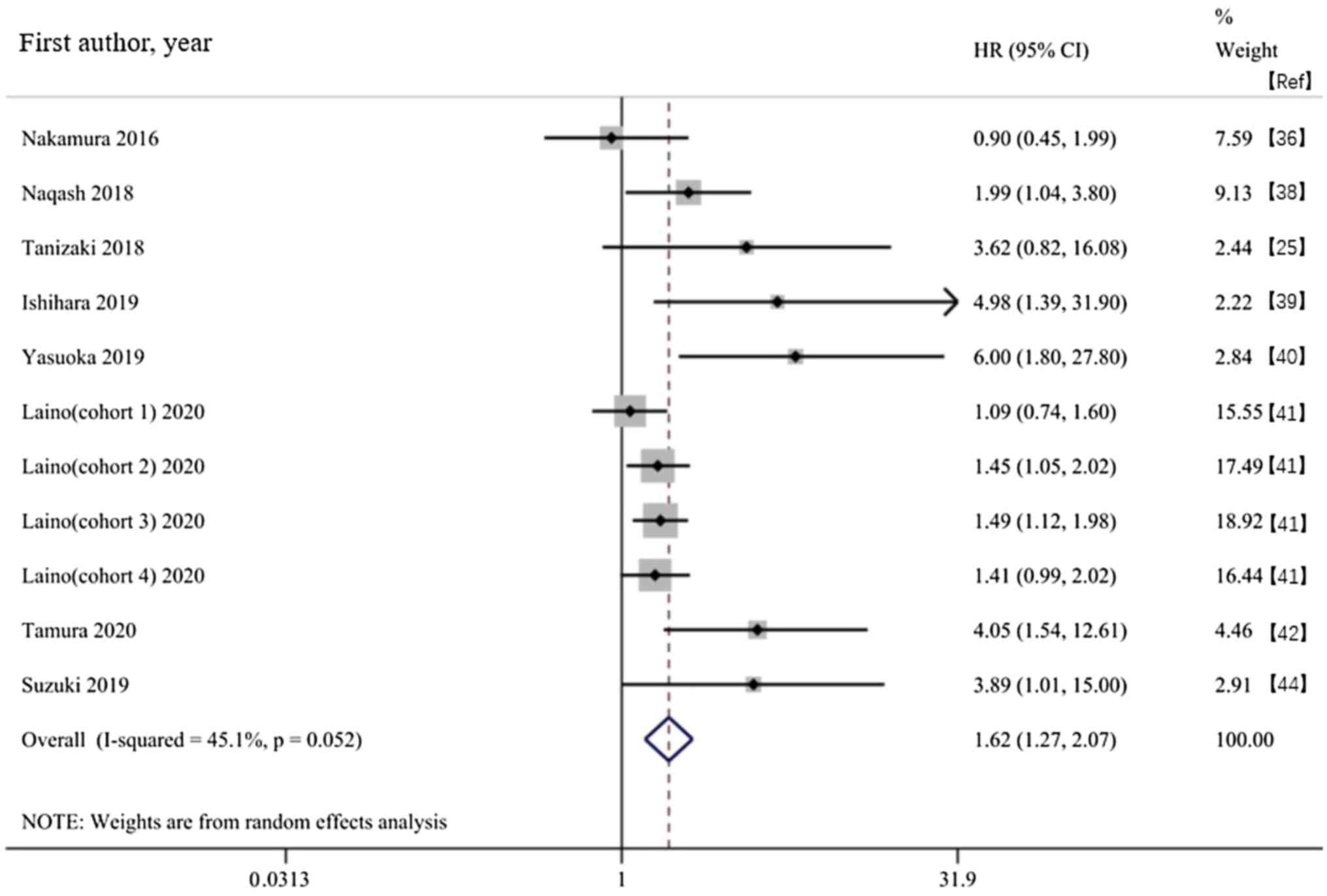
Survival outcome. Association between
the baseline CRP and OS in patients receiving ICIs
Of the 13 included studies, eight provided the
baseline CRP and OS. The random effects model showed a significant
association between high baseline CRP levels and shortened OS time
in patients receiving ICIs (HR, 1.62; 95% CI, 1.27-2.07;
P<0.001; Fig. 2). The subgroup
analysis based on type of cancer found that a high baseline CRP in
patients with multiple cancer types treated with ICIs was
associated with a poor OS. Subgroup analysis based on the CRP
cut-off value of 10 mg/l showed that an increased baseline CRP was
associated with poor OS regardless of whether the CRP levels were
>10 mg/l; prognosis of patients with CRP ≥10 mg/l was worse
(Table II).
 | Table IISubgroup analysis of overall
survival. |
Table II
Subgroup analysis of overall
survival.
| | Heterogeneity
test | | Meta-analysis | |
|---|
| Subgroup | Number of
studies | P-value | I2,
% | Effect model | Hazard ratio (95%
CI) | P-value | (Refs.) |
|---|
| NSCLC | 2 | 0.470 | 0 | Fixed | 2.19
(1.21-3.97) | 0.010 | (39,29) |
| RCC | 2 | 0.815 | 0 | Fixed | 4.32
(1.55-12.01) | 0.005 | (40,45) |
| UC | 2 | 0.655 | 0 | Fixed | 4.69
(2.04-10.79) | <0.001 | (41,43) |
| Melanoma | 2 | 0.555 | 0 | Fixed | 1.35
(1.15-1.59) | <0.001 | (37,42) |
| CRP cut-off,
mg/ml | | | | | | | |
|
<10 | 4 | 0.168 | 34.1 | Fixed | 1.40
(1.13-1.74) | 0.003 | (37,29,41,42) |
|
≥10 | 4 | 0.516 | 0 | Fixed | 2.76
(1.70-4.48) | <0.001 | (39,40,43,45) |
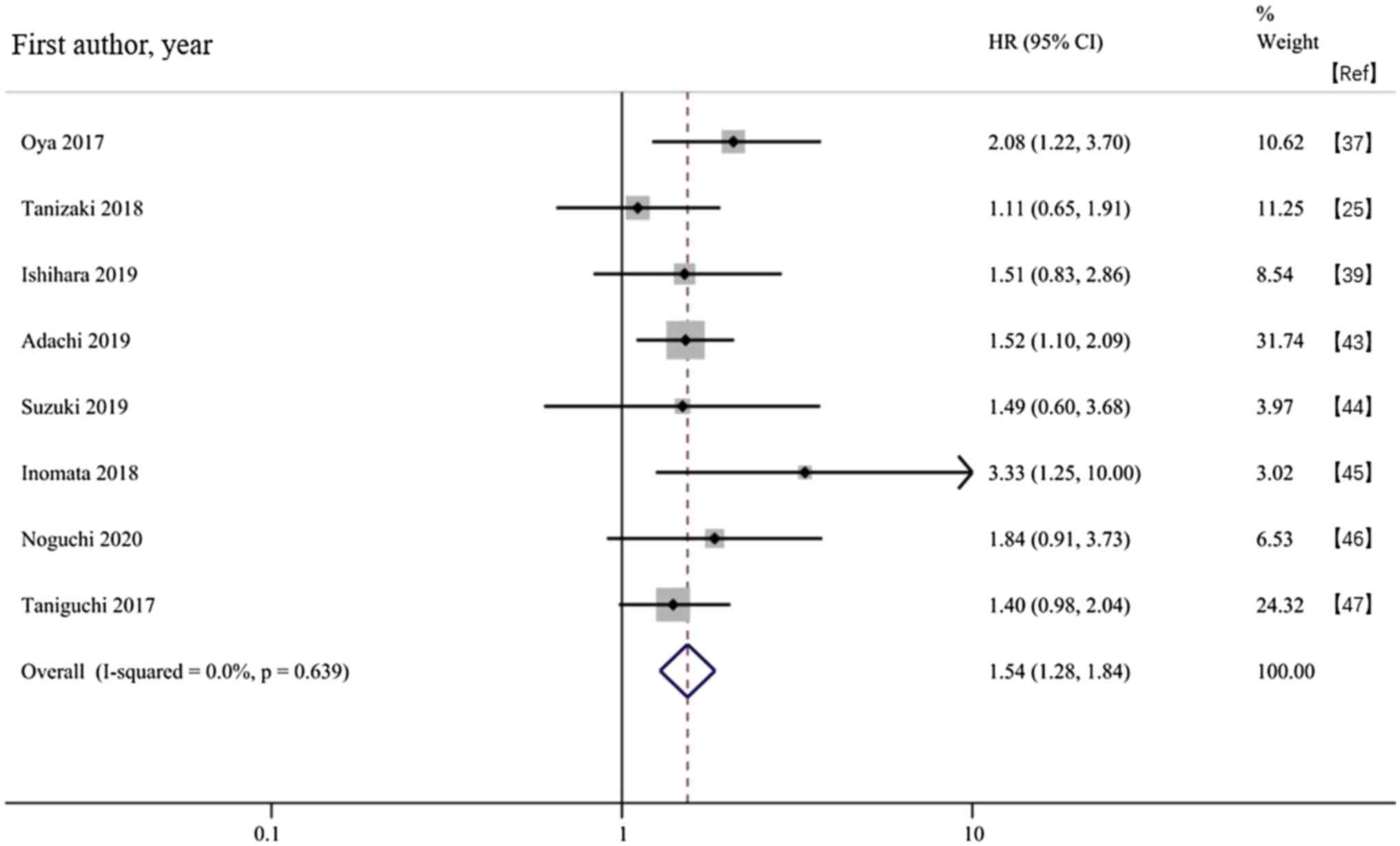
Association between the baseline CRP and PFS in
patients receiving ICIs. A total of eight studies evaluated PFS
outcomes. The fixed-effects model meta-analysis showed that an
increased baseline CRP was associated with a shorter PFS time in
patients treated with ICIs (HR, 1.54; 95% CI, 1.28-1.84;
P<0.001; Fig. 3). The subgroup
analysis stratified by cancer type showed consistent results with
OS, indicating that a high baseline CRP in patients with multiple
cancer types treated with ICIs was associated with a poor PFS time.
The subgroup analysis stratified by the CRP cut-off value showed
that CRP≥10 mg/l was associated with poor PFS. Although HR values
of PFS corresponding to CRP <10 mg/l were high, the difference
was not statistically significant (Table III).
 | Table IIISubgroup analysis of progression-free
survival. |
Table III
Subgroup analysis of progression-free
survival.
| | Heterogeneity
test | | Meta-analysis | |
|---|
| Subgroup | Number of
studies | P-value | I2,
% | Effect model | HR (95%CI) | P-value | (Refs.) |
|---|
| NSCLC | 5 | 0.296 | 18.6 | Fixed |
1.52(1.25,1.86) | <0.001 | (25,37,43,45,47) |
| RCC | 3 | 0.903 | 0.00 | Fixed |
1.61(1.06,2.44) | 0.024 | (39,44,46) |
| CRP cut-off,
mg/ml | | | | | | | |
|
<10 | 2 | 0.485 | 0.00 | Fixed |
1.30(0.96,1.76) | 0.089 | (25,47) |
|
≥10 | 6 | 0.723 | 0.00 | Fixed |
1.69(1.35,2.12) | <0.001 | (37,39,43,44,45,46) |
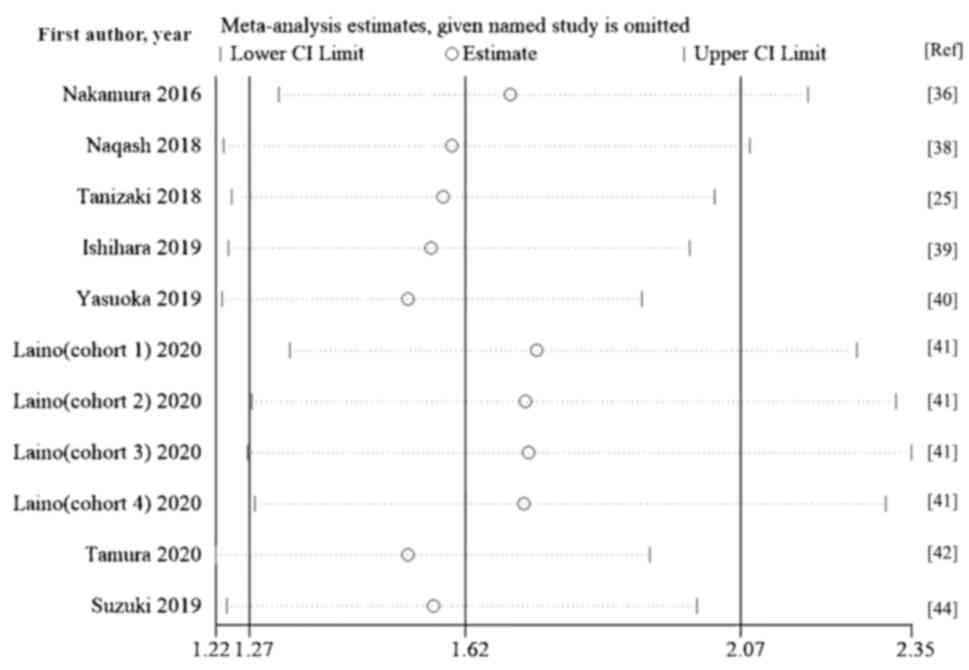
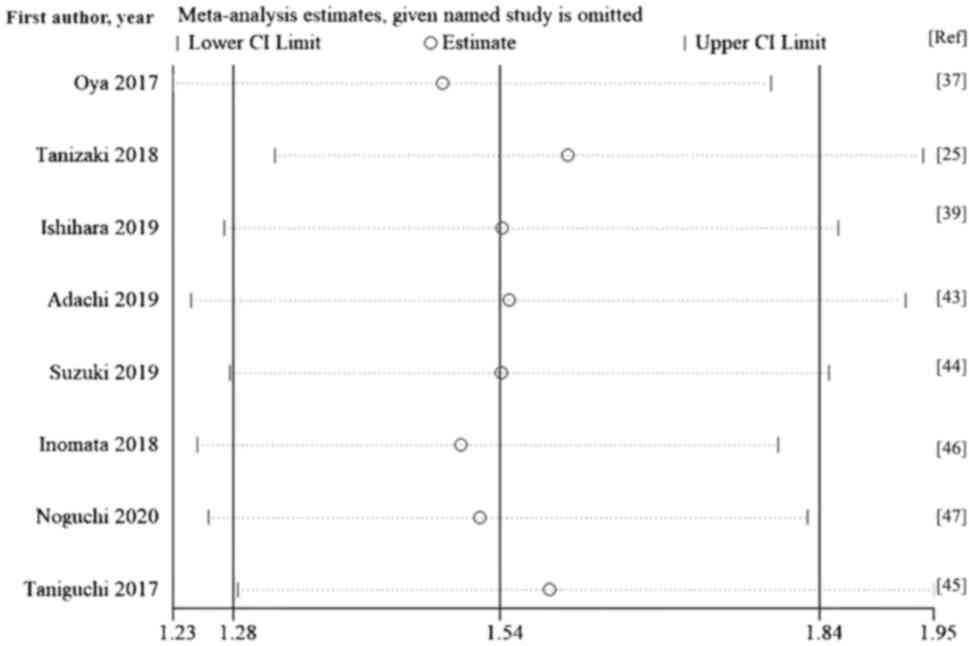
Sensitivity analysis
A sensitivity analysis was performed by excluding
each study individually. The pooled HR values of the remaining
studies ranged from 1.53-1.74 for OS and 1.48-1.60 for PFS, and the
lower and upper thresholds of the 95% CI were >1. The exclusion
of any study from the meta-analysis did not significantly change
the summary estimate, showing that the results were not driven by
any single study. The pooled HRs for OS and PFS were robust and the
present meta-analysis was reliable (Figs. 4 and 5)
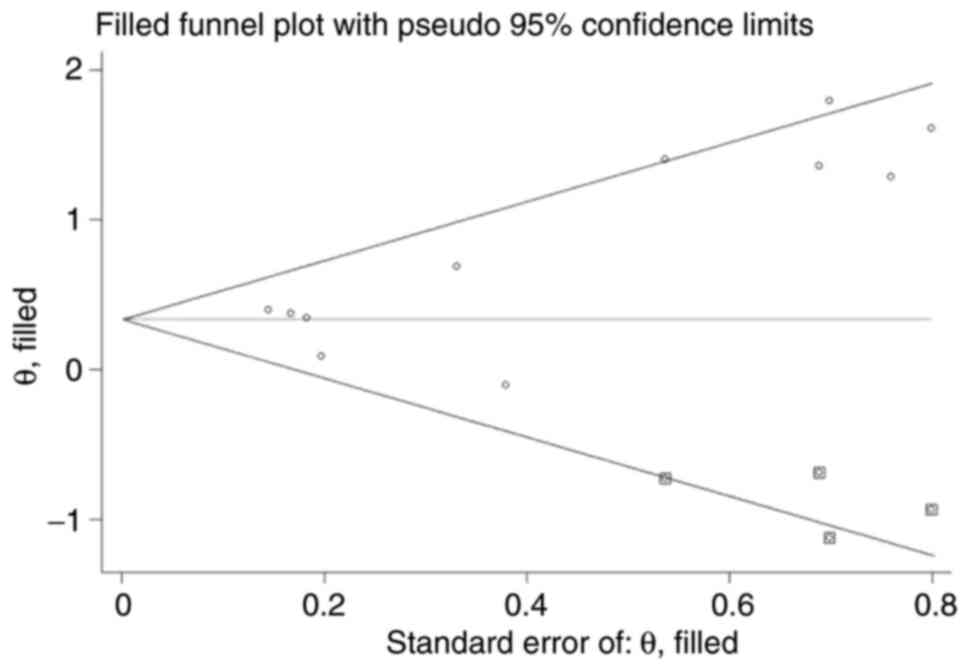
Publication bias
Begg's test funnel plot was drawn for the increased
baseline CRP and the outcome indicators of OS and PFS. The scatter
points were symmetrical, indicating a small possibility of
publication bias (OS, P=0.087; PFS, P=0.174). Egger's test
confirmed no publication bias in studies reporting the association
between baseline CRP levels and PFS (P=0.233), but the analysis of
the association between baseline CRP and OS suggested significant
publication bias (P=0.012).
The trim and fill methods were used to evaluate the
effect of publication bias on the meta-analysis outcomes. After
trimming and filling, the scatter points were symmetrical in the
funnel plot, indicating no publication bias (Fig. 6). For the pooled HRs for OS before
and after trimming and filling, the fixed effect model were 1.493
(95% CI, 1.282-1.738; P<0.001) and 1.398 (95% CI, 1.206-1.621;
P<0.001), and in the random effects model were 1.624 (95% CI,
1.272-2.074; P<0.001) and 1.410 (95% CI, 1.068-1.863; P=0.016),
respectively. After eliminating the influence of publication bias,
the result did not change significantly, suggesting that
publication bias had little effect on the results of the
meta-analysis.
Discussion
Inflammation is associated with all stages of cancer
development and increased levels of systemic inflammation are
associated with poor survival in patients with solid tumors
(48). CRP is an acute-phase serum
protein synthesized by hepatocytes and its expression is
significantly increased in inflammatory disease (49). Moreover, CRP is associated with the
prognosis of various cancer types (50). The association between cancer
prognosis and serum CRP levels may be due to tumorigenesis that
leads to increased CRP, which in turn promotes tumor progression.
Tumor cells can produce CRP themselves and may produce and release
cytokines and chemokines, such as IL-6 and IL-8, which increase the
serum CRP concentration. Tumor growth and invasion cause tissue
inflammation, leading to an increase in CRP levels. The innate and
adaptive immune systems may respond to tumor antigens by increasing
CRP levels (51). In addition, CRP
induces DNA damage and weakens the immune system, thereby promoting
carcinogenesis and tumor progression (22).
The present study analyzed current clinical evidence
to assess the prognostic value of baseline CRP levels in patients
with cancer in the context of immunotherapy. The meta-analysis
showed that increased baseline CRP levels were associated with poor
survival in patients with cancer treated with ICIs. Retrospective
analyses by Tong et al (52) and Minichsdorfer et al
(53) showed that increased
baseline CRP levels were associated with shorter median PFS and OS
in patients receiving immunotherapy. The present meta-analysis
combined PFS and OS to provide improved evidence for clarifying the
association between baseline CRP levels and prognosis in patients
with advanced cancer receiving immunotherapy. However, certain
studies showed opposite results, suggesting that an increased CRP
was not associated with decreased OS and PFS in patients with
melanoma and NSCLC treated with immunotherapy (35,36).
This disagreement may be due to the lower number of patients with
retrospective data, and different CRP level cut-off values used in
those studies. There is no uniform standard for the CRP cut-off
value, but a previous study suggested that the optimal cut-off
value for CRP as a prognostic marker is 10 mg/l, which was also the
upper limit of normal for CRP in most studies (23).
The present subgroup analysis based on cancer type
found that the elevated baseline CRP was associated with poor
survival outcomes in multiple cancers. Using two independent
multicenter real-world cohorts (discovery and validation cohorts),
Iivanainen et al (23)
found that the elevated baseline CRP was correlated with shortened
OS and PFS among patients treated with PD-1/PD-L1 in both cohorts.
In the present subgroup analysis based on cancer type, the
association between increased baseline CRP levels with survival
outcomes was significant in melanoma (two cohorts) and NSCLC
(validation cohort). Although not statistically significant, a
trend consistent with the general population was also observed in
renal cell and urothelial carcinoma, as well as with other cancer
types, which indicates the elevated baseline CRP was correlated
with poor OS and PFS times. Survival differences were similar among
all the studies (23). In the
current meta-analysis, the subgroup analysis based on the CRP
cut-off value of 10 mg/l found that both PFS and OS reported higher
mortality risk in patients with CRP ≥10 mg/l. CRP <10 mg/l was
also associated with poor OS, although the corresponding HR value
for PFS was increased and the difference was not statistically
significant. This may be due to the small number of studies with
CRP <10 mg/l included in the PFS analysis. A larger number of
high-quality studies should be included in the future to evaluate
the impact of CRP cut-off values on the prognosis of patients
treated with ICIs and CRP cut-off levels should be further
validated in future clinical applications. CRP levels may reflect a
specific biological tumor characteristic associated with
insensitivity to immunotherapy, which would prompt physicians to
use a therapeutic strategy targeting CRP in combination with ICIs.
A recent study showed that blocking synthesis and/or activity of
CRP in combination with ICIs improves response and survival in
patients with melanoma (41).
Larger studies are needed to find the best immunotherapy strategy.
The present study had limitations. All the included studies were
retrospective with several confounding factors; moreover, the
number of studies and the sample size were limited, which may lead
to potential bias. Furthermore, the included studies were
heterogeneous in terms of CRP cut-off values and ICI drugs. The
present meta-analysis only focused on the association between
baseline CRP levels and prognosis; the impact of post-treatment CRP
and dynamic changes in CRP on survival outcomes should be further
considered. Finally, although the trim and fill method confirmed
the results, there was some publication bias.
In summary, the current evidence suggested that
compared with patients with low baseline CRP levels, increased
baseline CRP levels were associated with poor OS and PFS in
patients receiving ICIs. Furthermore, a CRP≥10 mg/l indicated a
worse prognosis. Therefore, baseline CRP levels might serve as a
marker for the prognosis of patients with certain solid tumors
treated with ICIs. Due to the limited quality and quantity of the
included studies, a larger number of prospective well-designed
studies are required to verify the present findings.
Supplementary Material
Detailed information of studies
included in meta-analysis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX, JYW and KM conceived and designed the study. YX,
KM, FZ and MTM performed the experiments. LH, SJW, SPL, JYW and PPS
analyzed data and drafted the manuscript. JYW and SJW prepared the
figures. SJW, KM and PPS edited the manuscript. YX, JYW and SJW
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Duan J, Cui L, Zhao X, Bai H, Cai S, Wang
G, Zhao Z, Zhao J, Chen S, Song J, et al: Use of immunotherapy with
programmed cell death 1 vs programmed cell death ligand 1
inhibitors in patients with cancer: A systematic review and
meta-analysis. JAMA Oncol. 6:375–384. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
de Miguel M and Calvo E: Clinical
challenges of immune checkpoint inhibitors. Cancer Cell.
38:326–333. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ma K, Jin Q, Wang M, Li X and Zhang Y:
Research progress and clinical application of predictive biomarker
for immune checkpoint inhibitors Expert Rev Mol. Diagn. 19:517–529.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Powles T, van der Heijden MS, Castellano
D, Galsky MD, Loriot Y, Petrylak DP, Ogawa O, Park SH, Lee JL,
Giorgi UD, et al: Durvalumab alone and durvalumab plus tremelimumab
versus chemotherapy in previously untreated patients with
unresectable, locally advanced or metastatic urothelial carcinoma
(DANUBE): A randomised, open-label, multicentre, phase 3 trial.
Lancet Oncol. 21:1574–1588. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Felip E, Altorki N, Zhou C, Csőszi T,
Vynnychenko I, Goloborodko O, Luft A, Akopov A, Martinez-Marti A,
Kenmotsu H, et al: Adjuvant atezolizumab after adjuvant
chemotherapy in resected stage IB-IIIA non-small-cell lung cancer
(IMpower010): A randomised, multicentre, open-label, phase 3 trial.
Lancet. 398:1344–1357. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Weber JS, D'Angelo SP, Minor D, Hodi FS,
Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Miller WH Jr, Lao CD,
et al: Nivolumab versus chemotherapy in patients with advanced
melanoma who progressed after anti-CTLA-4 treatment (CheckMate
037): A randomised, controlled, open-label, phase 3 trial. Lancet
Oncol. 16:375–384. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brahmer JR, Rodríguez-Abreu D, Robinson
AG, Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A,
Cuffe S, et al: Health-related quality-of-life results for
pembrolizumab versus chemotherapy in advanced, PD-L1-positive NSCLC
(KEYNOTE-024): A multicentre, international, randomised, open-label
phase 3 trial. Lancet Oncol. 18:1600–1609. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hutarew G: PD-L1 testing, fit for routine
evaluation? From a pathologist's point of view. Memo. 9:201–206.
2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lisberg A and Garon EB: The value of PD-L1
testing in non-small-cell lung cancer. JAMA Oncol. 2:571–572.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jardim DL, Goodman A, de Melo Gagliato D
and Kurzrock R: The challenges of tumor mutational burden as an
immunotherapy biomarker. Cancer Cell. 39:154–173. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
David PC, Reck M, Luis PA, Creelan B, Horn
L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F, et
al: First-line nivolumab in stage IV or recurrent non-small-cell
lung cancer. N Engl J Med. 376:2415–2426. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hellmann MD, Nathanson T, Rizvi H, Creelan
BC, Sanchez-Vega F, Ahuja A, Ni A, Novik JB, Mangarin LMB,
Abu-Akeel M, et al: Genomic features of response to combination
immunotherapy in patients with advanced non-small-cell lung cancer.
Cancer Cell. 33:843–852.e4. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alborelli I, Leonards K, Rothschild SI,
Leuenberger LP, Prince SS, Mertz KD, Poechtrager S, Buess M,
Zippelius A, Läubli H, et al: Tumor mutational burden assessed by
targeted NGS predicts clinical benefit from immune checkpoint
inhibitors in non-small cell lung cancer. J Pathol. 250:19–29.
2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Marcus L, Fashoyin-Aje LA, Donoghue M,
Yuan M, Rodriguez L, Gallagher PS, Philip R, Ghosh S, Theoret MR,
Beaver JA, et al: FDA approval summary: Pembrolizumab for the
treatment of tumor mutational burden-high solid tumors. Clin Cancer
Res. 27:4685–4689. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Goodman A, Kato S, Bazhenova L, Patel SP,
Frampton GM, Miller V, Stephens PJ, Daniels GA and Kurzrock R:
Tumor mutational burden as an independent predictor of response to
immunotherapy in diverse cancers. Mol Cancer Ther. 16:2598–2608.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Palles C, Cazier JB, Howarth KM, Domingo
E, Jones AM, Broderick P, Kemp Z, Spain SL, Guarino E, Salguero I,
et al: Germline mutations in the proof-reading domains of POLE and
POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet.
45:136–144. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Gong J, Wang C, Lee PP, Chu P and Fakih M:
Response to PD-1 blockade in microsatellite stable metastatic
colorectal cancer harboring a POLE mutation. J Natl Compr Canc
Netw. 15:142–147. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Postow MA, Manuel M, Wong P, Yuan J, Dong
Z, Liu C, Perez S, Tanneau I, Noel M, Courtier A, et al: Peripheral
T cell receptor diversity is associated with clinical outcomes
following ipilimumab treatment in metastatic melanoma. J Immunother
Cancer. 23:10.1186/s40425-015-0070-4. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Snyder A, Nathanson T, Funt SA, Ahuja A,
Novik JB, Hellmann MD, Chang E, Aksoy BA, Al-Ahmadie H, Yusko E, et
al: Contribution of systemic and somatic factors to clinical
response and resistance to PD-L1 blockade in urothelial cancer: An
exploratory multi-omic analysis. PLoS Med.
14(e1002309)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tumeh PC, Harview CL, Yearley JH, Shintaku
IP, Taylor EJM, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu
V, et al: PD-1 blockade induces responses by inhibiting adaptive
immune resistance. Nature. 515:568–571. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Gao GD, Sun B, Wang XB and Wang SM:
Neutrophil to lymphocyte ratio as prognostic indicator for patients
with esophageal squamous cell cancer. Int J Biol Markers.
32:e409–e414. 2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Iivanainen S, Ahvonen J, Knuuttila A,
Tiainen S and Koivunen JP: Elevated CRP levels indicate poor
progression-free and overall survival on cancer patients treated
with PD-1 inhibitors. ESMO Open. 4(e000531)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chasseuil E, Saint-Jean M, Chasseuil H,
Peuvrel L, Quéreux G, Nguyen JM, Gaultier A, Varey E, Khammari A
and Dréno B: Blood predictive biomarkers for nivolumab in advanced
melanoma. Acta Derm Venereol. 98:406–410. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tanizaki J, Haratani K, Hayashi H, Chiba
Y, Nakamura Y, Yonesaka K, Kudo K, Kaneda H, Hasegawa Y, Tanaka K,
et al: Peripheral blood biomarkers associated with clinical outcome
in non-small cell lung cancer patients treated with nivolumab. J
Thorac Oncol. 13:97–105. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Muto Y, Kitano S, Tsutsumida A, Namikawa
K, Takahashi A, Nakamura Y, Yamanaka T, Yamamoto N and Yamazaki N:
Investigation of clinical factors associated with longer overall
survival in advanced melanoma patients treated with sequential
ipilimumab. J Dermatol. 46:498–506. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shibata Y, Kato T, Shimokawaji T and
Yamada K: P2.01-88 C-reactive protein (CRP) as a predictive marker
for survival in patients with advanced NSCLC treated with first
line Pembrolizumab Monotherapy. J Thorac Oncol. 10(13)2018.
|
|
28
|
Hernandez AV, Marti KM and Roman YM:
Meta-analysis. Chest. 158:S97–S102. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Higgins JPT, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ.
327:557–560. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
DerSimonian R and Laird N: Meta-analysis
in clinical trials. Control Clin Trials. 7:177–188. 1986.PubMed/NCBI View Article : Google Scholar
|
|
32
|
O'Rourke K, Shea B and Wells GA:
Meta-analysis of clinical trials(M)//Applied Statistics in the
Pharmaceutical Industry. Springer, New York, NY, pp397-424,
2001.
|
|
33
|
Egger M, Smith GD, Schneider M and Minder
C: Bias in meta-analysis detected by a simple, graphical test. BMJ.
315:629–634. 1997.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994.PubMed/NCBI
|
|
35
|
Duval S and Tweedie R: Trim and fill: A
simple funnel-plot-based method of testing and adjusting for
publication bias in meta-analysis. Biometrics. 56:455–463.
2000.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Nakamura Y, Kitano S, Takahashi A,
Tsutsumida A, Namikawa K, Tanese K, Abe T, Funakoshi T, Yamamoto N,
Amagai M and Yamazaki N: Nivolumab for advanced melanoma:
pretreatment prognostic factors and early outcome markers during
therapy. Oncotarget. 7:77404–77415. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Oya Y, Yoshida T, Kuroda H, Mikubo M,
Kondo C, Shimizu J, Horio Y, Sakao Y, Hida T and Yatabe Y:
Predictive clinical parameters for the response of nivolumab in
pretreated advanced non-small-cell lung cancer. Oncotarget.
8:103117–103128. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Naqash AR, Stroud CRG, Butt MU, Dy GK,
Hegde A, Muzaffar M, Yang LV, Hafiz M, Cherry CR and Walker PR:
Co-relation of overall survival with peripheral blood-based
inflammatory biomarkers in advanced stage non-small cell lung
cancer treated with anti-programmed cell death-1 therapy: Results
from a single institutional database. Acta Oncologica. 57:867–872.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Ishihara H, Tachibana H, Takagi T, Kondo
T, Fukuda H, Yoshida K, Iizuka J, Kobayashi H, Okumi M, Ishida H
and Tanabe K: Predictive impact of peripheral blood markers and
C-reactive protein in nivolumab therapy for metastatic renal cell
carcinoma. Target Oncol. 14:453–463. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Yasuoka S, Yuasa T, Nishimura N, Ogawa M,
Komai Y, Numao N, Yamamoto S, Kondo Y and Yonese J: Initial
experience of pembrolizumab therapy in Japanese patients with
metastatic urothelial cancer. Anticancer Res. 39:3887–3892.
2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Laino AS, Woods D, Vassallo M, Qian X,
Tang H, Wind-Rotolo M and Weber J: Serum interleukin-6 and
C-reactive protein are associated with survival in melanoma
patients receiving immune checkpoint inhibition. J Immunother
Cancer. 8(e000842)2020.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tamura D, Jinnouchi N, Abe M, Ikarashi D,
Matsuura T, Kato R, Maekawa S, Kato Y, Kanehira M, Takata R and
Obara W: Prognostic outcomes and safety in patients treated with
pembrolizumab for advanced urothelial carcinoma: Experience in
real-world clinical practice. Int J Clin Oncol. 25:899–905.
2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Adachi Y, Tamiya A, Taniguchi Y, Enomoto
T, Azuma K, Kouno S, Inagaki Y, Saijo N, Okishio K and Atagi S:
Predictive factors for progression- free survival in non-small cell
lung cancer patients receiving nivolumab based on performance
status. Cancer Med. 9:1383–1391. 2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Suzuki K, Terakawa T, Furukawa J, Harada
K, Hinata N, Nakano Y and Fujisawa M: C-reactive protein and the
neutrophil-to-lymphocyte ratio are prognostic biomarkers in
metastatic renal cell carcinoma patients treated with nivolumab.
Int J Clin Oncol. 25:135–144. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Inomata M, Hirai T, Seto Z, Tokui K, Taka
C, Okazawa S, Kambara K, Ichikawa T, Imanishi S, Yamada T, et al:
Clinical parameters for predicting the survival in patients with
squamous and non-squamous-cell NSCLC receiving PD-1 inhibitor
therapy. Pathol Oncol Res. 26:327–333. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Noguchi G, Nakaigawa N, Umemoto S,
Kobayashi K, Shibata Y, Tsutsumi S, Yasui M, Ohtake S, Suzuki T,
Osaka K, et al: C-reactive protein at 1 month after treatment of
nivolumab as a predictive marker of efficacy in advanced renal cell
carcinoma. Cancer Chemother Pharmacol. 86:75–85. 2020.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Taniguchi Y, Tamiya A, Isa SI, Nakahama K,
Okishio K, Shiroyama T, Suzuki H, Inoue T, Tamiya M, Hirashima T,
et al: Predictive factors for poor progression-free survival in
patients with non-small cell lung cancer treated with nivolumab.
Anticancer Res. 37:5857–5862. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Roxburgh CSD and McMillan DC: Role of
systemic inflammatory response in predicting survival in patients
with primary operable cancer. Future Oncol. 6:149–163.
2010.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Yoshida T, Ichikawa J, Giuroiu I, Laino
AS, Hao Y, Krogsgaard M, Vassallo M, Woods DM, Hodi FS and Weber J:
C reactive protein impairs adaptive immunity in immune cells of
patients with melanoma. J Immunother Cancer.
8(e000234)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Shrotriya S, Walsh D, Bennani-Baiti N,
Thomas S and Lorton C: C-reactive protein is an important biomarker
for prognosis tumor recurrence and treatment response in adult
solid tumors: A systematic review. PLoS One.
10(e0143080)2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Li W, Luo X, Liu Z, Chen Y and Li Z:
Prognostic value of C-reactive protein levels in patients with bone
neoplasms: A meta-analysis. PLoS One. 13(e0195769)2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Tong MT: A retrective cohort study to
explore the correlaition between peripheral blood markers and
survival in solid tumor treated with Atezolizumab monotheraphy
(unpublished PhD thesis). Zhejiang University, 2018.
|
|
53
|
Minichsdorfer C, Gleiss A, Aretin MB,
Schmidinger M and Fuereder T: 124P Serum parameters as prognostic
biomarkers for anti PD-1/PD-L1 therapy in patients with solid
tumours: A retrospective data analysis. Ann Oncol. 31:S289–S290.
2020.
|