Introduction
With an incidence of 0.14-0.32 per 100,000
individuals in 1999, aneurysmal bone cyst (ABC) is an uncommon bone
tumor that composes 1-2% of all primary bone tumors. Of these, ~70%
are primary bone tumors, and 30% are secondary (1). ABC typically affects teenagers up to
the age of 20 and is present in the femur, tibia, fibula, humerus,
skull and spine (2). The
pathogenesis of ABC is still unclear, and currently, the leading
theories are vascular, trauma and genetic factors (3). Vascular injury resulting in reactive
vascular malformations may be linked to the development of ABC, and
arteriovenous fistula or intramedullary artery embolism are also
considered to be risk factors for the onset of ABC (4). According to a previous study,
rearrangement of the ubiquitin-specific protease 6 gene is directly
associated with ABC (5).
The clinical symptoms of ABC mainly include pain,
swelling and masses at the disease site, and the primary
therapeutic options are as follows: i) Surgery, including local
curettage and internal fixation of the bone graft and whole segment
resection and reconstruction of the bone; ii) cryotherapy; iii)
sclerotherapy; iv) radionuclide ablation; v) arterial embolization;
and vi) chemotherapy, which is the primary choice for surgical
treatment of ABC (6).
Subsequently, 20-30% of ABC cases relapse following treatment;
thus, high-risk factors for malignant transformation include
recurrent recurrence, radiation therapy history and several
surgical and internal fixation procedures, and osteosarcoma is the
most prevalent malignant transformation (7).
In 1991, a case of recurrent malignant
transformation of the ABC of the distal tibia into osteosarcoma was
documented, and the patient was eventually treated with amputation
(8). By using electron microscopy,
Aho et al (9) examined four
patients with ABC and discovered that one of them went on to
develop malignant osteosarcoma seven years after receiving
radiation. In another case report, the patient did not receive
radiation therapy but developed osteosarcoma after receiving
repeated surgical treatments, highlighting the potential for
malignancy due to repeated surgery and internal fixation
stimulation (10). In summary, the
treatment of ABC malignant transformation into osteosarcoma is
mainly lumpectomy and reconstruction, and if the disease continues
to deteriorate in the later stage, amputation is the final
treatment with a low survival rate.
With a deeper knowledge of malignant bone tumors,
therapy for ABC malignant transition into osteosarcoma is now more
standard. Expanded resection of the tumor is the primary treatment
option, and it is supplemented by preoperative neoadjuvant
chemotherapy and a full course of postoperative chemotherapy. With
this course of treatment, the likelihood of limb preservation and
5-year survival rates improved from ~10-20% to ~60-70% in patients
without metastases and to ~20-30% in patients with metastatic
cancer (11). The present study
intends to improve the knowledge of whether ABC has the potential
to transform to other malignancies and provide clinical experience
in terms of clinical and imaging presentation, diagnosis, treatment
and prognosis.
Case summary
A 44-year-old Chinese man was sent to a local
hospital in February 2015 with intermittent pain in the left hip,
and an X-ray examination revealed a lytic lesion in the left
femoral neck. It was advised that the patient be transferred to a
hospital with a higher level of treatment. In March 2015, the
patient was admitted to the 940th Hospital of the Joint Logistics
Support Force of the Chinese People's Liberation Army (Lanzhou,
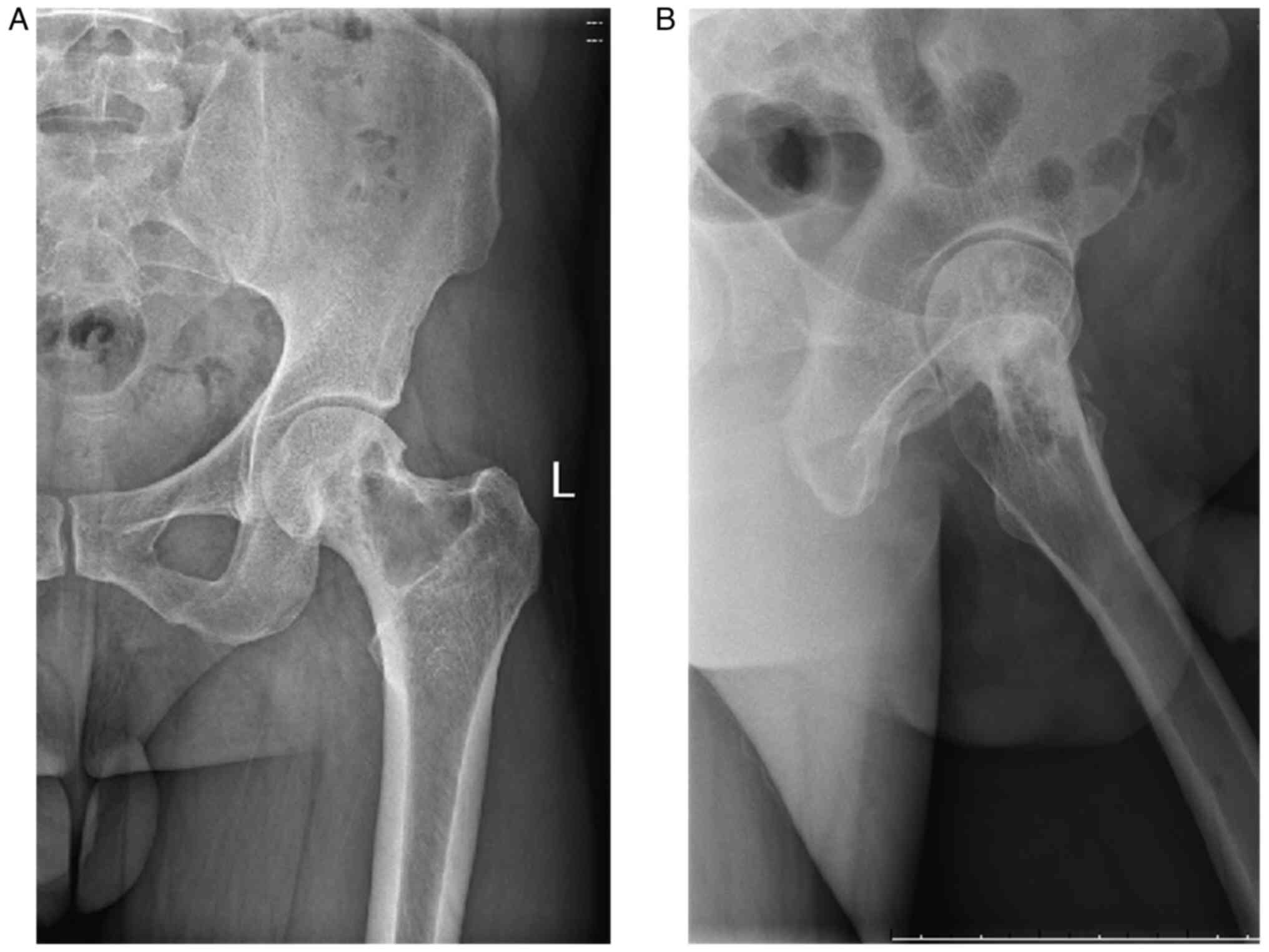
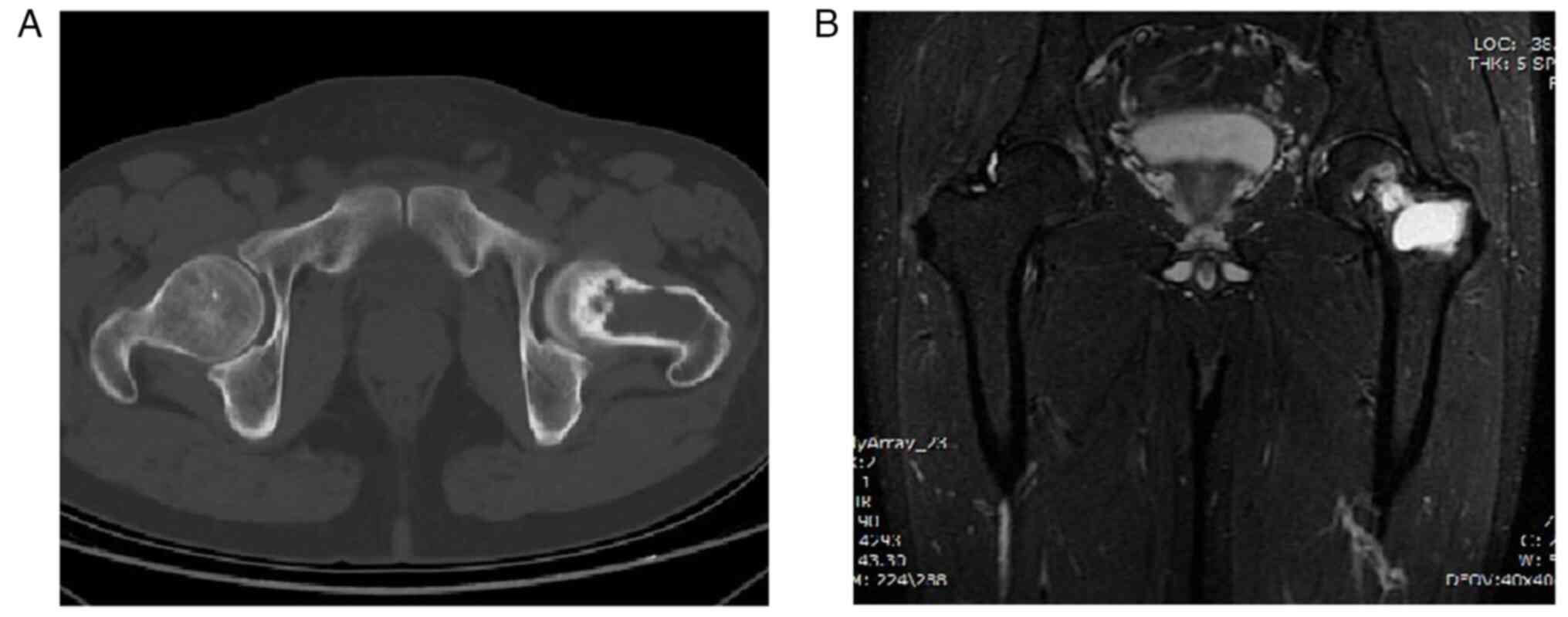
China). X-rays (Fig. 1), CT scans
and magnetic resonance imaging (MRI) revealed a proximal lesion of
the left femur (Fig. 2), which is
typically considered a benign bone disease with a high probability
of aneurysmal bone cyst.
After excluding contraindications to surgery, the
patient underwent curettage of the left femoral neck tumor, iliac
bone harvesting, tipped fibula grafting and internal fixation of
the plate in March 2015 at the 940th Hospital of the Joint
Logistics Support Force of the Chinese People's Liberation Army
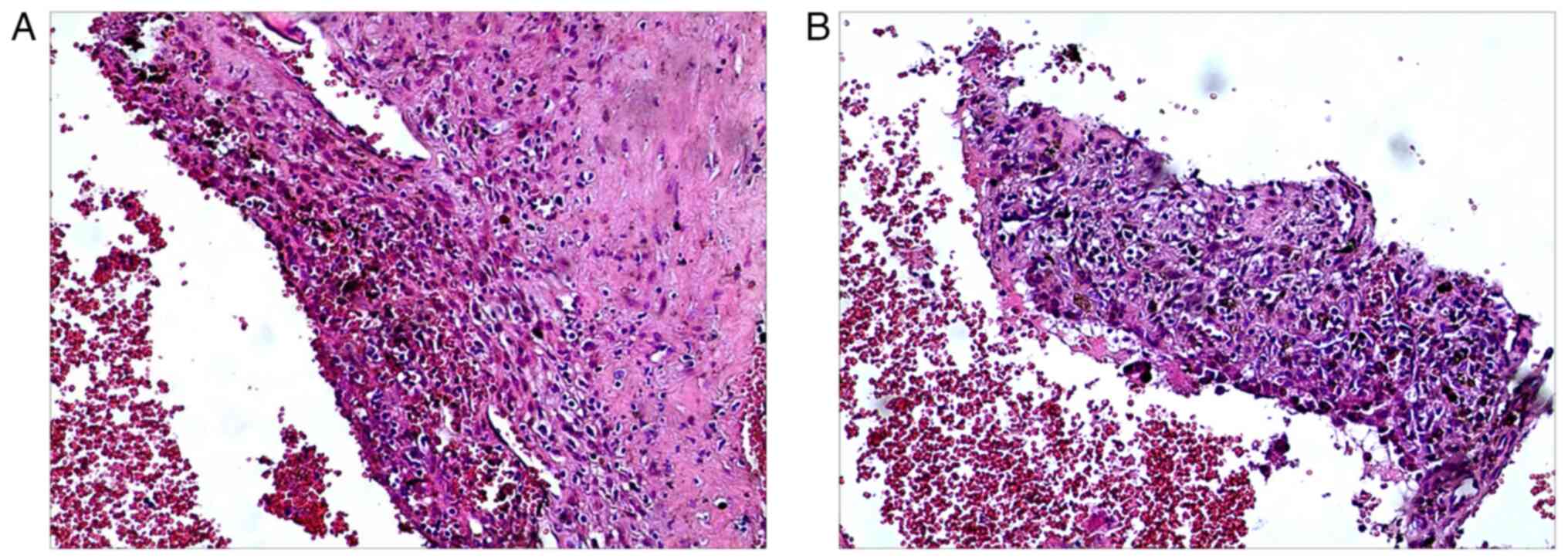
(Lanzhou, China). The postoperative histopathological examination
revealed that it was consistent with an aneurysmal bone cyst and
recovered well after surgery (Fig.
3). The internal fixation was removed at our department in
April 2016, and no evidence of recurrence was discovered. In June
2018, the patient consulted the Department of Joint Surgery again
for left hip pain, considering a recurrence of aneurysmal bone
cyst, and the patient underwent repeat left femoral neck aneurysm
bone cyst curettage, iliac bone harvesting and plate internal
fixation. Postoperative pathology showed recurrence of the aneurysm
bone cyst (Fig. 4). In May 2019,
the internal fixation was removed and no signs of recurrence were
evident.
The patient returned to our Department of Joint
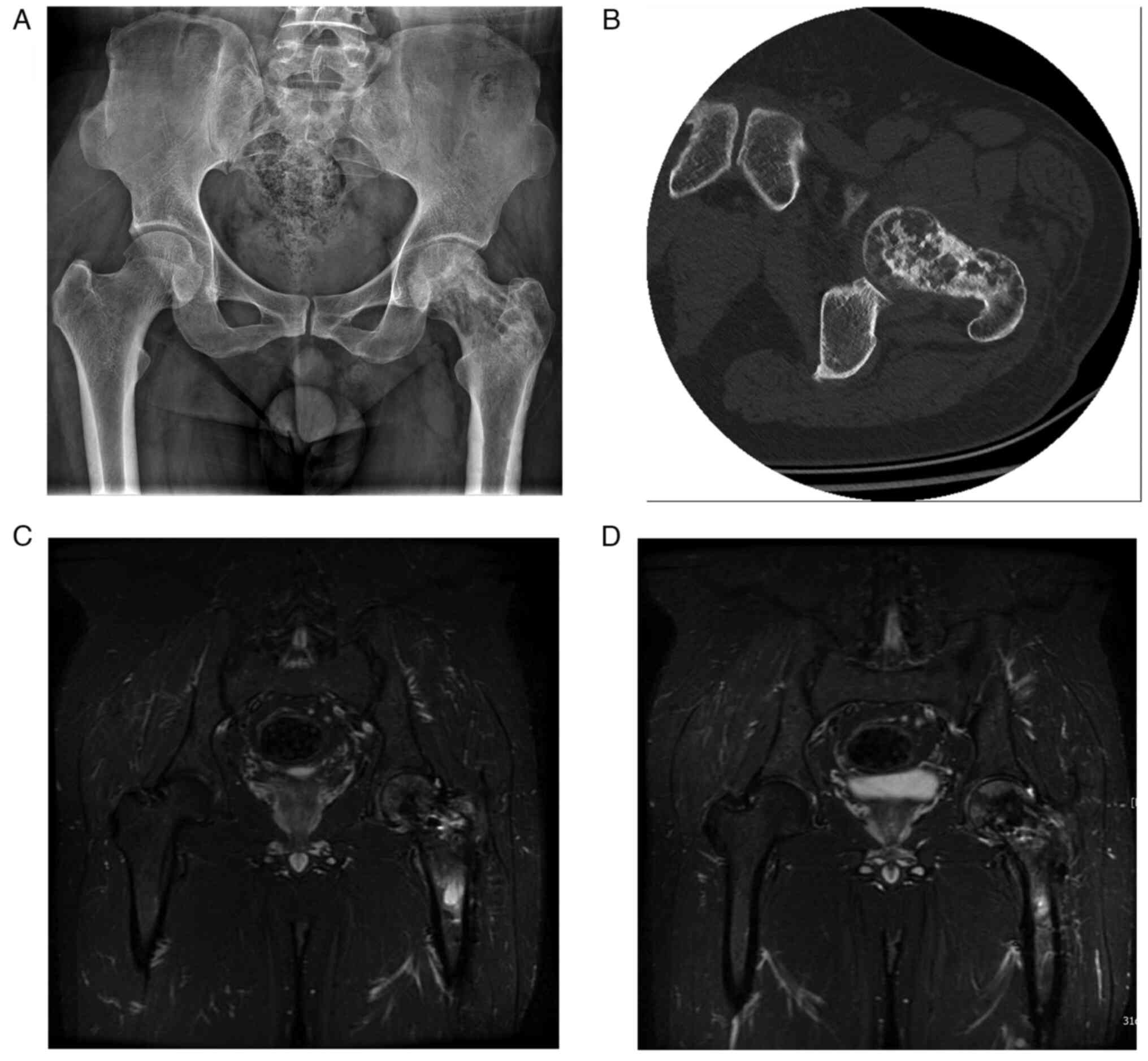
Surgery in August 2020, suffering from left hip pain. The X-ray
revealed that the bone density of the femoral neck was uneven, and
the CT examination showed worm-eaten bone destruction. The tumor
capsule showed sclerotic bands and the femoral head was normal in
shape. According to the MRI results, the left femoral head and neck
had a heterogeneous signal with a mixed long T2 signal shadow, and
the upper femur medullary cavity had an irregular long T1 and long
T2 signal with a morphology of ~1.8-cm. DWI also revealed a high
signal. Based on the CT and MRI results of the patient and a
history of repeated recurrences and multiple surgeries, the
possibility of malignancy in the ABC was strongly suspected
(Fig. 5). A surgical puncture
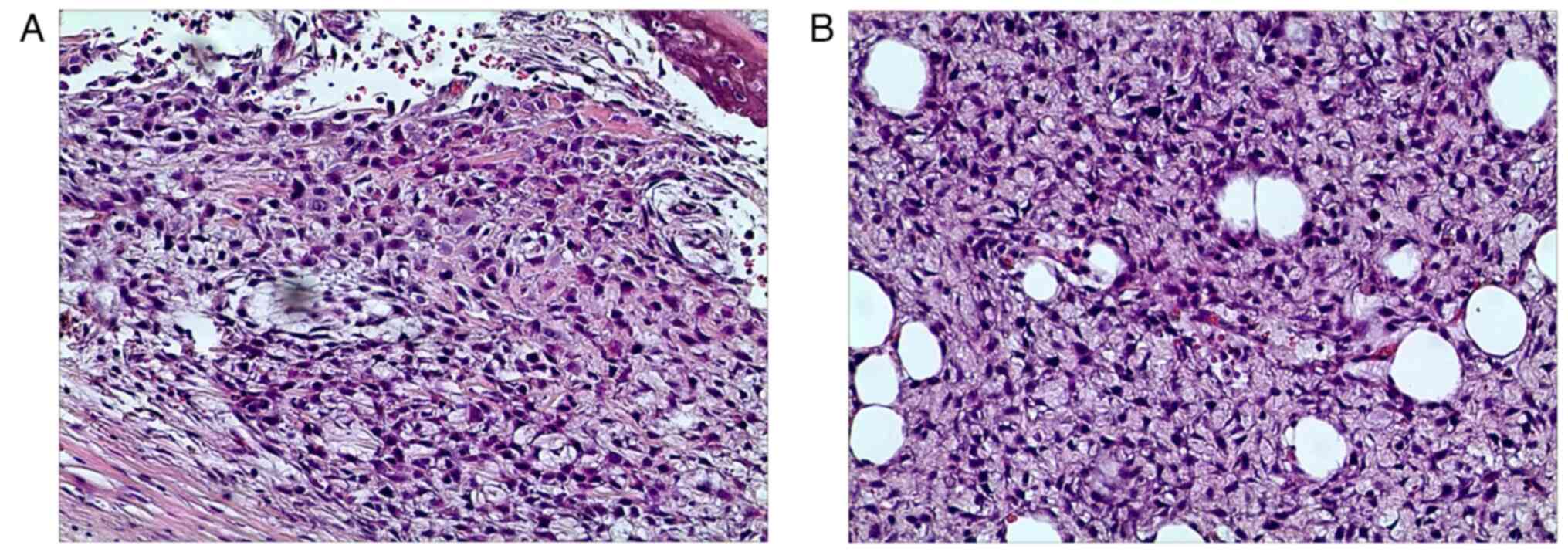
biopsy was performed, and the pathological results showed high
grade osteosarcoma of the left proximal femur (Fig. 6). Multitherapy, including
neoadjuvant chemotherapy, surgery and postoperative chemotherapy,
was chosen as the treatment plan after the patient and family
completed the necessary informed consent forms and were informed of
the present condition and treatment options.
The most widely used staging system in clinical
practice is the surgical staging system proposed by Enneking, which
has a good correlation with tumor prognosis and is adopted by the
Musculoskeletal Tumor Society (MSTS) and the International Society
for Limb Preservation, also known as MSTS surgical staging
(12). This system stages limited
malignant bone tumors according to the histological grade of the
tumor (low malignancy, stage I; high malignancy, stage II) and the
extent of local involvement (A, intra-interstitial; B,
extra-interstitial), with the interstitial status of the tumor
depending on whether the tumor breaks through the bone cortex.
Patients with distant metastases (M1) are considered stage III.
Based on the imaging of the patient, the patient had a stage IIA
Enneking Surgical Staging System, which is highly malignant
osteosarcoma; therefore, preoperative neoadjuvant chemotherapy and
postoperative chemotherapy was administered. A total of four
neoadjuvant chemotherapy sessions were performed before surgery
[patient height, 170 cm; weight, 81 kg; body surface area, 1.95
m2; protocol, injectable isocyclophosphamide (IFO) 12
g/m2 continuous pumping for D1-3 days + erlotinib
hydrochloride capsule 12 mg 1/day orally for 3 weeks; cisplatin 110
mg/m2 intravenously + liposome doxorubicin 34
mg/m2 intravenously]. Prior to neoadjuvant chemotherapy,
three days of hydrotherapy were started.
As recommended in the Chinese Society of Clinical
Oncology clinical guidelines for the management of classic
osteosarcoma, two alternating chemotherapy regimens of eight doses
at three-week intervals. During neoadjuvant chemotherapy, the
disease is evaluated as follows: i) Stable, with no local
recurrence or distant metastasis; ii) progressive, local recurrence
or proximal metastasis; iii) deterioration, local recurrence of the
tumor with proximal and distal metastasis or systemic metastasis. A
change in chemotherapy treatment or early surgery may be needed if
the disease worsens. Mestinon sodium injection (4.8 g) was supplied
before chemotherapy, with the addition of IFO, and after
chemotherapy to reduce urinary tract toxicity during IFO
chemotherapy. During chemotherapy, the blood count of the patient
was 2.51-2.98x109 cells/l, lymphocyte count was
0.61-0.75x109 cells/l and neutrophil count was
1.59-1.73x109 cells/l, indicating a degree II
myelosuppression. After receiving recombinant human
granulocyte-stimulating factor, diethylstilbestrol and caffeic acid
pills, the condition of the patient reverted to normal. The patient
also experienced gastrointestinal reactions, such as nausea and
vomiting, which subsided after receiving 8 mg of ondansetron
hydrochloride intravenously.
The status of the patient was determined to be
stable and progression-free following the neoadjuvant chemotherapy
course, with no local recurrence or distant metastases. The scope
of tumor resection was determined according to the preoperative MRI
lesion range to ensure a safe tumor margin (>5 cm of the lesion
range), and then left proximal femoral resection and artificial hip
tumor prosthesis replacement were performed. During the operation,
the proximal femur was removed and the removed proximal femur was
incised and the post-operative changes of the femoral neck scraping
and implantation were seen. A small amount of bleeding was seen in
the cross-section and the tissue was fish-like with unclear
borders, a fish-like tissue with indistinct borders measuring
~0.5x0.5 cm at ~11 cm from the femoral ridge, same as preoperative
imaging for high-grade osteosarcoma. The bone marrow tissue of the
distal femoral cavity was removed and sent for pathological
examination. The results of frozen section examination revealed no
sign of tumor tissue.
Postoperative X-ray shows suitable external
prosthesis and equal length of both lower limbs (Fig. 7). Postoperative treatment included
the prevention of infection (cefuroxime sodium 1.5 g IV 2/day for 3
days), prevention of thrombosis (sodium heparin calcium 3075 iu
subcutaneously), analgesia (parecoxib injection 40 mg
intramuscularly) and functional hip exercises (for example, hip
movement, muscle training, upright walking training). After the
healing of the surgical incision, 8 rounds of chemotherapy were
administered (protocol: injectable isocyclophosphamide 12
g/m2 IV 24 h continuous pumping for 3 days + anrotinib
hydrochloride capsule 12 mg orally 1/day for 3 weeks; cisplatin 110
mg/m2 IV + liposomal doxorubicin 34 mg/m2 IV,
hydrotherapy for 3 days before chemotherapy). The two regimens were
used alternately at 2-week intervals for a total of 8 sessions. The
responses and side effects listed above occurred during
postoperative chemotherapy and were treated symptomatically using
the same strategy, and the chemotherapy process went well. The
patient had good hip movement, no major pain, good walking ability,
suitability for everyday life and employment and good alignment of
the prosthesis without loosening or sinking on X-ray inspection at
regular follow-up appointments two years following surgery
(Fig. 8). The results of the
review indicated that the tumor did not exhibit either local
recurrence or distant metastasis
Discussion
A benign bone tumor that often occurs in adolescence
and whose pathogenesis is yet unknown, ABC is an uncommon bone
tumor with expansive, destructive and hemorrhagic characteristics
(13). The histology of ABC is
characterized by multiple blood-filled interstitial spaces, usually
lined by a variety of cellular components, including multinucleated
giant cells and fibroblast-like cells (14). Imaging can be used to diagnose ABC,
but histopathology analysis is what determines the ultimate
determination. The majority of ABCs are primary, and some are
secondary, but both primary and secondary ABCs can develop into
osteosarcoma, and certain benign or malignant tumors may be
combined with ABCs (15). Despite
the rarity of reports, the malignant transformation of ABC into
osteosarcoma is more frequently linked to radiotherapy stimulation
(16). Malignancy has also been
reported in association with surgical treatment and stimulation by
internal fixation (17), and a few
reports have overlooked the possibility of coexistence with other
tumors at the early stage of treatment (18).
In terms of the size of the lesion, the integrity of
the bone shell and the internal calcification, CT has high
diagnostic accuracy for ABC imaging diagnosis, and it can also
display the cystic area and fluid plane of the aneurysmal bone cyst
and the cystic septum (19). It
can also show the cystic area, fluid level and interscapular space
of aneurysmal bone cysts. Compared with CT, MRI has a higher
resolution of soft tissues and can observe patients from multiple
angles in coronal, cross-sectional and sagittal planes and is more
sensitive to hemorrhage, so that the internal structure and tissue
hierarchy of bone cysts can be observed more clearly. In addition,
the rate of fluid-fluid plane detection and cystic cavity detection
is significantly higher in MRI compared with in CT, and the
detection rate of pathological fractures is also higher in MRI
compared with CT (20).
The ABC diagnosis was corroborated by prior imaging
in the current case report, and all postoperative pathology samples
sent out for analysis likewise revealed aneurysmal bone cysts.
Imaging was abnormal after the second recurrence, with X-rays
showing a heterogeneous density of the femoral neck, possibly
related to the surgery. CT displayed worm-eaten destruction of the
left femoral head and neck and wall of the aneurysm. Typical ABCs
in MRI can show internal cystic cavities, low signal intervals of
varying size and signal intensity, fluid planes within the lesion,
cortical bone fractures and pushing at the border of the soft
tissue, with equal or slightly low signal in T1WI sequences and
mixed little high or high movement in T2WI. Reviewing the MRI
revealed a heterogeneous signal in the femoral head and femoral
neck bone, with a long mixed long T2 signal and a 1.8-cm irregular
long T1 and long T2 signal in the medullary cavity of the upper
femur. Therefore, after combining the CT and MRI results at the
time of the evaluation of the patient, we hypothesized that the
tumor could have undergone malignant transformation.
The malignant factors that emerged during the
treatment of this case and a review of the relevant literature are
considered as follows. First, there is the possibility of
previously combined osteosarcoma, but the absence of osteosarcoma
tissue in the first two pathological examinations was due to the
osteosarcoma being in an early stage, the imaging presentation
being unusual, the tumor being small and contained, or the
inability to collect pathological sections (21). Second, it was considered that the
first two pathological examinations were probably telangiectatic
osteosarcoma, whose histological morphology was highly similar to
ABC. Misdiagnosis or missing information might exist (22). The third factor was potential
cancer caused by frequent irritation from surgery and internal
fixation treatment (23).
Therefore, the paraffin-embedded tissues from the two previous
biopsies were once more subjected to histological examination to
exclude the possibility of underdiagnosis or misdiagnosis. There
was no osteosarcoma or telangiectatic osteosarcoma, and the patient
had not received radiation therapy or other treatments. In
conclusion, we hypothesized that the malignancy in the present case
may be linked to several operations and ongoing irritation of the
internal fixation.
There are various treatment options for ABC,
including surgery, freezing, radiotherapy and medication, but the
treatment modality changes immediately after malignant
transformation into osteosarcoma. At this stage, the primary
treatment modalities for osteosarcoma are extensive tumor
resection, preoperative neoadjuvant chemotherapy and postoperative
standardized chemotherapy. Preoperative neoadjuvant chemotherapy
has been demonstrated to significantly increase patient survival,
and, in a retrospective study, Hong et al (24) determined that patients who received
preoperative neoadjuvant chemotherapy have a significantly improved
5-year survival rate. High-dose methotrexate, doxorubicin,
cisplatin and isocyclophosphamide are still the major medications
used in neoadjuvant chemotherapy; however, in the present case,
anlotinib, a novel chemotherapeutic agent, and liposomal
doxorubicin were also administered.
Doxorubicin, a classical chemotherapeutic agent for
osteosarcoma, inhibits tumor cells by disrupting the structure and
function of mitochondria (25).
However, it has drawbacks, including difficulty in keeping blood
levels of the drug stable, significant cardiotoxicity, high
cardiotoxicity and resistance of tumor cells (26). Therefore, in recent years,
liposomal doxorubicin (L-DOX) has been used instead of doxorubicin.
Haghiralsadat et al (27)
demonstrated that L-DOX has a higher sensitivity and more stable
blood levels of chemotherapy drugs compared with doxorubicin in the
treatment of osteosarcoma. Another study showed less cardiotoxicity
(L-DOX) (28). The main side
effects of L-DOX are bone marrow suppression, gastrointestinal
reactions, hypoproteinemia, stomatitis and transient sinus
arrhythmias (29).
Anrotinib, a novel chemotherapeutic agent, is a
multitargeted tyrosine kinase inhibitor that suppresses the
activation of downstream signaling pathways, phosphorylation of
epithelial-mesenchymal transition and vascular endothelial growth
factor receptor 2, tumor development and osteosarcoma
chemosensitivity (30). In a
retrospective study analyzed by Cai et al (31), the overall remission rate (ORR) and
disease control rate (DCR) for osteosarcoma treated with the
anlotinib regimen were 19 and 71%, respectively, with a median
progression-free survival (PFS) of 5.4 months and overall survival
(OS) of 17.9 months, which had higher ORR and DCR and possessed
higher PFS and OS compared with the anlotinib chemotherapy regimen
alone. This suggests that anlotinib in combination with other
chemotherapies shows efficacy and tolerability in the treatment of
refractory bone and soft tissue sarcoma. In the present case, the
patient was assessed at the end of the neoadjuvant chemotherapy
course, and the tumor was progression-free and metastasis-free for
2 years. With positive outcomes and no evidence of recurrence or
distant metastases at follow-up, the chemotherapy regimen was
continued postoperatively.
In summary, aneurysmal bone cysts should be treated
with standardization, including in-depth intraoperative curettage
of the lesion and routine postoperative examination. Patients with
recurrent recurrence, radiation therapy and multiple surgeries
should be cautioned of the potential for malignant transformation
in clinical practice. Transformation of ABC in osteosarcoma is
malignant process and it should also be treated in a standardized
manner. Preoperative neoadjuvant chemotherapy and postoperative
standardized chemotherapy are recommended, which can greatly
improve patient survival and reduce recurrence and metastasis.
Acknowledgements
Not applicable.
Funding
Funding: The present case report was supported by the Key
Research and Development Program of Gansu Province (grant no.
21YF5FA154), Youth Science and Technology Foundation of Gansu
Province (grant no. 20JR5RA588), Youth Science and Technology
Foundation of Gansu Province (grant no. 21JR7RA014) and Military
Special Cultivation Project (grant no. 2021YXKY014).
Availability of data and materials
Not applicable.
Authors' contributions
XS, YQ and SZ conceived the study, participated in
the design of the study, collected the data and images and drafted
the manuscript. XS, YQ, HZ and LZ contributed to the revision of
the article and the processing of the data and images. LS, JL, XY
and HL participated in the study design and oversaw the manuscript
drafting process. All authors have read and approved the final
manuscript. XS and YQ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
The study was conducted with the consent of the
patient, who signed an informed consent form.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of this case report and any accompanying
image.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rapp TB, Ward JP and Alaia MJ: Aneurysmal
bone cyst. J Am Acad Orthop Surg. 20:233–241. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Burch S, Hu S and Berven S: Aneurysmal
bone cysts of the spine. Neurosurg Clin N Am. 19:41–47.
2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kransdorf MJ and Sweet DE: Aneurysmal bone
cyst: Concept, controversy, clinical presentation, and imaging. AJR
Am J Roentgenol. 164:573–580. 1995.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nasri E and Reith JD: Aneurysmal bone
cyst: A review. J Pathol Transl Med. 57:81–87. 2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hermann A, Polivka M, Loit MP, Guichard JP
and Bousson V: Aneurysmal bone cyst of the frontal bone-A
radiologic-pathologic correlation. J Radiol Case Rep. 12:16–24.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cottalorda J and Bourelle S: Current
treatments of primary aneurysmal bone cysts. J Pediatr Orthop B.
15:155–167. 2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Cruz GS, Cuevas-Suárez CE, Saavedra JPA,
Giorgis R, Teixeira MRK and Muniz FWMG: Percutaneous treatments of
primary aneurysmal bone cysts: Systematic review and meta-analysis.
Eur J Orthop Surg Traumatol. 31:1287–1295. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kyriakos M and Hardy D: Malignant
transformation of aneurysmal bone cyst, with an analysis of the
literature. Cancer. 68:1770–1780. 1991.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Aho HJ, Aho AJ and Einola S: Aneurysmal
bone cyst, a study of ultrastructure and malignant transformation.
Virchows Archiv A Pathol Anat Histol. 395:169–179. 1982.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mei J, Gao Y, Wang S and Cai X: Malignant
transformation of aneurysmal bone cysts: A case report. Chin Med J
(Engl). 122:110–112. 2009.PubMed/NCBI
|
|
11
|
Zhu W, Zhu L, Bao Y, Zhong X, Chen Y and
Wu Q: Clinical evaluation of neoadjuvant chemotherapy for
osteosarcoma. J BUON. 24:1181–1185. 2019.PubMed/NCBI
|
|
12
|
Murphey MD and Kransdorf MJ: Staging and
classification of primary musculoskeletal bone and soft-tissue
tumors according to the 2020 WHO update, from the AJR special
series on cancer staging. AJR Am J Roentgenol. 217:1038–1052.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cottalorda J and Bourelle S: Modern
concepts of primary aneurysmal bone cyst. Arch Orthop Trauma Surg.
127:105–114. 2007.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Purdue E: Aneurysmal bone cysts: Denosumab
extends its reach. Transl Res. 164:135–138. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mendenhall WM, Zlotecki RA, Gibbs CP,
Reith JD, Scarborough MT and Mendenhall NP: Aneurysmal bone cyst.
Am J Clin Oncol. 29:311–315. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kansagra AP, Wan JJ, Devulapalli KK,
Horvai AE, O'Donnell RJ and Link TM: Malignant transformation of an
aneurysmal bone cyst to fibroblastic osteosarcoma. Am J Orthop
(Belle Mead NJ). 45:E367–E372. 2016.PubMed/NCBI
|
|
17
|
Wuisman P, Roessner A, Blasius S, Grunert
J, Vestering T and Winkelmann W: High malignant surface
osteosarcoma arising at the site of a previously treated aneurysmal
bone cyst. J Cancer Res Clin Oncol. 119:375–378. 1993.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hsu CC, Wang JW, Huang CH and Chen WJ:
Osteosarcoma at the site of a previously treated aneurysmal bone
cyst. A case report. J Bone Joint Surg Am. 87:395–398.
2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Uhl M and Herget GW: Tumor-like bony
lesions of the skeleton. Orthopäde. 49:825–838. 2020.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
20
|
Sullivan RJ, Meyer JS, Dormans JP and
Davidson RS: Diagnosing aneurysmal and unicameral bone cysts with
magnetic resonance imaging. Clin Orthop Relat Res. 366:186–190.
1999.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Janevska V, Spasevska L, Samardziski M,
Nikodinovska V, Zhivadinovik J and Trajkovska E: From aneurysmal
bone cyst to telangiectatic osteosarcoma with metastasis in
inguinal lymph nodes-case report. Med Pregl. 68:127–132.
2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Mascard E, Gomez-Brouchet A and Lambot K:
Bone cysts: Unicameral and aneurysmal bone cyst. Orthop Traumatol
Surg Res. 101(Suppl 1):S119–S127. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Bello Báez A, López Pino MÁ, Azorín
Cuadrillero D and Sirvent Cerdá S: Aneurysmatic bone cyst
coexisting with osteosarcoma. Radiopathologic discussion. Discusión
radiopatológica. Radiología. 52:247–250. 2010.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
24
|
Hong KT, Park HJ, Kim BK, An HY, Choi JY,
Cheon J, Park S, Kim H and Kang HJ: Favorable outcome of high-dose
chemotherapy and autologous hematopoietic stem cell transplantation
in patients with nonmetastatic osteosarcoma and low-degree
necrosis. Front Oncol. 12(978949)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Adamczyk-Grochala J, Bloniarz D, Zielinska
K, Lewinska A and Wnuk M: DNMT2/TRDMT1 gene knockout compromises
doxorubicin-induced unfolded protein response and sensitizes cancer
cells to ER stress-induced apoptosis. Apoptosis. 28:166–185.
2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Armstrong J and Dass CR: Doxorubicin
action on mitochondria: Relevance to osteosarcoma therapy? Curr
Drug Targets. 19:432–438. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Haghiralsadat F, Amoabediny G, Sheikhha
MH, Forouzanfar T, Helder MN and Zandieh-Doulabi B: A novel
approach on drug delivery: Investigation of a new nano-formulation
of liposomal doxorubicin and biological evaluation of entrapped
doxorubicin on various osteosarcoma cell lines. Cell J. 19:55–65.
2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Skubitz KM: Phase II trial of
pegylated-liposomal doxorubicin (Doxil) in sarcoma*. Cancer Invest.
21:167–176. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wen XZ, Pan QZ, Xu BS, Xiao W, Weng DS,
Zhao JJ, Xu HR, Huang Z, Niu XH and Zhang X: Phase I study of
pegylated liposomal doxorubicin and cisplatin in patients with
advanced osteosarcoma. Cancer Chemother Pharmacol. 89:209–215.
2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Liu Z, Gao S, Zhu L, Wang J, Zhang P, Li
P, Zhang F and Yao W: Efficacy and safety of anlotinib in patients
with unresectable or metastatic bone sarcoma: A retrospective
multiple institution study. Cancer Med. 10:7593–7600.
2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cai M, Zhu J and Zhou G: Efficacy and
safety of treating refractory bone and soft tissue sarcoma with
anlotinib in different treatment patterns. Comput Math Methods Med.
2022(3287961)2022.PubMed/NCBI View Article : Google Scholar
|