Introduction
The optic nerve is generated by various axons of
retinal ganglion cells (RGCs) located at the back of the eyeball
(1). Optic nerve injury, including
glaucoma, is a type of neurodegenerative disease that occurs in the
eye (2). Degeneration, necrosis
and apoptosis of RGCs and their axons are the pathological causes
of visual impairment following optic nerve injury (3). The main direct result of optic nerve
injury is the demise of RCGs, leading to blindness due to the
inability of neuronal regeneration (4). Therefore, it is essential to explore
novel methods to ameliorate the therapeutic efficacy of current
therapies for the treatment of optic nerve injury.
The Janus kinase (JAK)/STAT signaling pathway is a
common pathway of various cytokine signal transductions, which is
involved in cell proliferation, apoptosis, inflammation and other
processes (5). The activation of
the JAK/STAT pathway requires an extracellular ligand to bind to a
transmembrane receptor, leading to the activation of the
receptor-related JAKs. Subsequently, phosphorylated (p) tyrosine
kinases and their related receptors provide docking sites to the
STAT transcription factors (6).
STATs translocate to the nucleus and control the expression levels
of certain target genes (7). The
activation of the JAK/STAT pathway promotes the progression of
various diseases (8).
Concomitantly, activation of the JAK/STAT pathway is linked to the
occurrence of various neurological diseases (9). For instance, overexpression of p-JAK
and p-STAT induced by inflammatory factors takes part in the
pathogenesis of depression (10).
In addition, during neuroinflammation and in certain degenerative
diseases, inhibition of abnormal activation of the JAK/STAT pathway
is involved in ameliorating and attenuating the disease symptoms
(11). A previous study found that
JAK inhibitors could be an effective clinical treatment for
patients with Parkinson's disease (12).
The Cassia seed, also named ‘Juemingzi’ in Chinese,
belongs to the Cassia genus of Leguminosae (13). At present, the majority of the
studies mainly focused on anthraquinone compounds present in Cassia
seeds, which can be divided into free and conjugated
anthraquinones, including hesperidin, chrysophanol, emoin methyl
ether and aloe emoin (14).
Physcion is its main anthraquinone component and has a wide range
of pharmacological effects; moreover, when administered orally, it
was shown to be essentially non-toxic and able to cross the
blood-brain barrier (15).
Previous studies on the neuroprotective effects of physcion have
mainly focused on the prevention of ischemia, hypoxia and
ischemia-reperfusion brain injury (16). However, the effects of physcion on
optic nerve injury remain unclear.
The present study aimed to explore the possible
mechanism of physcion in alleviating microglia overactivation and
inflammatory response in rats with optic nerve injury. The results
may provide a new theoretical basis for clinical studies which aim
to examine the pathological mechanism and clinical treatment of
optic nerve injury.
Materials and methods
Cell culture and treatment
Rat HAPI microglial cell lines were purchased from
Shenzhen HaodiHuatuo Biological Technology Co., Ltd., and by DNA
species identification it was confirmed that the present HAPI cell
line was derived from rats. Cells were cultured in DMEM (cat. no.
11965092; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS
at 37˚C with 5% CO2. The cells were split into five
groups as follows: Control group; IFN-β-induced inflammation group;
and three physcion groups. The control group did not contain any
treatment except for DMEM. The inflammatory group cells were
treated with rat IFN-β recombinant protein (250 pg/ml; Thermo
Fisher Scientific, Inc.) for 24 h at 37˚C, andthe treatment group
cells were incubated with 50, 100 or 200 µmol/l physcion (Beijing
Solarbio Science & Technology Co., Ltd.) for 24 h at 37˚C
following pretreatment with IFN-β.
Cell Counting Kit-8 (CCK-8)
The cells were seeded into a 96-well plate at 5,000
cells/well and incubated overnight at 37˚C with 5% CO2.
Cells were then treated with different concentrations of physcion
for 24 h, as previously described. Subsequently, 10 µl CCK-8
reagent (Dojindo Molecular Technologies, Inc.) was added into each
well for incubation according to the manufacturer's instructions,
followed by assessment of the optical density value at 450 nm using
a microplate reader (Bio-Rad Laboratories, Inc.).
5-ethynyl-2'-deoxyuridine (EdU)
The cells were seeded into a 96-well plate at 5,000
cells/well and incubated overnight at 37˚C with 5% CO2.
When the cells had grown to 80-90% confluence, they were washed
with PBS and 0.05% trypsin was added for digestion. After 1 min,
the digestion was stopped and centrifugation was performed at 200 x
g for 3 min at room temperature. After removal of the supernatant,
the cells were washed with PBS, 2X EdU (dissolved in DMEM; Beyotime
Institute of Biotechnology) was added at room temperature for 30
min, and then fixation was performed with 4% polyoxymethylene for
15 min at room temperature. Finally, the cells were washed in PBS
with 3% BSA and treated with Triton-X-100 (1%) for 5 min at room
temperature. Subsequently, the cells were incubated with DAPI (10
µg/ml; Beyotime Institute of Biotechnology) for 10 min in the dark.
Finally, a fluorescence microscope was utilized to capture the
images. Five randomly selected fields were imaged and Image J
(Version 1.45; National Institutes of Health) was applied for
quantification.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from HAPI cells
(1x107) using TRIzol® reagent, and its
concentration and quality were determined using the
NanoDrop™ 2000 (both from Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. Subsequently,
total RNA was reverse transcribed into cDNA according to the
instructions of the PrimeScript™ RT Master Mix (Perfect
Real Time) Kit (Takara Biotechnology Co., Ltd.). The TB
Green® Fast qPCR Mix (Takara Biotechnology Co., Ltd.)
was used to perform the RT-PCR procedure. The primer was
synthesized by Shanghai Sangong Biotech. The primer sequences were
as follows: JAK2 forward, 5'-TGGGAATGGCTTGCCTTACA-3' and reverse,
5'-TTGGGTGGATACCAGATCCTT-3'; and STAT3 forward,
5'-CTGAGGTACAATCCCGCTCG-3' and reverse, 5'-TGGCTGCATATGCCCAATCT-3'.
Relative expression of mRNA was normalized to that of U6 and GAPDH,
respectively, using the 2-ΔΔCq method (17). The primer sequences were as
follows: U6 forward, 5'-TGCTGGCATTGGCAGTACAT-3' and reverse,
5'-AAACATGGAACGCCTCATGATTTG-3'; and GAPDH forward,
5'-GCATCTTCTTGTGCAGTGCC-3' and reverse,
5'-GATGGTGATGGGTTTCCCGT-3'.
Cell apoptosis analysis
Cell apoptosis was examined using flow cytometry
methods. Cells from the logarithmic growth phase were seeded into
6-well plates overnight and then treated separately with different
reagents. For each group, 3x105 cells were centrifuged
at 200 x g for 5 min at room temperature. The cells were washed and
stained with PI and Annexin V-FITC (Annexin V-FITC Apoptosis
Detection Kit; cat. no. C1062S; Beyotime Institute of
Biotechnology). Cell apoptosis was examined by FACSCaliber (BD
Biosciences) and analyzed by FlowJo software (version 10.0;
Treestar, Inc.).
Western blot analysis
Cells were incubated overnight and treated with
different reagents and then further incubated for 48 h. Cells were
collected by centrifugation at 200 x g for 5 min at room
temperature, and total protein was extracted using RIPA buffer
(BestBio). Total protein (30 µg/lane) was quantified with a BCA Kit
(Beyotime Institute of Biotechnology) and separated by SDS-PAGE on
a 10% gel. The separated proteins were subsequently transferred
onto a polyvinylidene membrane (MilliporeSigma). Subsequently, the
membranes were blocked with 5% BSA for 60 min at room temperature
and incubated overnight at 4˚C with the following primary
antibodies: Bcl-2 (1:1,000; cat. no. ab32124; Abcam); Bax (1:1,000;
cat. no. ab32503; Abcam); JAK2 (1:1,000; cat. no. ab108596; Abcam);
p-JAK2 (1:1,000; cat. no. ab32101; Abcam); STAT3 (1:1,000; cat. no.
ab68153; Abcam); p-STAT3 (1:1,000; cat. no. ab267373; Abcam); and
GAPDH (1:2,000; cat. no. ab9485; Abcam). Following primary
incubation, the membranes were incubated with horseradish
peroxidase-labeled secondary antibody (1:2,000; cat no. ab150077;
Abcam) for 1 h at room temperature. Protein bands were visualized
using Novex® ECL Chemiluminescent Substrate Reagent Kit
(Thermo Fisher Scientific, Inc.) and protein expression was
quantified by Gel-Pro analyzer 4.0 (Media Cybernetics, Inc.). GAPDH
was used as the loading control.
ELISA analysis
According to the instructions provided by the
manufacturer, the expression levels of IL-6, TNF-α, IL-1β, monocyte
chemoattractant protein-1 (MCP-1), superoxide dismutase (SOD),
peroxidase (POD) and catalase (CAT), and the concentration levels
of malondialdehyde (MDA) and nitric oxide (NO) in the cell
(1x104) supernatant were analyzed by measuring the
absorbance value at 450 nm using a microplate reader. The
expression levels of IL-6 were examined by Rat IL-6 ELISA Kit (cat.
no. PI328; Beyotime Institute of Biotechnology), TNF-α by Rat TNF-α
ELISA Kit (cat. no. PT516; Beyotime Institute of Biotechnology),
IL-1β by Rat IL-1β ELISA Kit (cat. no. PI303; Beyotime Institute of
Biotechnology), MCP-1 by Rat MCP-1 ELISA Kit (cat. no. PC128;
Beyotime Institute of Biotechnology), SOD by Rat SOD ELISA Kit
(cat. no. DS-496; Shanghai Guangrui Biological Technology Co.,
Ltd.), POD by Rat POD ELISA Kit (cat. no. CR102665; YCEXTRACT
Biotechnology; Wuxi Yuncui Biotechnology Co., Ltd.), CAT by Rat CAT
ELISA kit (cat. no. CSB-E13439r; Cusabio Technology, LLC), MDA by
Rat MDA ELISA Kit (cat. no. NDC-EKX-A1T9QG-96; Nordic BioSite AB)
and NO by NO Assay Kit (cat. no. S0021S; Beyotime Institute of
Biotechnology).
Intracellular reactive oxygen species
(ROS) detection
Cells were cultured at a density of
1.8x105 cells/ml, and then incubated for 24 h. The cells
with different treatments were then treated with
2',7'-dichlorodihydrofluorescein diacetate (10 µM; Beyotime
Institute of Biotechnology) in the dark, the cells were resuspended
in PBS, followed by an examination of the fluorescence intensity
using a microscope. Image J was applied for quantification.
Statistical analysis
The data are expressed as mean ± standard deviation.
SPSS version 19.0 (IBM Corp.) was implemented to analyze the data.
One-way ANOVA with Turkey's post hoc test was used for comparison
among multiple groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Physcion prevents IFN-β-induced HAPI
cell injury
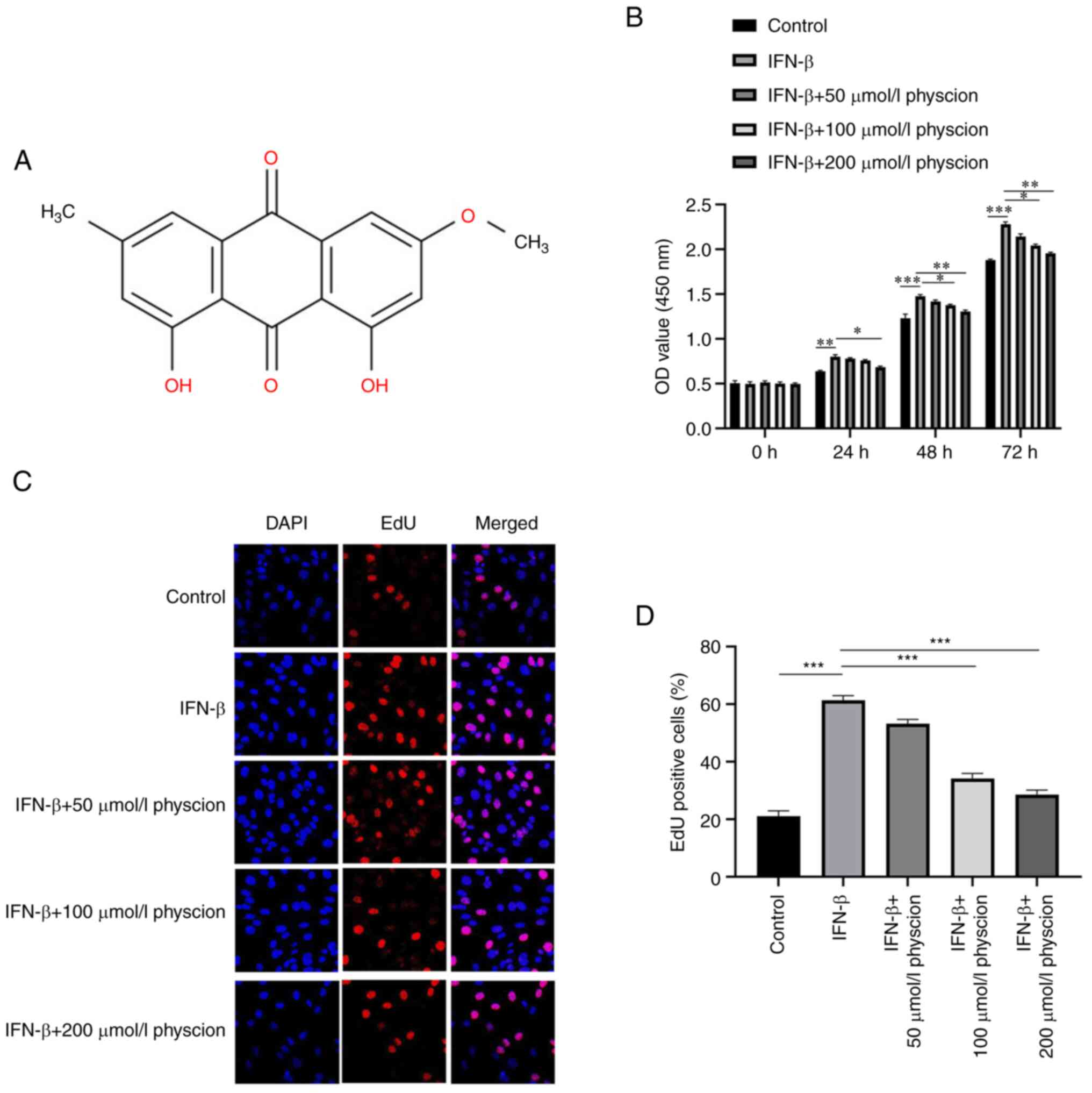
The molecular structure of physcion is shown in
Fig. 1A. The CCK-8 assay indicated
that IFN-β significantly increased the viability of HAPI cells;
however, this phenomenon was inhibited following treatment of the
cells with physcion (50, 100 or 200 µmol/l) in a dose-dependent
manner (Fig. 1B). Similarly, the
results of the EdU incorporation assay indicated that IFN-β induced
the proliferation of HAPI cells. Nevertheless, IFN-β-induced
proliferation in HAPI cells was reversed by physcion (50, 100 or
200 µmol/l) addition (Fig. 1C and
D).
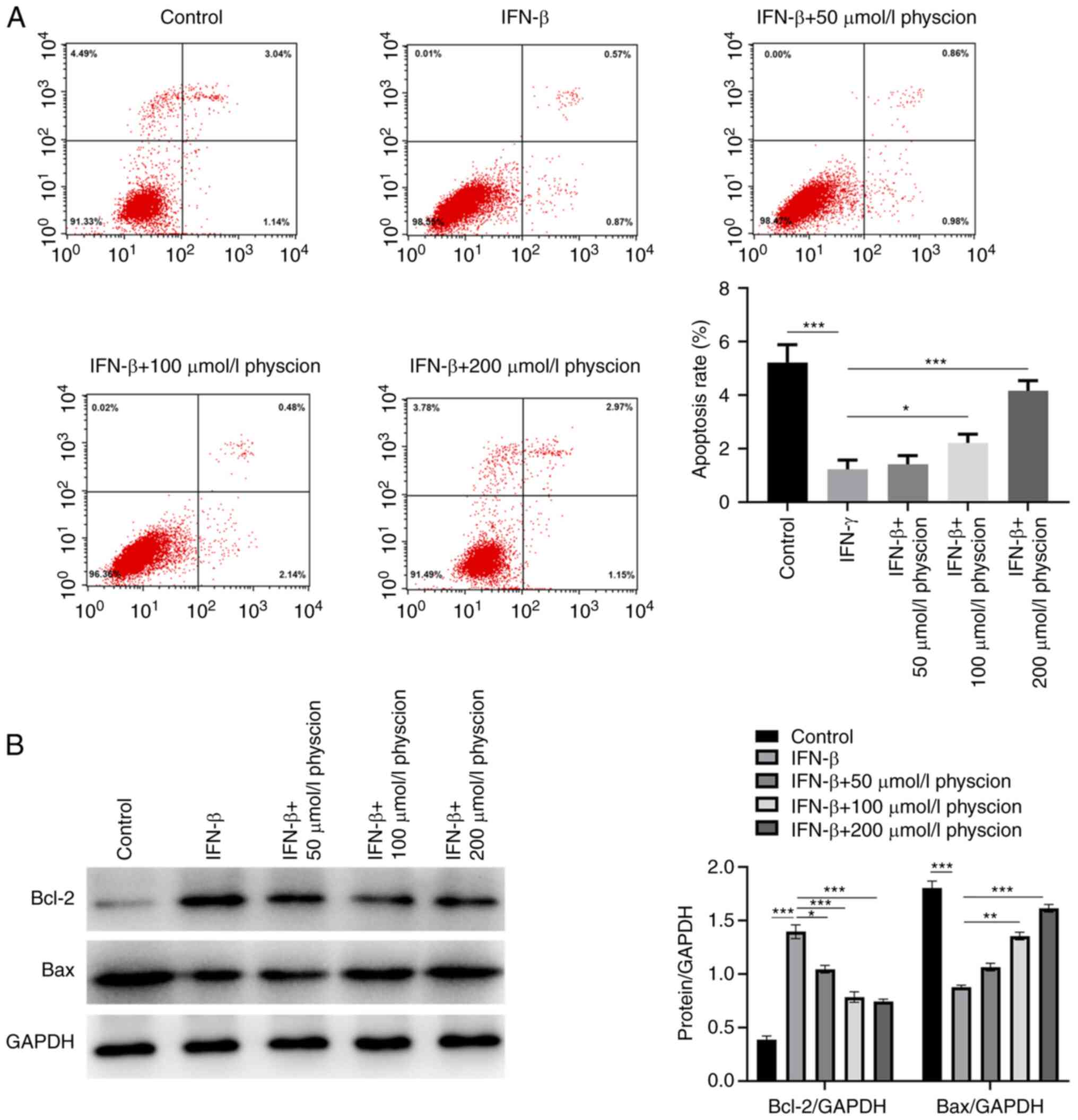
Subsequently, the effects of physcion on the
induction of apoptosis in IFN-β-treated HAPI cells were
investigated. Flow cytometry analysis demonstrated that IFN-β
reduced the extent of HAPI cell apoptosis, while physcion (50, 100
or 200 µmol/l) treatment could increase the proportion of apoptotic
cells (Fig. 2A). Concomitantly,
the apoptotic effect of physcion was confirmed using western blot
analysis. It was shown that IFN-β increased Bcl-2 levels and
decreased Bax levels; however, this effect was counteracted
following physcion (50, 100 or 200 µmol/l) treatment (Fig. 2B).
Physcion restrains the IFN-β-induced
inflammatory response in HAPI cells
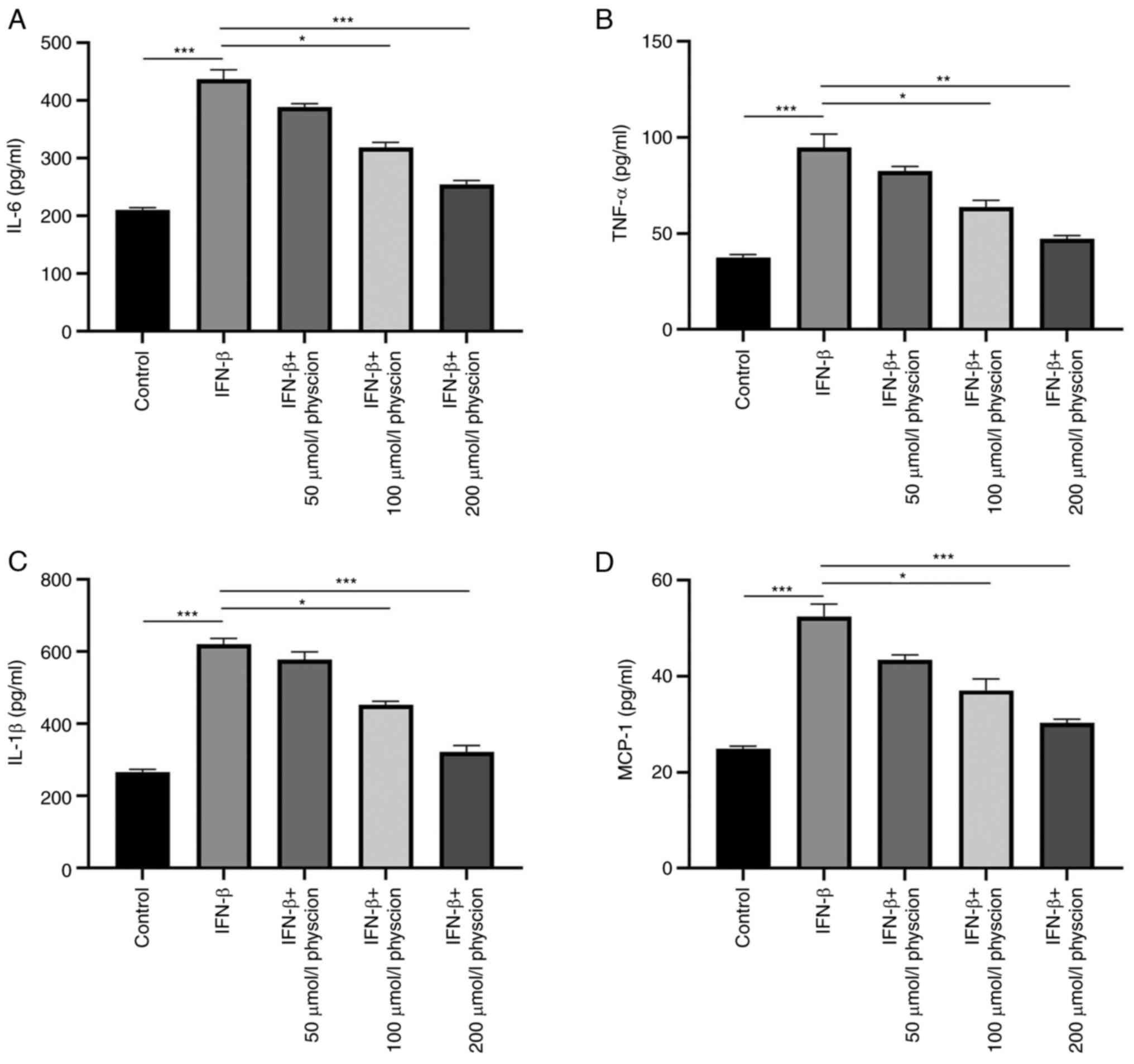
The effects of physcion on the IFN-β-induced
inflammatory response were assessed in HAPI cells. As shown in
Fig. 3A-D, the production of
inflammatory factors (IL-6, TNF-α, IL-1β and MCP-1) was enhanced in
HAPI cells following induction of IFN-β expression. Nonetheless,
treatment of the cells with physcion (50, 100 or 200 µmol/l)
restrained the production of these inflammatory factors.
Physcion alleviates IFN-β-induced
oxidative stress in HAPI cells
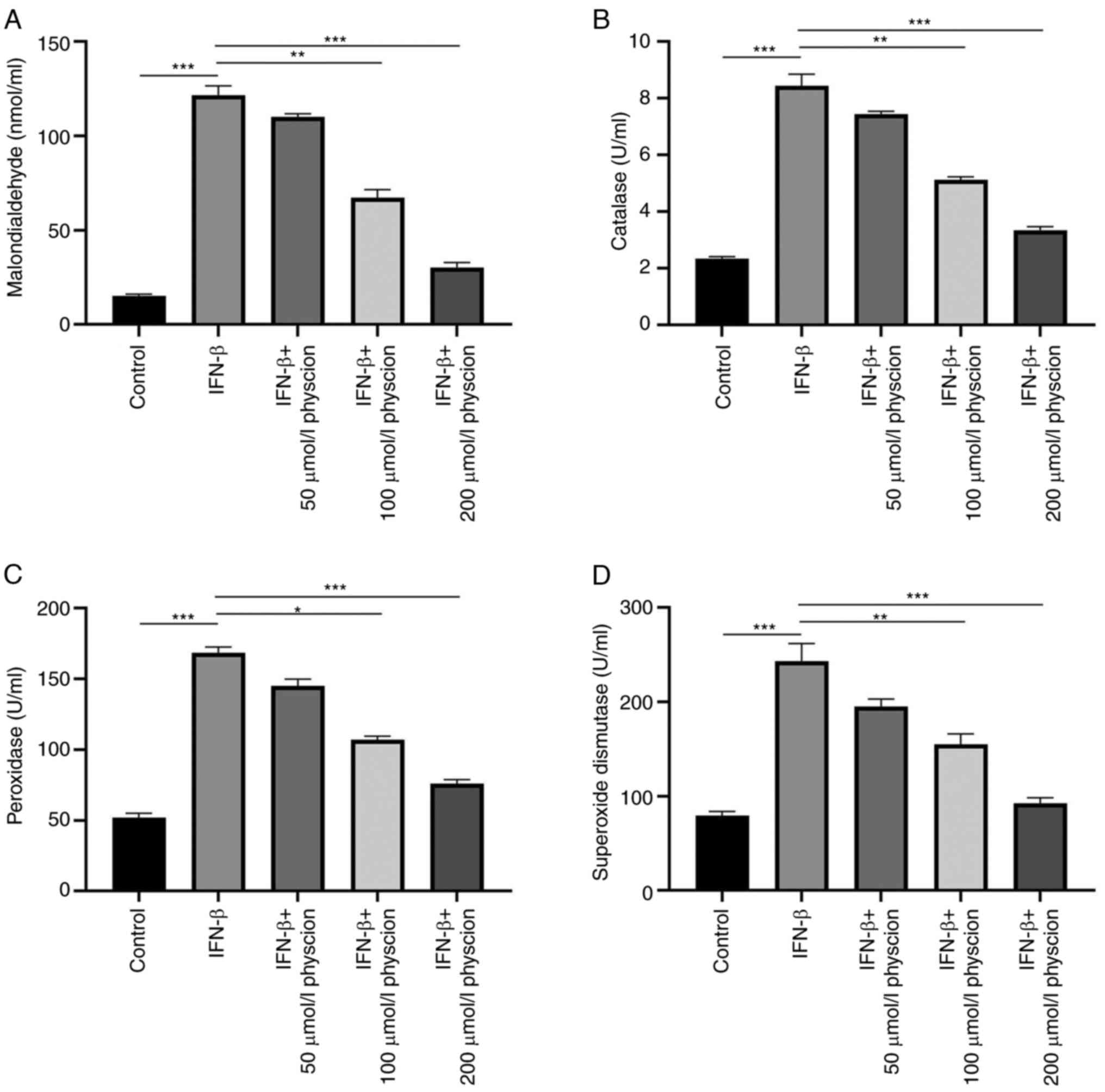
Furthermore, the effects of physcion were assessed
in HAPI cells following oxidative stress induced by IFN-β. The
activity levels of oxidant and antioxidant enzymes were evaluated.
The results indicated that IFN-β increased the activity levels of
SOD, POD and CAT and the concentration of MDA, respectively,
compared with those in the control cells. In addition, physcion
(50, 100 or 200 µmol/l) treatment counterbalanced the oxidative
stress induced by IFN-β as manifested by the decreased levels of
SOD, POD, CAT and MDA (Fig. 4A-D).
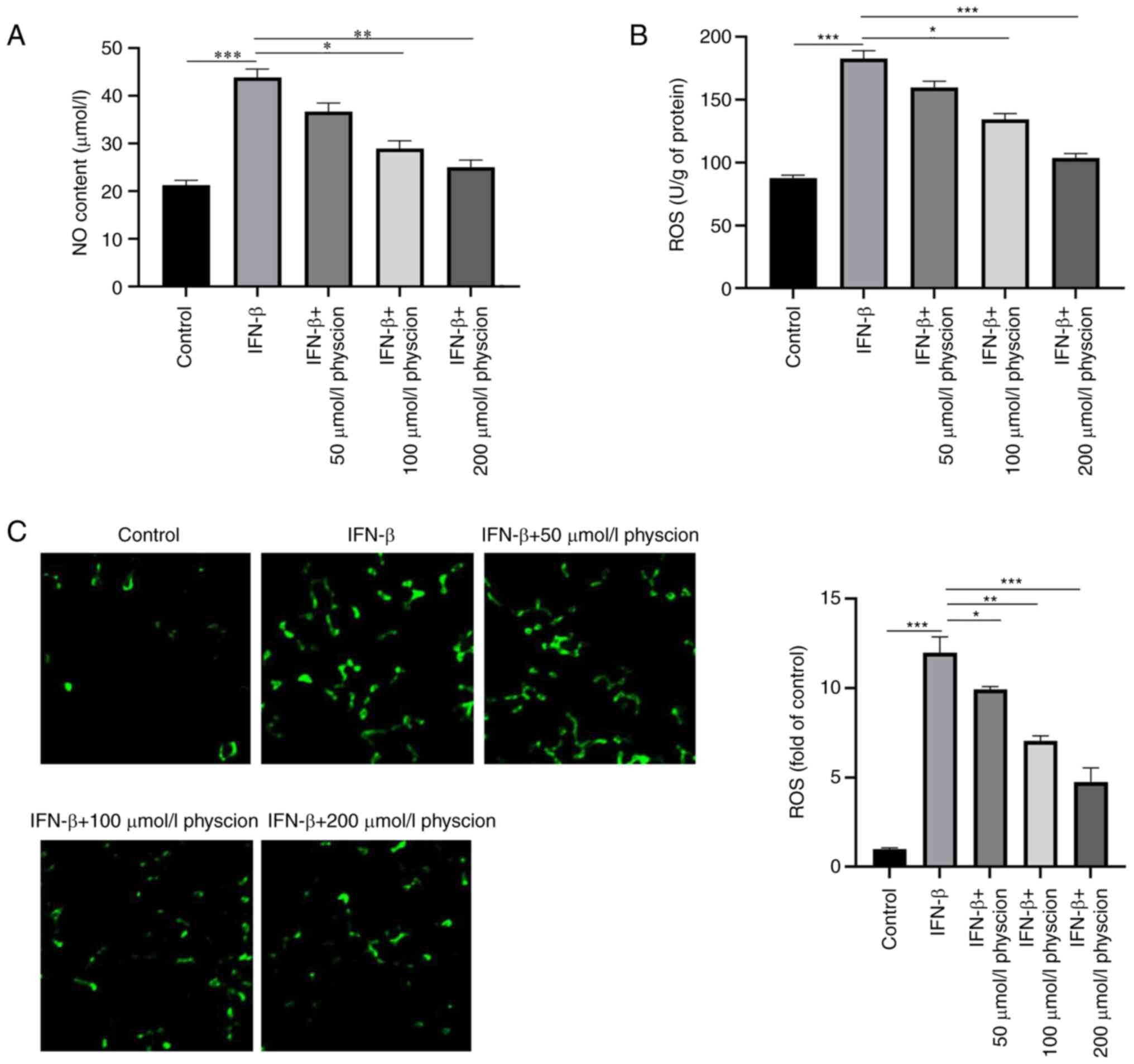
The data indicated that IFN-β significantly reduced oxygen-glucose
deprivation/reperfusion-induced NO content and ROS production in
HAPI cells; however, these changes were offset following physcion
treatment (Fig. 5A-C).
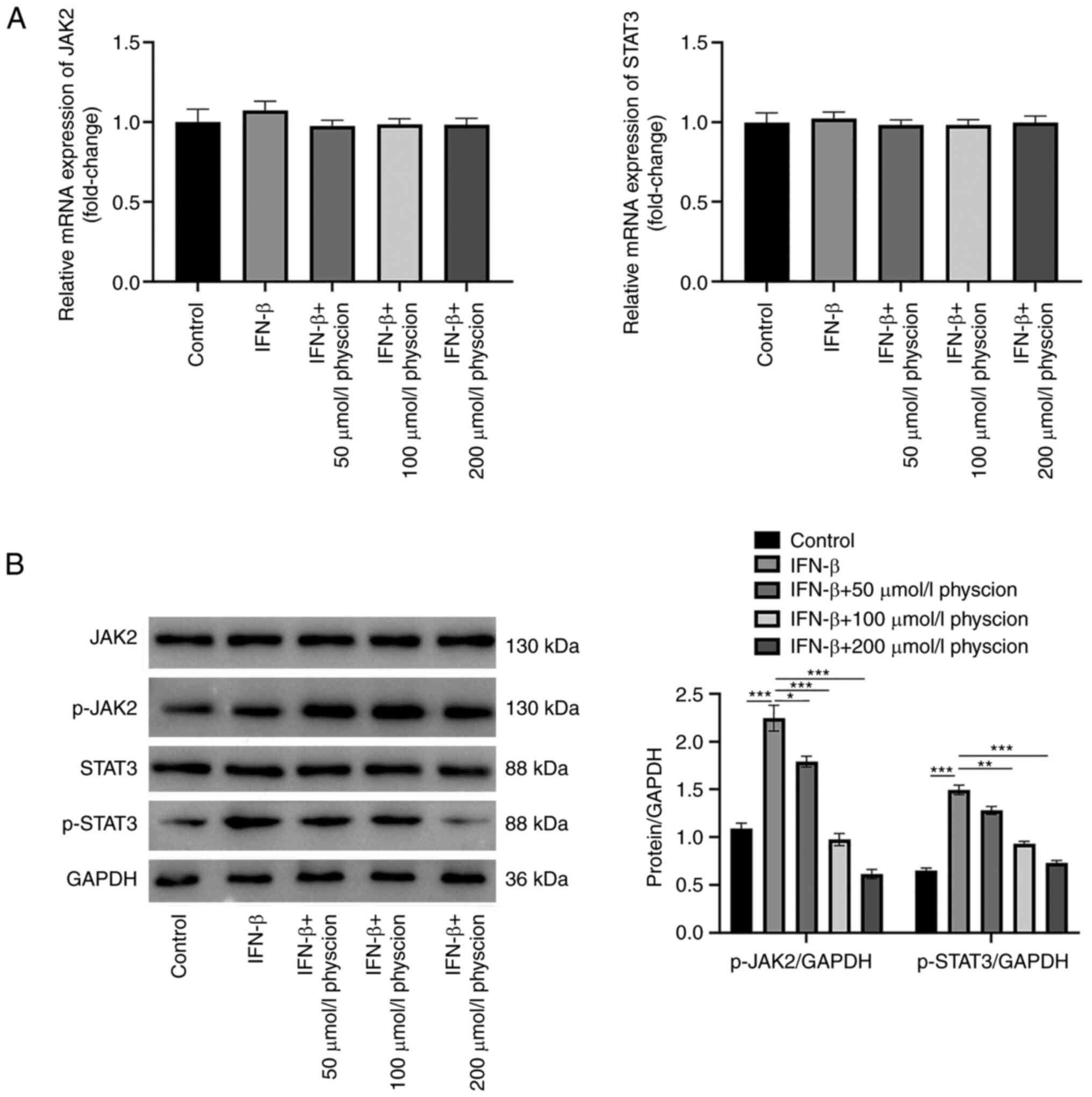
Physcion ameliorates IFN-β-induced
injury in HAPI cells by suppressing the JAK2/STAT3 pathway
A previous study showed that inhibition of the
JAK2/STAT3 pathway could improve central nervous system injury
(18). Therefore, to investigate
if physcion could improve IFN-β-induced HAPI cell injury via the
JAK2/STAT3 pathway, the mRNA and protein levels of key genes in
this pathway were detected. The results indicated that both IFN-β
and physcion did not affect the mRNA levels of JAK2 and STAT3
(Fig. 6A). However, the protein
levels of p-JAK2 and p-STAT3 were increased in HAPI cells treated
with IFN-β. These changes were counteracted following physcion
treatment (Fig. 6B).
Discussion
It is known that physcion exerts a therapeutic role
in a variety of diseases. Han et al (19) indicated that physcion hindered
colorectal cancer metastasis by regulating Sox2. A previous study
by Dong et al (20)
demonstrated that physcion inhibited cerebral ischemia-reperfusion
injury. However, its effect on optic nerve injury remains unclear.
The present study established an in vitro model of optic
nerve injury via the treatment of HAPI cells with IFN-β. The
present results demonstrated that physcion could ameliorate the
in vitro IFN-β-induced neuronal injury by inhibiting
microglia cell proliferation while increasing cell apoptosis.
Microglia cells belong to a resident type of immune
cells of the retina that can act as specialized scavenger cells to
respond to injury by regulating inflammation (21). Microglia activation is featured by
variations in cell morphology, signal transduction and gene
expression that alter the secretion of pro-inflammatory mediators
(22). A previous study showed
that TLR-9 exerted pivotal functions during the development of
optic nerve injury (23).
Moreover, it was reported that physcion could reduce lipogenesis
and alleviate inflammation in ethanol-induced liver injury
(24). Consistent with the
aforementioned reports, the present study indicated that physcion
restrained the IFN-β-induced inflammatory reaction in HAPI cells by
inhibiting the production of IL-6, TNF-α, IL-1β and MCP-1 in
IFN-β-treated HAPI cells.
Oxidative stress is a crucial factor involved in
optic nerve injury and it is involved in the development of this
disease (25). Oxidative stress
contributes to RGC loss in various ocular diseases, such as ocular
trauma and glaucoma (26). A
previous study by Zhang et al (27) suggested that physcion exerted an
anti-breast cancer function by regulating oxidative
stress-controlled mitochondrial apoptosis. The current study
indicated that physcion alleviated IFN-β-induced oxidative stress
in HAPI cells by decreasing the levels of SOD, POD, CAT, MDA, NO
and ROS.
JAK/STAT is an important inflammatory regulatory
pathway stimulated by multiple cytokines (6,28).
The JAK/STAT signaling pathway is active in neurological diseases,
such as stroke and traumatic brain injury (29). High expressions of JAK2 and STAT3
were observed in various neuronal injury models such as rat pain
models of sciatic nerve ligation (30) and experimental glaucoma optic nerve
injury models (31). Moreover,
inhibition of the expression of this factor could significantly
relieve nerve injury (32).
Relevant studies reported that neuropathy such as neuron damage
could lead to increased secretion of IL-6 and IL-21, as well as
activation of the JAK-STAT pathway and phosphorylation of STAT3,
which could induce immune response. Neuron damage could also
activate glial cells, including microglia and astrocytes, and
induce ATP, pro-inflammatory factors, induced ROS, NOS,
prostaglandin, excitatory amino acids and release of other
substances, causing persistent inflammation (33,34).
The present study indicated that the phosphorylation levels of JAK2
and STAT3 were upregulated in HAPI cells following their treatment
with IFN-β. In addition, physcion treatment enhanced JAK2 and STAT3
phosphorylation levels. These findings indicated that the
neuroprotective role of IFN-β may be linked to the JAK2/STAT3
signaling pathway.
Taken together, the results indicated that physcion
exhibited a neuroprotective effect against optic nerve injury by
relieving neuroinflammation and oxidative stress via inactivation
of the JAK2/STAT3 pathway. Therefore, physcion may be a potential
agent for the treatment of optic nerve injury. However, the present
study only investigated the effect of physcion treatment on HAPI
cell proliferation and apoptosis, and the relationship between
JAK2/STAT3 and inflammatory response and oxidative stress was only
examined on a cellular level. This is a limitation of the present
study and subsequent animal experiments should be performed in
future studies.
Acknowledgements
Not applicable.
Funding
Funding: This study was funded by the Scientific Research
Project of Nantong Health and Family Planning Commission (grant no.
MB2020004), Special Project of Clinical Medicine of Nantong
University (grant no. 2022LZ002) and the 2022 Scientific Research
Development Fund of Kangda College of Nanjing Medical University
(grant no. KD2022KYJJZD018).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JJL conducted most of the experiments and wrote the
manuscript, YaZ, MDX and YuZ conducted some of the experiments and
performed the data analysis. PPL, YS and QC designed the study,
provided the funding for the study and revised the manuscript. YaZ
and MDX confirm the authenticity of all the raw data. All authors
have read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yazdankhah M, Shang P, Ghosh S, Hose S,
Liu H, Weiss J, Fitting CS, Bhutto IA, Zigler JS Jr, Qian J, et al:
Role of glia in optic nerve. Prog Retin Eye Res.
81(100886)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang J, Struebing FL and Geisert EE:
Commonalities of optic nerve injury and glaucoma-induced
neurodegeneration: Insights from transcriptome-wide studies. Exp
Eye Res. 207(108571)2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Liu YF, Liang JJ, Ng TK, Hu Z, Xu C, Chen
S, Chen SL, Xu Y, Zhuang X, Huang S, et al: CXCL5/CXCR2 modulates
inflammation-mediated neural repair after optic nerve injury. Exp
Neurol. 341(113711)2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cai XF, Lin S, Geng Z, Luo LL, Liu YJ,
Zhang Z, Liu WY, Chen X, Li X, Yan J and Ye J: Integrin CD11b
deficiency aggravates retinal microglial activation and RGCs
degeneration after acute optic nerve injury. Neurochem Res.
45:1072–1085. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xin P, Xu X, Deng C, Liu S, Wang Y, Zhou
X, Ma H, Wei D and Sun S: The role of JAK/STAT signaling pathway
and its inhibitors in diseases. Int Immunopharmacol.
80(106210)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Hu X, Li J, Fu M, Zhao X and Wang W: The
JAK/STAT signaling pathway: From bench to clinic. Signal Transduct
Target Ther. 6(402)2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lashgari NA, Roudsari NM, Momtaz S,
Sathyapalan T, Abdolghaffari AH and Sahebkar A: The involvement of
JAK/STAT signaling pathway in the treatment of Parkinson's disease.
J Neuroimmunol. 361(577758)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Salas A, Hernandez-Rocha C, Duijvestein M,
Faubion W, McGovern D, Vermeire S, Vetrano S and Vande Casteele N:
JAK-STAT pathway targeting for the treatment of inflammatory bowel
disease. Nat Rev Gastroenterol Hepatol. 17:323–337. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ruganzu JB, Zheng Q, Wu X, He Y, Peng X,
Jin H, Zhou J, Ma R, Ji S, Ma Y, et al: TREM2 overexpression
rescues cognitive deficits in APP/PS1 transgenic mice by reducing
neuroinflammation via the JAK/STAT/SOCS signaling pathway. Exp
Neurol. 336(113506)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Malemud CJ and Miller AH: Pro-inflammatory
cytokine-induced SAPK/MAPK and JAK/STAT in rheumatoid arthritis and
the new anti-depression drugs. Expert Opin Ther Targets.
12:171–183. 2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Oh YC, Li W and Choi JG: Saussureae radix
attenuates neuroinflammation in LPS-stimulated mouse BV2 microglia
via HO-1/Nrf-2 induction and inflammatory pathway inhibition.
Mediators Inflamm. 2021(6687089)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Qin H, Buckley JA, Li X, Liu Y, Fox TH
III, Meares GP, Yu H, Yan Z, Harms AS, Li Y, et al: Inhibition of
the JAK/STAT pathway protects against α-synuclein-induced
neuroinflammation and dopaminergic neurodegeneration. J Neurosci.
36:5144–5159. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rodrigues-Junior AG, Santos MTA, Hass J,
Paschoal BSM and De-Paula OC: What kind of seed dormancy occurs in
the legume genus Cassia? Sci Rep. 10(12194)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang J, Ye H, Lai H, Li S, He S, Zhong S,
Chen L and Peng A: Separation of anthraquinone compounds from the
seed of Cassia obtusifolia L. using recycling counter-current
chromatography. J Sep Sci. 35:256–262. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Adnan M, Rasul A, Hussain G, Shah MA,
Sarfraz I, Nageen B, Riaz A, Khalid R, Asrar M, Selamoglu Z, et al:
Physcion and physcion 8-O-β-D-glucopyranoside: Natural
anthraquinones with potential anticancer activities. Curr Drug
Targets. 22:488–504. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li RR, Liu XF, Feng SX, Shu SN, Wang PY,
Zhang N, Li JS and Qu LB: Pharmacodynamics of five anthraquinones
(aloe-emodin, emodin, rhein, chysophanol, and physcion) and
reciprocal pharmacokinetic interaction in rats with cerebral
ischemia. Molecules. 24(1898)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kretz A, Happold CJ, Marticke JK and
Isenmann S: Erythropoietin promotes regeneration of adult CNS
neurons via Jak2/Stat3 and PI3K/AKT pathway activation. Mol Cell
Neurosci. 29:569–579. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Han YT, Chen XH, Gao H, Ye JL and Wang CB:
Physcion inhibits the metastatic potential of human colorectal
cancer SW620 cells in vitro by suppressing the transcription factor
SOX2. Acta Pharmacol Sin. 37:264–275. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Dong X, Wang L, Song G, Cai X, Wang W,
Chen J and Wang G: Physcion protects rats against cerebral
ischemia-reperfusion injury via inhibition of TLR4/NF-kB signaling
pathway. Drug Des Devel Ther. 15:277–287. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Prinz M, Jung S and Priller J: Microglia
biology: One century of evolving concepts. Cell. 179:292–311.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Voet S, Prinz M and van Loo G: Microglia
in central nervous system inflammation and multiple sclerosis
pathology. Trends Mol Med. 25:112–123. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang L and Li X: Toll-like receptor-9
(TLR-9) deficiency alleviates optic nerve injury (ONI) by
inhibiting inflammatory response in vivo and in vitro. Exp Cell
Res. 396(112159)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yao Y, Zuo A, Deng Q, Liu S, Zhan T, Wang
M, Xu H, Ma J and Zhao Y: Physcion protects against ethanol-induced
liver injury by reprogramming of circadian clock. Front Pharmacol.
11(573074)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Levkovitch-Verbin H, Harris-Cerruti C,
Groner Y, Wheeler LA, Schwartz M and Yoles E: RGC death in mice
after optic nerve crush injury: Oxidative stress and
neuroprotection. Invest Ophthalmol Vis Sci. 41:4169–4174.
2000.PubMed/NCBI
|
|
26
|
Dammak A, Huete-Toral F, Carpena-Torres C,
Martin-Gil A, Pastrana C and Carracedo G: From oxidative stress to
inflammation in the posterior ocular diseases: Diagnosis and
treatment. Pharmaceutics. 13(1376)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhang L, Dong R, Wang Y, Wang L, Zhou T,
Jia D and Meng Z: The anti-breast cancer property of physcion via
oxidative stress-mediated mitochondrial apoptosis and immune
response. Pharm Biol. 59:303–310. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
O'Shea JJ, Pesu M, Borie DC and Changelian
PS: A new modality for immunosuppression: Targeting the JAK/STAT
pathway. Nat Rev Drug Discov. 3:555–564. 2004.PubMed/NCBI View
Article : Google Scholar
|
|
29
|
Simon LS, Taylor PC, Choy EH, Sebba A,
Quebe A, Knopp KL and Porreca F: The Jak/STAT pathway: A focus on
pain in rheumatoid arthritis. Semin Arthritis Rheum. 51:278–284.
2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tang J, Li ZH, Ge SN, Wang W, Mei XP, Wang
W, Zhang T, Xu LX and Li JL: The inhibition of spinal astrocytic
JAK2-STAT3 pathway activation correlates with the analgesic effects
of triptolide in the rat neuropathic pain model. Evid Based
Complement Alternat Med. 2012(185167)2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lozano DC, Choe TE, Cepurna WO, Morrison
JC and Johnson EC: Early optic nerve head glial proliferation and
Jak-Stat pathway activation in chronic experimental glaucoma.
Invest Ophthalmol Vis Sci. 60:921–932. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Luo JM, Cen LP, Zhang XM, Chiang SW, Huang
Y, Lin D, Fan YM, van Rooijen N, Lam DS, Pang CP and Cui Q:
PI3K/akt, JAK/STAT and MEK/ERK pathway inhibition protects retinal
ganglion cells via different mechanisms after optic nerve injury.
Eur J Neurosci. 26:828–842. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li T, Li L, Peng R, Hao H, Zhang H, Gao Y,
Wang C, Li F, Liu X, Chen F, et al: Abrocitinib attenuates
microglia-mediated neuroinflammation after traumatic brain injury
via inhibiting the JAK1/STAT1/NF-κB pathway. Cells.
11(3588)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Dong Y, Hu C, Huang C, Gao J, Niu W, Wang
D, Wang Y and Niu C: Interleukin-22 plays a protective role by
regulating the JAK2-STAT3 pathway to improve inflammation,
oxidative stress, and neuronal apoptosis following cerebral
ischemia-reperfusion injury. Mediators Inflamm.
2021(6621296)2021.PubMed/NCBI View Article : Google Scholar
|