Introduction
Primary nephrotic syndrome (PNS) is a chronic kidney
disease (CKD) commonly seen in children, with an estimated
incidence of 20-70 cases per 1,000,000 individuals and a prevalence
of 160 per million individuals (1). Its age of onset is typically around
3-5 years of age, and it occurs more frequently in boys than in
girls. Steroid therapy is effective in 85-90% of children with PNS
and these children do not progress to renal failure. As its course
is prolonged, steroid-resistant nephrotic syndrome (SRNS) is prone
to develop to end-stage renal disease (ESRD). ESRD has an incidence
of 4.0-17.5 per million individuals and a prevalence of 4.9-38.7
per million individuals in children (2). The ESRD-related mortality rate ranges
from 25.0-27.1% (3). Additionally,
ESRD is a significant economic burden on society and families.
Certain children with PNS gradually progress to ESRD given the
insidious nature of the early stages of the disease and a lack of
specific predictors. Following long-term therapy, children with PNS
may develop mineral metabolism disorder (4).
Fibroblast growth factor (FGF)23 is a phosphaturic
hormone that plays a pivotal role in mineral metabolism
homeostasis. FGF23 predominantly binds to FGF receptor 1 (FGFR1)
(5). The transmembrane protein
klotho is a co-factor that enhances cell surface interactions
(primarily with FGFR1) in vivo by increasing the affinity of
FGFR1 for FGF23(6). FGF23 reduces
phosphate reabsorption by the proximal renal tubules and phosphate
absorption by the intestinal tract, ultimately leading to a
reduction in phosphate levels (7).
Further, FGF23 inhibits 1-α-hydroxylase activity in the kidneys,
consequently reducing 25-hydroxyvitamin D (25-OH-D) levels
(8). According to Meir et
al (9), parathyroid hormone
(PTH) activates nuclear receptor-related protein-1 through PTH
receptors and induces FGF23 transcription in bone cells. In another
study, mice with overexpression of FGF23 exhibited
hyperparathyroidism (10) and
clinical studies have revealed that FGF23 increases the synthesis
and release of PTH in adults (11). In adult patients with CKD, FGF23
has been shown to directly inhibit bone mineralization (12). Furthermore, amongst adults with
renal insufficiency, increased FGF23 levels have been found to be
related to morbidity and mortality (11). The risk of progression of nephrotic
disease to ESRD in children may be reduced through earlier
detection of and intervention for renal injury. Hypertension,
proteinuria, and a low glomerular filtration rate (GFR) may lead to
the progression of PNS to ESRD.
It is well established that FGF23 is significantly
elevated in adult ESRD and is associated with the disturbance of
mineral metabolism (13-15).
However, it is unclear whether FGF23 is elevated in early renal
disease in children and whether FGF23 levels reflect abnormal
mineral metabolism and can be used for early detection. It was
hypothesized that FGF23 is involved in mineral metabolism disorders
and the progression of childhood kidney disease.
Materials and methods
Study participants and inclusion
criteria
Three groups of children (<18 years of age) were
included in the present study: Children with PNS, ESRD, and a
control group. For the PNS group, children with PNS, defined by a
GFR >15 ml/min/1.73 m2 were included unless they had
congenital and secondary nephrotic syndrome. The diagnostic
criteria for PNS were: i) Proteinuria with urinary protein levels
≥50 mg/kg for 24 h or a urine protein/urinary creatinine ratio
(mg/mg) >2.0, and ii) hypoalbuminemia with serum albumin levels
≤25 g/l. The PNS proteinuric phase was characterized by
proteinuria, with a urinary protein to creatinine ratio >2.0 or
positive results for protein on a urine test for 3 consecutive
days. The PNS remission phase was characterized by the absence of
edema and protein-free urine for at least 3 consecutive days.
Hypoalbuminemia affects calcium concentration and is common in PNS
during the proteinuric phase. Albumin-corrected calcium (cCa)
levels were calculated using the following equation: cCa in
mmol/l=total Ca + [0.02 x (40-albumin [in g/l])] (16). For the ESRD group, children were
included if they had GFR <15 ml/min/1.73 m2. The
exclusion criteria for the ESRD group were Henoch-Schönlein purpura
nephritis, hepatitis B virus-related nephritis, drug-induced
nephritis, Alport syndrome, systemic lupus erythematosus nephritis,
congenital urinary tract malformation, or other diagnoses which led
to the children developing ESRD. All control participants had
normal renal function, no renal disease or family history of renal
disease, and no metabolic diseases. The patients were screened
according to the inclusion criteria by two senior attending
physicians.
A total of 36 children with kidney disease were
identified, including 24 patients with PNS and 12 patients with
ESRD. Of the 24 children with PNS, 7 were not in the remission
stage. Therefore, 17 children with PNS in both the proteinuria
phase and remission phase were included. Among the 12 patients with
ESRD, 8 were excluded as the primary disease was not nephrotic
syndrome. Finally, 21 children with kidney disease (17 with PNS
with proteinuria and normal renal function and 4 with ESRD) and 20
healthy children were selected for a cross-sectional observational
study. The median ages of the PNS, ESRD, and control groups were
3.80, 10.53, and 2.75 years, respectively. There were 14 males and
3 females, 3 females and 1 male, and 12 males and 8 females in the
PNS, ESRD, and control groups, respectively. Patients with PNS and
ESRD were admitted to the Department of Pediatric Nephrology, the
First Affiliated Hospital of Jinan University, Guangzhou, China,
and the 20 healthy children were recruited from the outpatient
department of the same hospital. The study was conducted in
accordance with the Declaration of Helsinki and approved by the
Medical Ethics Committee of the First Affiliated Hospital of Ji Nan
University (approval no. 2017-017). Written informed consent was
obtained from the parents and guardians of all patients.
Sample collection
A 2 ml sample of venous blood was collected from
each child who met the inclusion criteria. The blood samples were
centrifuged at 2,415 x g at 4˚C for 15 min. Serum samples were
divided into four parts and stored at -80˚C: Two parts were used
for the analysis of PTH, calcium, phosphate, and 25-OH-D levels at
the Department of Clinical Laboratory of the First Affiliated
Hospital of Jinan University. PTH levels were detected using an
immunoassay analyzer (UniCel DxI800; Beckman Coulter, Inc.). Serum
25-OH-D levels were detected by the LIAISON®
25-hydroxyvitamin D Assay (cat. no. 310600; DiaSorin, Inc.), which
uses chemiluminescent immunoassay technology. The other two parts
of the serum samples were used for the detection of FGF23 levels.
FGF23 levels were determined using human FGF23 ELISA (cat. no.
EZHFGF23-32K; MilliporeSigma) according to the manufacturer's
protocol. The absorbance was measured using a microplate reader at
wavelengths of 450 and 590 nm (Shenzhen Highcreation Technology
Co., Ltd.). Clinical data entry, statistics, and experimental
procedures were performed and confirmed by two physicians.
Statistical analysis
Statistical analysis was performed using SPSS 22
(IBM Corp.). Normally distributed data are presented as the mean ±
standard deviation. Skewed data are presented as the median and
interquartile range. Differences between multiple groups were
compared using a Kruskal-Wallis test followed by a Dunn-Bonferroni
post hoc test.
Categorical data were analyzed using a Fisher's
exact test. Spearman's correlation analysis was used to analyze the
correlation between the FGF23 levels and mineral metabolism
parameters. FGF23, PTH, and 25-OH-D levels were log-transformed for
further analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
The median ages of the PNS, ESRD, and control groups
were 3.80 (2.70-8.40), 10.53 (7.35-12.54), and 2.75 (1.63-5.48)
years, respectively. There was a significant difference in age
between the control and ESRD groups (P=0.036). There were 14 males
and 3 females, 3 females, and 1 male, and 12 males and 8 females in
the PNS, ESRD, and control groups, respectively; the distribution
of sex amongst the groups did not differ significantly (P>0.05).
Table I shows the characteristics
of each group. In the control group, taking the median age as the
time node, the serum FGF23 levels of those >2.75 years was 2.12
(1.60,4.61), and that of those <2.75 years was 2.14 (1.48,4.00;
<2.75 years vs. >2.75 years: Z=−0.11, P=0.91). There was no
association between FGF23 levels and age in the healthy control
group (r=-0.14, P=0.55) (data not shown).
 | Table ICharacteristics of the study
participants. |
Table I
Characteristics of the study
participants.
| Characteristic | PNS, n=17 | ESRD, n=4 | Control, n=20 |
|---|
| Age, median years
(P25-P75) | 3.80 (2.70-8.40) | 10.53
(7.35-12.54) | 2.75 (1.63-5.48) |
| Sex, n (%) | | | |
|
Female | 3(18) | 1(25) | 8(40) |
|
Male | 14(82) | 3(75) | 12(60) |
| Medication, n
(%) | | | |
|
Vitamin
D | 7(41) | 1(25) | - |
|
Calcitriol | 1(6) | 2(50) | - |
|
Calcium
administration | 7(41) | 3(75) | |
|
Glucocorticoid | 8(47) | 2(50) | - |
|
Immunosuppressant | 5(29) | 0 (0) | - |
|
Hemodialysis | 0 (0) | 1(25) | - |
|
Peritoneal
dialysis | 0 (0) | 3(75) | - |
The median FGF23 serum levels in the control, PNS,
and ESRD groups were 2.12 (1.48-3.30), 6.98 (2.24-11.12), and
1,866.00 (341.51-3,225.25) pg/ml, respectively (Table II). The serum FGF23 levels of the
PNS group were significantly higher than that of the control group
(P=0.038). FGF23 levels were significantly higher in the ESRD group
than in the remission stage of PNS (P=0.014) and control group
(P=0.001). PTH levels were significantly higher in the ESRD group
than in the PNS group (P=0.025) and control group (P=0.006). The
PNS group had significantly lower serum calcium and 25-OH-D levels
than the control group (P=0.0001). The serum 25-OH-D levels in the
control group were significantly higher than that in the remission
stage of PNS (P=0.008). The serum Ca levels in the ESRD group were
significantly lower than that in the control group (P=0.017). In
the PNS subgroup analysis, the median serum FGF23 levels were not
significantly higher in patients in the proteinuric phase than in
those in the remission phase (6.98 and 2.99 pg/l, respectively;
P=0.867). Serum calcium levels were significantly higher in the
remission phase compared with that in the proteinuric phase
(P=0.001). These results are shown in Table II and Fig. 1A-E.
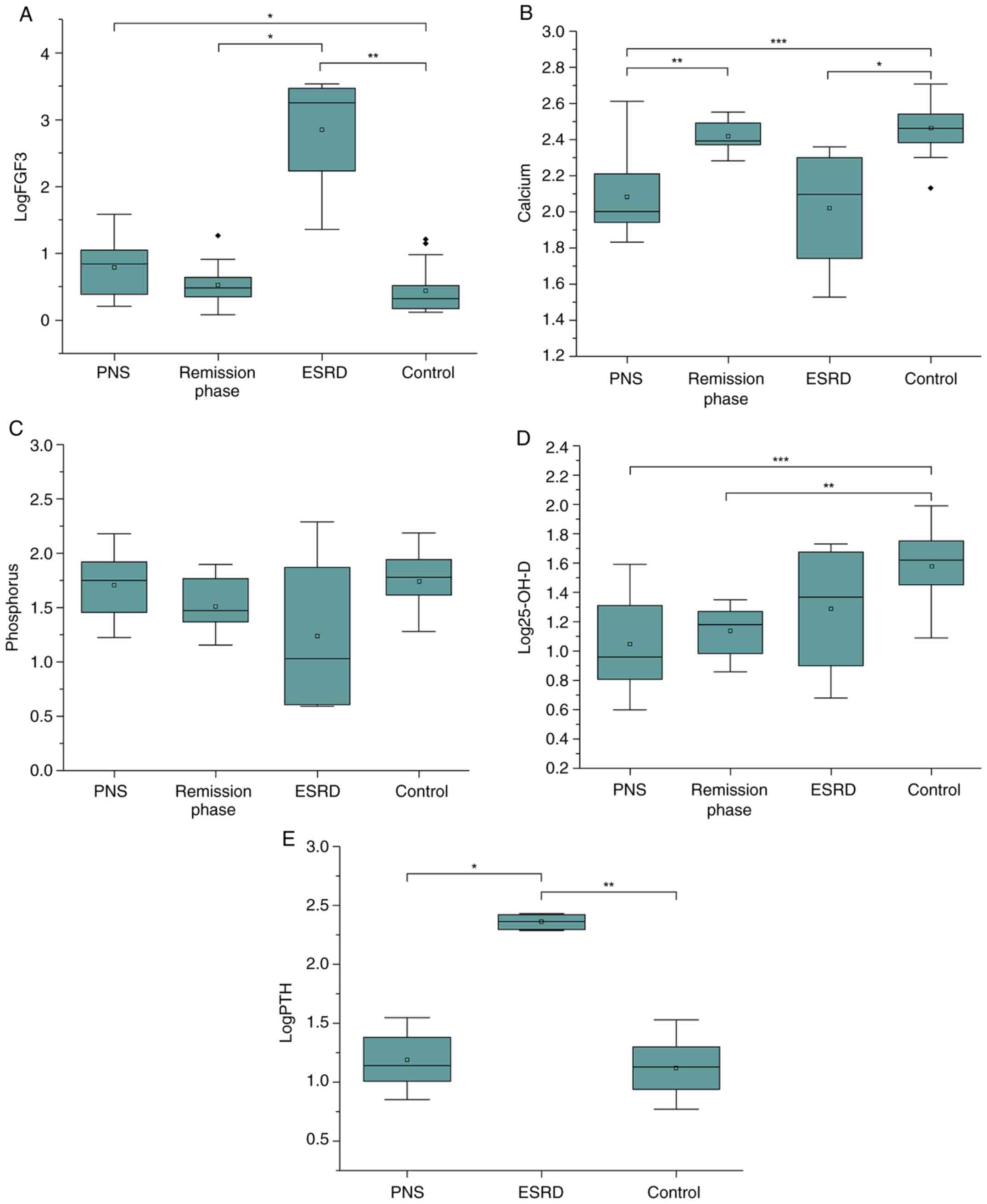 | Figure 1(A) The serum FGF23 levels of the PNS
group were significantly higher than that of the control group
(P=0.038). FGF23 levels were significantly higher in the ESRD group
than in the remission stage of PNS (P=0.014) and control group
(P=0.001). (B) Serum calcium levels were significantly increased in
the remission phase and control groups compared with those in the
PNS group (P=0.001 and P=0.001, respectively). The serum calcium
levels in the ESRD group were significantly lower than that in
control group (P=0.017). (C) There were no significant differences
in the serum phosphate levels of each group. (D) The PNS group and
the remission stage had significantly lower 25-OH-D levels than the
control group (P=0.0001 and P=0.008, respectively). (E) PTH levels
were significantly higher in the ESRD group compared with the PNS
and control groups (P=0.025 and P=0.006, respectively). 25-OH-D,
Serum 25-hydroxy vitamin D; ESRD, end-stage renal disease; FGF23,
fibroblast growth factor 23; PTH, parathyroid hormone; PNS, primary
nephrotic syndrome. *P≤0.05, **P≤0.01,
***P≤0.001. |
 | Table IIFGF23 and mineral metabolism
parameters of the participants. |
Table II
FGF23 and mineral metabolism
parameters of the participants.
| Group | FGF23, pg/ml
(P25-P75) | Calcium, mmol/l | Phosphorus,
mmol/l | 25-OH-D, ng/ml
(P25-P75) | PTH, pg/ml
(P25-P75) |
|---|
| PNS, n=17 | 6.98
(2.24-11.12)a |
2.08±0.21a | 1.67±0.24 | 9.22
(6.14-20.79)a | 13.72
(10.10-25.62)b |
| Remission phase,
n=17 | 2.99
(2.23-4.37)c |
2.42±0.09d | 1.51±0.25 | 15.20
(8.98-19.55)e | - |
| ESRD, n=4 | 1,866.0
(341.51-3,225.25)f |
2.02±0.37f | 1.24±0.08 | 27.55
(6.86-51.19) | 234.53
(195.89-266.38)f |
| Control, n=20 | 2.12
(1.48-3.30) | 2.46±0.13 | 1.74±0.27 | 42.10
(26.7-57.66) | 13.56
(8.5-20.03) |
FGF23 levels were significantly positively
correlated with serum calcium and cCa levels (r=0.518, P=0.033, and
r=0.648, P=0.005, respectively) in the PNS remission phase. For the
control group, FGF23 and 25-OH-D levels were significantly
positively correlated (r=0.505, P=0.046), but FGF23 and iPTH levels
were significantly negatively correlated (r=-0.651, P=0.006). FGF23
was not found to exert any effects on mineral metabolism factors in
the PNS proteinuric phase; these results are shown in Table III.
 | Table IIICorrelation between logFGF23 and
mineral metabolism parameters in patients with PNS and the control
group. |
Table III
Correlation between logFGF23 and
mineral metabolism parameters in patients with PNS and the control
group.
| | Proteinuric
phasea | Remission
phasea |
Controla |
|---|
| Ca, mmol/l | -0.178 (0.494) | 0.518 (0.033) | 0.262 (0.282) |
| cCa, mmol/l | 0.115 (0.660) | 0.648 (0.005) | - |
| P, mmol/l | 0.241 (0.351) | 0.003 (0.992) | 0.467 (0.051) |
| 25-OH-D, ng/ml | -0.007 (0.978) | 0.230 (0.473) | 0.505 (0.046) |
| Log PTH, pg/ml | -0.022 (0.935) | - | -0.651 (0.006) |
| cCa*P,
mg2/dl2 | 0.231 (0.372) | 0.156 (0.551) | - |
Discussion
Adult patients with ESRD exhibit elevated levels of
FGF23; however, data on FGF23 in childhood PNS and patients who
have progressed to ESRD is scarce. The results of the present study
showed that FGF23 levels begin to rise in early-stage PNS with
normal GFR, serum phosphate, and calcium levels. In contrast, Van
Husen et al (17) and
Bacchetta et al (18)
reported elevated levels of FGF23 in children with Stage 3 and
Stage 2 CKD, respectively. De Seigneux et al (19) found that the concentration of FGF23
at the time of relapse in eight children with PNS was higher than
that in remission; patients in this study were treated with
corticosteroids, which may have influenced the results. Yadav et
al (20) reported reduced
levels of vitamin D and found that urinary losses may lead to lower
levels of FGF23 in adults with untreated PNS. Bacchetta et
al (18) showed that
corticosteroid therapy was associated with increased FGF23 levels
in children with CKD. In the present study, FGF23 levels in the PNS
subgroup in remission were lower than those in the subgroup with
proteinuria. Therefore, the reason for the increase in FGF23 levels
in the proteinuria stage of PNS may be related to proteinuria or
PNS disease itself.
FGF23 levels were significantly increased in
patients who progressed to ESRD. Decreased kidney function may lead
to increased FGF23 levels in ESRD and higher levels correlate with
more severe kidney disease. In adult CKD, high levels of FGF23 are
considered a risk factor for progression to ESRD (21). Although FGF23 levels began to
increase in patients with PNS with normal GFR, they increased
significantly in patients who progressed to ESRD in this study,
indicating that high FGF23 levels may also be a risk factor for
disease progression in children.
Despite significantly elevated FGF23 levels, it was
found that serum phosphate levels were normal in children with PNS.
Several factors, including calcium and vitamin D levels, dietary
phosphate intake, and skeletal conditions can affect serum
phosphate levels. Trautvetter et al (22) demonstrated that high phosphate
intake leads to elevated FGF23 levels, but there has been limited
research on the mechanism of action of FGF23 on phosphate
metabolism in children with PNS. Siomou et al (23) observed a positive correlation
between serum phosphate and FGF23 levels, whilst another study
demonstrated no association (24).
The present study found no significant correlation between serum
phosphate and FGF23 levels in children with kidney disease. Our
results suggested that serum phosphate levels may not be a
sensitive measure of early-stage CKD in children.
FGF23 levels in the PNS remission phase positively
correlated with serum calcium and cCa levels. Shimada et al
(25) found that dietary calcium
supplementation increased the mRNA expression of FGF23 in mice
lacking the vitamin D receptor, suggesting that calcium has an
independent effect on FGF23 levels. Furthermore, previous studies
have found that patients receiving hemodialysis with dialysis
solution containing high levels of calcium, and/or taking oral
calcitriol had high serum levels of FGF23(26). However, the mechanisms underlying
the relationship between FGF23, and ionized calcium are yet to be
fully elucidated.
Hyperphosphatemia and low 25-OH-D levels in patients
with ESRD can result in increased PTH levels, which often leads to
secondary hyperparathyroidism (14). In the present study, FGF23 and PTH
levels in children who progressed to ESRD were increased. There is
a negative feedback loop between FGF23 and PTH, whereby PTH
signaling activates protein kinase A, which increases FGF23
expression and secretion, and the secretion of PTH is inhibited by
FGF23(27). In ESRD, expression of
the klotho receptor is decreased and FGFRs are downregulated which
can result in the resistance of parathyroid cells to FGF23(28). Here, it was found that patients
with PNS had lower serum 25-OH-D levels. In agreement with this,
Pavix et al (29) found a
decrease in 25-OH-D levels during the early stage of kidney
disease. Lower levels of 25-OH-D in children with PNS may be due to
lower levels of outdoor activity and reduced vitamin D synthesis as
compared to healthy children. In children with PNS with
proteinuria, vitamin D and calcium-binding proteins are lost
through urine (30) and there is a
decrease in the activity of 1-α hydroxylase, which is essential for
converting 25(OH)D3 into
1,25(OH)2D3 (13).
In the present study, both FGF23 and 25-OH-D levels
were significantly altered in early renal disease, which may
indicate the involvement of FGF23 and 25-OH-D in mineral metabolism
disorders during their early stages. Whilst there was no
correlation between FGF23 and mineral metabolism-related factors in
the PNS group, this may have been affected by abnormal calcium and
phosphate metabolism. This result suggests that FGF23 levels may be
influenced by the disease itself, in addition to mineral
metabolism.
In the control group, FGF23 levels of children
>2.75 years old compared to those <2.75 years old were not
significantly different. There was no correlation between FGF23
levels and age in the control group. Bacchetta et al
(18) also found that FGF23 serum
levels increased after 15 years of age as phosphate decreased. In
this study, all children were <15 years of age; age may have
little effect on serum FGF23 levels in young children with CKD.
The present study has some limitations. First, as a
single-center study, the sample size was small. Second, as the
study was cross-sectional and observational, it was not possible to
examine the mechanisms underlying the relationships between
variables. Third, the ELISA kit used initially in this study was
discontinued, so the number of patients in the study could not be
increased to compensate for any potential differences.
In conclusion, the present study showed that FGF23
levels began to rise in children with PNS before Stage 1 CKD, and
FGF23 levels may be associated with the progression and the
severity of nephrosis in children. This study suggests that serum
FGF23 levels are useful for the early detection of abnormal mineral
metabolism in children with PNS.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Medical Science
Foundation of Jinan University (grant no. 88016-013037).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
FY, SZ and ZG performed the clinical diagnosis and
treatment. SP and DL collected the specimens. DL and FY performed
the experiments. DL and SP analyzed the data. DL and FY wrote the
manuscript. DL and FY confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
Declaration of Helsinki and was approved by the First Affiliate
Ethics Committee of Jinan University Affiliated Hospital
(Guangzhou, China; approval no. 2017-017).
Patient consent for publication
The parents and/or legal guardians of all study
participants provided written informed consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Eddy AA and Symons JM: Nephrotic syndrome
in childhood. Lancet. 362:629–639. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Shi XM, Liu BN, Zhong XH, Wang F and Ding
J: Epidemiology of chronic kidney disease in children. Zhonghua Er
Ke Za Zhi. 57:721–724. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
3
|
United States Renal Data System: 2018
USRDS annual data report: ESRD among children, adolescents, and
young adults (EB/OL) 2018.
|
|
4
|
Aggarwal A, Yadav AK, Ramachandran R,
Kumar V, Kumar V, Sachdeva N, Khandelwal N and Jha V: Bioavailable
vitamin D levels are reduced and correlate with bone mineral
density and markers of mineral metabolism in adults with nephrotic
syndrome. Nephrology (Carlton). 21:483–489. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Takashi Y and Fukumoto S: FGF23 beyond
phosphotropic hormone. Trends Endocrinol Metab. 29:755–767.
2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kurosu H and Kuro-O M: The Klotho gene
family as a regulator of endocrine fibroblast growth factors. Mol
Cell Endocrinol. 299:72–78. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Edmonston D and Wolf M: FGF23 at the
crossroads of phosphate, iron economy and erythropoiesis. Nat Rev
Nephrol. 16:7–19. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Perwad F, Zhang MY, Tenenhouse HS and
Portale AA: Fibroblast growth factor 23 impairs phosphorus and
vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin
D-1alpha-hydroxylase expression in vitro. Am J Physiol Renal
Physiol. 293:F1577–F1583. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Meir T, Durlacher K, Pan Z, Amir G,
Richards WG, Silver J and Naveh-Many T: Parathyroid hormone
activates the orphan nuclear receptor Nurr1 to induce FGF23
transcription. Kidney Int. 86:1106–1115. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bai X, Miao D, Li J, Goltzman D and
Karaplis AC: Transgenic mice overexpressing human fibroblast growth
factor 23 (R176Q) delineate a putative role for parathyroid hormone
in renal phosphate wasting disorders. Endocrinology. 145:5269–5279.
2004.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Razzaque MS and Lanske B: The emerging
role of the fibroblast growth factor-23-klotho axis in renal
regulation of phosphate homeostasis. J Endocrinol. 194:1–10.
2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Liu S and Quarles LD: How fibroblast
growth factor 23 works. J Am Soc Nephrol. 18:1637–1647.
2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gutierrez O, Isakova T, Rhee E, Shah A,
Holmes J, Collerone G, Jüppner H and Wolf M: Fibroblast growth
factor-23 mitigates hyperphosphatemia but accentuates calcitriol
deficiency in chronic kidney disease. J Am Soc Nephrol.
16:2205–2215. 2005.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Stubbs J, Liu S and Quarles LD: Role of
fibroblast growth factor 23 in phosphate homeostasis and
pathogenesis of disordered mineral metabolism in chronic kidney
disease. J Semin Dial. 20:302–308. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Park SY, Jeong KH, Moon JY, Lee SH, Ihm
CG, Rhee SY, Woo JT, Oh IH and Lee TW: The relationship between
circulating fibroblast growth factor 23 and bone metabolism factors
in Korean hemodialysis patients. Clin Exp Nephrol. 14:239–243.
2010.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Figge J, Jabor A, Kazda A and Fencl V:
Anion gap and hypoalbuminemia. Crit Care Med. 26:1807–1810.
1998.PubMed/NCBI View Article : Google Scholar
|
|
17
|
van Husen M, Fischer AK, Lehnhardt A,
Klaassen I, Möller K, Müller-Wiefel DE and Kemper MJ: Fibroblast
growth factor 23 and bone metabolism in children with chronic
kidney disease. Kidney Int. 78:200–206. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bacchetta J, Dubourg L, Harambat J,
Ranchin B, Abou-Jaoude P, Arnaud S, Carlier MC, Richard M and
Cochat P: The influence of glomerular filtration rate and age on
fibroblast growth factor 23 serum levels in pediatric chronic
kidney disease. J Clin Endocrinol Metab. 95:1741–1748.
2010.PubMed/NCBI View Article : Google Scholar
|
|
19
|
de Seigneux S, Courbebaisse M, Rutkowski
JM, Wilhelm-Bals A, Metzger M, Khodo SN, Hasler U, Chehade H, Dizin
E, Daryadel A, et al: Proteinuria increases plasma phosphate by
altering its tubular handling. J Am Soc Nephrol. 26:1608–1618.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Yadav AK, Ramachandran R, Aggarwal A,
Kumar V, Gupta KL and Jha V: Fibroblast growth factor 23 in
untreated nephrotic syndrome. Nephrology (Carlton). 23:362–365.
2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Isakova T, Xie H, Yang W, Xie D, Anderson
AH, Scialla J, Wahl P, Gutiérrez OM, Steigerwalt S, He J, et al:
Fibroblast growth factor 23 and risks of mortality and end-stage
renal disease in patients with chronic kidney disease. JAMA.
305:2432–2439. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Trautvetter U, Jahreis G, Kiehntopf M and
Glei M: Consequences of a high phosphorus intake on mineral
metabolism and bone remodeling in dependence of calcium intake in
healthy subjects-a randomized placebo-controlled human intervention
study. Nutr J. 15:1–11. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Siomou E, Challa A, Printza N, Giapros V,
Petropoulou F, Mitsioni A, Papachristou F and Stefanidis CJ: Serum
osteoprotegerin, RANKL and fibroblast growth factor-23 in children
with chronic kidney disease. Pediatr Nephrol. 26:1105–1114.
2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wesseling-Perry K, Tsai EW, Ettenger RB,
Jüppner H and Salusky IB: Mineral abnormalities and long-term graft
function in pediatric renal transplant recipients: A role for
FGF-23. Nephrol Dial Transplant. 26:3779–3784. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shimada T, Yamazaki Y, Takahashi M,
Hasegawa H, Urakawa I, Oshima T, Ono K, Kakitani M, Tomizuka K,
Fujita T, et al: Vitamin D receptor-independent FGF23 actions in
regulating phosphate and vitamin D metabolism. Am J Physiol Renal
Physiol. 289:F1088–F1095. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cancela AL, Oliveira RB, Graciolli FG, dos
Reis LM, Barreto F, Barreto DV, Cuppari L, Jorgetti V, Carvalho AB,
Canziani ME, et al: Fibroblast growth factor 23 in hemodialysis
patients: effects of phosphate binder, calcitriol and calcium
concentration in the dialysate. Nephron Clin Pract. 117:c74–c82.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lanske B and Razzaque MS: Molecular
interactions of FGF23 and PTH in phosphate regulation. Kidney Int.
86:1072–1074. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Galitzer H, Ben-Dov IZ, Silver J and
Naveh-Many T: Parathyroid cell resistance to fibroblast growth
factor 23 in secondary hyperparathyroidism of chronic kidney
disease. Kidney Int. 77:211–218. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pavik I, Jaeger P, Ebner L, Wagner CA,
Petzold K, Spichtig D, Poster D, Wüthrich RP, Russmann S and Serra
AL: Secreted Klotho and FGF23 in chronic kidney disease stage 1 to
5: A sequence suggested from a cross-sectional study. Nephrol Dial
Transplant. 28:352–359. 2013.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sato KA, Gray RW and Lemann J: Urinary
excretion of 25-hydroxyvitamin D in health and the nephrotic
syndrome. J Lab Clin Med. 99:325–330. 1982.PubMed/NCBI
|















