Introduction
Almost all cases of HCV infection have been cured
since the worldwide introduction of direct-acting antivirals
(DAAs), including Japan (1-7),
and sustained virologic response (SVR) induced by DAA treatment for
HCV results in significantly lower all-cause mortality and lower
incidence rates of hepatocellular carcinoma (HCC) (8). Though marked improvements in the
treatment and prognosis of patients with HCV infection have been
achieved, certain patient populations, for example, those having a
serious underlying disease, have not received the benefits of the
advancements made in HCV treatment. Patients with psychiatric
disorders are one such population. Unfortunately, they have been
excluded from antiviral treatment with interferon-based regimens
because of their neuropsychiatric side effects, insufficient
treatment compliance or, more likely, due to re-infection from
their continued intravenous drug use (9-12).
In the era of interferon-based regimen treatment, only a few
patients with psychiatric disorders were treated successfully and
achieved SVR (9). Although
psychiatric disorders may inhibit to starting treatment for
HCV-infected patients, it is necessary to treat HCV infection in
patients with psychiatric disorders, because HCV infection itself
was associated with psychiatric disorders (13-15)
and psychiatric disorders are common co-morbidities of individuals
with HCV infections (9). DAA
treatment is more effective and tolerated than interferon-based
treatment for HCV infection. Therefore, DAA treatment for patients
with psychiatric disorders has to be considered to decrease HCV
infection in this vulnerable population and achieve HCV eradication
for everyone, including vulnerable individuals. To the best of our
knowledge, although there are limited reports on DAA treatment in
patients with HCV and psychiatric disorders in Europe and the
United States (16-18),
none were reported in Asia, especially Japan.
In the current study, data were analyzed and
compared between patients from patients with HCV treated with DAAs
who presented or did not present psychiatric disorders. The
efficacy and safety of DAA treatment for patients with psychiatric
disorders were also examined.
Patients and methods
Patients
This was an observational, single-center study in
which data were retrospectively collected from the medical records
of Suzuka General Hospital (Japan) between September 2014 and
December 2021. Patients with HCV infection who had been started on
DAA treatment for their HCV infection at the Department of
Gastroenterology of the hospital were included. In total, 15 (7
males and 8 females) and 209 (110 males and 99 females) patients
with HCV and psychiatric disorders (P) or with non-psychiatric
disorders (NP), respectively, were started on DAA treatment for HCV
infection. The 15 cases of HCV in Group P included 2 cases with
chronic hepatitis after acute HCV infection (19), and, of the 209 cases of HCV cases
in Group NP, 1 had chronic hepatitis after acute HCV infection.
Oral informed consent, including a statement of agreement to the
use of their samples in scientific research, was obtained from each
patient at the first medical examination in the Outpatient
Department of Suzuka General Hospital (http://www.miekosei.or.jp/2_sch/privacy.html).
Informed consent was also obtained in the form of an opt-out on the
website. The present study was approved by the Ethics Committee of
Suzuka General Hospital (approval no. 284).
Diagnosis
HCV infection was diagnosed based on a patient being
positive for both anti-HCV antibody (HCV-Ab) and HCV RNA. Chronic
hepatitis infection, cirrhosis and HCC were diagnosed using
ultrasonography [Aloka Arietta 850 (Hitachi, Ltd.), Aplio a550
(Canon Medical Systems Corporation Co., Ltd.)], CT (Aquilion
TSX-101A and Aquilion PRIME (Canon Medical Systems Corporation Co.,
Ltd.)] and/or MRI scan [Ingenia Elition 3.0T and Achieva 1.5T
(Koninklijke Philips N.V.)]. Serum HCV-Ab levels were measured
using a chemiluminescent enzyme immunoassay (cobas e801, cat. no.
30916; Roche Diagnostics). Blood specimens were obtained by drawing
blood from each patient, and the serum fraction was obtained by
centrifugation (2,100 x g at room temperature for 5 min) of the
blood specimens for HCV-Ab level analysis. Measurements of
α-fetoprotein (AFP), protein induced by vitamin K absence or
antagonist-II (PIVKAII), HCV RNA, serogroup and genotype analyses
were performed at LSI Medience Corporation. HCV genotyping was
performed when the HCV serotype could not be determined. The
detection of HCV amino acid substitutions was performed at SRL,
Inc. or the Division of Virology, Department of Infection and
Immunity, Jichi Medical University School of Medicine (Tochigi,
Japan). Aspartate aminotransferase (AST), alanine aminotransferase
(ALT), alkaline phosphatase (ALP), total bilirubin (T-Bil), albumin
(Alb) and creatinine (Crea) levels were measured using the
Cobas® 8000 modular analyzer series (Roche Diagnosis
K.K.) or the Labospect 008 (Hitachi High-Technology Corporation).
ALP levels were measured using the Japan Society of Clinical
Chemistry (JSCC) method (20). The
platelet count was measured using the Sysmex® XE2100I™
hematology automated analyzer (Sysmex Corporation). The prothrombin
time (PT) values were measured using the Sysmex CS-2500 automated
coagulation analyzer (Sysmex Corporation). Blood samples were
obtained from each patient. The serum fraction obtained via
centrifugation (2,100 x g at room temperature for 5 min) of the
blood specimens were used for the analysis of AST, ALT, ALP, T-Bil,
Alb and Crea serum levels. Whole blood samples were used for the
platelet count analysis. The plasma that was separated using a
blood-collecting container with sodium citrate was used for the
analysis of the PT.
Statistical analysis
Statistical analysis was performed using BellCurve
v3.20 (Social Survey Research Information Co., Ltd.) for Excel
(Microsoft Corporation). Fisher's exact test, Mann-Whitney U test
and Friedman's test were used for the comparisons between groups.
These methods of analysis were performed one time for each
comparison.
Results
Characteristics of HCV patients with
and without psychiatric disorders
Of the 209 patients in the group NP, nine were
re-treated after post-DAA treatment relapse or because did not
respond to DAA treatment; eight cases were retreated once, while
one case was retreated twice using other DAA agents. No cases of
HCV/other virus co-infection were found. The co-morbid psychiatric
disorders are listed in Table
I.
 | Table IList of psychiatric disorders in
hepatitis virus C RNA-positive patients with psychiatric
disorders. |
Table I
List of psychiatric disorders in
hepatitis virus C RNA-positive patients with psychiatric
disorders.
| Psychiatric
disorder | Number of cases |
|---|
| Alcoholism | 1 |
| Cenesthopathy | 1 |
| Dementia | 1 |
| Delirium | 1 |
| Depression | 7 |
| Dissociative
disorder | 1 |
| Epileptic
psychosis | 1 |
| Insomnia | 6 |
| Panic disorder | 1 |
| Schizophrenia | 2 |
| Stimulant
psychosis | 1 |
All 15 cases in group P had at least one psychiatric
disease, as shown in Table I. The
drugs prescribed for these cases were alprazolam, aripiprazole,
brotizolam, carbamazepine, chlorpromazine, donepezil, duloxetine,
ethyl loflazepate, etizolam, flunitrazepam, haloperidol,
levetiracetam, lorazepam, milnacipran, mirtazapine, nitrazepam,
olanzapine, sodium valproate, sulpiride and/or zolpidem.
The details of the characteristics before DAA
treatment of both groups are presented in Fig. 1. Group P was younger than group NP
(55±13.9 years vs. 68±13.0 years, respectively; P=0.04). However,
no significant differences in the male/female ratio, history of
interferon therapy, chronic hepatitis/cirrhosis ratio, DAA
re-treatment and laboratory data were observed between the two
groups. Cirrhosis was present in 3 patients (20%) in group P and 43
(20.6%) in group NP (P>0.999).
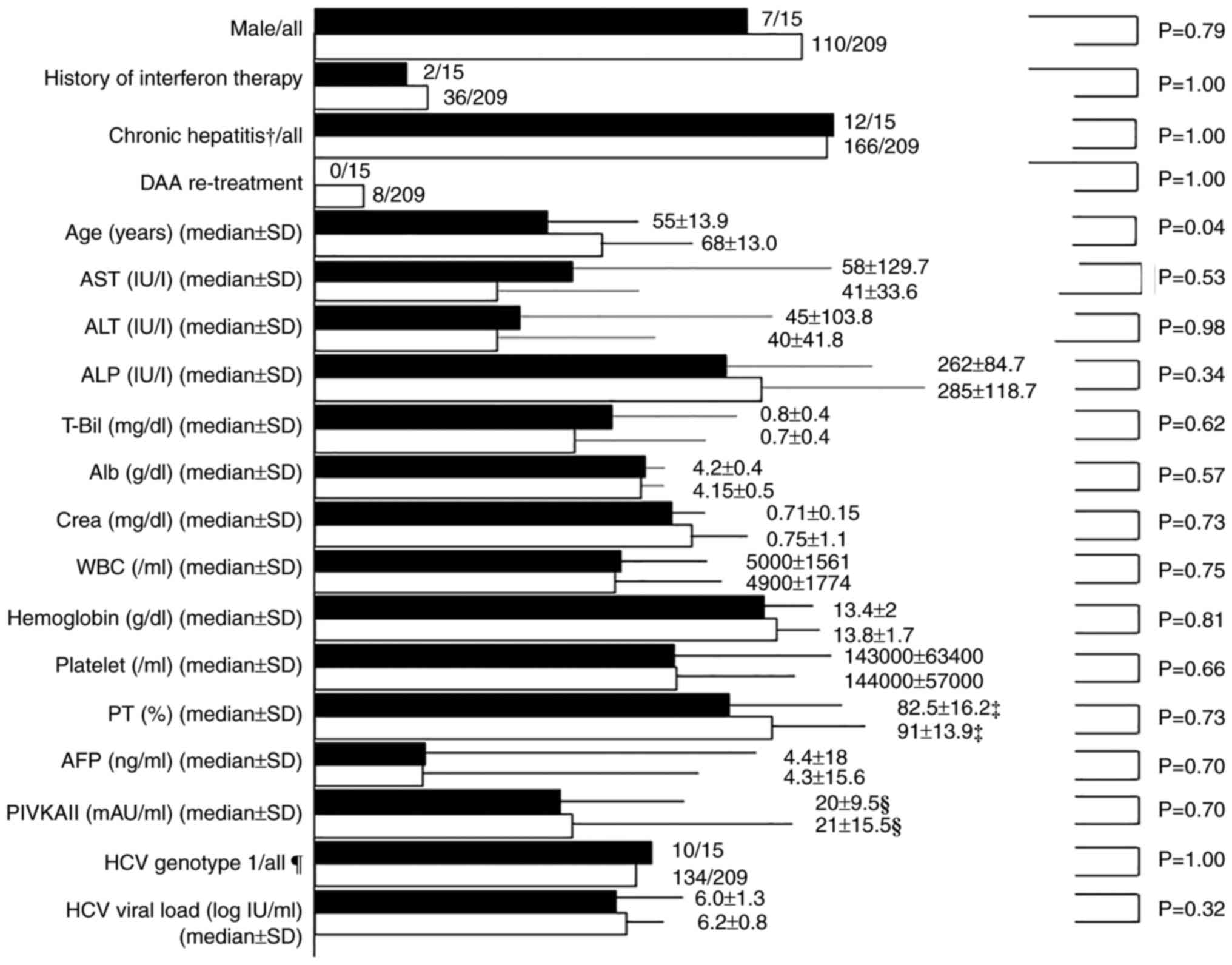 | Figure 1Characteristics of pre-direct-acting
antiviral treatment in the P and NP group. The black column
indicates the P group and the white column indicates the NP group.
†Chronic cases after the acute hepatitis Course were
included in chronic hepatitis Cases. ‡Exclusion of
anticoagulant therapy cases. §Exclusion of warfarin
therapy cases. Genotype1 includes serotype1, genotype1a, and
genotype1b and genotype2 includes serotype2, genotype2a and
genotype2b. Genotypes other than type 1 and 2 were not detected in
the patients. DDA, direct-acting antiviral; P, psychiatric
disorder; NP, nonpsychiatric disorder; AST, aspartate
aminotransferase; ALT, alanine transaminase; ALP, alkaline
phosphatase; T-Bil, total bilirubin; Alb, albumin; Crea, cotinine;
WBC, white blood cells; PT, prothrombin time; AFP, α-fetoprotein;
PIVKAII, protein-induced vitamin K absence or antagonist-II;
hepatitis C virus. |
Amino acid substitutions, DAA
treatment initiation and rate of completion of planned DAA
treatment
Of the 144 genotype 1 cases, 117 were analyzed for
nonstructural protein (NS)4A and NS5A amino acid substitutions
before treatment with asunaprevir (ASV) + daclatasvir (DCV),
ombitasvir (OBV) + paritaprevir (PTV) + ritonavir (R), ledipasvir
(LDV) + sofosbuvir (SOF), or daclatasvir (DCV) + asunaprevir (ASV)
+ beclabuvir (BCV). Of these 117 cases, HCV amino acid
substitutions were detected in 21, as follows: 17 cases of Y93 in
NS5A, two cases of L31 in NS5A and two cases of Q80 in NS4A. One of
the Q80 in NS4A cases was detected in an HCV RNA genotype 1a case
(group NP). A total of two cases who had not had any amino acid
substitutions before their first DAA treatment were found to have
amino acid substitutions (Y93 or L31) after relapse to the first
DAA treatment with ASV + DCV. All cases with Y93 substitutions in
NS5A, except for one case in group P, were treated with LDV + SOF.
The two cases with amino acid substitutions (Y93 or L31) after
relapse to the first DAA treatment with ASV + DCV belonged to the
group NP and were re-treated by DCV + ASV + BCV.
The distribution of DAA treatments for the group P
was as follows: Four cases of ASV + DCV; three cases of OBV + PTV +
R; four cases of SOF + ribavirin (RBV); 1 case of LDV + SOF; and 3
cases of glecaprevir (GLE) + pibrentasvir (PIB). The distribution
of DAA treatments for the group NP was as follows: 58 cases of ASV
+ DCV; 12 cases of OBV + PTV + R; 44 cases of SOF + RBV; 42 cases
of LDV + SOF; 45 cases of GLE + PIB two cases of DCV + ASV + BCV;
three cases of elbasvir (EBV) + grazoprevir (GZR); two cases of SOF
+ velpatasvir (VEL); and one case of SOF + VEL + RBV (Table II). The planned dosage period of
each DAA treatment was as follows: ASV + DCV for 24 weeks, OBV +
PTV + R for 12 weeks, SOF + RBV for 12 weeks, LDV + SOF 12 for
weeks, EBV + GZR for 12 weeks, GLE + PIB for 8 or 12 weeks, DCV +
ASV + BCV for 12 weeks, SOF + VEL for 12 weeks and SOF + VEL + RBV
for 24 weeks. Concerning treatment with GLE + PIB, cases without
cirrhosis were treated for 8 weeks, while cases with cirrhosis were
treated for 12 weeks. Of the 15 cases in group P, three patients
had incomplete treatment because of T-Bil elevation (one case, 1
week of treatment), aggravation of daily living activities (one
case, 4 weeks of treatment) and treatment discontinuation requested
by the patient (one case, 18 weeks of treatment). Of the 209 cases
in the group NP, 13 had incomplete treatment because of the
development of hepatocellular carcinoma (two cases, 10 and 20 weeks
of treatment, respectively), skin rash (two cases, 2 and 7 weeks of
treatment, respectively), the elevation of creatine (one case, 16
weeks of treatment), development of ascites (one case, 4 weeks of
treatment), continuous detectable HCV RNA level in the serum at 12
weeks after DAA treatment start (one case, 12 weeks of treatment),
treatment discontinuation requested by the patient (one case, 10
weeks of treatment), the elevation of ALP (one case, 4 weeks of
treatment), vomiting (one case, 1 week of treatment), the onset of
malignant lymphoma (one case, 20 weeks of treatment), general
fatigue (one case, 19 weeks of treatment) and the onset of herpes
zoster (one case, 5 weeks of treatment). The rate of completion of
the DAA treatment planned was compared between groups P and NP
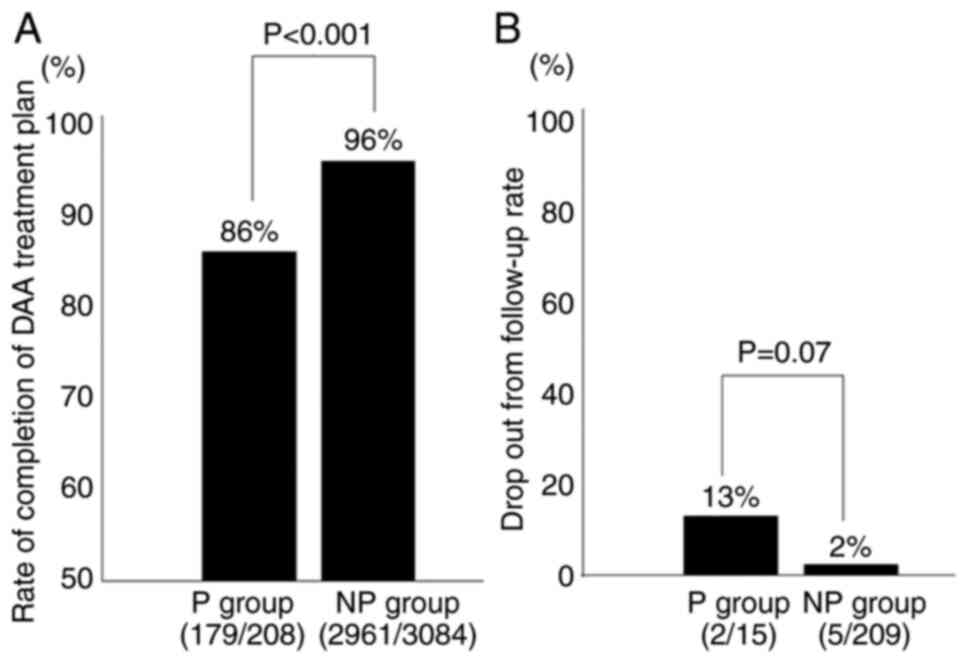
(Fig. 2A). Completion of DAA
treatment as scheduled was less achieved in group P (86%) than in
group NP (96%) (P<0.001). On the other hand, of the patients
treated with DAAs, two in the group P and 5 in group NP were
lost-to-follow-up; however, there was no significant difference
between the two groups (Fig. 2B).
There was neither discontinuation nor reduction of medications for
psychiatric disorders during DAA treatment in the group P. In
addition, there were no new psychiatric changes and no
exacerbations of existing psychiatric disorders during DAA
treatment in both groups.
 | Table IIDAA treatment for hepatitis virus C
infection for patients in the P and NP groups. |
Table II
DAA treatment for hepatitis virus C
infection for patients in the P and NP groups.
| DAA treatment | Group P, n | Group NP, n | Total, n |
|---|
| ASV/DCV | 4 | 58 | 62 |
|
Ombitasvir/paritaprevir/ritonavir | 3 | 12 | 15 |
| Ledipasvir/SOF | 1 | 42 | 43 |
| SOF/RBV | 4 | 44 | 48 |
|
DCV/ASV/beclabuvir | 0 | 2 | 2 |
|
Elbasvir/grazoprevir | 0 | 3 | 3 |
|
Glecaprevir/pibrentasvir | 3 | 45 | 48 |
| SOF/VEL | 0 | 2 | 2 |
| SOF/VEL/RBV | 0 | 1 | 1 |
| Total | 15 | 209 | 224 |
Sustained virologic response at 24
weeks (SVR-24) in patients with and without a psychiatric
disorder
Of all 224 cases, 200 cases (89.2%) achieved SVR at
24 weeks (SVR-24) after the end of DAA treatment (Table III). Of the 15 group P cases, 12
achieved SVR-24 and of the 209 group NP cases, 188 achieved SVR-24.
The SVR-24 rate was 80 and 90% in groups P and NP, respectively,
with no significant difference (Table III). The three group P cases who
had not achieved SVR included two drop-out cases and the 21 group
NP cases who had not achieved SVR included five drop-out cases.
Since these drop-out patients had completed anti-viral treatment,
but they did not come to the hospital after anti-viral treatment
completion, it was not possible to determine whether they achieved
SVR 24. In addition, the reasons for stopping their visits to the
hospital could also not be ascertained. Excluding lost-to-follow-up
cases, SVR-24 was achieved in 12/13 (92.3%) group P cases vs.
188/204 (92.1%) group NP cases, with no significant difference
between the two groups (P>0.99).
 | Table IIIComparison of the SVR rate between P
and NP groups. |
Table III
Comparison of the SVR rate between P
and NP groups.
| Group | Number of SVR | Number of
non-SVR | SVR/total | SVR rate, % |
|---|
| P | 12 | 3 | 12/15 | 80.0a |
| NP | 188 | 21 | 188/209 | 90.0a |
| Total | 200 | 24 | 200/224 | 89.2 |
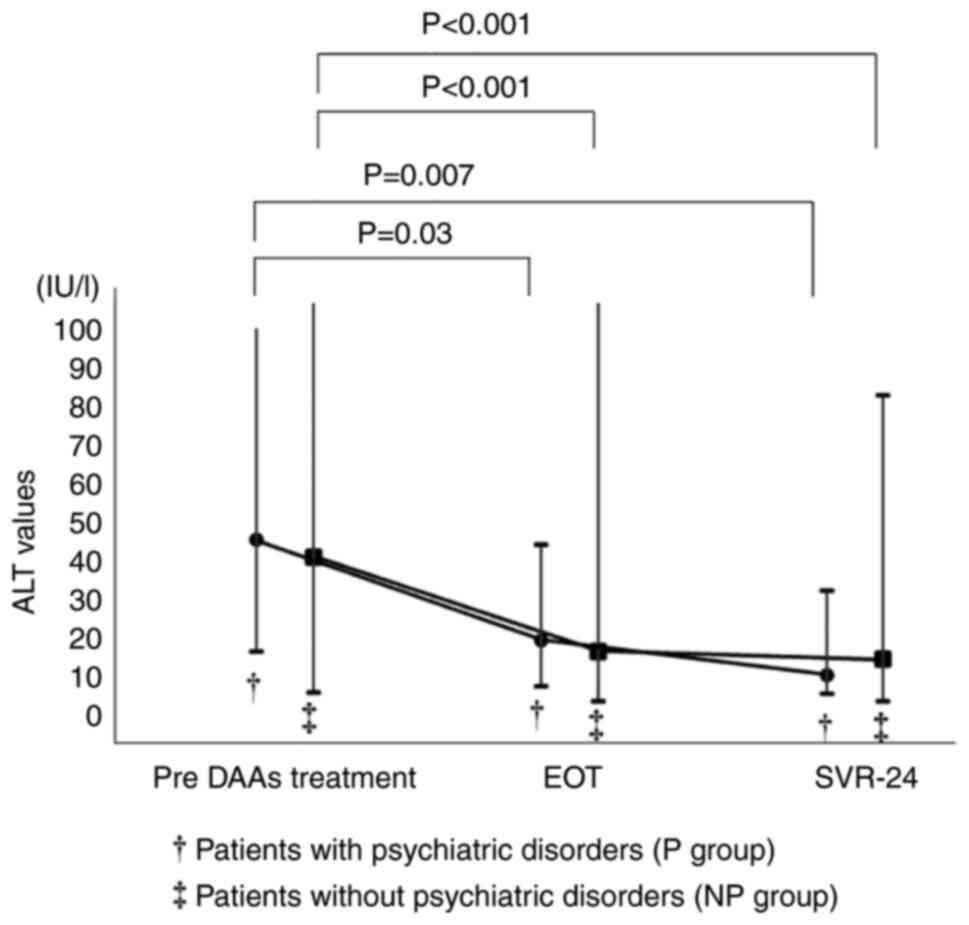
When the chronological changes of ALT values in the
SVR-24 achievement cases in both groups were compared, the ALT
values were decreased after DAA treatment (Fig. 3). The chronological changes of ALT
values were significant in both groups. However, the laboratory
data including ALT values were not significantly different between
the two groups at the end of DAA treatment (EOT) (Fig. 4). Similarly, the laboratory data at
SVR-24, except for the ALT values, were not significantly different
between the two groups (Fig.
5).
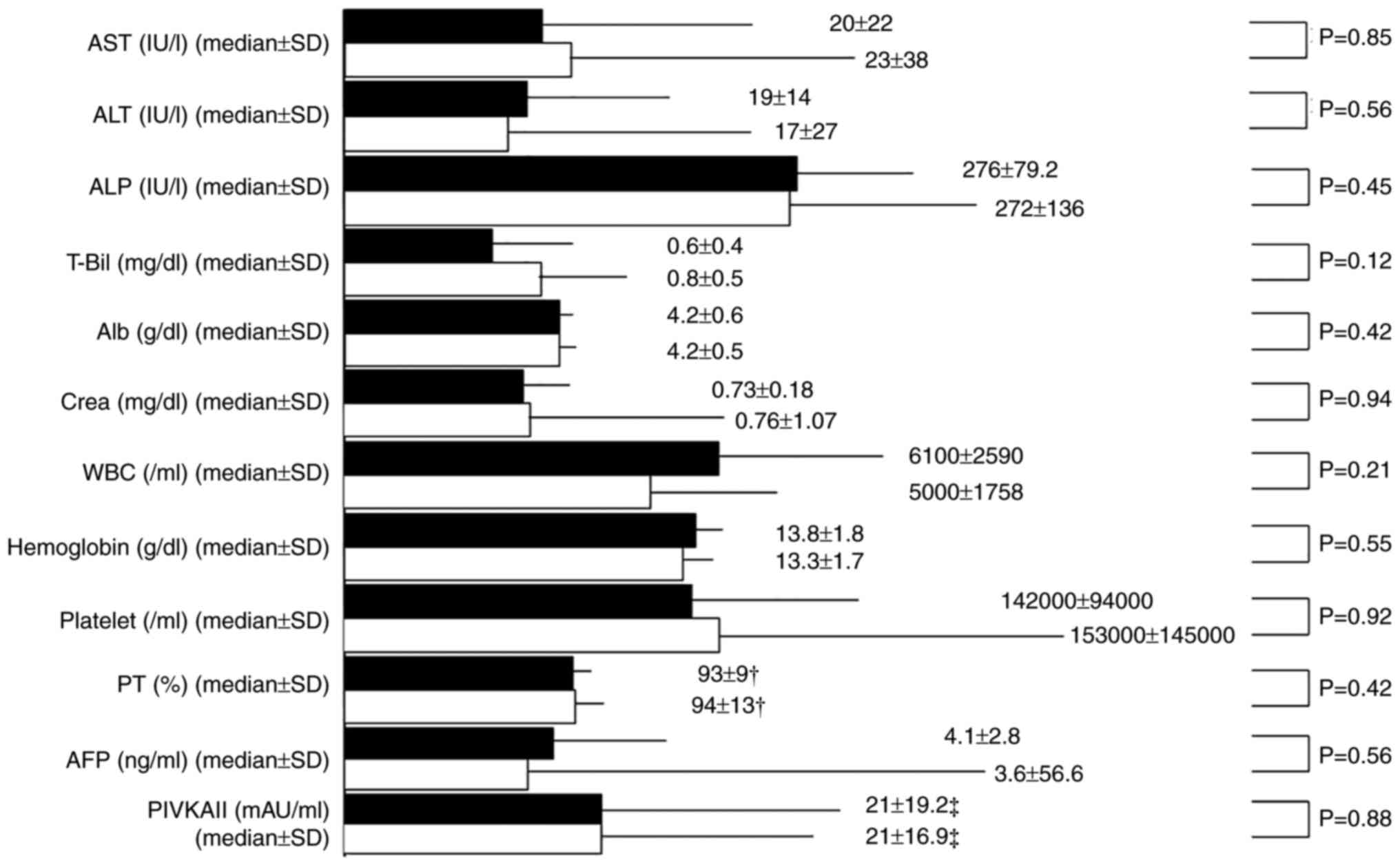 | Figure 4Comparison of the laboratory data at
the end of DAA treatment (EOT) between the psychiatric disorder
group (P group) and the non-psychiatric disorder group (NP group).
The black columns represent the P group and the white columns
represent the NP group. †Exclusion of anticoagulant
(including warfarin) therapy cases. ‡Exclusion of
warfarin therapy cases. P, psychiatric disorder; NP, nonpsychiatric
disorder; AST, aspartate aminotransferase; ALT, alanine
transaminase; ALP, alkaline phosphatase; T-Bil, total bilirubin;
Alb, albumin; Crea, cotinine; WBC, white blood cells; PT,
prothrombin time; AFP, α-fetoprotein; PIVKAII, protein-induced
vitamin K absence or antagonist-II. |
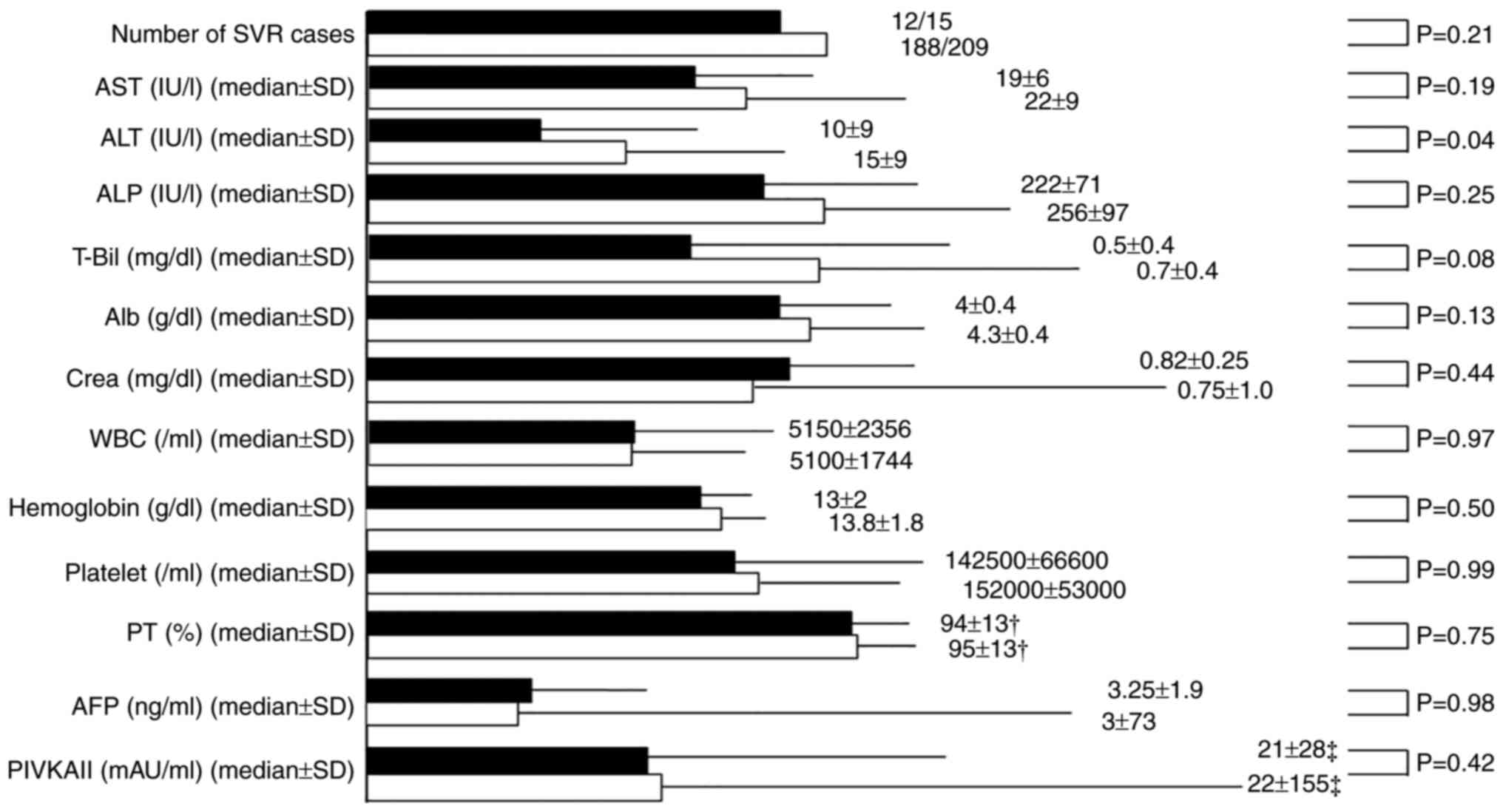 | Figure 5Comparison of the laboratory data on
the sustained virological response (SVR) between the psychiatric
disorder group (P group) and the non-psychiatric disorder group (NP
group). The black columns represent the P group and the white
columns represent the NP group. †Exclusion of
anticoagulant (including warfarin) therapy cases.
‡Exclusion of warfarin therapy cases. P, psychiatric
disorder; NP, nonpsychiatric disorder; AST, aspartate
aminotransferase; ALT, alanine transaminase; ALP, alkaline
phosphatase; T-Bil, total bilirubin; Alb, albumin; Crea, cotinine;
WBC, white blood cells; PT, prothrombin time; AFP, α-fetoprotein;
PIVKAII, protein-induced vitamin K absence or antagonist-II. |
Discussion
Since interferon-free DAA therapy became available
in 2014, SVR has been achieved in 78-100% of patients with chronic
HCV infection in several studies and real-world clinical practice
(7,21). In the present study, ~90% of HCV
infection cases achieved SVR-24. Therefore, the HCV population
including cases with psychiatric disorders in the present study
tended to be similar to that reported in other studies. The SVR-24
rate was slightly less in group P than in group NP, but there was
no significant difference. The SVR-24 rate of the two groups was
similar, 92.3 and 92.1%, respectively, when the analysis excluded
the drop-out cases. All cases lost-to-follow-up with psychiatric
disorders occurred after DAA treatment completion and no adverse
events leading to DAA discontinuation and DAA-related serious
adverse events were found in the lost-to-follow-up cases. Because
hepatic enzyme values decreased after DAA treatment and DAA
treatment also ameliorated hepatitis in both groups, it appears
that DAA treatment for HCV infection in patients with psychiatric
disorders was as effective, tolerable and safe, as it was for
patients without psychiatric disorders. Sackey et al
(18) also reported that treatment
with DAAs was not associated with psychiatric decompensation in
patients with hepatitis C virus infection and pre-existing mental
illnesses. It was suggested that HCV positivity is a potential risk
factor for the development of psychiatric disorders (13-15);
therefore, DAA treatment for patients with psychiatric disorders
should be started as soon as possible.
Although there was no significant difference between
the two groups, there were more cases lost to follow-up in group P
than in group NP. If the number of cases in group P was to
increase, more lost-to-follow-up cases would occur and adversely
affect the drop-out rate or the SVR-24 rate. Unfortunately,
medication nonadherence is common in patients with psychiatric
disorders (22-24).
The patient-psychiatrist relationship could be particularly
relevant for adherence in patients with a psychiatric disorder
(24). In addition, Sakamaki et
al (25) reported the
appearance of psychiatric symptoms in patients with underlying
psychiatric problems after DAA treatment and suggested that close
monitoring is necessary for these patients (25). In the present study, no mental
status changes or psychiatric problems appeared during and after
DAA treatment in both cases with and without psychiatric disorders.
However, hepatologists should seriously consider the patients'
mental status for DAA treatment and follow-up while contacting a
psychiatrist frequently. In the present study, T-Bil elevation
appeared just after the start of DAA treatment in one group P case.
After cessation of DAA treatment, the T-Bil level recovered to the
normal range. Whether this elevation of T-Bil was attributed to the
interaction of DAAs with psychiatric medications was unclear. No
abnormal liver function enzyme values were detected during DAA
treatment in other cases, but psychiatric patients should be
carefully followed up during DAA treatment in coordination with a
pharmacologist and a psychiatrist.
The present study has some limitations. The sample
size was small and the study was conducted in a single center. In
addition, this was a retrospective study. However, the results of
this study were obtained from real-world clinical practice and this
is the first report of DAA treatment for HCV infection in
psychiatric patients in Asia including Japan. More cases need to be
evaluated in a prospective, randomized study in the future. In
addition, more detailed analyses of psychiatric changes after DAA
treatment, especially after SVR, may be important in collaboration
with psychiatrists. Since individuals with psychiatric diseases
have high HCV seroprevalence (26)
and the Asian region has a large population and higher HCV
infection rate than other regions (27), it is expected that several patients
with latent psychiatric diseases need treatment for HCV in
Asia.
In conclusion, the results of DAA treatment for HCV
infection in psychiatric patients were analyzed and its
effectiveness, tolerability, and safety were similar in psychiatric
patients and nonpsychiatric patients. DAA treatment can result in a
high SVR rate for psychiatric patients with HCV infection.
Therefore, DAA treatment should be aggressively started for
psychiatric patients with HCV infection and eliminate HCV.
Moreover, progression to end-stage liver diseases, namely,
hepatocellular carcinomas, liver cirrhosis, or liver failure should
be prevented.
Acknowledgements
The authors would like to thank Professor Hiroaki
Okamoto and colleagues (Division of Virology, Department of
Infection and Immunity, Jichi Medical University School of
Medicine) for searching for HCV amino acid substitutions.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HO was involved in the conception and design of the
study, and the writing and preparation of the manuscript and
tables. TT, HA, ST, HK, YI, HT, SM, TSase, TSaito, KM and AN
collected the data. HO and AN confirm the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Suzuka General Hospital.
Patient consent for publication
Oral informed consent, including the statement of
agreement to the use of their samples in scientific research was
obtained from each patient at the first medical examination in the
Outpatient Department of Suzuka General Hospital.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kumada H, Suzuki Y, Ikeda K, Toyota J,
Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K, et
al: Daclatasvir plus asunaprevir for chronic HCV genotype 1b
infection. Hepatology. 59:2083–2091. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Omata M, Nishiguchi S, Ueno Y, Mochizuki
H, Izumi N, Ikeda F, Toyoda H, Yokosuka O, Nirei K, Genda T, et al:
Sofosbuvir plus ribavirin in Japanese patients with chronic
genotype 2 HCV infection: An open-label, phase 3 trial. J Viral
Hepat. 21:762–768. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chayama K, Notsumata K, Kurosaki M, Sato
K, Rodrigues L Jr, Setze C, Badri P, Pilot-Matias T, Vilchez RA and
Kumada H: Randomized trial of interferon- and ribavirin-free
ombitasvir/paritaprevir/ritonavir in treatment-experienced
hepatitis C virus-infected patients. Hepatology. 61:1523–1532.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mizokami M, Yokosuka O, Takehara T,
Sakamoto N, Korenaga M, Mochizuki H, Nakane K, Enomoto H, Ikeda F,
Yanase M, et al: Ledipasvir and sofosbuvir fixed-dose combination
with and without ribavirin for 12 weeks in treatment-naive and
previously treated Japanese patients with genotype 1 hepatitis C:
An open-label, randomised, phase 3 trial. Lancet Infect Dis.
15:645–653. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Suda G, Furusyo N, Toyoda H, Kawakami Y,
Ikeda H, Suzuki M, Arataki K, Mori N, Tsuji K, Katamura Y, et al:
Daclatasvir and asunaprevir in hemodialysis patients with hepatitis
C virus infection: A nationwide retrospective study in Japan. J
Gastroenterol. 53:119–128. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Akuta N, Sezaki H, Suzuki F, Kawamura Y,
Hosaka T, Kobayashi M, Kobayashi M, Saitoh S, Suzuki Y, Arase Y, et
al: Favorable efficacy of daclatasvir plus asunaprevir in treatment
of elderly Japanese patients infected with HCV genotype 1b aged 70
and older. J Med Virol. 89:91–98. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Falade-Nwulia O, Suarez-Cuervo C, Nelson
DR, Fried MW, Segal JB and Sulkowski MS: Oral direct-acting agent
therapy for hepatitis C virus infection: A systematic review. Ann
Intern Med. 166:637–648. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Backus LI, Belperio PS, Shahoumian TA and
Mole LA: . Impact of sustained virologic response with
direct-acting antiviral treatment on mortality in patients with
advanced liver disease. Hepatology. 69:487–497. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hauser P and Kern S: Psychiatric and
substance use disorders co-morbidities and hepatitis C: Diagnostic
and treatment implications. World J Hepatol. 7:1921–1935.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Leutscher PD, Lagging M, Buhl MR, Pedersen
C, Norkrans G, Langeland N, Mørch K, Färkkilä M, Hjerrild S,
Hellstrand K, et al: Evaluation of depression as a risk factor for
treatment failure in chronic hepatitis C. Hepatology. 52:430–435.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Dalgard O, Bjøro K, Hellum K, Myrvang B,
Skaug K, Gutigard B, Bell H and Construct Group: Treatment of
chronic hepatitis C in injecting drug users: 5 years' follow-up.
Eur Addict Res. 8:45–49. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hosoda S, Takimura H, Shibayama M,
Kanamura H, Ikeda K and Kumada H: Psychiatric symptoms related to
interferon therapy for chronic hepatitis C: Clinical features and
prognosis. Psychiatry Clin Neurosci. 54:565–572. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zignego AL and Craxì A: Extrahepatic
manifestations of hepatitis C virus infection. Clin Liver Dis.
12:611–636. 2008.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Schaefer M, Capuron L, Friebe A,
Diez-Quevedo C, Robaeys G, Neri S, Foster GR, Kautz A, Forton D and
Pariante CM: Hepatitis C infection, antiviral treatment and mental
health: a European expert consensus statement. J Hepatol.
57:1379–1390. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cheng JS, Hu JH, Chang MY, Lin MS, Ku HP,
Chien RN and Chang ML: Hepatitis C-associated late-onset
schizophrenia: A nationwide, population-based cohort study. J
Psychiatry Neurosci. 46:E583–E591. 2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Back D, Belperio P, Bondin M, Negro F,
Talal AH, Park C, Zhang Z, Pinsky B, Crown E, Mensa FJ and Marra F:
Efficacy and safety of glecaprevir/pibrentasvir in patients with
chronic HCV infection and psychiatric disorders: An integrated
analysis. J Viral Hepat. 26:951–960. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
de Gennaro N, Diella L, Monno L, Angarano
G, Milella M and Saracino A: Efficacy and tolerability of DAAs in
HCV-monoinfected and HCV/HIV-coinfected patients with psychiatric
disorders. BMC Infect Dis. 20(196)2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sackey B, Shults JG, Moore TA, Rogers R,
Mehvar M and King JG: Evaluating psychiatric outcomes associated
with direct-acting antiviral treatment in veterans with hepatitis C
infection. Ment Health Clin. 8:116–121. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Okano H, Murakami M, Asakawa H, Tsuruga S,
Tochio T, Kumazawa H, Sakuno T, Isono Y, Tanaka H, Matsusaki S, et
al: Acute hepatitis C virus infections in spouses: The utility of a
genetic analysis of the hepatitis C virus hypervariable region
sequence for identifying the infectious source. Arch Clin Med Case
Rep. 5:537–548. 2021.
|
|
20
|
Yamadate S and Nakayama T: The future
topics of discussion on JSCC recommended methods. Rinsho Byori.
64:544–549. 2016.PubMed/NCBI(In Japanese).
|
|
21
|
Tahata Y, Sakamori R and Takehara T:
Treatment progress and expansion in Japan: From interferon to
direct-acting antiviral. Glob Health Med. 3:321–334.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Phan SV: Medication adherence in patients
with schizophrenia. Int J Psychiatry Med. 51:211–219.
2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Gaudiano BA, Weinstock LM and Miller IW:
Improving treatment adherence in bipolar disorder: A review of
current psychosocial treatment efficacy and recommendations for
future treatment development. Behav Modif. 32:267–301.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lazary J, Pogany L, De Las Cuevas C,
Villasante-Tezanos GA and De Leon J: Adherence to psychiatric
medications: Comparing patients with schizophrenia, bipolar
disorder and major depression. Neuropsychopharmacol Hung.
23:363–373. 2021.PubMed/NCBI
|
|
25
|
Sakamaki A, Kamimura K, Fukui N, Watanabe
H, Sakai N, Tominaga K, Mizuno K, Takamura M, Kawai H, Sugai T, et
al: A case report of psychiatric symptoms following direct-acting
antiviral and ribavirin combination therapy for chronic hepatitis C
in a patient with innate anxiety. BMC Gastroenterol.
19(85)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Braude MR, Phan T, Dev A and Sievert W:
Determinants of hepatitis C virus prevalence in people with serious
mental illness: A systematic review and meta-analysis. J Clin
Psychiatry. 83(21r14079)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sarin SK, Kumar M, Eslam M, George J, Al
Mahtab M, Akbar SMF, Jia J, Tian Q, Aggarwal R, Muljono DH, et al:
Liver diseases in the Asia-Pacific region: A lancet
gastroenterology & hepatology commission. Lancet Gastroenterol
Hepatol. 5:167–228. 2020.PubMed/NCBI View Article : Google Scholar
|