Introduction
Bupivacaine (BUP) is a widely used local anesthetic
for regional anesthesia and pain management. However, it is
associated with potential neurotoxic effects, which may lead to
neurological complications in intraspinal anesthesia and systemic
toxicity when used as a local anesthetic, even when administered
within the clinically approved dose range (1). Our previous studies have shown that
BUP-induced neurotoxicity is associated with apoptosis, activation
of the nucleotide-binding oligomerization domain-like receptor
family pyrin domain-containing 3 (NLRP3) inflammasome and
ferroptosis (2-4).
Nevertheless, the molecular mechanism underlying BUP-induced
neurotoxicity remains incompletely understood due to its
complexity.
The endoplasmic reticulum (ER) is an essential
cellular organelle that plays a crucial role in protein synthesis,
which is essential for normal cellular functions and cell survival
(5). However, ER stress can arise
when an overload of calcium ions or the accumulation of unfolded
proteins occurs, which activates the unfolded protein response
(UPR) to restore protein homeostasis. The UPR pathway involves the
signaling of three types of transmembrane proteins on the ER,
namely inositol-requiring enzyme 1 (IRE1), protein kinase RNA-like
ER kinase (PERK) and activating transcription factor 6(6). Specifically, the PERK signaling
pathway can inhibit new protein synthesis, which may promote cell
survival or death; notably, when ER stress is prolonged, the PERK
signaling pathway may ultimately result in apoptosis (7). A previous study demonstrated that ER
stress is a critical contributor to BUP-induced neurotoxicity
(8). In our previous in
vivo study, the results indicated that BUP induces neurotoxic
effects by activating apoptosis via the mitochondrial pathway
(9). In our subsequent in
vitro study, it was found that activation of the
PERK-eukaryotic translation initiation factor 2 α
(eIF2α)-activating transcription factor 4 (ATF4) pathway leads to
apoptosis and contributes to BUP-induced spinal neurotoxicity in
rats (10). However, the precise
molecular mechanism by which BUP induces activation of the
PERK-eIF2α-ATF4 signaling pathway is currently not fully understood
and requires further investigation.
Sirtuin 1 (SIRT1) is a nicotinamide adenine
dinucleotide-dependent enzyme that belongs to the class III histone
deacetylase family. SIRT1 exerts protective effects against
cellular damage via the deacetylation of downstream substrates and
modulation of biological processes such as metabolism regulation,
DNA damage repair, cell cycle control, stress response and
apoptosis (11). Previous studies
have suggested that the downregulation of SIRT1 is a major
contributor to the pathogenesis of neurological disorders such as
Parkinson's disease, Alzheimer's disease, spinal cord injury and
cerebral ischemia-reperfusion injury (12-14).
A recent study elucidated the involvement of SIRT1 in the
neurotoxicity caused by local anesthesia (15). Furthermore, the inhibition of ER
stress has been established as an important mechanism via which
SIRT1 exerts protective effects in various diseases, such as
myocardial ischemia reperfusion injury, hepatic steatosis, chronic
obstructive pulmonary disease and inflammatory bowel diseases
(16-20).
Also, a study demonstrated that SIRT1 stimulates growth-plate
chondrogenesis by attenuating the PERK-eIF2α-CHOP pathway (21). However, the potential association
between SIRT1 and the PERK-eIF2α-ATF4 pathway in BUP-induced
neurotoxicity has not yet been investigated.
Resveratrol (RSV) is a natural polyphenol present in
various plant sources, including grapes, nuts, wine and berries,
which has been shown to yield neuroprotective effects through the
activation of SIRT1(22). In our
previous study, compelling evidence was provided that RSV inhibits
ER stress, reduces neuronal apoptosis and alleviates BUP-induced
spinal neurotoxicity in rats via the upregulation of SIRT1
expression and suppression of PERK-eIF2α-ATF4 pathway activation
(23). The aim of the present
study was to determine the association between SIRT1 and PERK
signaling pathways in the context of the RSV-mediated attenuation
of BUP-induced cytotoxicity in PC12 cells. The findings of this
study may provide a new perspective on the potential of RSV as a
targeted therapeutic approach for the treatment of BUP-induced
neurotoxicity.
Materials and methods
Materials
PC12 rat adrenal pheochromocytoma cells were
obtained from Icell Bioscience Inc., Shanghai. BUP hydrochloride
and RSV were purchased from Sigma-Aldrich (Merck KGaA), while
CCT020312 and EX527 were obtained from MedChemExpress. High-glucose
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
(FBS) were purchased from Gibco (Thermo Fisher Scientific, Inc.).
The Cell Counting Kit-8 (CCK-8) was supplied by Biosharp Life
Sciences, and the Annexin V/7-aminoactinomycin D (7-AAD) apoptosis
detection kit was provided by BD Biosciences. Antibodies against
SIRT1 (cat. no. ab110304) and caspase-12 (cat. no. ab62484) were
purchased from Abcam; antibodies against PERK (cat. no. 3192S),
phosphorylated (p)-eIF2α (cat. no. 3398T), eIF2α (cat. no. 5324T),
CHOP (cat. no. 5554T) and ATF4 (cat. no. 11815) were supplied by
Cell Signaling Technology, Inc.; antibodies against Bax (cat. no.
60267-1-Ig), Bcl-2 (cat. no. 26593-1-AP), glucose-regulated protein
78 (GRP78; cat. no. 11587-1-AP) and GAPDH (cat. no 10494-1-AP) were
provided by Proteintech Group, Inc.; and antibodies against cleaved
caspase-3 (cat. no. WL02117) and p-PERK (cat. no. WL05295) were
obtained from Wanleibio Co., Ltd.
Cell culture and treatment
PC12 cells were cultured in high-glucose DMEM
supplemented with 10% FBS and 1% penicillin-streptomycin at 37˚C in
a 5% CO2 incubator. The culture medium was refreshed
every day. To establish the BUP-induced cytotoxicity model, PC12
cells were incubated with 0-1.0 mM BUP for 24 h at 37˚C. In the RSV
+ BUP groups, 0-20 µM RSV was applied to PC12 cells for 2 h at
37˚C, followed by cotreatment 0.8 mM BUP for 24 h at 37˚C.
Moreover, in the EX527 + RSV + BUP and CCT + RSV + BUP pretreatment
groups, the cells were pretreated with SIRT1 inhibitor EX527 (10
µM) and PERK activator CCT020312 (4 µM) for 30 min at 37˚C,
followed by co-treatment with RSV (20 µM) and BUP (0.8 mM) for 24 h
at 37˚C. PC12 cells not exposed to any experimental treatments or
interventions were regarded as the control group.
Cell viability
The CCK-8 assay was used to determine the viability
of PC12 cells following the manufacturer's instructions. Cells were
seeded at a density of 3x103 cells per well in 96-well
plates and incubated for 24 h. After treatment, cells were
incubated with 10% CCK-8 solution for 1 h. An enzyme-linked
immunometric meter was used to measure the average optical density
at 450 nm.
Light microscopy
PC12 cells were seeded in 24-well plates at a
density of 2x104 cells/well and incubated for 24 h.
Subsequently, the PC12 cells were subjected to various
interventions. An inverted phase-contrast microscope (Leica
Microsystems GmbH) was used to examine cell morphology, and images
were captured at x200 magnification.
Flow cytometry
To measure the apoptosis rate, flow cytometry was
performed with the Annexin V/7-AAD apoptosis detection kit in
accordance with the manufacturer's instructions. PC12 cells were
seeded in 6-well plates at a density of 2x105 cells per
well. After various interventions, the cells were collected and
resuspended in 500 µl binding buffer for 5 min. After double
staining the cell preparations with Annexin V and 7-AAD for 5 min
at room temperature in the dark, cell analysis was performed using
a CytoFLEX Flow Cytometer (Beckman Coulter, Inc.). The acquired
data were subsequently analyzed using FlowJo software v. 10.8.1
(FlowJo LLC).
Immunofluorescence assay
PC12 cells were first seeded onto 14-mm round
coverslips in a 24-well plate. After rinsing with
phosphate-buffered saline (PBS), the cells on the coverslips were
fixed in 4% paraformaldehyde for 15 min at room temperature.
Following permeabilization with 0.5% Triton-X100 for 15 min, the
cells were blocked with 10% goat serum (BIOSS) for 40 min at room
temperature. The cells were incubated overnight at 4˚C with primary
antibodies against SIRT1 (diluted 1:500) and ATF4 (diluted 1:300).
After rinsing with PBS, the cells were incubated with secondary
antibodies (diluted 1:300) labeled with Alexa Fluor 488 (cat. no.
ab150133; Abcam) or Alexa Fluor 594 (cat. no. ab150080; Abcam) for
1 h at room temperature. The cells were then stained with DAPI
(diluted 1:1,000) for 30 min at room temperature to stain the
nuclei. Finally, images were captured using a fluorescent
microscope (BX53; Olympus Corporation) at x200 magnification, and
the mean fluorescence intensity was determined using ImageJ
software v.1.53 (National Institutes of Health).
Western blot analysis
PC12 cells were lysed with RIPA lysis buffer
(Beijing Solarbio Science & Technology Co., Ltd.), and the
protein concentration was determined using the BCA method. An equal
amount (20 µg) of protein was loaded per lane and subjected to
electrophoresis on a 10% SDS-PAGE gel. The separated proteins were
then transferred to PVDF membranes. After blocking with 5% skimmed
milk for 1 h at room temperature, the cells were incubated
overnight at 4˚C with primary antibodies against p-PERK, PERK,
ATF4, CHOP, p-eIF2α, eIF2α, caspase-12, Bcl-2, cleaved caspase-3,
GRP78, Bax and GAPDH (all diluted 1:1,000). After washing three
times with Tris-buffered saline with 0.05% Tween 20, the membranes
were incubated with infrared-labeled goat anti-rabbit or goat
anti-mouse secondary antibodies (1:10,000; Invitrogen; Thermo
Fisher Scientific, Inc.) for 1 h at 4˚C. An LI-COR Odyssey Infrared
imaging system (Li-Cor Biosciences) was used to obtain the array
image. The protein blot intensities were quantified using ImageJ
software v.1.53 (National Institutes of Health) and normalized to
the protein levels of the GAPDH loading control.
Statistical analysis
SPSS version 25.0 (IBM Corp.) was used to perform
the statistical analyses of the data presented in the study. Three
independent experiments were conducted for all assays. Data are
presented as the mean ± SEM. Differences among groups were analyzed
using one-way ANOVA followed by Tukey's post hoc tests. P<0.05
was considered to indicate a statistically significant
difference.
Results
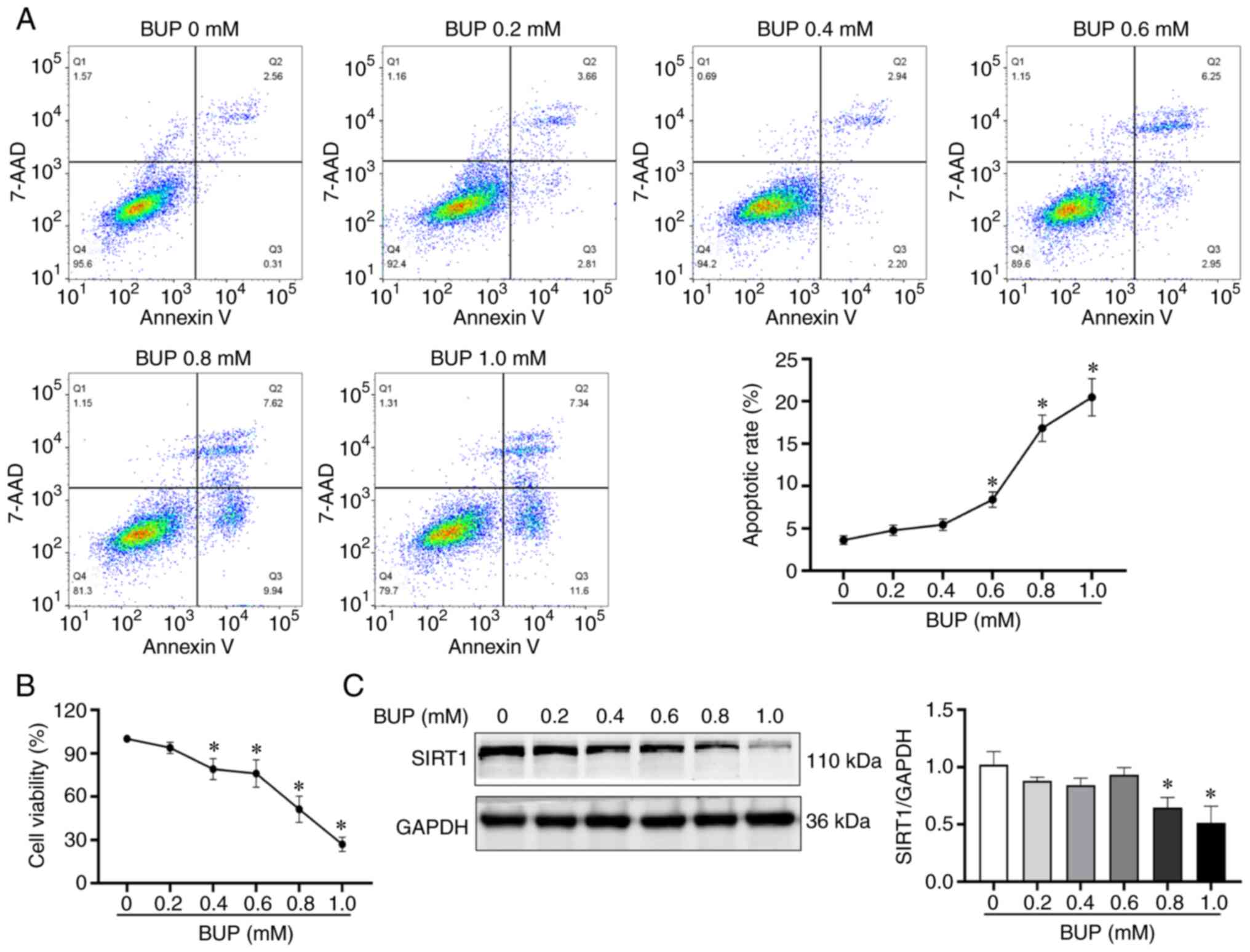
BUP downregulates SIRT1, decreases
viability and increases apoptosis in PC12 cells
To investigate the mechanisms underlying the
cytotoxicity of BUP on PC12 cells, PC12 cells were exposed to
various concentrations of BUP for 24 h. The cell viability,
apoptosis rate and SIRT1 protein expression levels of the cells
were evaluated using CCK-8, flow cytometry and western blot assays,
respectively. As shown in Fig. 1,
BUP induced apoptosis, reduced SIRT1 protein levels and decreased
cell viability in PC12 cells in a concentration-dependent manner.
The cell viability in the 0.2, 0.4, 0.6, 0.8 and 1.0 mM BUP groups
was 95.89±3.66, 76.01±2.79, 73.70±5.90, 54.55±4.37 and 28.87±2.63%,
respectively, compared with that in the control group. Based on
these results, 0.8 mM BUP was selected as the optimal concentration
for BUP-induced PC12 cell cytotoxicity induction in subsequent
experiments.
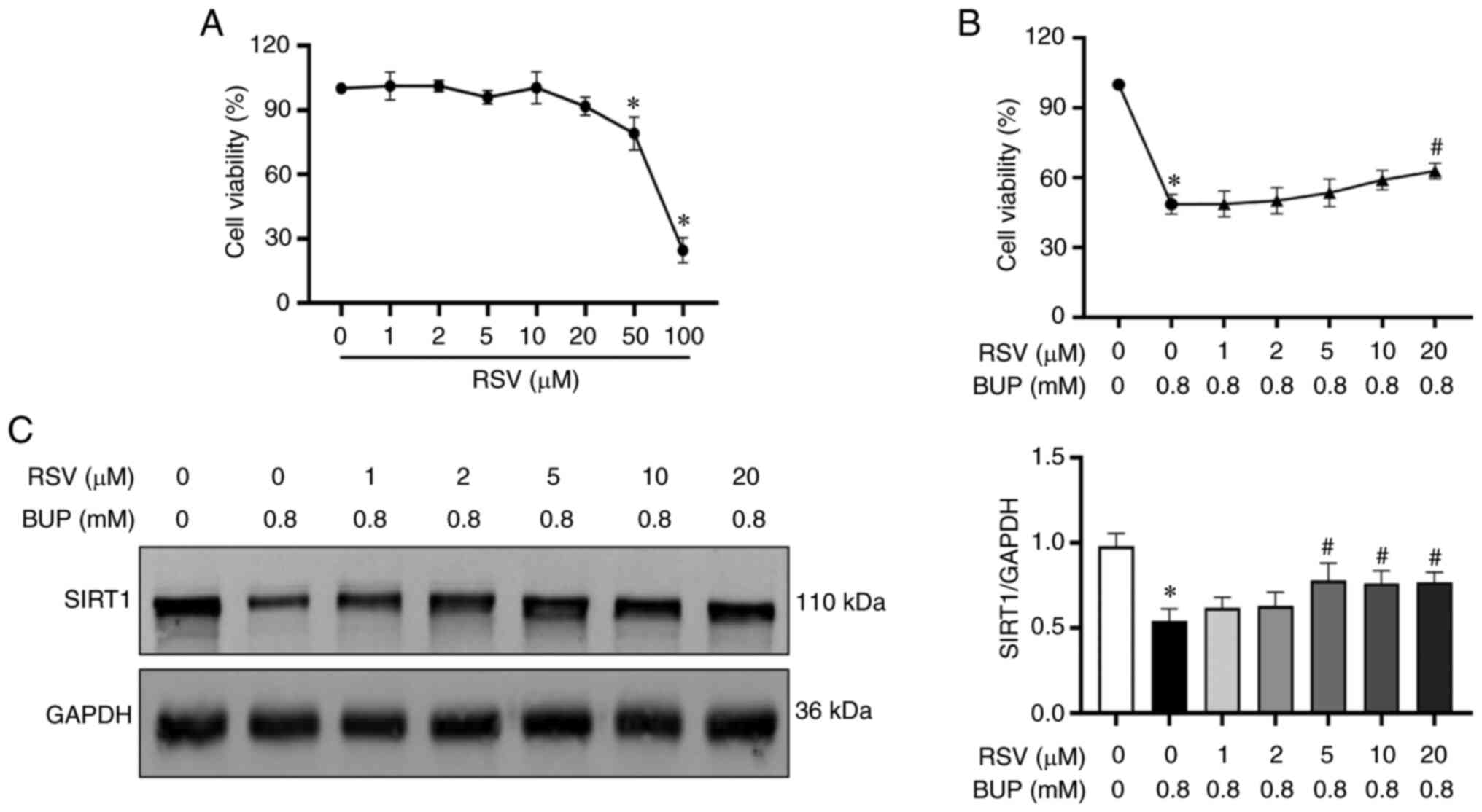
RSV treatment alleviates BUP-induced
cytotoxicity in PC12 cells
To determine the protective effect of RSV on
BUP-induced cytotoxicity, PC12 cells were incubated with various
concentrations of RSV for 24 h. Cell viability was then evaluated
via the CCK-8 assay. As shown in Fig.
2A, no significant change in cell viability was observed in
cells treated with RSV concentrations of 0-20 µM. However, cells
treated with 50 or 100 µM RSV exhibited significantly decreased
viability compared with untreated cells. Subsequently, cells were
treated with different concentrations (0, 1, 2, 5, 10 and 20 µM) of
RSV for 2 h, followed by cotreatment with 0.8 mM BUP for 24 h to
evaluate the protective effect of RSV against BUP-induced cell
injury. As shown in Fig. 2B and
C, treatment with 5, 10 and 20 µM
RSV upregulated SIRT1 protein expression in PC12 cells compared
with that in the cells treated with BUP alone, and treatment with
20 µM RSV restored cell viability. Accordingly, the optimal
concentration of RSV was identified to be 20 µM, which was used to
investigate the protective effect on BUP-induced cytotoxicity in
subsequent experiments.
RSV protects PC12 cells against
BUP-induced apoptosis via upregulation of SIRT1 protein
expression
To investigate the mechanism underlying the
RSV-mediated protection of PC12 cells against BUP-induced
cytotoxicity, the role of SIRT1 was evaluated using the SIRT1
inhibitor EX527. First, the optimal concentration of EX527 was
determined using a CCK-8 assay. Based on the results shown in
Fig. 3A, 10 µM was selected as the
optimal treatment concentration. PC12 cells were then treated with
BUP alone or in combination with RSV, or pretreated with EX527
followed by RSV and BUP cotreatment. As shown in Fig. 3B-E, morphological analysis revealed
that BUP induced cellular shrinkage, membrane blebbing and the
retraction of protrusions in PC12 cells, which was accompanied by
decreased cell viability and SIRT1 protein levels compared with
those in the control group. RSV attenuated the BUP-induced
reductions in SIRT1 protein expression and cell viability. It also
mitigated the BUP-induced pathological changes. Furthermore,
pretreatment with EX527 reversed the RSV-induced change in the
expression levels of SIRT1 and abolished the protective effect of
RSV against BUP-induced cytotoxicity. These results suggest that
the upregulation of SIRT1 mediates the protective effect of RSV
against BUP-induced cytotoxicity in PC12 cells.
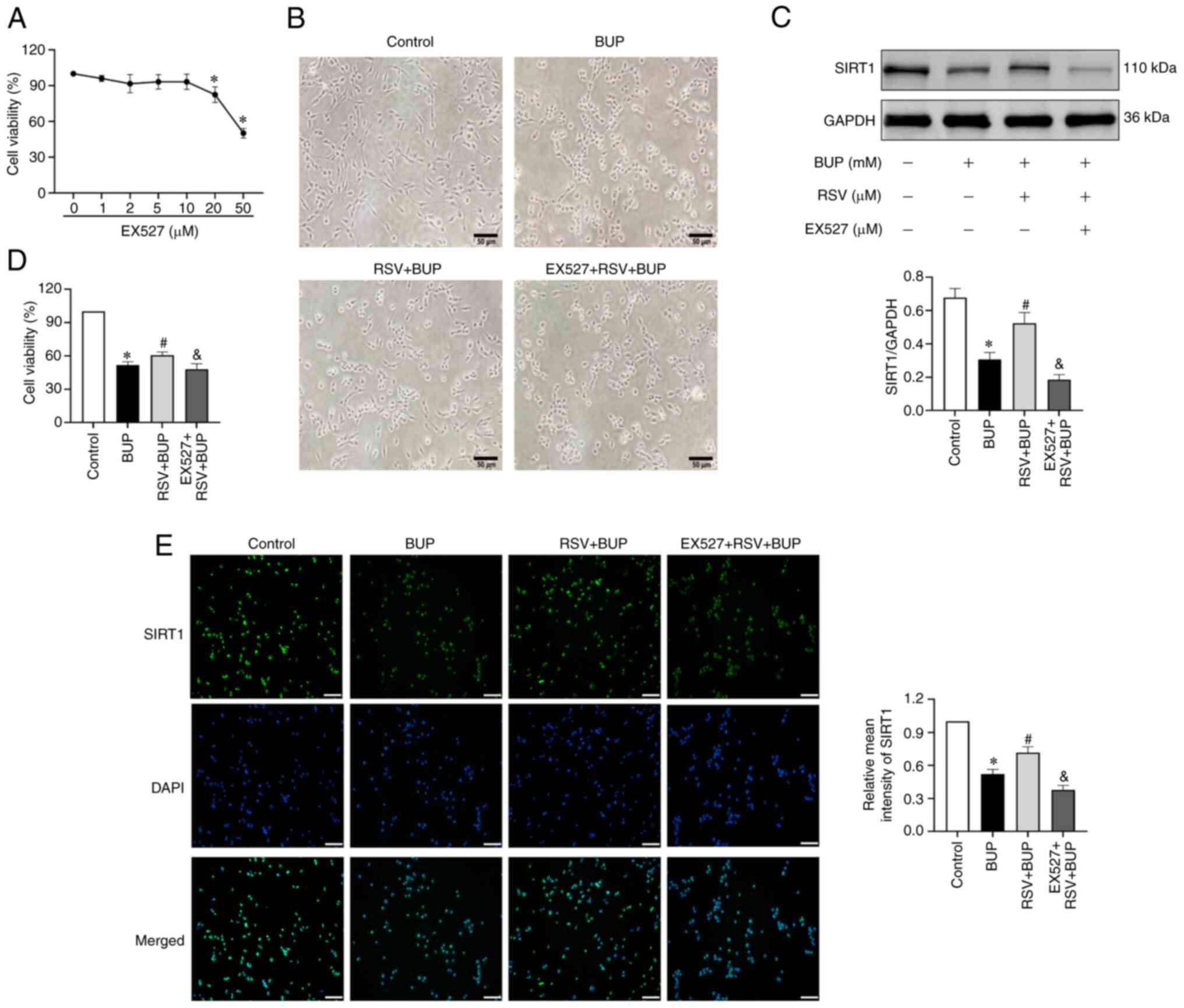 | Figure 3RSV protects PC12 cells against
BUP-induced cytotoxicity via SIRT1 upregulation. (A) Cell viability
in PC12 cells exposed to increasing concentrations of EX527. (B)
Morphology of PC12 cells in the control, BUP, RSV + BUP and EX527 +
RSV + BUP groups observed under a phase-contrast microscope
(magnification, x200; scale bar, 50 µm). (C) Representative western
blot images and semi-quantification of SIRT1 protein levels in each
group. (D) Cell viability in each group. (E) Representative
immunofluorescence images of SIRT1 (green) and cell nuclei (blue)
staining (scale bar, 50 µm) and the relative mean intensity of
SIRT1 immunofluorescence in each group. Data are presented as the
mean ± SEM (n=3). *P<0.05 vs. the control group;
#P<0.05 vs. the BUP group; &P<0.05
vs. the RSV + BUP group. RSV, resveratrol; BUP, bupivacaine, SIRT1,
sirtuin 1; EX527, SIRT1 inhibitor. |
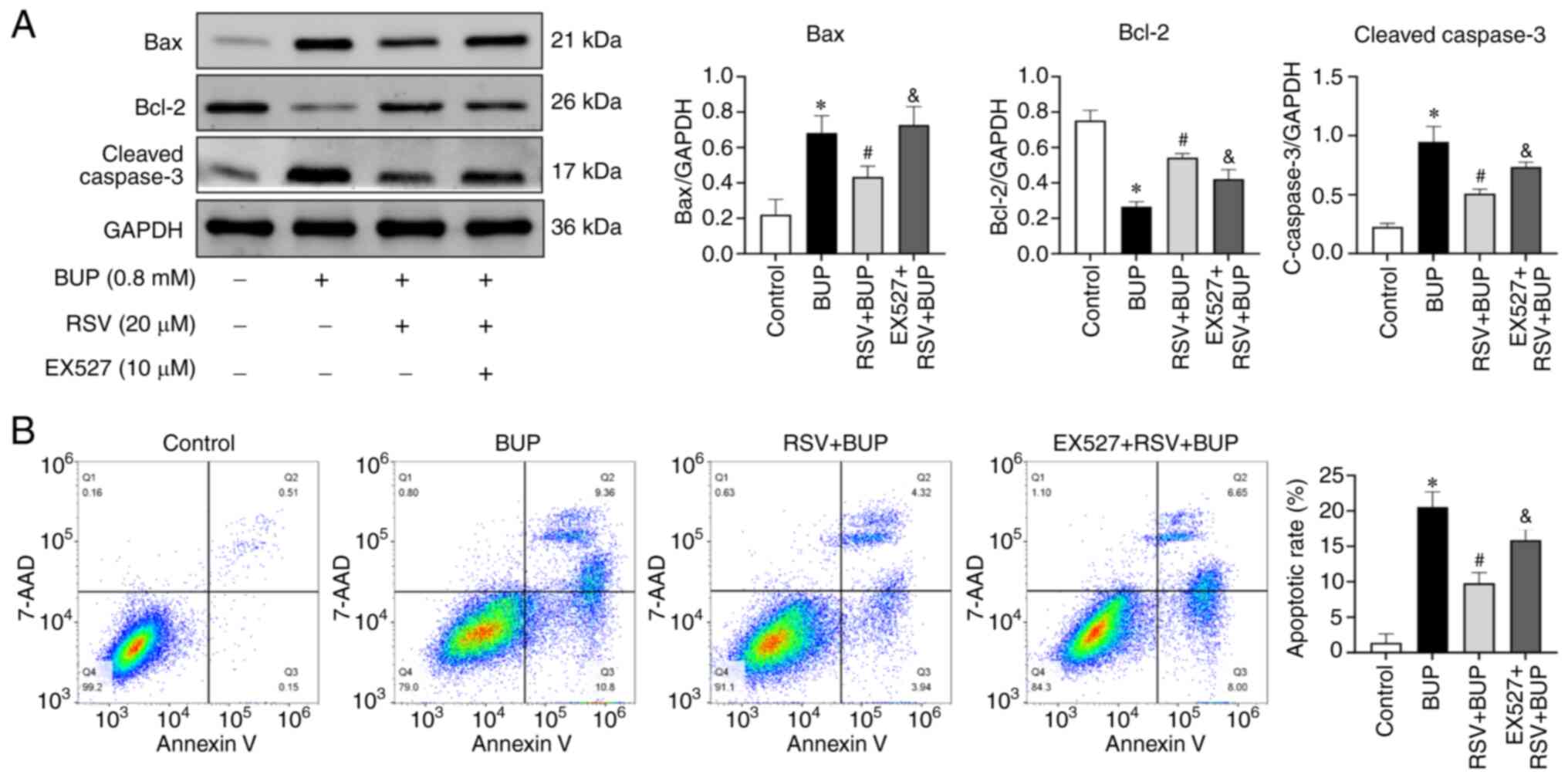
To further investigate whether SIRT1 is involved in
the protective effect of RSV against BUP-induced apoptosis, the
expression levels of apoptotic proteins and the apoptosis rates of
the PC12 cells were detected by western blotting and flow
cytometry, respectively. As shown in Fig. 4, BUP increased pro-apoptotic Bax
and cleaved caspase-3 protein expression and decreased
anti-apoptotic Bcl-2 protein expression, resulting in an increased
apoptosis rate compared with that in the control group. In the RSV
+ BUP group compared with the BUP group, the Bax and cleaved
caspase-3 protein levels and apoptosis rate were decreased, while
the expression of Bcl-2 was increased. However, pretreatment with
EX527 attenuated the effects of RSV on apoptotic protein expression
and increased the apoptosis rate compared with that in the RSV +
BUP group. These results suggest that the protective effect of RSV
against BUP-induced apoptosis in PC12 cells is mediated by the
upregulation of SIRT1.
RSV protects against BUP-induced
apoptosis through PERK pathway inhibition
To further elucidate the molecular mechanisms
underlying the protective effect of RSV on BUP-induced apoptosis,
the expression levels of proteins associated with the PERK
signaling pathway and ER stress were analyzed. The role of the PERK
signaling pathway was evaluated using the PERK activator CCT020312.
First, the optimal concentration of CCT020312 was determined using
a CCK-8 assay. Based on the results shown in Fig. 5A, 4 µM was selected as the optimal
treatment concentration for subsequent experiments. The western
blotting and CCK-8 assay results shown in Fig. 5B-D indicate that BUP induced ER
stress and activated the PERK pathway, as evidenced by increased
levels of the ER stress marker proteins GRP78, caspase-12 and CHOP,
as well as the PERK pathway-associated proteins p-PERK, p-eIF2α and
ATF4 in the BUP group compared with the control group. However,
compared with those in the BUP group, the levels of GRP78,
caspase-12, CHOP, p-PERK, p-eIF2α and ATF4 proteins were decreased
in the RSV + BUP group. Pretreatment with 4 µM CCT020312 increased
the protein levels of p-PERK, p-eIF2α, ATF4, caspase-12 and CHOP
compared with those in the RSV + BUP group. These data indicate
that RSV inhibits BUP-induced ER stress and PERK/eIF2α/ATF4 pathway
activation.
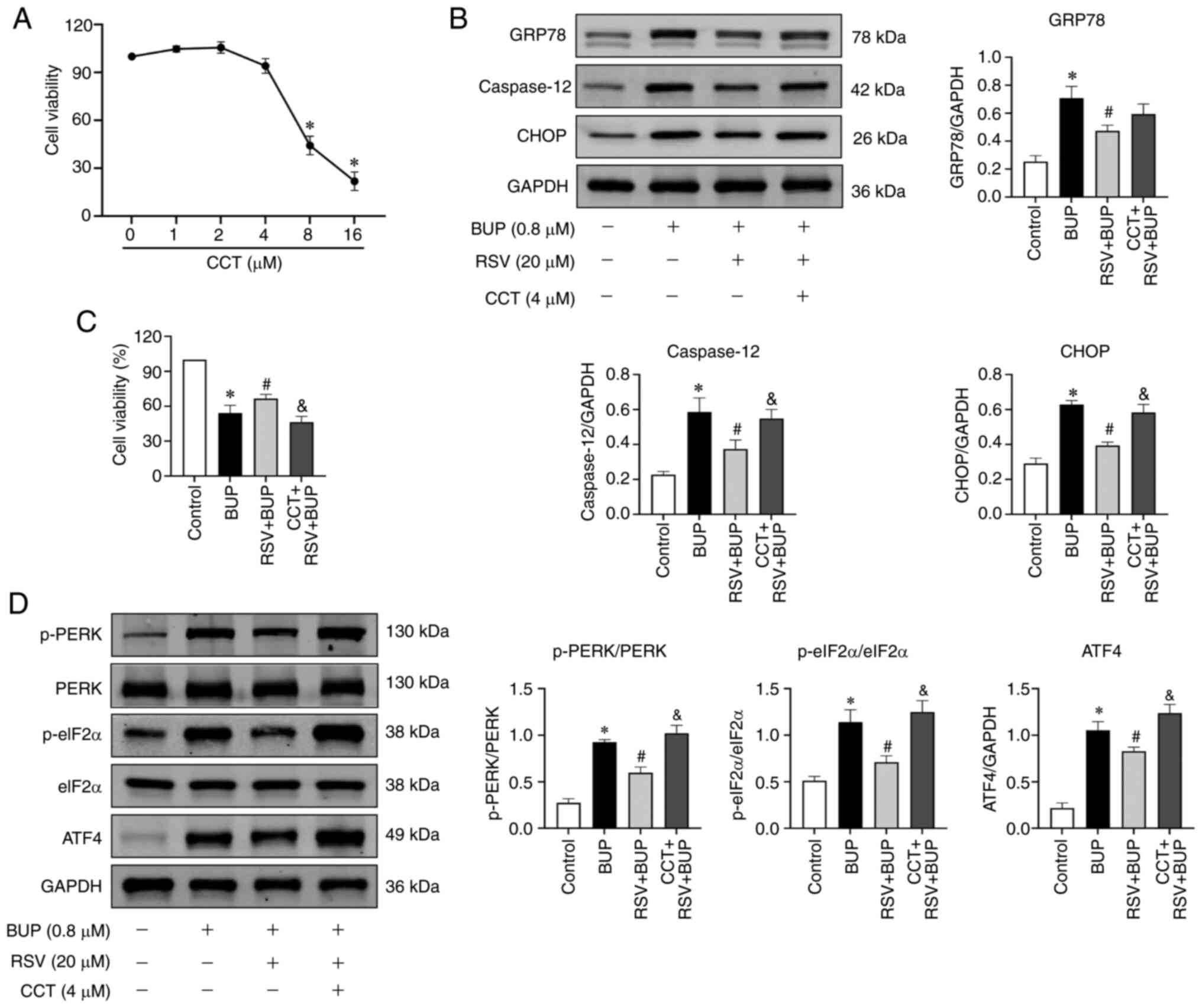 | Figure 5RSV inhibits BUP-induced endoplasmic
reticulum stress and PERK-eIF2α-ATF4 pathway activation. (A) Cell
viability of PC12 cells exposed to increasing concentrations of
CCT. (B) Representative western blot images and semi-quantification
of the GRP78, caspase-12 and CHOP protein levels of PC12 cells in
the control, BUP, RSV + BUP and CCT + RSV + BUP groups. (C) Cell
viability and (D) p-PERK/PERK, p-eIF2α/eIF2α and ATF4 protein
levels in each group. Data are presented as the mean ± SEM (n=3).
*P<0.05 vs. the control group; #P<0.05
vs. the BUP group; &P<0.05 vs. the RSV + BUP
group. RSV, resveratrol; BUP, bupivacaine; PERK, protein kinase
RNA-like ER kinase; eIF2α, eukaryotic translation initiation factor
2 α; ATF4, activating transcription factor 4; CCT, CCT020312 (PERK
activator); GRP78, glucose-regulated protein 78; p-,
phosphorylated. |
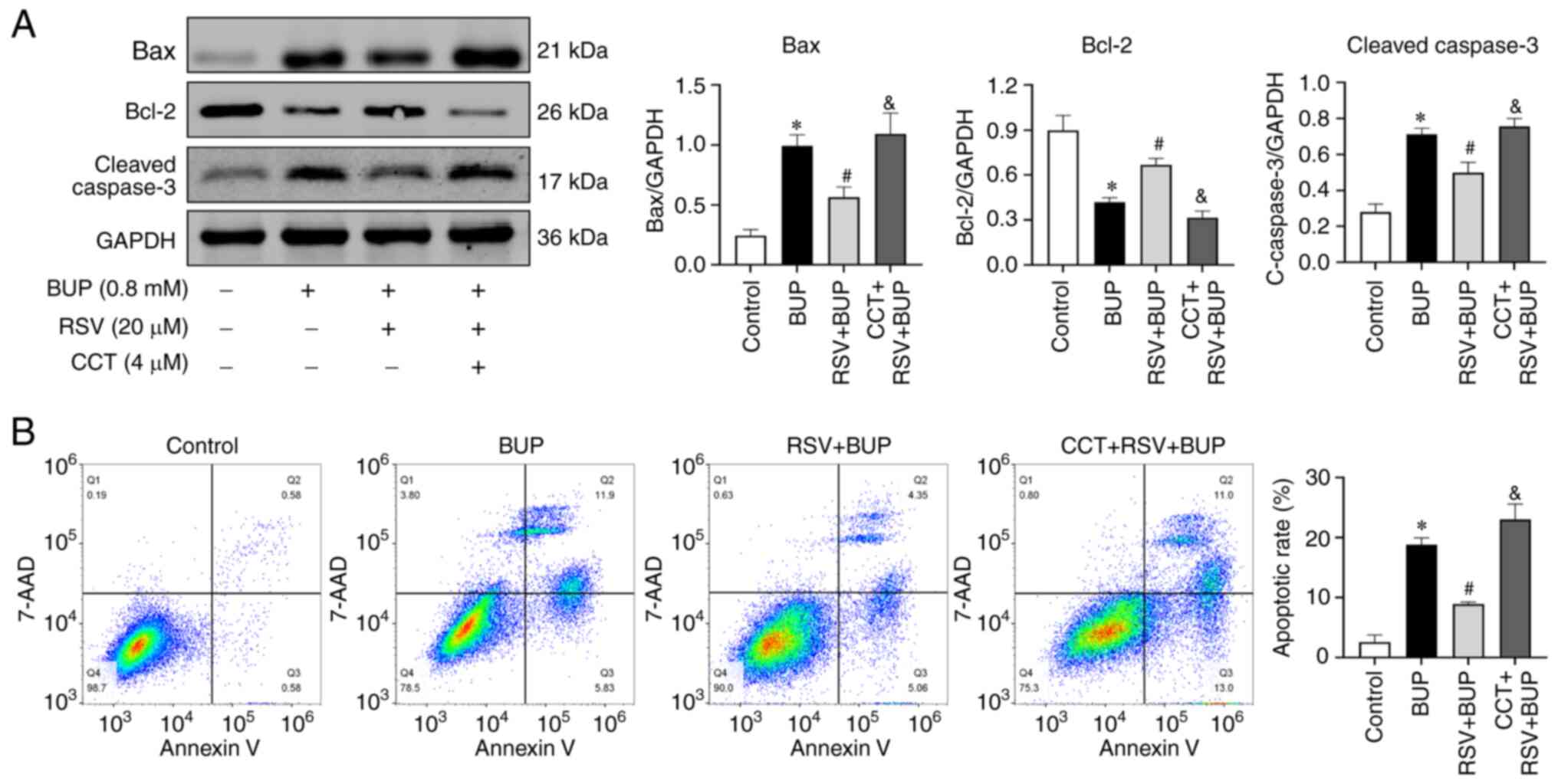
To further elucidate the role of the PERK-eIF2α-ATF4
pathway in the protective effects of RSV against BUP-induced
apoptosis, the expression levels of apoptotic proteins and the rate
of apoptosis were assessed in each experimental group using western
blotting and flow cytometry, respectively. As shown in Fig. 6, the Bax and cleaved caspase-3
proteins levels were elevated, Bcl-2 protein levels were reduced
and the rate of apoptosis was increased in the CCT + RSV + BUP
group compared with the RSV + BUP group. These findings suggest
that RSV mitigates BUP-induced apoptosis by inhibiting the
PERK-eIF2α-ATF4 pathway in PC12 cells.
RSV inhibits the PERK-eIF2α-ATF4
pathway by increasing SIRT1 expression
To investigate the potential role of SIRT1 in the
inhibitory effect of RSV on the PERK-eIF2α-ATF4 pathway, the
protein levels of p-PERK, p-eIF2α, and ATF4 were detected in the
EX527 + RSV + BUP group using western blotting and
immunofluorescence assays. As shown in Fig. 7, pretreatment with 10 µM EX527
significantly reversed the inhibitory effect of RSV on p-PERK,
p-eIF2α and ATF4 protein levels when compared with the RSV + BUP
group. These findings suggest that RSV may inhibit the
PERK-eIF2α-ATF4 pathway via the upregulation of SIRT1 expression in
BUP-exposed PC12 cells.
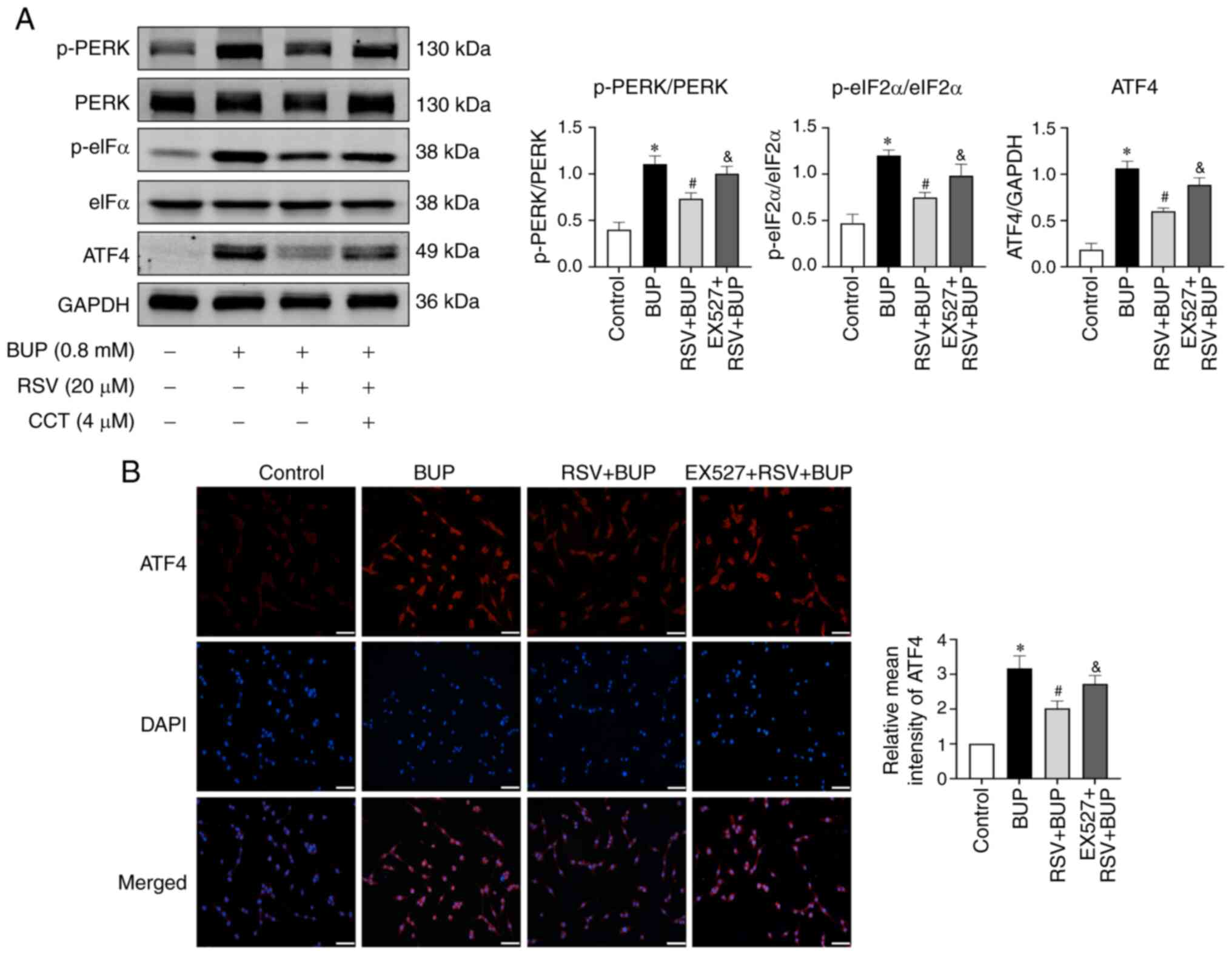 | Figure 7RSV inhibits the PERK-eIF2α-ATF4
pathway via the upregulation of SIRT1 expression in BUP-treated
PC12 cells. (A) Representative western blot images and
semi-quantification of p-PERK/PERK, p-eIF2α/eIF2α and ATF4 protein
levels in the control, BUP, RSV + BUP and EX527 + RSV + BUP groups.
(B) Representative immunofluorescence images of ATF4 (red) and cell
nuclei (blue) staining (scale bar, 50 µm) and relative mean
intensity analysis of ATF4 immunofluorescence in each group. Data
are presented as the mean ± SEM (n=3). *P<0.05 vs.
the control group; #P<0.05 vs. the BUP group;
&P<0.05 vs. the RSV+BUP group. RSV, resveratrol;
PERK, protein kinase RNA-like ER kinase; eIF2α, eukaryotic
translation initiation factor 2 α; ATF4, activating transcription
factor 4; SIRT1, sirtuin 1; BUP, bupivacaine; p-, phosphorylated;
EX527, SIRT1 inhibitor. |
Discussion
In the present study, the changes in the viability
of PC12 cells treated with BUP were evaluated at a 24-h time point.
This time point was selected based on the results of preliminary
experiments and consideration of previous studies (24-29).
Additional experiments using a SIRT1 inhibitor and PERK pathway
agonist were then conducted to evaluate the regulatory relationship
between SIRT1 and the PERK pathway in the context of the
RSV-mediated attenuation of BUP-induced neurotoxicity. The main
findings of the present study are as follows. Firstly, it provides
the first evidence of the involvement of SIRT1 in BUP-induced
neurotoxicity. By using a SIRT1 inhibitor, EX527, it was confirmed
that the neuroprotective effect of RSV against BUP-induced
neurotoxicity is achieved through the upregulation of SIRT1
expression. Secondly, it reveals that a PERK pathway agonist can
attenuate the ability of RSV to reduce BUP-induced apoptosis in
PC12 cells. This finding suggests that the PERK pathway plays a
crucial role in mediating the protective effects of RSV against
BUP-induced neurotoxicity. Finally, the research findings suggest
that RSV blocks the PERK-eIF2α-ATF4 pathway of ER stress by
increasing SIRT1 expression in BUP-exposed PC12 cells. These
findings contribute to an improved understanding of the molecular
mechanisms involved in BUP-induced neurotoxicity and highlight the
neuroprotective potential of RSV through its regulatory effect on
the SIRT1 and PERK signaling pathways.
SIRT1 is a well-studied member of the sirtuin family
that has been shown to be associated with the modulation of various
physiological and pathological conditions involving aging,
metabolism, oxidative stress, autophagy and inflammation (30-33).
As a positive regulator in cellular response, SIRT1 can activate
different substrates, including p53, FOXO3 and NF-κB, to alleviate
cell damage caused by various factors (34-36).
There is an increasing consensus that SIRT1 is a vital player in
the protection of cells from apoptosis following injury (14,37,38).
RSV is a natural SIRT1 agonist that has shown protective effects
against neurotoxicity and neurodegeneration through the activation
of SIRT1 (39-42).
The present study sought to explore the mechanisms underlying
BUP-induced neurotoxicity and the potential protective effects of
RSV against the effects of BUP on PC12 cells. The findings
demonstrate that BUP significantly reduced cell viability and SIRT1
expression levels in a concentration-dependent manner. Furthermore,
RSV treatment inhibited BUP-induced apoptosis via the
downregulation of the expression of pro-apoptotic proteins
caspase-3 and Bax and upregulation of the expression of Bcl-2.
Moreover, to evaluate the involvement of SIRT1 in the protective
functions of RSV, EX527, a specific inhibitor of SIRT1 was used in
the present study. The results demonstrate that EX527 increased the
apoptosis rate and expression levels of caspase-3 and Bax, and
decreased the expression of Bcl-2 in the cells cotreated with RSV
and BUP, which partially abolished the protective effect of RSV
against apoptosis. These data suggest that RSV inhibits BUP-induced
apoptosis in PC12 cells via the activation of SIRT1.
ER stress has been linked to the pathogenesis of
BUP-induced neurotoxicity in a previous study, in which the
inhibition of ER stress was shown to attenuate the apoptosis caused
by BUP (43). In the present
study, it was discovered that BUP increased the expression of ER
stress marker proteins GRP78, caspase-12 and CHOP, while RSV
treatment significantly decreased the BUP-induced increases in the
expression of these proteins, indicating that RSV suppressed
BUP-induced ER stress. Under severe ER stress conditions, PERK
dissociates from the molecular chaperone GRP78 and undergoes
autophosphorylation through dimerization, leading to the
phosphorylation of eIF2α and subsequent upregulation of ATF4
protein expression. It is now understood that CHOP, one of the
target genes of ATF4, triggers cell apoptosis by upregulating the
expression of the pro-apoptosis protein Bax, while the expression
of the anti-apoptosis protein Bcl-2 is downregulated (44). Furthermore, a recent study
demonstrated that RSV protects against acrolein-induced ferroptosis
and insulin secretion dysfunction via the ER-stress-associated PERK
pathway (45). Therefore, the PERK
agonist CCT020312 was used in the present study to examine whether
the PERK signaling pathway mediates the anti-apoptotic effects of
RSV against BUP-induced apoptosis in PC12 cells. The results
substantiated that BUP activated the PERK pathway in PC12 cells, as
demonstrated by increased levels of p-PERK, p-eIF2α and ATF4, which
is consistent with the findings of preliminary experiments in the
present study. Treatment with RSV significantly decreased the
p-PERK/PERK and p-eIF2α/eIF2α ratios and decreased ATF4 protein
expression in BUP-treated PC12 cells. However, pretreatment with
CCT020312 increased the p-PERK/PERK and p-eIF2α/eIF2α ratios and
the expression levels of ER stress marker proteins caspase-12 and
CHOP, indicating that CCT020312 reversed the inhibitory effect of
RSV on ER stress. Furthermore, western blotting and flow cytometry
were used to quantify the expression of apoptosis-related proteins
and the apoptosis rate. The results indicated that CCT020312
partially abolished the protective effect of RSV against
BUP-induced apoptosis. Therefore, these results support the
hypothesis that the protective effect of RSV against BUP-induced
injury in PC12 cells is mediated through the inhibition of the
PERK-eIF2α-ATF4 pathway, which thereby exerts an anti-apoptotic
effect.
Previous studies have provided evidence to suggest
that SIRT1 plays a crucial role in mediating the suppressive
effects of RSV on ER stress (46,47).
SIRT1 has been shown to block the activation of IRE1 and X-box
binding protein 1 (XBP1) through deacetylation under ER stress
conditions. For instance, Chou et al (48) reported that RSV or the
overexpression of SIRT1 significantly decreased cadmium-induced
activation of the IRE-1α/spliced XBP1 pathway and NLRP3
inflammasome, along with pyroptosis. It has been reported that
SIRT1 directly interacts with lysine on PERK and eIF2α to regulate
PERK activation (49,50). Notably, RSV has been reported to
restore cardiac function and reduce cardiomyocyte apoptosis via
SIRT1-mediated inhibition of the PERK/eIF2α pathway (50). Therefore, rescue experiments were
conducted in the present study to investigate the involvement of
SIRT1 in the inhibitory effects of RSV on the PERK signaling
pathway. The results showed that EX527 significantly increased the
p-PERK/PERK and p-eIF2α/eIF2α ratios and decreased the expression
of ATF4 in the cells treated with RSV and BUP, indicating that
SIRT1 mediates the suppressive effects of RSV on the
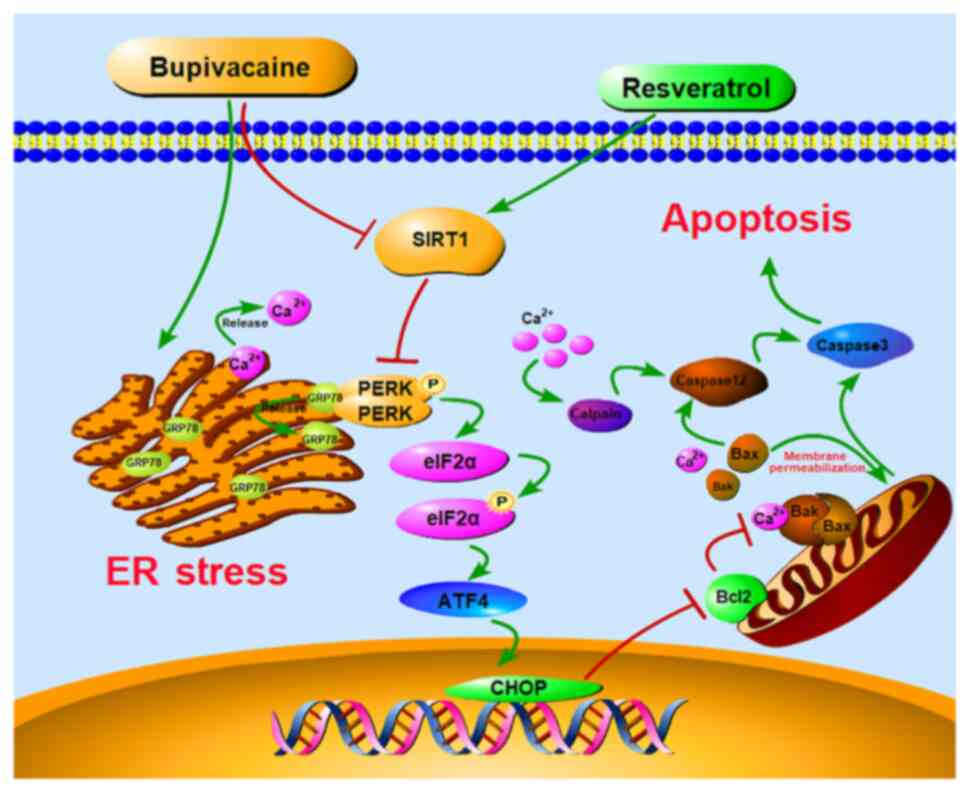
PERK-eIF2α-ATF4 pathway. A graphical image depicting the proposed
mechanism in which RSV-mediated SIRT1 upregulation protects against
BUP-induced neurotoxicity via inhibition of the PERK-eIF2α-ATF4
pathway is shown in Fig. 8.
The present study has three limitations that should
be acknowledged. Firstly, the changes in cell viability were only
evaluated at a single time point (24 h) after the treatment of PC12
cells with BUP. The effects of BUP, RSV and EX527 on PC12 cell
viability were not investigated at different time points. Secondly,
GAPDH was used as a loading control to quantify the target bands in
the western blot analysis, with the aim of standardizing the
quantification. The expression of full-length caspase-3 protein was
not analyzed for comparison with cleaved caspase-3, which could
have provided a more accurate assessment. Thirdly, the interaction
between SIRT1 and the proteins in the PERK signaling pathway was
not directly investigated. Further research is necessary to
identify the specific proteins within the PERK signaling pathway
that are targeted by SIRT1 deacetylation in BUP-induced
neurotoxicity.
In summary, the present study reveals that SIRT1
plays a pivotal role in the pathogenesis of BUP-induced
neurotoxicity by modulating activation of the PERK-eIF2α-ATF4
signaling pathway. Compelling evidence that RSV protects against
BUP-induced PC12 cell apoptosis via the upregulation of SIRT1
expression and subsequent inhibition of the PERK-eIF2α-ATF4
signaling pathway is provided. These findings highlight potential
therapeutic targets and strategies for the treatment of BUP-induced
neurotoxicity.
Acknowledgements
Not applicable.
Funding
Funding: This research was funded by the Innovation Project of
Guangxi Graduate Education (grant no. YCBZ2022091).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL was responsible for data curation, investigation,
conceptualization and writing the original draft of the manuscript.
NH was responsible for data curation, software and visualization.
YZ was responsible for investigation, data curation and validation.
JLa was responsible for investigation, data curation and
validation. XL was responsible for data curation, validation,
software and investigation. JLi was responsible for project
administration, conceptualization, writing, reviewing and editing
the manuscript, visualization and validation. YL and JLi confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen X, Xu Z, Lin R and Liu Z: Persistent
cauda equina syndrome after cesarean section under combined
spinal-epidural anesthesia: A case report. J Clin Anesth.
27:520–523. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ji J, Yan X, Li Z, Lai Z and Liu J:
Therapeutic effects of intrathecal versus intravenous
monosialoganglioside against bupivacaine-induced spinal
neurotoxicity in rats. Biomed Pharmacother. 69:311–316.
2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lai J, Ji JM, Chen MY, Luo YP, Yu Y, Zhou
G, Wei LL, Huang LS and Liu JC: Melatonin ameliorates
bupivacaine-induced spinal neurotoxicity in rats by suppressing
neuronal NLRP3 inflammasome activation. Neurosci Lett.
772(136472)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zhao Y, Luo Y, Liu Z, Chen Y, Wei L, Luo
X, Zhou G, Lai J, Ji J, Lin Y and Liu J: Ferrostatin-1 ameliorates
bupivacaine-induced spinal neurotoxicity in rats by inhibiting
ferroptosis. Neurosci Lett. 809(137308)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Marciniak SJ, Chambers JE and Ron D:
Pharmacological targeting of endoplasmic reticulum stress in
disease. Nat Rev Drug Discov. 21:115–140. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ghemrawi R and Khair M: Endoplasmic
reticulum stress and unfolded protein response in neurodegenerative
diseases. Int J Mol Sci. 21(6127)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hetz C, Zhang K and Kaufman RJ:
Mechanisms, regulation and functions of the unfolded protein
response. Nat Rev Mol Cell Biol. 21:421–438. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Li L, Zhang QG, Lai LY, Wen XJ, Zheng T,
Cheung CW, Zhou SQ and Xu SY: Neuroprotective effect of ginkgolide
B on bupivacaine-induced apoptosis in SH-SY5Y cells. Oxid Med Cell
Longev. 2013(159864)2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Liang Y, Ji J, Lin Y, He Y and Liu J: The
Ganglioside GM-1 inhibits bupivacaine-induced neurotoxicity in
mouse neuroblastoma Neuro2a cells. Cell Biochem Funct. 34:455–462.
2016.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Liu B, Ji J, Feng Q, Luo X, Yan X, Ni Y,
He Y, Mao Z and Liu J: Monosialoganglioside protects against
bupivacaine-induced neurotoxicity caused by endoplasmic reticulum
stress in rats. Drug Des Devel Ther. 13:707–718. 2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Liu H, Xu S, Wang C, Deng Y, Xu B, Yang T,
Sun J and Liu W: The beneficial role of sirtuin 1 in preventive or
therapeutic options of neurodegenerative diseases. Neuroscience.
504:79–92. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Tang BL and Chua CE: SIRT1 and neuronal
diseases. Mol Aspects Med. 29:187–200. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Fangma Y, Wan H, Shao C, Jin L and He Y:
Research progress on the role of sirtuin 1 in cerebral ischemia.
Cell Mol Neurobiol. 43:1769–1783. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yu X, Zhang S, Zhao D, Zhang X, Xia C,
Wang T, Zhang M, Liu T, Huang W and Wu B: SIRT1 inhibits apoptosis
in in vivo and in vitro models of spinal cord injury
via microRNA-494. Int J Mol Med. 43:1758–1768. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Zheng LN, Guo FQ, Li ZS, Wang Z, Ma JH,
Wang T, Wei JF and Zhang WW: Dexmedetomidine protects against
lidocaine-induced neurotoxicity through SIRT1
downregulation-mediated activation of FOXO3a. Hum Exp Toxicol.
39:1213–1223. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang X, Yuan B, Cheng B, Liu Y, Zhang B,
Wang X, Lin X, Yang B and Gong G: Crocin alleviates myocardial
ischemia/reperfusion-induced endoplasmic reticulum stress via
regulation of miR-34a/Sirt1/Nrf2 pathway. Shock. 51:123–130.
2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li YP, Wang SL, Liu B, Tang L, Kuang RR,
Wang XB, Zhao C, Song XD, Cao XM, Wu X, et al: Sulforaphane
prevents rat cardiomyocytes from hypoxia/reoxygenation injury in
vitro via activating SIRT1 and subsequently inhibiting ER stress.
Acta Pharmacol Sin. 37:344–353. 2016.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zheng X, Xu F, Liang H, Cao H, Cai M, Xu W
and Weng J: SIRT1/HSF1/HSP pathway is essential for
exenatide-alleviated, lipid-induced hepatic endoplasmic reticulum
stress. Hepatology. 66:809–824. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
He B, Zhang W, Qiao J, Peng Z and Chai X:
Melatonin protects against COPD by attenuating apoptosis and
endoplasmic reticulum stress via upregulating SIRT1 expression in
rats. Can J Physiol Pharmacol. 97:386–391. 2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Melhem H, Hansmannel F, Bressenot A,
Battaglia-Hsu SF, Billioud V, Alberto JM, Gueant JL and
Peyrin-Biroulet L: Methyl-deficient diet promotes colitis and
SIRT1-mediated endoplasmic reticulum stress. Gut. 65:595–606.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kang X, Yang W, Wang R, Xie T, Li H, Feng
D, Jin X, Sun H and Wu S: Sirtuin-1 (SIRT1) stimulates growth-plate
chondrogenesis by attenuating the PERK-eIF-2α-CHOP pathway in the
unfolded protein response. J Biol Chem. 293:8614–8625.
2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Shaito A, Posadino AM, Younes N, Hasan H,
Halabi S, Alhababi D, Al-Mohannadi A, Abdel-Rahman WM, Eid AH,
Nasrallah GK and Pintus G: Potential adverse effects of
resveratrol: A literature review. Int J Mol Sci.
21(2084)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Luo Y, Zhao Y, Lai J, Wei L, Zhou G, Yu Y
and Liu J: Resveratrol suppresses bupivacaine-induced spinal
neurotoxicity in rats by inhibiting endoplasmic reticulum stress
via SIRT1 modulation. Biomed Res Int. 2023(1176232)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fan X, Bian W, Liu M, Li J and Wang Y:
MiRNA-429 alleviates ketamine-induced neurotoxicity through
targeting BAG5. Environ Toxicol. 36:620–627. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hao R, Ge J, Song X, Li F, Sun-Waterhouse
D and Li D: Cadmium induces ferroptosis and apoptosis by modulating
miR-34a-5p/Sirt1axis in PC12 cells. Environ Toxicol. 37:41–51.
2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shayan M, Mehri S, Razavi BM and
Hosseinzadeh H: Minocycline protects PC12 cells against
cadmium-induced neurotoxicity by modulating apoptosis. Biol Trace
Elem Res. 201:1946–1954. 2023.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Tang XP, Guo XH, Geng D and Weng LJ:
d-Limonene protects PC12 cells against corticosterone-induced
neurotoxicity by activating the AMPK pathway. Environ Toxicol
Pharmacol. 70(103192)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang Y, He Y, Deng N, Chen Y, Huang J and
Xie W: Protective effect of resveratrol against
corticosterone-induced neurotoxicity in PC12 cells. Transl
Neurosci. 10:235–240. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang Z, Hu S, He Y and Ji L: LINC00665
rescues bupivacaine induced neurotoxicity in human neural cell of
SH-SY5Y through has-miR-34a-5p. Brain Res Bull. 177:210–216.
2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jęśko H, Wencel P, Strosznajder RP and
Strosznajder JB: Sirtuins and their roles in brain aging and
neurodegenerative disorders. Neurochem Res. 42:876–890.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang Y, Li T, Pan M, Wang W, Huang W,
Yuan Y, Xie Z, Chen Y, Peng J, Li X and Meng Y: SIRT1 prevents
cigarette smoking-induced lung fibroblasts activation by regulating
mitochondrial oxidative stress and lipid metabolism. J Transl Med.
20(222)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ren Q, Hu Z, Jiang Y, Tan X, Botchway BOA,
Amin N, Lin G, Geng Y and Fang M: SIRT1 protects against apoptosis
by promoting autophagy in the oxygen glucose
deprivation/reperfusion-induced injury. Front Neurol.
10(1289)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Jiao F and Gong Z: The beneficial roles of
SIRT1 in neuroinflammation-related diseases. Oxid Med Cell Longev.
2020(6782872)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chen H, Lin X, Yi X, Liu X, Yu R, Fan W,
Ling Y, Liu Y and Xie W: SIRT1-mediated p53 deacetylation inhibits
ferroptosis and alleviates heat stress-induced lung epithelial
cells injury. Int J Hyperthermia. 39:977–986. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen L, Li S, Zhu J, You A, Huang X, Yi X
and Xue M: Mangiferin prevents myocardial infarction-induced
apoptosis and heart failure in mice by activating the Sirt1/FoxO3a
pathway. J Cell Mol Med. 25:2944–2955. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kaewmool C, Kongtawelert P, Phitak T,
Pothacharoen P and Udomruk S: Protocatechuic acid inhibits
inflammatory responses in LPS-activated BV2 microglia via
regulating SIRT1/NF-κB pathway contributed to the suppression of
microglial activation-induced PC12 cell apoptosis. J Neuroimmunol.
341(577164)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Mao H, Wang L, Xiong Y, Jiang G and Liu X:
Fucoxanthin attenuates oxidative damage by activating the
Sirt1/Nrf2/HO-1 signaling pathway to protect the kidney from
ischemia-reperfusion injury. Oxid Med Cell Longev.
2022(7444430)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang F, Ma J, Wang J, Chen M, Xia H, Yao S
and Zhang D: SIRT1 ameliorated septic associated-lung injury and
macrophages apoptosis via inhibiting endoplasmic reticulum stress.
Cell Signal. 97(110398)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang H, Dong X, Liu Z, Zhu S, Liu H, Fan
W, Hu Y, Hu T, Yu Y, Li Y, et al: Resveratrol suppresses
rotenone-induced neurotoxicity through activation of SIRT1/Akt1
signaling pathway. Anat Rec (Hoboken). 301:1115–1125.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
40
|
Tang X, Zhao Y, Zhou Z, Yan J, Zhou B, Chi
X, Luo A and Li S: Resveratrol mitigates sevoflurane-induced
neurotoxicity by the SIRT1-dependent regulation of BDNF expression
in developing mice. Oxid Med Cell Longev.
2020(9018624)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Bai L, Liu R, Wang R, Xin Y, Wu Z, Ba Y,
Zhang H, Cheng X, Zhou G and Huang H: Attenuation of Pb-induced Aβ
generation and autophagic dysfunction via activation of SIRT1:
Neuroprotective properties of resveratrol. Ecotoxicol Environ Saf.
222(112511)2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gomes BAQ, Silva JPB, Romeiro CFR, Dos
Santos SM, Rodrigues CA, Gonçalves PR, Sakai JT, Mendes PFS, Varela
ELP and Monteiro MC: Neuroprotective mechanisms of resveratrol in
Alzheimer's disease: Role of SIRT1. Oxid Med Cell Longev.
2018(8152373)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Li L, Ye XP, Lu AZ, Zhou SQ, Liu H, Liu
ZJ, Jiang S and Xu SY: Hyperglycemia magnifies bupivacaine-induced
cell apoptosis triggered by mitochondria dysfunction and
endoplasmic reticulum stress. J Neurosci Res. 91:786–798.
2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Iurlaro R and Muñoz-Pinedo C: Cell death
induced by endoplasmic reticulum stress. FEBS J. 283:2640–2652.
2016.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Zhang X, Jiang L, Chen H, Wei S, Yao K,
Sun X, Yang G, Jiang L, Zhang C, Wang N, et al: Resveratrol
protected acrolein-induced ferroptosis and insulin secretion
dysfunction via ER-stress-related PERK pathway in MIN6 cells.
Toxicology. 465(153048)2022.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Shati AA: Resveratrol protects against
cadmium chloride-induced hippocampal neurotoxicity by inhibiting ER
stress and GAAD 153 and activating sirtuin 1/AMPK/Akt. Environ
Toxicol. 34:1340–1353. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhu H, Li X, Qiao M, Sun X and Li G:
Resveratrol alleviates inflammation and ER stress through
SIRT1/NRF2 to delay ovarian aging in a short-lived fish. J Gerontol
A Biol Sci Med Sci. 78:596–602. 2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chou X, Ding F, Zhang X, Ding X, Gao H and
Wu Q: Sirtuin-1 ameliorates cadmium-induced endoplasmic reticulum
stress and pyroptosis through XBP-1s deacetylation in human renal
tubular epithelial cells. Arch Toxicol. 93:965–986. 2019.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Zhang Y, He L, Tu M, Huang M, Chen Y, Pan
D, Peng J and Shen X: The ameliorative effect of terpinen-4-ol on
ER stress-induced vascular calcification depends on SIRT1-mediated
regulation of PERK acetylation. Pharmacol Res.
170(105629)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Prola A, Pires Da Silva J, Guilbert A,
Lecru L, Piquereau J, Ribeiro M, Mateo P, Gressette M, Fortin D,
Boursier C, et al: SIRT1 protects the heart from ER stress-induced
cell death through eIF2α deacetylation. Cell Death Differ.
24:343–356. 2017.PubMed/NCBI View Article : Google Scholar
|