Introduction
Aggressive fibromatosis (AF), a borderline soft
tissue tumor arising from fibrous connective tissue, occurs
throughout the body. The incidence of AF is significantly higher in
females compared to male patients (1,2).
Emerging evidence has suggested that the etiology of this disease
has several aspects, including genetic, endocrine and physical
factors. Surgical trauma may accelerate the development of AF, the
pathogenesis of which remains unknown. Two principal types of AF
have been identified, namely the sporadic and the genetic type. The
sporadic type is more common and its pathogenesis is associated
with mutations in the β-catenin gene (3). In addition, the genetic type of AF is
more common in familial adenomatous polyposis and Gardner syndrome
(4). The genetic type is often
intraperitoneally associated with an adenomatous polyposis coli
disease gene mutation (5). AF can
impact functionality and cause treatment-related morbidity and
mortality. AF is a complex condition with numerous recognised
treatments, including active observation, hormonal therapy,
chemotherapy, radiotherapy and surgical resection. Hormonal agents
and nonsteroidal anti-inflammatory drugs have benign side effect
profiles but generally limited efficacy. Among patients with
progressive, refractory or symptomatic AF, sorafenib significantly
prolonged progression-free survival.
Case report
Case
In the present study, the case of a 36-year-old
female with AF who underwent cervical spinal cord ependymoma
surgery is reported. AF developed in the soft tissue of the neck
adjacent to the incision site. The patient did not have any genetic
family history of AF. In June 2019, the patient visited the
China-Japan Union Hospital of Jilin University (Changchun, China),
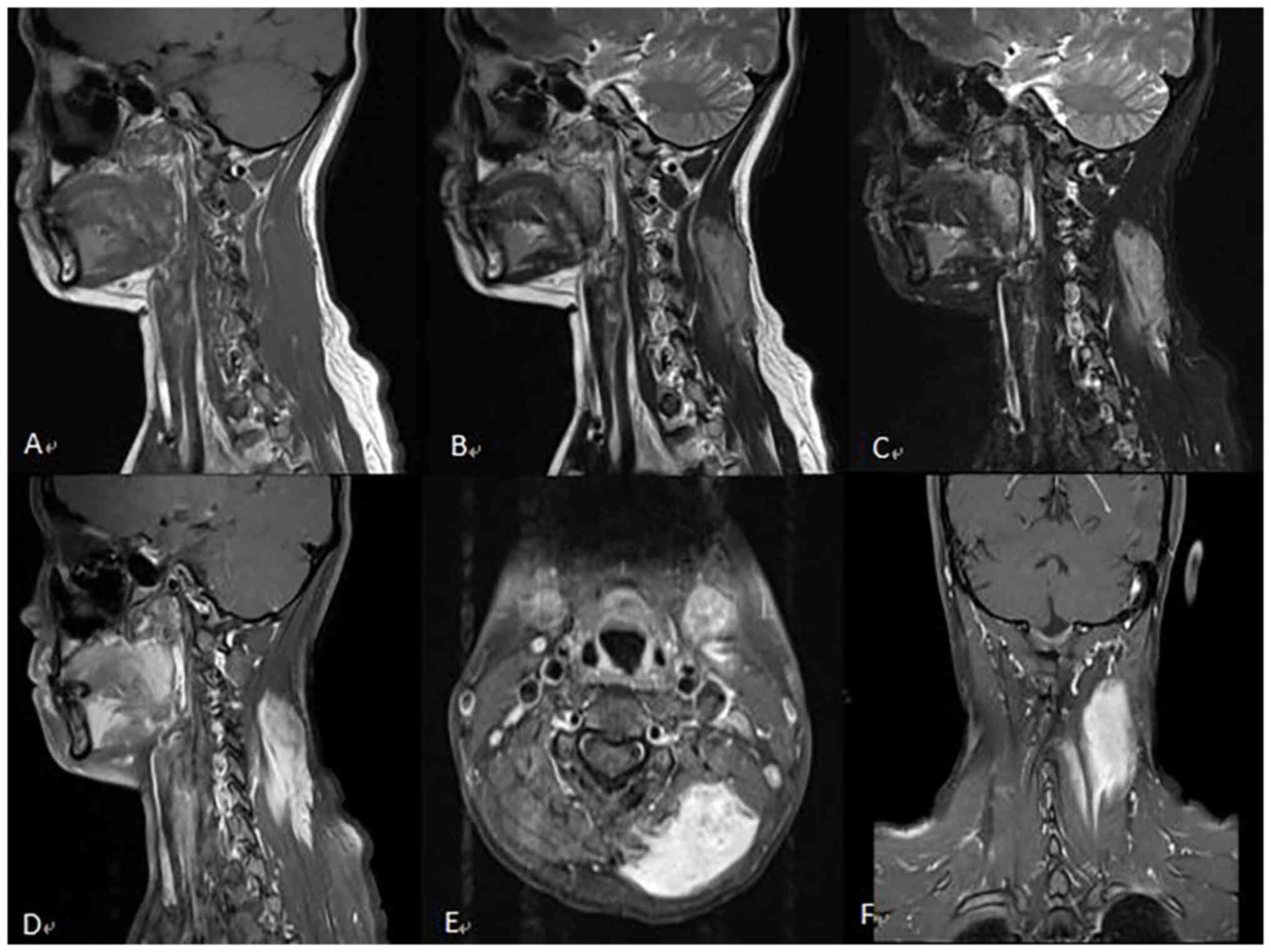
complaining of neck and shoulder pain for >3 months. An enhanced
magnetic resonance imaging (MRI) scan of the cervical spine was
then performed. The MRI scan showed uneven enhanced masses in the
spinal cord at the medulla-C3 vertebral level (Fig. 1).
The patient was otherwise in good health and
self-reported that she had no history of hypertension, coronary
heart disease, diabetes, cerebrovascular disease, hepatitis,
malaria or tuberculosis. The patient also denied any known familial
genetic disease history. In August 2019, the patient underwent a
posterior median approach at the Beijing Tiantan Hospital (Beijing,
China) for complete removal of the medulla-C3 vertebral level
intramedullary tumor. The patient had a good prognosis and the
7x1.5x2-cm mass was completely resected, with no residual lesions
(Fig. 2). Postoperative
pathological findings classified the mass as a World Health
Organization II grade ependymoma (Fig.
3A). Immunohistochemical analysis revealed that the tumor was
positive for glial fibrillary acidic protein (Fig. 3B) and S-100 (Fig. 3C), and negative for oligodendrocyte
transcription factor 2, epithelial membrane antigen (data not
shown), synaptophysin (Fig. 3D)
and progesterone receptor expression (data not shown). The Ki67
positive nuclear expression rate was 2% (data not shown).
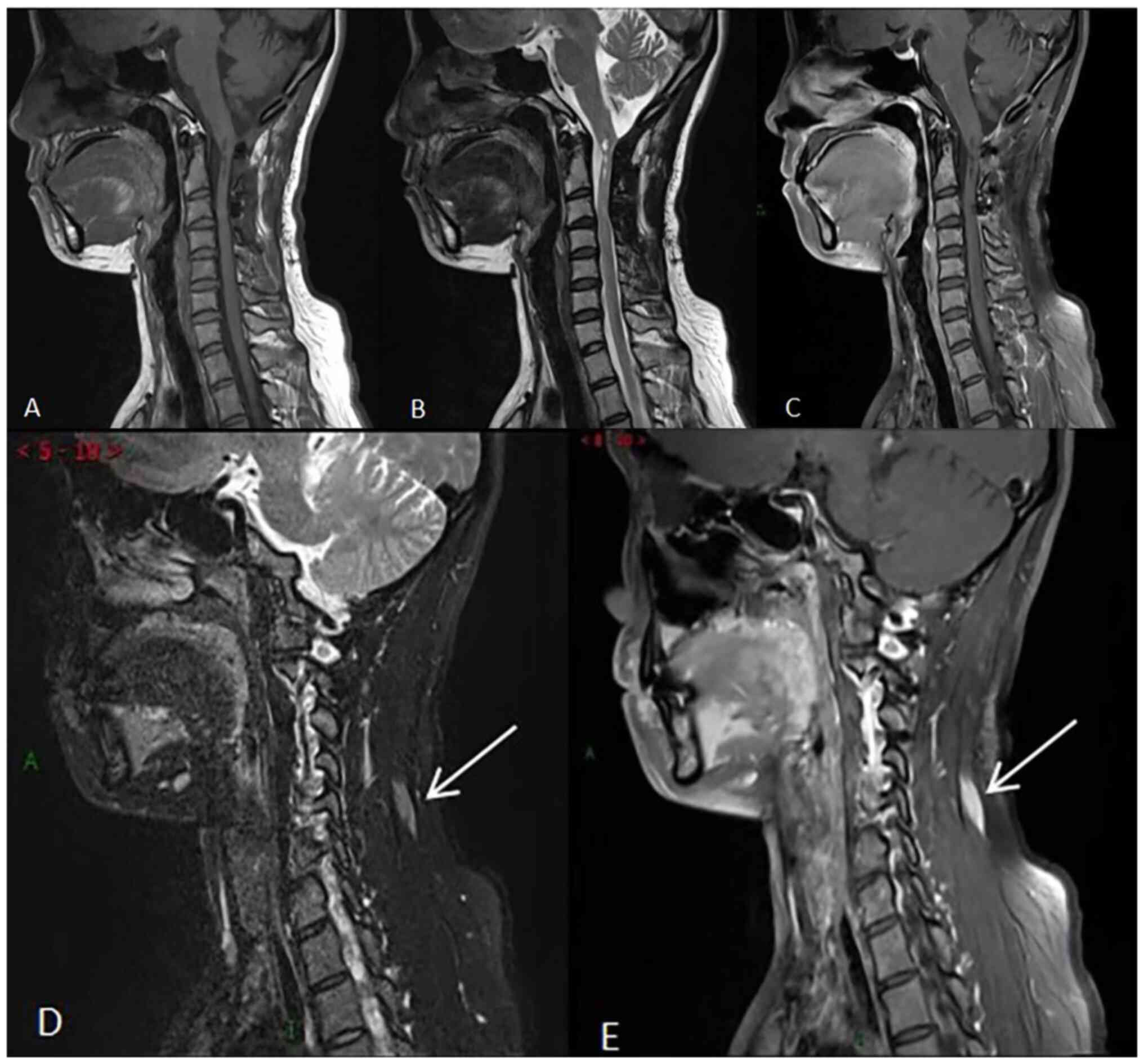
Postoperatively, the female patient underwent a
routine cervical spine plain MRI scan and was then fully reviewed
at the China-Japan Union Hospital of Jilin University (Changchun,
China) in July 2020 (Fig. 4). As
indicated in Fig. 4A-C,
postoperative changes of the medulla-C3 vertebral level ependymoma
were observed with no clear signs of recurrence. In addition,
strips of abnormal enhancement were revealed in the soft tissue of
the left side of the neck (Fig. 4D
and E). A follow-up review or
further examination were recommended. The patient did not
experience any discomfort during follow-up. Therefore,
re-examination was performed at two years after the last follow-up,
while the patient was not treated for the soft tissue lesions in
the neck. In July 2022, the patient arrived at the China-Japan
Union Hospital of Jilin University (Changchun, China) for
re-examination of the cervical vertebra by plain MRI scan with
enhancement (Fig. 5). The results
revealed abundant blood supply and occupying space in the left
cervical semispinosus and splenius capitis muscles. The lesion
showed infiltrative growth, while its volume was significantly
increased compared with that in the previous scan. The clinicians
recommended that the patient should undergo an ultrasound-guided
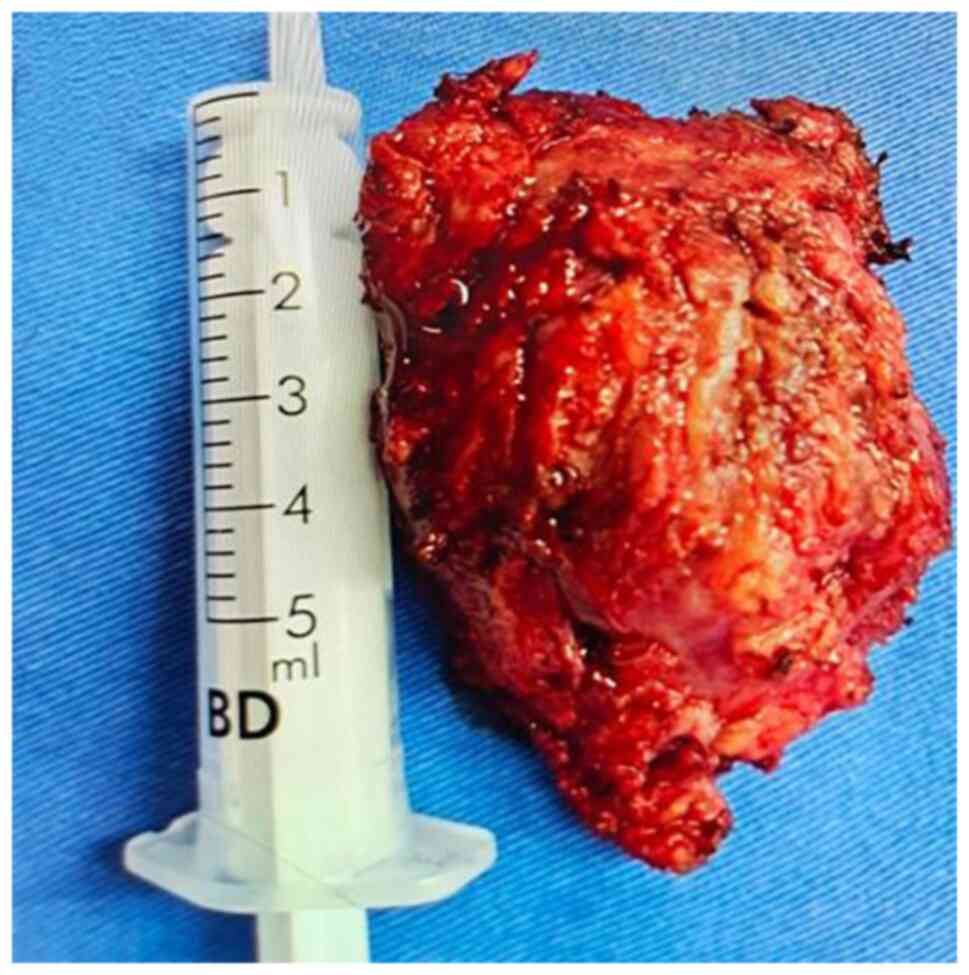
puncture biopsy of the posterior neck. After one month, the lesion
on the back of the left side of the patient's neck was surgically
removed. The intraoperative tumor was located in the muscle space
with apparent adhesion into the muscular layer with a complete
capsule covering its surface. The tumor, 5x8x7 cm in size, was
completely removed (Fig. 6). A
needle biopsy revealed spindle-cell lesions originating from
mesenchymal tissue. The nuclei were blunt and rounded at both ends,
and atypia was not obvious. Division of the nuclei was rare. The
postoperative pathology suggested that the mass consisted of
spindle cells (including fibroblasts and myofibroblasts), and
infiltrated the fat and the rhabdoid tissue. The postoperative
pathology combined with immunohistochemical results indicated AF
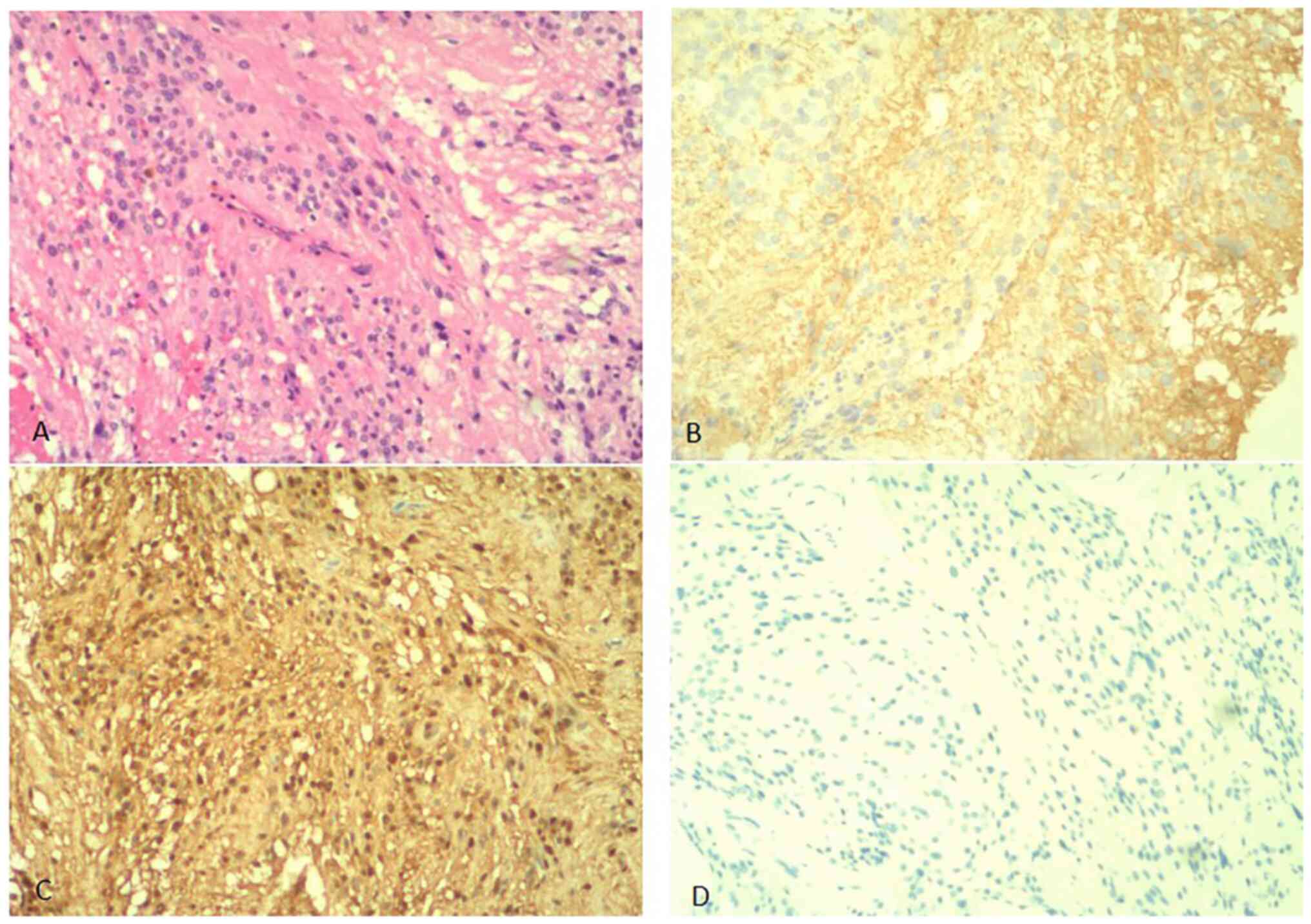
(Fig. 7). For
immunohistochemistry, 5-µm tissue sections of formalin-fixed and
paraffin-embedded material were prepared on glass slides. The
relevant markers were detected by the immunohistochemical EnVision
method (Agilent Technologies, Inc.). The immunohistochemical
analysis results demonstrated that the tumor was positive for
β-catenin (nucleoplasm) (Fig. 7B),
smooth muscle actin (SMA) (Fig.
7C), calponin (Fig. 7D),
vimentin, Ki-67 (1%) and desmin, and negative for CD34, actin,
S-100, mucin 4 and Bcl-2 (data not shown). During the follow-up,
the patient underwent cervical MRI half a year after surgery. The
results suggested AF recurrence and the patient was re-admitted for
radical surgery and volumetric modulated arc therapy. An MRI scan
of the neck was recommended semi-annually after discharge.
Methods
Histological analysis and immunohistochemical
staining for EnVision were performed according to standard
protocols. The following primary antibodies were used:
Anti-β-catenin (cat. no. ab32572; working solution), anti-SMA (cat.
no. ab108424; working solution), anti-Calponin (cat. no. ab227667;
working solution), anti-Vimentin (cat. no. ab92547; working
solution), anti-Ki-67 (cat. no. ab92742; working solution),
anti-Desmin (cat. no. ab32362; workingsolution), anti-CD34 (cat.
no. ab81289; working solution), anti-Actin (cat. no. ab32575;
working solution), anti-S-100 (cat. no. ab197896; working
solution), anti-MUC4 (cat. no. ab243921; working solution),
anti-Bcl-2 (cat. no. ab238042; working solution), anti-GFAP (cat.
no. ab68428; working solution), anti-Olig2 (cat. no. ab109186;
working solution), anti-MUC1 (cat. no. ab109185; working solution),
anti-Synaptophysin (cat. no. ab32127; working solution) and
anti-Progesterone Receptor (cat. no. ab32085; working solution)
(all Abcam).
Discussion
The incidence of AF is higher in women during
pregnancy and postpartum, while the abdominal wall scar is the most
common tumor site. Trauma and endocrine factors may be associated
with AF. Previous studies demonstrated that positive estrogen
receptor β and cyclin D1-mediated immune responses were associated
with a high proliferation rate of AF, and estrogen receptor
upregulation was able to predict postoperative tumor recurrence
(6,7). It was therefore hypothesized that
estrogen could modulate AF. However, evidence of the efficacy of
antiestrogen therapy in AF has been limited to case series and
single-arm trials (8). Treatment
guidelines do not routinely recommend treatment with hormones and
non-steroidal anti-inflammatory drugs (NSAIDs), including
celecoxib, which are commonly used for pain control. Although
treatment approaches for AF continue to evolve, several directions
are clear. For the majority of patients with AF, surgery is no
longer the preferred primary therapeutic approach, which has been
replaced by active surveillance (9).
In the present case, following cervical spinal cord
ependymoma surgery, the lesion was located in the soft tissue of
the neck adjacent to the incision site. Within two years after
surgery, the lesion had rapidly grown and caused marked discomfort
for the patient. The tumor could be associated with the massive
reactive proliferation of fibroblasts and myofibroblasts triggered
by the external impact and intrinsic injury of the neck muscles and
fascia after surgery. Therefore, surgical treatment was applied.
During follow-up, the patient underwent cervical MRI half a year
after surgery. The MRI scanning results revealed abnormal signals
within the soft tissue of the neck and therefore, further
examination was recommended. The results suggested AF recurrence
and the patient was re-admitted for radical surgery and volumetric
modulated arc therapy. More specifically, the pathology suggested
recurrence of invasive fibromatosis with infiltrative growth in
striated muscles and adipose tissues. Through the follow-up of this
case, the diagnosis and treatment patterns of AF were thoroughly
explored. The choice of treatment modality may depend on the extent
and location of the lesion, and the capacity of the medical
facility. Based on the clinical condition and patients'
preferences, any of the aforementioned treatment options may be
potentially applied as a first- or second-line therapy. In general,
surgery is not considered a first-line treatment strategy. Among
patients with progressive AF, those with symptoms, systemic
therapies, surgery and ablative therapies are considered. Systemic
therapy is commonly applied as a first-line therapy. However, the
choice of other treatment approaches, such as surgical resection
and medical or ablative therapies, partially depends on the
location of the tumor (10).
In a previous case report, unresectable AF of the
neck was successfully treated with chemotherapy combined with
NSAIDs for ~21 months (11). The
most recent National Comprehensive Cancer Network guidelines
recommend the systemic treatment of tumors with symptoms and
impaired or threatened functions with common chemotherapy drugs,
including sorafenib and methotrexate combined with vinorelbine, and
methotrexate combined with vinblastine (12). The treatment of AF has gradually
changed from surgical resection with active intervention to
conservative treatment with follow-up monitoring.
Accurate diagnosis is crucial for AF treatment. In
terms of rare AF in the neck, a differential diagnosis should be
made to exclude the possibility of a solitary fibrous tumor or
nodular fasciitis. In the case of solitary fibrous tumors, the
incidence in the pleural cavity is >50%. The incidence of
extrapleural disease is only 0.6% and rarely affects the head and
neck (13). In terms of MRI scan,
T1-weighted imaging (T1WI) showed an equivalent signal, T2WI an
uneven hypersignal and enhancement a moderate to obvious
enhancement. It was difficult to distinguish a solitary fibrous
tumor from AF on the image. Therefore, imaging should be combined
with a review of the patient's medical history, pathological
findings and immunohistochemistry. In the case of the present
study, immunohistochemical staining showed that the solitary
fibrous tumor was mostly positive for CD34, vimentin, Bcl-2 and
CD99, and negative for S-100 and SMA. In addition, the female
patient had a clear history of neck surgery, thus assisting the
preoperative differential diagnosis. In nodular fasciitis, the
benign lesion originates from the fascia tissue and its essence is
considered a benign hyperplasia of the fibrous tissue. It most
commonly occurs in the extremities, followed by the trunk and the
head and neck (14). Nodular
fasciitis is an isolated lesion, with a generally small size, which
is characterized by rapid growth for no more than three months.
Nodular fasciitis of the head and neck, which is commonly caused by
local trauma and inflammation, is relatively rare (15,16).
It is usually treated by local complete excision. Previous reports
have suggested that it may spontaneously resolve, while
preoperative diagnosis is difficult. The diagnosis of nodular
fasciitis requires a combination of imaging findings, pathological
diagnosis and immunohistochemical expression results (17,18).
In conclusion, AF near the incision site after
cervical spinal cord ependymoma surgery is relatively rare.
However, imaging examination alone cannot diagnose AF. In the case
of the present study, immunohistochemical examination of AF
indicated that the tumor cells expressed vimentin and SMA in the
cytoplasm and β-catenin in the nucleus. The diagnosis of AF
requires pathological results combined with immunohistochemical
results.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XZ, SL and BY conceived the study, participated in
the design of the study, and collected the data and the images. XZ,
GC, SL and BY drafted the manuscript. JD participated in the data
acquisition and interpretation, was involved in drafting the
manuscript and critically revised the manuscript. GC, TL and YG
collected the clinical data and performed the literature research.
XZ and TL confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The patient provided written informed consent for
publication of the medical data and images for this case.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
de Camargo VP, Keohan ML, D'Adamo DR,
Antonescu CR, Brennan MF, Singer S, Ahn LS and Maki RG: Clinical
outcomes of systemic therapy for patients with deep fibromatosis
(desmoid tumor). Cancer. 116:2258–265. 2010.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bertario L, Russo A, Sala P, Eboli M,
Giarola M, D'amico F, Gismondi V, Varesco L, Pierotti MA and Radice
P: Hereditary Colorectal Tumours Registry. Hereditary Colorectal
Tumours Registry. Genotype and phenotype factors as determinants of
desmoid tumors in patients with familial adenomatous polyposis. Int
J Cancer. 95:102–107. 2001.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Escobar C, Munker R, Thomas JO, Li BD and
Burton GV: Update on desmoid tumors. Ann Oncol. 23:562–569.
2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tayeb Tayeb C, Parc Y, Andre T and
Lopez-Trabada Ataz D: Polypose adénomateuse familiale, tumeurs
desmoïdes et syndrome de Gardner (Familial adenomatous polyposis,
desmoid tumors and Gardner syndrome). Bull Cancer. 107:352–358.
2020.PubMed/NCBI View Article : Google Scholar : (In French).
|
|
5
|
Nieuwenhuis MH, Casparie M, Mathus-Vliegen
LM, Dekkers OM, Hogendoorn PC and Vasen HF: A nation-wide study
comparing sporadic and familial adenomatous polyposis-related
desmoid-type fibromatoses. Int J Cancer. 129:256–261.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fiore M, Coppola S, Cannell AJ, Colombo C,
Bertagnolli MM, George S, Le Cesne A, Gladdy RA, Casali PG, Swallow
CJ, et al: Desmoid-type fibromatosis and pregnancy: A
multi-institutional analysis of recurrence and obstetric risk. Ann
Surg. 259:973–978. 2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Santti K, Ihalainen H, Rönty M, Karlsson
C, Haglund C, Sampo M, Tarkkanen M and Blomqvist C: Estrogen
receptor beta expression correlates with proliferation in desmoid
tumors. J Surg Oncol. 119:873–879. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Riedel RF and Agulnik M: Evolving
strategies for management of desmoid tumor. Cancer. 128:3027–3040.
2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
National Comprehensive Cancer Network
(NCCN). NCCN clinical practice guidelines in oncology (NCCN
Guidelines). Soft Tissue Sarcoma. Version 1.2021.
|
|
10
|
Prendergast K, Kryeziu S and Crago AM: The
evolving management of desmoid fibromatosis. Surg Clin North Am.
102:667–677. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Longhi A, Errani C, Battaglia M,
Alberghini M, Ferrari S, Mercuri M and Molinari M: Aggressive
fibromatosis of the neck treated with a combination of chemotherapy
and indomethacin. Ear Nose Throat J. 90:E11–E15. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
National Comprehensive Cancer Network
(NCCN).NCCN Clinical Practice Guidelines in Oncology (NCCN
Guidelines). Soft Tissue Sarcoma. Version 2.2023.
|
|
13
|
Gengler C and Guillou L: Solitary fibrous
tumour and haemangiopericytoma: Evolution of aconcept.
Histopathology. 48:63–74. 2006.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Jo VY and Fletcher CD: WHO classification
of soft tissue tumours: An update based on the 2013 (4th) edition.
Pathology. 46:95–104. 2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Singh S, Paul S, Dhall K and Khichy S:
Nodular fasciitis: A diagnostic challenge. Indian J Pathol
Microbiol. 56:288–290. 2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Reitzen SD, Dogan S and Har-El G: Nodular
fasciitis: A case series. J Laryngol Otol. 123:541–544.
2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
de Carli ML, Sá Fernandes K, dos Santos
Pinto D Jr, Witzel AL and Martins MT: Nodular fasciitis of the oral
cavity with partial spontaneous regression (nodular fasciitis).
Head Neck Pathol. 7:69–72. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kang A, Kumar JB, Thomas A and Bourke AG:
A spontaneously resolving breast lesion: imaging and cytological
findings of nodular fasciitis of the breast with FISH showing USP6
gene rearrangement. BMJ Case Rep.
2015(bcr2015213076)2015.PubMed/NCBI View Article : Google Scholar
|