Introduction
In China, middle-aged and elderly individuals ≥60
years old, accounted for 18.7% of all cases in the 7th census data.
The proportion of elderly is growing, and the incidence of
degenerative lumbar spinal stenosis (DLSS) is increasing annually
(1).
DLSS refers to a chronic lumbar disease in which
secondary degenerative changes of the vertebral body,
intervertebral disc and paraspinal soft tissue occur due to stress
imbalances in the lumbar spine, resulting in a series of back and
leg pain and neurological symptoms caused by spinal canal volume
change and dural sac stenosis (2).
Parameters related to the sagittal position of the spine and pelvis
can be used as criteria to evaluate the state of physical balance
(3). The ‘cone of economy’, first
described by Dubousset (4),
indicates that the normal spinal and pelvic shape curve can enable
individuals to fulfill the needs of physiological posture and daily
activities with minimum energy consumption. Once the sagittal
alignment of the spine is altered, balances of the spine require
more energy from the surrounding tissues to maintain, resulting in
muscle fatigue and paravertebral pain. Artificial muscle removal
experiments have demonstrated that the lumbar spine can appear
unstable under very mild loading without the support of the
corresponding muscles (5).
Therefore, paravertebral muscle mass is an important factor for the
entire process of lumbar degeneration. Paravertebral muscle
degeneration is associated with the development of a variety of
lumbar diseases and the emergence of postoperative complications
(6-9).
Previous studies on DLSS have mostly investigated
the sagittal imbalance of the spine-pelvis or the degeneration of
paravertebral muscles (10-13).
However, the combination of the two has not been explored, to the
best of the authors’ knowledge, resulting in imperfect treatment
options and ultimately affecting therapeutic outcomes. Therefore,
the literature on DLSS, spinal-pelvic sagittal imbalance, and
paravertebral muscle degeneration was reviewed and the present
study was designed to analyze the link between lumbar paravertebral
muscles and spinal-pelvic parameters in patients with DLSS by
measuring the parameters associated with lumbar paravertebral
muscles and spinal-pelvic sagittal position. The results obtained
in the present study may provide a basis for the subsequent
treatment and prognosis of DLSS.
Materials and methods
Study design
A total of 165 patients and healthy volunteers with
lumbar spinal stenosis who were admitted to Ordos Central Hospital
for treatment between January 2020 and January 2022 were included.
Among all the patients (n=165) who participated in the present
study, there were 72 men and 93 women, ranging in age from 53 to 80
years old, including 95 patients in the experimental group
(patients with DLSS) and 70 patients in the control group (healthy
volunteers). The present study was approved by the Ethics Committee
of Ordos Central Hospital (Ordos, China; approval no. 2022-012).
Informed consent was obtained from all subjects and/or their legal
guardians for the present study.
Inclusion and exclusion criteria
The inclusion criteria consisted of a clinical
diagnosis of DLSS including spondylolisthesis (I˚ or II˚), with
lumbar magnetic resonance imaging (MRI) and defined as
single-segment stenosis, including central canal stenosis and/or
lateral recess stenosis, spinal x-ray, radiating pain in the lower
extremity and/or neurogenic claudication after a single trip of
less than 100 m.
The exclusion criteria consisted of other spinal and
soft tissue diseases, such as spinal trauma, spinal infection,
spinal metastatic lesions, spondylolisthesis (III˚ or IV˚), a
history of spinal surgery, severe osteoarthritis of the hip and
knee, lower limb paralysis, Parkinson's disease, multiple
sclerosis, soft tissue tumors, or infections.
The inclusion criteria for the control group were
individuals with lumbar MRI and spinal X-ray. No history of any
lumbar disease, low back pain, radiation pain in the lower
extremities, and neurogenic claudication. The exclusion criteria
were the same as in the experimental group.
Imaging examination. Spinal X-ray
A universal digital radiography system (General
Medical Merate S.p.A.) was used to obtain anteroposterior and
lateral radiographs of the full-length spine. Lateral images were
imported into Surgimap (v2.3.2.1; Nemaris, Inc.) to measure and
calculate each sagittal parameter.
Lumbar MRI
MRI was performed using a Signa HDxt 3.0T magnetic
resonance scanner (GE Healthcare). For conventional MRI scans of
the sagittal fat-suppressed FSE T2WI, the following settings were
used: Echo time (TE)=42.72; repetition time (TR)=3,246; display
field of view (DFOV)=31x31 cm; slice thickness=4, interslice
distance=5 and number of excitations (NEX)=2. For cross-sectional
T2WI the following settings were used: TE=123.66; TR=2854;
DFOV=20x20 cm; slice thickness=4; interslice distance=5; NEX=2. In
the present study, the location and degree of compression of spinal
stenosis were observed primarily through multiple angles in the
axial and sagittal planes. The responsible lesion was confirmed in
accordance with the medical history and clinical signs of the
patients, and the cross-sectional MRI T2WI corresponding to the
lesion was selected as the baseline image for measurement.
Evaluation indicators. Spine-pelvic
parameters
Surgimap allowed the measurement of spine-related
parameters. Lafage et al (14) confirmed that the application of
Surgimap to calculate the relevant parameters had the advantages of
shorter processing periods, less errors and easier data storage
compared with traditional manual methods, and was thus suitable for
clinical use. Full-length X-ray lateral images of the standard
spine as JPG files were imported from the Radiology Department into
Surgimap to measure the spinal sagittal parameters separately
according to the corresponding operating criteria (Figs. 1 and 2).
 | Figure 1Measurement diagram developed using
Surgimap. (A) LL, the angle formed by the tangent of the upper edge
of the L1 vertebral body and the tangent of the upper endplate of
the S1 vertebral body. (B) TK, the angle formed by the tangent of
the upper edge of the T4 vertebral body and the tangent of the
lower edge of the T12 vertebral body. (C) PT, passing through the
middle of the upper endplate of S1, a straight line between the
point and the midpoint of the line connecting the centers of the
bilateral femoral heads was added in order to exhibit the angle
formed by the straight line and the long axis of the body. SS, the
angle formed by the tangent line of the upper endplate of S1 and
the horizontal line. PI, a straight line through the midpoint of
the line connecting the midpoint of the upper endplate of S1 and
the center of the bilateral femoral heads was added. The angle
formed by the vertical line of the upper endplate of S1 is
depicted. (D) SVA, the distance between the plumb line of the
seventh cervical vertebra and the posterior upper angle of the
first sacrum. LL, lumbar lordosis; TK, thoracic kyphosis; PT,
pelvic tilt; SS, sacral slope; PI, pelvic incidence; SVA, sagittal
vertical axis. |
 | Figure 2Spine-pelvic parameters measured using
Surgimap. (A) TK, the angle formed by the tangent of the upper edge
of the T4 vertebral body and the tangent of the lower edge of the
T12 vertebral body. SVA, the distance between the plumb line of the
seventh cervical vertebra and the posterior upper angle of the
first sacrum. (B) LL, the angle formed by the tangent of the upper
edge of the L1 vertebral body and the tangent of the upper endplate
of the S1 vertebral body. PT, passing through the middle of the
upper endplate of S1, a straight line between the point and the
midpoint of the line connecting the centers of the bilateral
femoral heads was created, and the angle formed by the straight
line and the long axis of the body is depicted. SS, the angle
formed by the tangent line of the upper endplate of S1 and the
horizontal line. PI, a straight line through the midpoint of the
line connecting the midpoint of the upper endplate of S1 and the
center of the bilateral femoral heads was added. The angle formed
by the vertical line of the upper endplate of S1 is revealed. LL,
lumbar lordosis; TK, thoracic kyphosis; PT, pelvic tilt; SS, sacral
slope; PI, pelvic incidence; SVA, sagittal vertical axis. |
Paraspinal muscle parameters
After image selection, a region of interest was
drawn using ImageJ (v1.53c; National Institutes of Health)
(Fig. 3), and the bilateral
paravertebral muscles cross-sectional area (CSA) of the upper
vertebral body at the lesion space, and subcutaneous fat extent
were demonstrated and analyzed. The relative CSA (RCSA) was
calculated using the following formula: Paravertebral muscle
area/vertebral body area x 100% (the interindividual difference
mostly decreased using this ratio). Furthermore, the software
threshold technique (15) was used
to measure the gray values of paravertebral muscles and the
subcutaneous fat (Fig. 4), which
were subsequently imported into Microsoft® Excel for Mac
(v.16.48; Microsoft Corporation) and line graphs were created for
analysis (Fig. 5). The ratio of
the gray values of the coincident parts of the two to the gray
values of paravertebral muscles was used to calculate the fatty
infiltration ratio (FIR) (16). It
is important to note that if the areas of interest of the
multifidus and erector spinalis muscles cannot be drawn on the
lumbar MRI, the case will be excluded. All parameters were measured
using an independent attending physician.
Statistical analysis
Statistical analysis was performed using IBM SPSS
Statistics (v.26.0; IBM Corp.). Continuous data are presented as
the mean ± SD. An independent samples Student's t-test was used for
comparison between two groups (two-tailed tests). A paired samples
Student's t-test was used for comparison within a group (two-tailed
tests). A one-way ANOVA was used for comparison between multiple
groups (one-tailed tests). If the difference was statistically
significant, Tukey's post hoc test was used for pound-wise
comparison after the fact. Categorical data have been presented as
frequencies (percentages). A Pearson's χ2 test was used
for comparison of the distribution between two groups (two-tailed
tests). A Spearman's rank correlation analysis was used for
correlation analysis (two-tailed tests). P<0.05 was considered
to indicate a statistically significant difference.
Results
Analysis of general data results
A total of 165 subjects were included in the present
study, including 70 subjects in the control group and 95 in the
experimental group. The mean age of the experimental group (L4-5
group, 67.44±10.98 years; L5-S1 group, 64.17±4.9 years). The age
range was 57-83 years. There were no statistically significant
differences in age, sex, or body mass index amongst the three
groups (P>0.05; Table I). In
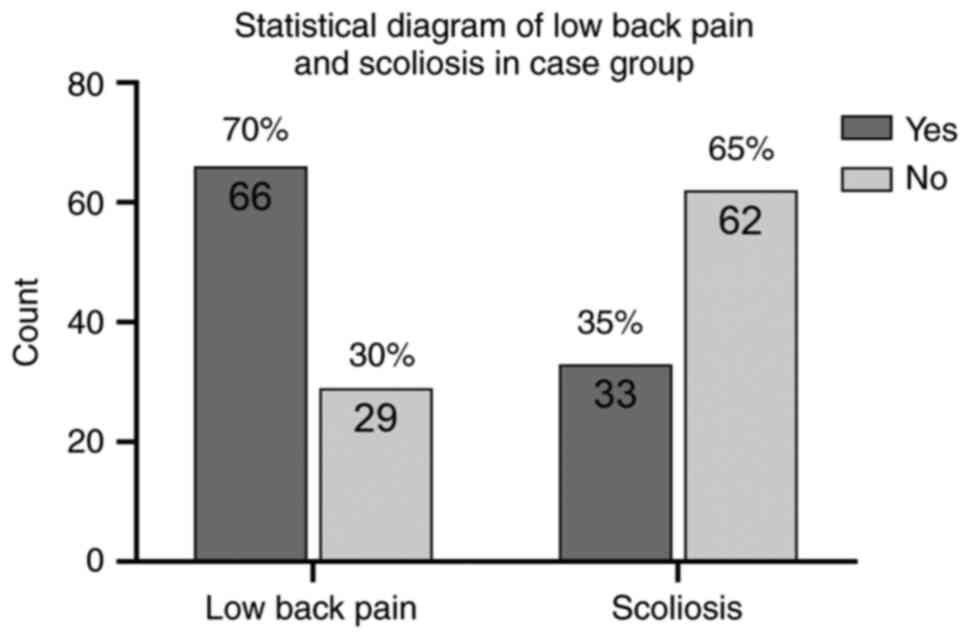
the experimental group, 66 patients had symptoms of low back pain,
accounting for ~70% of the whole experimental group, and 33
patients revealed scoliosis on the spinal X-ray (Fig. 6).
 | Table IClinicopathological characteristics
of the study population |
Table I
Clinicopathological characteristics
of the study population
| Clinicopathological
characteristics | DLSS (L4-5) | DLSS (L5-S1) | Control group |
χ2/F | P-value |
|---|
| Male, n (%) | 22 (44.9) | 21 (45.7) | 29 (41.4) | 0.246 | 0.884 |
| Female, n (%) | 27 (55.1) | 25 (54.3) | 41 (58.6) | | |
| Age, years | 67.44±10.98 | 64.17±4.9 | 63.91±8.97 | 2.272 | 0.111 |
| Body mass index
(kg/m2)a | 21.68±3.3 | 22.66±2.83 | 22.95±2.32 | 2.651 | 0.074 |
Analysis of the spine-pelvis
parameters
Pelvic incidence (PI), pelvic tilt (PT) and sagittal
vertical axis (SVA) values were significantly higher in patients
with DLSS compared with the controls, in contrast, lumbar lordosis
(LL) was significantly lower. Post-hoc test results revealed that
there were no significant differences in all indexes between L4-5
and L5-S1 patients (all P>0.05; Table II).
 | Table IISpinal-pelvic sagittal parameters in
the DLSS patients and control group. |
Table II
Spinal-pelvic sagittal parameters in
the DLSS patients and control group.
| | DLSS patient
group | |
|---|
| Indicator | L4-5 | L5-S1 | Control group |
|---|
| SVA, mm |
63.95±28.31a |
64.3±42.56a | 37.07±22.77 |
| TK, ˚ | 32.49±13.77 | 30.73±4.87 | 31.9±10.85 |
| LL, ˚ | 39.
20±11.83a |
38.92±8.89a | 46.83±10.72 |
| PI, ˚ |
52.63±10.14a |
49.62±8.92a | 41.07±10 |
| PT, ˚ |
22.39±6.94a |
21.54±7.97a | 11.64±7.28 |
| SS, ˚ | 29.88±6.27 | 31.01±4 | 29.42±7.2 |
Analysis of paravertebral muscle
parameters
There were no significant differences in the
bilateral multifidus (MF), the ratio of fat infiltration in the
erector Spinus muscle (ES FIR), and RCSA within the control group
(P>0.05).
Both right MF FIR and right ES FIR were
significantly higher in patients with DLSS (L4-5) than in the
ipsilateral controls, and the right MF FIR was higher than its
contralateral (all P<0.05). The left and right MF-RCSA were
significantly lower in patients with DLSS (L4-5) than in the
ipsilateral ES-RCSA (P<0.05). In addition, left and right MF
RCSA were significantly lower in the control group than in the
ipsilateral ES RCSA as well (P<0.05). There was no significant
difference in the CSA of the upper vertebral body between the DLSS
patient group (L4-5) and the control group (P>0.05) (Table III).
 | Table IIIParavertebral muscle parameters in
the DLSS patients (L4-5) and control group. |
Table III
Paravertebral muscle parameters in
the DLSS patients (L4-5) and control group.
| | DLSS patient group
(L4-5) | Control group |
|---|
| Indicator | Right | Left | Right | Left |
|---|
| MF-FIR, % |
19.23±5.12a,b | 15.23±7.38 | 15.45±3.82 | 14.89±3.86 |
| ES-FIR, % |
18.86±7.62a | 12.6±13.46 | 12.70±2.26 | 12.53±1.00 |
| MF-CSA,
mm2 | 738.95±307.57 | 707.46±295.31 | 927.47±167.98 | 948.92±219.73 |
| ES-CSA,
mm2 |
1,092.77±389.81 |
1,099.69±337.68 | 898.4±110.42 | 932.76±107.48 |
| MF-RCSA, % | 41.08±21.44 | 43.17±16.64 | 39.64±7.95 | 40.25±7.87 |
| ES-RCSA, % | 52.65±12.84 | 56.73±16.41 | 48.64±8.47 | 49.99±5.43 |
| Upper vertebral
body CSA, mm2 |
1,931.89±388.23 | - |
1,881.14±279.68 | - |
The right MF-FIR in the DLSS (L5-S1) patients was
significantly higher than that in the ipsilateral side of the
control group (P<0.05). MF RCSA in both the left and right sides
in DLSS patients was significantly higher than that in the
ipsilateral side ES RCSA (P<0.05) MF RCSA in both the left and
right sides in the control group was significantly higher than that
in the ipsilateral ES RCSA (P<0.05). There was no significant
difference in the upper vertebral body CSA values between patients
with DLSS (L5-S1) and the control group (P>0.05) (Table IV).
 | Table IVParavertebral muscle parameters in
DLSS patients (L5-S1) and control group. |
Table IV
Paravertebral muscle parameters in
DLSS patients (L5-S1) and control group.
| | DLSS patient group
(L5-S1) | Control group |
|---|
| Indicator | Right | Left | Right | Left |
|---|
| MF-FIR, % |
19.59±6.56a | 18.42±3.41 | 14.98±3.43 | 15.84±2.82 |
| ES-FIR, % | 13.00±7.00 | 14.13±5.59 | 13.67±2.04 | 14.53±2.41 |
| MF-CSA,
mm2 | 696.32±200.41 | 857.71±242.24 | 840.43±146.76 | 851.95±123.32 |
| ES-CSA,
mm2 |
1,039.28±269.05 |
1,044.17±313.66 | 817.07±122.82 | 834.6±134.77 |
| MF-RCSA, % | 58.99±30.10 | 62.87±21.96 | 47.35±6.75 | 48.24±7.00 |
| ES-RCSA, % | 39.92±14.04 | 41.01±22.74 | 36.44±8.04 | 27.48±9.61 |
| Upper vertebral
body CSA, mm2 |
1,914.92±310.66 | - |
1,781.91±251.36 | - |
Correlation analysis of the paraspinal
parameters with the spinal-pelvic sagittal parameters
In the DLSS (L4-5) patient group, right ES FIR was
negatively correlated with thoracic kyphosis (TK). Left MF RCSA was
positively correlated with TK, whilst the left ES RCSA was
negatively correlated with SVA (all P<0.05). In the DLSS (L5-S1)
group, there was a significant positive correlation between the
right MF RCSA and right ES RCSA with TK (both P<0.05) (Table V).
 | Table VCorrelation analysis between the
paravertebral muscle parameters and spinal-pelvic parameters in
patients with DLSS . |
Table V
Correlation analysis between the
paravertebral muscle parameters and spinal-pelvic parameters in
patients with DLSS .
| | SVA | TK | LL | PI | PT | SS |
|---|
| Indicator | L4-5 | L5-S1 | L4-5 | L5-S1 | L4-5 | L5-S1 | L4-5 | L5-S1 | L4-5 | L5-S1 | L4-5 | L5-S1 |
|---|
| MF-FIR, % | | | | | | | | | | | | |
|
Right | -0.087 | -0.252 | 0.000 | -0.21 | 0.082 | 0.189 | 0.238 | 0.140 | 0.077 | -0.105 | 0.294 | -0.091 |
|
Left | -0.075 | 0.231 | -0.056 | 0.245 | 0.000 | -0.035 | -0.309 | 0.357 | -0.372 | 0.336 | -0.054 | -0.315 |
| ES-FIR, % | | | | | | | | | | | | |
|
Right | 0.346 | -0.021 | -0.536a | 0.392 | -0.411 | 0. 224 | 0.172 | -0.252 | 0.099 | 0.133 | 0.304 | -0.063 |
|
Left | 0.054 | -0.14 | -0.456 | 0.371 | -0.272 | 0.566 | 0.119 | -0.133 | 0.091 | 0.119 | 0.216 | 0.329 |
| MF-RCSA, % | | | | | | | | | | | | |
|
Right | 0.297 | 0.308 | 0.121 | 0.685a | 0.156 | -0.252 | -0.241 | -0.35 | -0.376 | 0.245 | 0.118 | 0.308 |
|
Left | -0.224 | -0.217 | 0.502a | -0.329 | 0.300 | -0.028 | 0.021 | -0.266 | -0.146 | 0.098 | -0.023 | 0.175 |
| ES-RCSA, % | | | | | | | | | | | | |
|
Right | 0.126 | -0.028 | 0.182 | 0.615a | 0.1 74 | -0.112 | -0.097 | -0.203 | -0.374 | -0.042 | 0.077 | 0.343 |
|
Left | -0.504a | -0.168 | 0.393 | 0.154 | 0.1 50 | -0.056 | -0.186 | 0.231 | -0.044 | -0.238 | -0.200 | 0.105 |
| Upper vertebral
body CSA, mm2 | -0.103 | -0.098 | 0.097 | -0.315 | -0.044 | -0.252 | -0.009 | -0.343 | 0.262 | -0.035 | -0.118 | -0.434 |
Discussion
DLSS is affected by spine-pelvis sagittal imbalances
since the beginning and during the progression and outcome of the
disease. The study of the relationship between DLSS and
spine-pelvis sagittal imbalances is important for the prediction of
the occurrence, development, prognosis, improvement and therapeutic
management of the condition. A previous study demonstrated that
spinal-pelvic sagittal balance should satisfy the following values:
SVA <40 mm; PI-LL <10˚ and PT <20˚ (17). The quality of life scores of the
patients were higher when SVA was <50 mm. However, when SVA was
≥50 mm, patients exhibited severe clinical symptoms, and quality of
life scores also decreased notably. Thus, SVA ≥50 mm was considered
indicative of spinopelvic sagittal imbalance. In the present study,
patients with DLSS had an SVA value of >50 mm (64.10±34.40 mm),
a PI-LL value of >10˚ (11.91˚±16.17˚) and a PT value of >20˚
(22.02˚±7.27˚), thus, indicating significant sagittal imbalance.
This was inconsistent with Lim and Kim (18) who obtained normal PI values and
favorable spinal-pelvic sagittal balance in patients with DLSS
during comparative analysis of spinal-pelvic sagittal balance
parameters between degenerative spondylolisthesis and patients with
DLSS, possibly due to ethnic differences and differing lifestyles.
The present study also observed that patients with DLSS had a
larger PI. Amongst the sagittal parameters, PI is of special
interest. Mac-Thiong et al (19) identified that PI values were
constant after skeletal development was completed in each person
and that they did not change the posture in the receptor position.
In the present study, the patient group had a larger PI value,
indicating that a larger PI value may be one of the risk factors
for DLSS.
In the present study, it was also revealed that
patients with DLSS had a larger SVA, PT, PI and smaller LL than the
control group, which was consistent with the study conducted by
Barrey et al (20), and
again demonstrated that patients with DLSS are likely to exhibit
sagittal imbalances. Three bulges of the human spine, cervical
anterior, and TK, can be clearly observed from lateral radiographs
of the whole spine in normal population, and these are associated
with the pelvis through LL. The lumbar spine is the link between
the spine and the pelvis, and the imbalance of the spine in the
sagittal position eventually affects the changes in pelvic
parameters through the conduction of LL, thus, it is important to
maintain the balance of the spinopelvic LL in the sagittal plane.
Based on the results of the present study, LL was decreased
compared with the healthy individuals; if LL is smaller, the
physiological curvature of the lumbar spine is straighter in
patients, which is reflected in the body posture by significant
anteversion and forward movement of the center of gravity; the body
has to compensate for pelvic retroversion in order to correct this
posture, thus PT is increased. The physiological curvature of the
lumbar spine disappears, the lumbar regions bear more burden from
the body, and degeneration of the lumbar spine, facet joint
hyperplasia, and ligamentum flavum hypertrophy occur over time. The
results of the present study highlight certain avenues for future
surgical treatment of DLSS, and surgery should not only decompress
the spinal canal at the affected level, but also appropriately
correct the imbalances in the sagittal position.
The psoas major muscle (PS) of the anterior group
and MF and ES of the posterior group in the paravertebral muscles
are often referred to as spinal dynamic stabilizers (21,22).
PS maintains lumbar anteversion and curvature (23), MF aids rotational motion of the
lumbar spine (24), and ES
participates in lumbar flexion and extension (25). A previous study demonstrated that
PS shows no obvious signs of fatty infiltration in either normal
subjects or patients with lower back pain (26), thus, only MF and ES for FIR and
RCSA were measured and compared. Paravertebral muscle degeneration,
including decreased muscle fibers and increased fatty infiltration,
is associated with the development and progression of a variety of
lumbar diseases and the development of postoperative complications
(9,27). The physiological function of an
individual muscle is reflected in muscle CSA and density (28). Denervation and disuse decrease
muscle CSA while increased fatty infiltration decreases muscle
density (29). In the present
study, it was identified that the right MF FIR was significantly
higher in patients with DLSS than in ipsilateral controls, which is
consistent with the findings of Lee et al (30) who exhibited a significantly higher
degree of fatty infiltration in the paravertebral muscles of
patients with spinal degeneration than in healthy subjects. Within
muscle per unit area, the higher the degree of fat infiltration,
the fewer the muscle fibers, and the lower the muscle strength. The
maintenance of lumbar stability is inseparable from the action of
paravertebral muscles. When the muscle strength decreases to a
point where the muscle is insufficient to maintain lumbar
stability, pain and discomfort are experienced in the lumbar
region. Lower back pain was the primary symptom in 70% of the cases
included in the present study. It was also observed that at the
L4-5 group, the right MF FIR in patients with DLSS was higher than
that in the contralateral side, indicating that the right MF muscle
strength was lower than that in the left side, which is similar to
the results of Jiang et al (16). However, Shafaq et al
(31) revealed in their study that
there were no significant differences in the CSA of bilateral MF
and the degree of fat infiltration in patients with DLSS alone.
Thus, it was hypothesized by the authors of the present study that
when there is a different degree of fatty infiltration in the left
and right lumbar muscles, the strength of the muscles on both sides
is inconsistent, and this will result in significant left and right
tilt in the lumbar region, followed by scoliosis and coronal
imbalance. A total of 33 patients who were included in the present
study, exhibited significant scoliosis on admission. A
retrospective study observed that both DLSS (L4-5) and degenerative
spondylolisthesis (L4-5) patients had a smaller PS CSA, MF CSA, and
ES CSA at the lower edge of L3, L4, and L5 vertebral bodies than in
the controls. However, the CSA studied failed to exclude deviations
caused by individual body size. In the present study, the RCSA was
calculated using an adjusted calculation method described by
Urrutia et al (29), thus,
eliminating the effect of individual differences on the results.
However, no significant difference in RCSA was identified between
the patient group and the control group, indicating that the
degeneration of the paravertebral muscles was primarily due to
fatty infiltration, and the RCSA of the muscles did not change
notably. Patients with DLSS exhibited a greater degree of severe
paravertebral muscle degeneration (greater degree of fatty
infiltration), and lower functional scores (32,33).
The results of the present study also demonstrated that right and
left MF RCSA were significantly lower than ipsilateral ES RCSA at
L4-5, while right and left MF RCSA was significantly higher than
ipsilateral ES RCSA at L5-S1 in both patients with DLSS and
controls, and this finding may be associated with natural
morphological changes in human MF and ES. Fortin et al
(33) observed similar results in
their study.
A previous study revealed that standardized exercise
of the paravertebral muscles slowed the progression of DLSS
(34). The early symptoms of
discomfort in patients with DLSS can be relieved by exercising the
lower back muscles, using acupuncture, massaging, and other
traditional Chinese medicine treatment methods to relieve
paravertebral muscle fatigue, with small swallow fly and other
movements to strengthen the strength of the core muscle groups in
the lower back. Preoperative exercise of the lower back muscles can
reduce the early clinical symptoms of patients with DLSS and the
frequency of the disease. Postoperative exercises of the lower back
muscles can improve the prognosis and improve the quality of life
of patients. Notably, exercising the lower back muscles improves
lumbar degenerative diseases, whilst healthy individuals should
also strengthen the lower back muscles to prevent the occurrence of
lumbar degenerative diseases.
The relationship between spinal-pelvic sagittal
imbalance and paravertebral muscle degeneration has become a
research hotspot in recent years. The results of the present study
showed that the ratio of fat infiltration in the right ES FIR was
negatively associated with TK in patients with DLSS at L4-5,
similar to that observed by Jun et al (35), in which imaging data from 50
elderly patients were analyzed. They concluded that paravertebral
muscle FIR was associated with TK. Thus, the imbalance in the
sagittal position of the body (increased TK) requires greater
muscle strength to correct, and greater muscle strength can only be
demonstrated when the muscle FIR is smaller. Hiyama et al
(36) detected that the mean CSAs
of PS at L4 and L5 were negatively associated with PT by analyzing
data from 140 patients with DLSS. Although PS was not studied in
detail, MF RCSA and ES RCSA were identified to be positively
associated with TK in patients with DLSS patients in the present
study. When the lower lumbar spine loses its physiological
curvature, LL becomes smaller, the body shows significant
anteversion, the center of gravity moves forward, SVA and TK
increase in order to maintain the overall balance of the body in
the sagittal position, the pelvis compensates for retroversion.
However, the pelvic retroversion is controlled by the paravertebral
muscles, and the strength producing ability of the muscles is
related to their physical size, and greater muscle strength is
required to ensure the stability of the lumbar spine. Thus, when TK
increases, the lower lumbar spine requires a larger RCSA. Overall,
to the best of the authors' knowledge, there are no studies
investigating the relationship between spinal-pelvic sagittal
imbalance and paravertebral muscle degeneration in patients with
DLSS, and it is expected that the results obtained in the present
study will highlight novel avenues for the improvement of the
clinical basis for the treatment of DLSS.
The present study has several limitations. Due to
the slow onset and long course of DLSS, the majority of patients
opt for conservative treatment to manage the symptoms. However,
surgical treatment is usually required for multi-segmental spinal
stenosis. Therefore, fewer patients with single-level spinal
stenosis were enrolled in the present study, and subsequent studies
should include more cases and consider each type of DLSS. A
complete study would include simple to complex DLSS. Through the
results of the present study, the general path of the occurrence
and development of DLSS was determined. However, treatment could
not be performed based on a single aspect, and other factors
related to DLSS are key to treatment. When the patient is
determined to have surgical treatment, the patient should be
informed of the correct way to perform lumbar and dorsal muscle
exercises, and initiate these exercises sometime before the
operation. In order to increase the chances of rapid postoperative
recovery, the patients should continue to perform long-term
postoperative lumbar and dorsal muscle exercises to strengthen the
rehabilitation effect. The correction of the sagittal position line
and lumbodorsal muscle fat removal are the two aspects of fusion
treatment, which may result in an improved rehabilitation effect.
However, the relationship between the start time of preoperative
lumbar and dorsal muscle exercises and the time of elective surgery
needs to be determined. Under the premise of ensuring no delay in
treatment, preoperative lumbar and dorsal muscle exercise should be
assisted to increase the chances of a quicker recovery and fine
treatment of single-stage/multi-stage spinal stenosis.
In conclusion, FIR and RCSA in the paraspinal
muscles of patients with DLSS were associated with TK. Therefore, a
comprehensive assessment of the individual differences in
performance is necessary for the prevention and treatment of DLSS.
For patients requiring surgical treatment, a detailed surgical plan
should be developed prior to surgery. The correction angle of the
spinal and pelvic-related parameters is critical, and reasonable
post-operative core muscle exercises are particularly
important.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Inner Mongolia
Autonomous Region Health Science and Technology Program (grant no.
202202376) and the Ordos City Science and Technology Program
Project.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KZ and WY prepared and revised the manuscript, KZ
and TB contributed to the collection and analysis of the data. KZ
and TB confirm the authenticity of all the raw data. CW, BX, TW,
FG, QZ, HL, XT, TZ and GG contributed to the collection and
classification of the data. YW and WY designed and supervised the
overall research and revised the manuscript. All authors have read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
All methods were carried out in accordance with the
relevant guidelines and regulations. All experimental protocols
were approved by the Ethics Committee of Ordos Central Hospital,
Inner Mongolia Medical University (Ordos, China; approval no.
2022-012). Informed consent was obtained from all subjects and/or
their legal guardians for the present study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xu C, Zhang Y, Dong M, Wu H, Yu W, Tian Y,
Cao P, Chen H, Wang X, Shen X, et al: The relationship between
preoperative cervical sagittal balance and clinical outcome of
laminoplasty treated cervical ossification of the posterior
longitudinal ligament patients. Spine J. 20:1422–1429.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kreiner DS, Baisden J, Mazanec DJ, Patel
RD, Bess RS, Burton D, Chutkan NB, Cohen BA, Crawford CH 3rd,
Ghiselli G, et al: Guideline summary review: An evidence-based
clinical guideline for the diagnosis and treatment of adult isthmic
spondylolisthesis. Spine J. 16:1478–1485. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Aizenshtein A, Kachel E, Liza GR, Hijazi B
and Blum A: Effects of preoperative WBC count on post-CABG surgery
clinical outcome. South Med J. 113:305–310. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Dubousset J: Three-dimentional analysis of
the scoliotic deformity. The Pediatric Spine: Principles and
Practice 1994.
|
|
5
|
Panjabi MM: The stabilizing system of the
spine. Part I. Function, dysfunction, adaptation, and enhancement.
J Spinal Disord. 5:383–389. 1992.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ogon I, Takebayashi T, Takashima H, Morita
T, Yoshimoto M, Terashima Y and Yamashita T: Magnetic resonance
spectroscopic analysis of multifidus muscles lipid content and
association with spinopelvic malalignment in chronic low back pain.
Br J Radiol. 90(20160753)2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sun D, Liu P, Cheng J, Ma Z, Liu J and Qin
T: Correlation between intervertebral disc degeneration, paraspinal
muscle atrophy, and lumbar facet joints degeneration in patients
with lumbar disc herniation. BMC Musculoskelet Disord.
18(167)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hyun SJ, Kim YJ and Rhim SC: Patients with
proximal junctional kyphosis after stopping at thoracolumbar
junction have lower muscularity, fatty degeneration at the
thoracolumbar area. Spine J. 16:1095–1101. 2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Kalichman L, Hodges P, Li L, Guermazi A
and Hunter DJ: Changes in paraspinal muscles and their association
with low back pain and spinal degeneration: CT study. Eur Spine J.
19:1136–1144. 2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vives MJ: The paraspinal muscles and their
role in the maintenance of global spinal alignment. Another wrinkle
in an already complex problem. Spine J. 16:459–461. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Suh DW, Kim Y, Lee M, Lee S, Park SJ and
Yoon B: Reliability of histographic analysis for paraspinal muscle
degeneration in patients with unilateral back pain using magnetic
resonance imaging. J Back Musculoskelet Rehabil. 30:403–412.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ravindra VM, Senglaub SS, Rattani A, Dewan
MC, Härtl R, Bisson E, Park KB and Shrime MG: Degenerative lumbar
spine disease: Estimating global incidence and worldwide volume.
Global Spine J. 8:784–794. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pratali RR, Battisti R, Oliveira C,
Maranho DAC and Herrero C: Correlation between the severity of the
lumbar degenerative disease and sagittal spinopelvic alignment. Rev
Bras Ortop (Sao Paulo). 57:41–46. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lafage R, Ferrero E, Henry JK, Challier V,
Diebo B, Liabaud B, Lafage V and Schwab F: Validation of a new
computer-assisted tool to measure spino-pelvic parameters. Spine J.
15:2493–2502. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Fortin M and Battié MC: Quantitative
paraspinal muscle measurements: inter-software reliability and
agreement using OsiriX and ImageJ. Phys Ther. 92:853–864.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Jiang J, Wang H, Wang L, Zhang B, Guo Q,
Yuan W and Lu X: Multifidus degeneration, a new risk factor for
lumbar spinal stenosis: A case-control study. World Neurosurg.
99:226–231. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Schwab F, Patel A, Ungar B, Farcy JP and
Lafage V: Adult spinal deformity-postoperative standing imbalance:
how much can you tolerate? An overview of key parameters in
assessing alignment and planning corrective surgery. Spine (Phila
Pa 1976). 35:2224–2231. 2010.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lim JK and Kim SM: Comparison of sagittal
spinopelvic alignment between lumbar degenerative spondylolisthesis
and degenerative spinal stenosis. J Korean Neurosurg Soc.
55:331–336. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Mac-Thiong JM, Labelle H and Roussouly P:
Pediatric sagittal alignment. Eur Spine J. 20 (Suppl 5):S586–S590.
2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Barrey C, Jund J, Noseda O and Roussouly
P: Sagittal balance of the pelvis-spine complex and lumbar
degenerative diseases. A comparative study about 85 cases. Eur
Spine J. 16:1459–1467. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Freeman MD, Woodham MA and Woodham AW: The
role of the lumbar multifidus in chronic low back pain: A review.
PM R. 2:142–146. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wagner H, Anders Ch, Puta Ch, Petrovitch
A, Mörl F, Schilling N, Witte H and Blickhan R: Musculoskeletal
support of lumbar spine stability. Pathophysiology. 12:257–265.
2005.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Regev GJ, Kim CW, Tomiya A, Lee YP,
Ghofrani H, Garfin SR, Lieber RL and Ward SR: Psoas muscle
architectural design, in vivo sarcomere length range, and passive
tensile properties support its role as a lumbar spine stabilizer.
Spine (Phila Pa 1976). 36:E1666–E1674. 2011.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Andersson EA, Grundström H and
Thorstensson A: Diverging intramuscular activity patterns in back
and abdominal muscles during trunk rotation. Spine (Phila Pa 1976).
27:E152–E160. 2002.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ng JK, Richardson CA and Jull GA:
Electromyographic amplitude and frequency changes in the
iliocostalis lumborum and multifidus muscles during a trunk holding
test. Phys Ther. 77:954–961. 1997.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ropponen A, Videman T and Battié MC: The
reliability of paraspinal muscles composition measurements using
routine spine MRI and their association with back function. Man
Ther. 13:349–356. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Buckinx F, Reginster JY, Dardenne N,
Croisiser JL, Kaux JF, Beaudart C, Slomian J and Bruyère O:
Concordance between muscle mass assessed by bioelectrical impedance
analysis and by dual energy X-ray absorptiometry: A cross-sectional
study. BMC Musculoskelet Disord. 16(60)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hicks GE, Simonsick EM, Harris TB, Newman
AB, Weiner DK, Nevitt MA and Tylavsky FA: Cross-sectional
associations between trunk muscle composition, back pain, and
physical function in the health, aging and body composition study.
J Gerontol A Biol Sci Med Sci. 60:882–887. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Urrutia J, Besa P, Lobos D, Campos M,
Arrieta C, Andia M and Uribe S: Lumbar paraspinal muscle fat
infiltration is independently associated with sex, age, and
inter-vertebral disc degeneration in symptomatic patients. Skeletal
Radiol. 47:955–961. 2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lee JC, Cha JG, Kim Y, Kim YI and Shin BJ:
Quantitative analysis of back muscle degeneration in the patients
with the degenerative lumbar flat back using a digital image
analysis: Comparison with the normal controls. Spine (Phila Pa
1976). 33:318–325. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shafaq N, Suzuki A, Matsumura A, Terai H,
Toyoda H, Yasuda H, Ibrahim M and Nakamura H: Asymmetric
degeneration of paravertebral muscles in patients with degenerative
lumbar scoliosis. Spine (Phila Pa 1976). 37:1398–1406.
2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Fortin M, Omidyeganeh M, Battié MC, Ahmad
O and Rivaz H: Evaluation of an automated thresholding algorithm
for the quantification of paraspinal muscle composition from MRI
images. Biomed Eng Online. 16(61)2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fortin M, Lazáry À, Varga PP, McCall I and
Battié MC: Paraspinal muscle asymmetry and fat infiltration in
patients with symptomatic disc herniation. Eur Spine J.
25:1452–1459. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Fritz JM, Lurie JD, Zhao W, Whitman JM,
Delitto A, Brennan GP and Weinstein JN: Associations between
physical therapy and long-term outcomes for individuals with lumbar
spinal stenosis in the SPORT study. Spine J. 14:1611–1621.
2014.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Jun HS, Kim JH, Ahn JH, Chang IB, Song JH,
Kim TH, Park MS, Chan Kim Y, Kim SW, Oh JK and Yoon DH: The effect
of lumbar spinal muscle on spinal sagittal alignment: Evaluating
muscle quantity and quality. Neurosurgery. 79:847–855.
2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hiyama A, Katoh H, Sakai D, Tanaka M, Sato
M and Watanabe M: The correlation analysis between sagittal
alignment and cross-sectional area of paraspinal muscle in patients
with lumbar spinal stenosis and degenerative spondylolisthesis. BMC
Musculoskelet Disord. 20(352)2019.PubMed/NCBI View Article : Google Scholar
|