1. Introduction
Acute pancreatitis is a common acute abdominal
disease characterized by abnormal activation of pancreatic
proteases and a secondary local inflammatory response in the
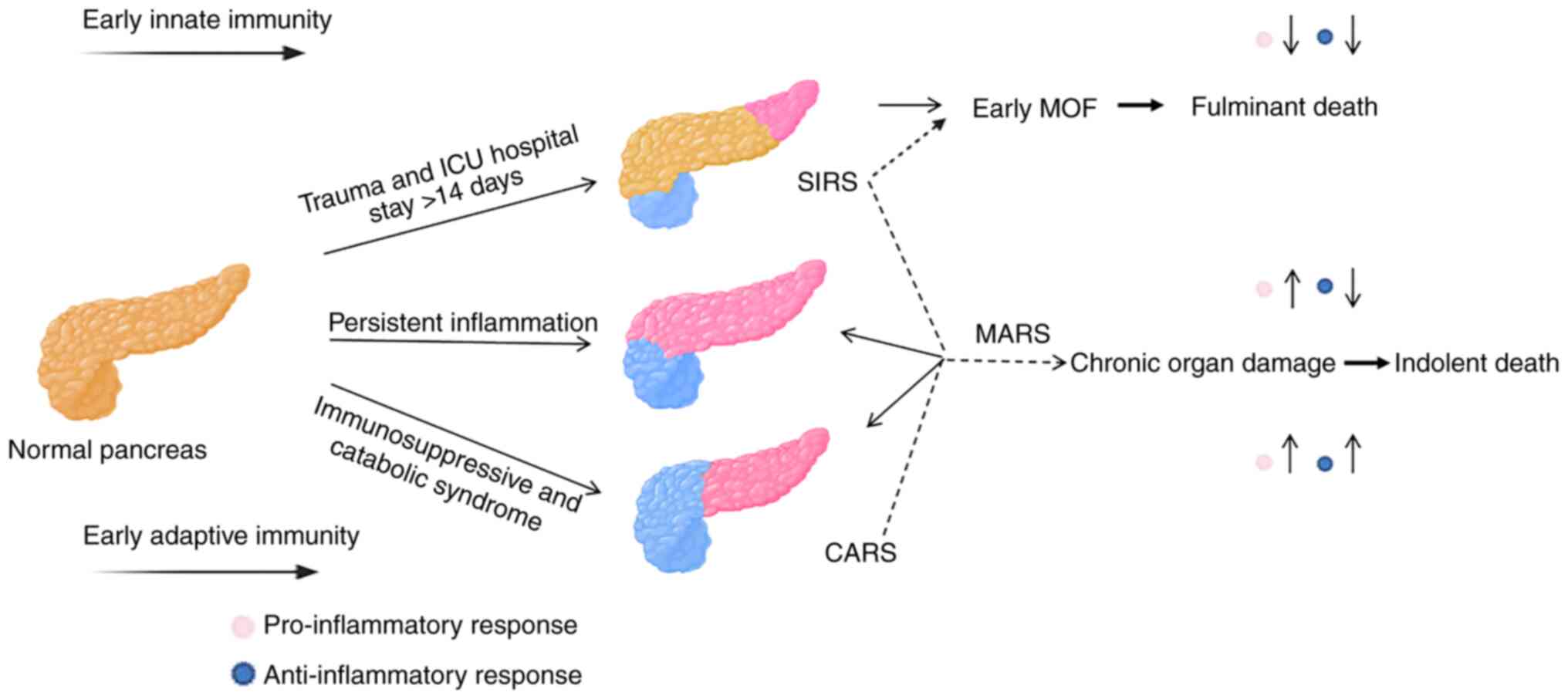
pancreas due to multiple etiologies (1). Systemic inflammatory response
syndrome (SIRS) and multiple organ dysfunction syndrome (MODS) can
occur from onset to 2 weeks after onset (2). Among acute pancreatitis cases, 80%
are mild acute pancreatitis and 20% can progress to severe acute
pancreatitis (SAP), which can lead to persistent inflammation,
immunosuppression and catabolism syndrome (PICS) in the later stage
due to abdominal infection (2-3). The
immune system is preactivated after the onset of SAP, resulting in
SIRS and antagonistic compensatory anti-inflammatory response
syndrome (CARS) (4). If the
disease is uncontrolled, CARS and SIRS antagonize each other and
progressively worsen, resulting in mixed antagonistic response
syndrome (MARS) (5). Progression
on the ‘SIRS-CARS-MARS’ immune axis continues, and the control of
the proinflammatory response slowly declines, with ~43.1% of
patients developing PICS at a later stage (6). The immune model of SIRS-CARS-MARS is
formed after trauma. After further development, the advantages of
the pro-inflammatory response against the anti-inflammatory
response are gradually reversed, and persistent inflammation and
severe immunosuppression are finally formed (6). The evolution of PICS is shown in
Fig. 1. Organ dysfunction syndrome
refers to the occurrence of dysfunction or failure of more than two
organs or systems successively or simultaneously after various
traumas and infections, so that the body cannot maintain the
stability of the internal environment (7). PICS can be regarded as a new clinical
subtype of late organ dysfunction syndrome, a chronic critical
illness (CCI) characterized by immune paralysis (8). CARS was first used to describe
phenomena that could explain the initial response of the host to a
variety of infectious and noninfectious conditions (8). CARS was proposed to follow SIRS and
appeared to explain this increased susceptibility to infection and
bimodal distribution of multiorgan failure (4,9).
CARS was viewed as progressive suppression in adaptive immunity,
resulting in secondary infections (8). PICS came after CARS and was used to
summarize the refractory CCI population with immune paralysis as
the main feature, which is characterized by persistent severe
immune paralysis and high catabolism (7). Even with good treatment and
nutritional support, it is inevitable to have repeated nosocomial
infection, malnourishment and other problems, which eventually lead
to death from chronic organ failure (10). Compared with CARS, PICS has a more
complex mechanism and a worse prognosis (11). There are numerous similarities
between PICS and CCI, for example, both focus on the
pathophysiological process of critically ill patients after 14 days
of onset. Inflammation, nutrition and high catabolism are the
common concerns of both (10). CCI
covers a wider range of problems, including ventilator dependence,
brain dysfunction, neuromuscular dysfunction, neuroendocrine
disorders and malnutrition, which are related to the chronic phase
of critically ill patients (6).
PICS focuses on inflammation, immunity and nutrition, which are
closely related to infection, and its core feature is immune
paralysis. Therefore, to a certain extent, PICS is a special type
of CCI, and it is a CCI with immune paralysis (7). Patients with PICS are prone to
pulmonary insufficiency, acquired muscle weakness, decreased
immunity, malnutrition and dyspnea, which in turn affects the
quality of life of patients (11).
PICS is the key point difficulty in the clinical treatment of CCI
due to its high susceptibility to infection and organ damage and
poor prognosis; early warning and treatment of PICS is the key to
improve the long-term prognosis of patients with CCI (11). Over the years, previous studies
have explored treatment methods for SAP and its complications,
which consume enormous medical resources and are associated with
prolonged intensive care unit (ICU) hospitalization and poor
mortality rates (12,13). The term PICS is used to describe
the newly observed phenotypes of persistent inflammation,
immunosuppression and proteolytic metabolism, which represents the
next challenge in surgical intensive care (11). It is important for clinical staff
to establish a collaborative partnership with patients and to help
them identify complications. The purpose of the present review is
to elucidate the pathogenesis, diagnosis and possible treatment
methods of SAP combined with PICS.
2. Pathogenesis of PICS in SAP
Trypsinization
The early activation of the enzyme for any reason is
the initiating factor for SAP (13). Abnormal activation of trypsin can
not only cause bleeding and necrosis of the pancreas itself but
also disrupt the vascular endothelial barrier through venous
reflux, causing a large amount of blood to seep out of the blood
vessels, leading to capillary leakage syndrome, multiple organ
bleeding and abdominal fluid accumulation (13). Some protein kinases can induce the
release of various pro-inflammatory factors through transduction
signals, regulate inflammatory reactions, promote inflammatory
reactions generated by pancreatic enzyme digestion and ultimately
lead to PICS (14).
Inflammatory factor release
Activated trypsin can stimulate mononuclear
macrophages and damaged pancreatic acini in the pancreas to produce
inflammatory mediators, leading to a cascade of white blood cell
overactivation and increased inflammatory mediators levels referred
to as the ‘waterfall effect’ (13). If not corrected in a timely manner,
the ‘waterfall effect’ can further develop into a persistent
inflammatory response state, ultimately leading to PICS (14). TNF-α and IL-6 are the earliest and
core inflammatory factors that are upregulated during the onset of
acute pancreatitis (15). They can
act on various inflammatory cells, promote the release of
inflammatory mediators such as IL-1 and IL-8, exacerbate
inflammatory reactions, and cause tissue damage (14).
Immunosuppression
In the early stage of SAP, the body is in a state of
coexistence of SIRS and CARS, which is a mixed type of
anti-inflammatory response syndrome, leading to immune suppression
(16). With the progression of
SAP, the number of peripheral blood CD4+ T lymphocytes
and the CD4+/CD8+ ratio decreases, the
balance of T helper (Th)1/Th2 cells shifts in the direction of Th2
cells, and when Th2 cells are dominant, they secrete inflammatory
response inhibitors such as IL-4 and IL-10, which leads to a
decline in cellular immune function, a systemic inflammatory
response state, and PICS (17,18).
In patients with SAP, the expression of human leukocyte antigen on
the surface of monocyte macrophages is markedly reduced, antigen
presentation is impaired, and the levels of the proinflammatory
factors IL-1 and IL-6 are reduced, all of which impair the
activation of the immune response of the body, leading to
immunosuppression (19).
Intestinal bacterial
translocation
Intestinal bacterial translocation is the main cause
of SAP, and the destruction of the intestinal barrier is a
prerequisite for bacterial translocation (20). The main mechanisms of intestinal
bacterial translocation are intestinal wall ischemia, hypoxia and
ischemia-reperfusion injury (20).
Following the translocation of intestinal bacteria and their
endotoxin, the activated mononuclear-macrophages are further
stimulated to produce more inflammatory factors, which constitute a
‘second strike’ to organs such as the pancreas and even aggravate
MODS (13,21). Inflammatory factors produced by
intestinal bacterial translocation and MODS aggravate each other.,
forming a vicious cycle and exacerbating the sustained inflammatory
response state of the body, eventually leading to PICS (21).
Microcirculation disturbance
Early SAP is often accompanied by pathological
changes, including capillary ischemia, increased permeability and
microthrombus formation (13).
Excessive activation of neutrophils and macrophages promotes the
secretion of inflammatory factors, which not only induces capillary
leakage syndrome but also disrupts the endothelial function of
capillaries, leading to impaired coagulation mechanisms. This is
closely related to inflammatory cascade reactions, including
coagulation factor Xa promoting the excessive release of
inflammatory factors such as IL-6 and IL-8, increasing the number
of adhesion factors, and activating inflammatory reactions
(13). When the pancreatic artery
is complicated with spasm and embolism, it can lead to ischemia and
necrosis of the pancreatic segment supplied by the blood vessel,
resulting in microcirculation disturbance (13).
Internal relationship
Patients with PICS have impaired immune function,
which makes it difficult to clear infectious pathogens or induce
recurrent infections. Multidrug-resistant infections often occur,
resulting in pathogen-associated molecular patterns (PAMP) or
persistent antigen release. Furthermore, PAMP or antigen acts on
the pattern recognition receptors (PRR) of immune cells or induces
adaptive immune activation, leading to a persistent inflammatory
response (22). When the
inflammatory response persists, not only a large number of
pro-inflammatory factors are released, but also anti-inflammatory
factors and high expression of immunosuppressive molecules are
secreted to try to restore the balance with the pro-inflammatory
response (23). However, improper
synthesis, secretion or expression of these anti-inflammatory
factors and immunosuppressive molecules can lead to or aggravate
the immunosuppression of the body (23). Myeloid-derived suppressor cells are
an important mechanism mediating immune dysfunction in PICS
(24). When activated,
myeloid-derived suppressor cells synthesize a large amount of
TNF-α, IL-10, nitric oxide and reactive oxygen species, inhibits
the function of various T cells (mainly CD4+ and
CD8+ T cells), promotes the proliferation of regulatory
T cells, and exerts pro-inflammatory and immunosuppressive effects
(22,25). Clarifying the relationship between
immunity and inflammation in PICS is crucial for the implementation
of goal-directed immunomodulatory therapy targets. Persistent
inflammation in PICS can lead to the imbalance of substance and
energy metabolism. During inflammation, a large number of cytokines
and stress-related hormones are released, resulting in high
catabolism, increased energy consumption and muscle protein
decomposition. High catabolism is mainly manifested as
malnutrition, weight loss and hypoproteinemia (11). Pro-inflammatory cytokines can act
on mitochondria to reduce the activity of respiratory chain
enzymes, resulting in insufficient energy metabolism, reduced
production of adenosine triphosphate, and aggravation of the
imbalance of energy supply and demand, which may lead to tissue and
cell damage (26,27). High catabolism leads to muscle
protein and fat decomposition, muscle atrophy, and muscle weakness
(11). Tissue decomposition can
induce the release of damage associated molecular patterns (DAMP)
and induce the inflammatory response via the DAMP-pattern
recognition receptors (PRRs) signaling pathway (28,29).
Therefore, regulation of inflammation and intervention of
metabolism are expected to become novel targets for PICS treatment.
Metabolic abnormalities in PICS are not only limited to solid cells
in organs and tissues of the whole body, but also involve various
immune cells (30). A previous
study (31) has reported that
after peripheral blood monocytes were incubated with
lipopolysaccharide or Candida albicans, not only was the
secretion of TNF-α, IL-1β and IL-6 markedly reduced, but the
activities of the glycolysis pathway, oxidative phosphorylation and
fatty acid β oxidation pathway of monocytes were also markedly
reduced, and the metabolic pathway activity was restored after
recovery. Patients with malnutrition lack the corresponding
metabolic substrates and enzymes, resulting in insufficient ATP
production and aggravating immunosuppression (32). Immune imbalance in PICS can induce
metabolic disorders (30).
Abnormal metabolism of immune cells may mediate immune inflammatory
response disorders; however, to the best of our knowledge, the
metabolic patterns of immune cells in PICS, such as metabolic
substrates, pathways, key enzymes and regulatory mechanisms, remain
to be elucidated. Targeted intervention of immune cell metabolic
molecules may open up a novel field of immunomodulatory
therapy.
3. Diagnosis
Due to advances in intensive care technology, an
increasing number of patients with SAP have avoided early death
(33). However, despite the
comprehensive treatment of active nutritional support and minimally
invasive or surgical drainage, there are still critically ill
patients who stay in the ICU for a prolonged period of time and
develop CCI (11). Therefore,
identifying and appropriately managing patients at high risk of
death directly affects the prognosis of PICS (34). Elderly patients with systemic
infection and severe trauma are at high risk of PICS (35). Elderly individuals are susceptible
to PICS due to multiple common diseases and a gradual decline in
immune function as they age (6,36).
Therefore, with an aging population, PICS will become a novel
challenge for critically ill patients. In addition, patients with
obesity and biliary pancreatitis have a higher incidence of PICS
than patients with pancreatitis of other etiologies (34,37).
Patients with SAP require long-term supportive care in the ICU and
are prone to ventilator dependency and malnutrition, which mainly
presents with recurrent nosocomial infections, acquired debility,
cognitive dysfunction and psychosomatic impairment (anxiety,
depression and sleep disorders) (38). The diagnosis of PICS relies on the
following four indicators (Table
I) (6,11,39,40):
i) ICU hospitalization >14 days; ii) persistent inflammatory
response: C-reactive protein (CRP) >3.0 mg/dl or retinol-binding
protein <10 mg/l; iii) immunosuppression: Lymphocyte count
<0.80x109/liter; and iv) catabolism: Serum albumin
<30 g/l, prealbumin <10 mg/dl, creatinine/height index
<80% or body mass decline during hospitalization >10% or body
mass index <18 kg/m2. The diagnostic criteria for
PICS have not been standardized (41), and PICS symptoms are numerous but
nonspecific. The selection of current indicators, such as
C-reactive protein, lymphocytes, neutrophils and albumin, is
reasonable although it has a certain degree of subjectivity. One
study applied this diagnosis in examining outcomes of SAP (14). Another study reported that the
criterion of day 14 CRP for PICS should be 3.0 mg/dl with lower
Barthel Index, albumin and total lymphocyte counts (39). We hypothesized that PICS diagnosis
needs to satisfy at least one of each aspect. ICU stay >14 days
is a prerequisite, inflammatory indicators such as CRP,
immunosuppression indicators such as lymphocytes and catabolism
indicators such as prealbumin should also be present. Molecular
biology detection technology has made great progress, and the
determination of the inflammatory response and immune status could
be realized by direct detection at the cellular and molecular
levels (42). Enzyme-linked
immunosorbent assays (ELISA) could reflect the inflammatory state
of the body by directly detecting the expression levels of IL-6,
IL-10, IL-1 receptor antagonist and soluble tumor necrosis factor
receptor 1 in plasma (43). Flow
cytometry could make the phenotype of the microcytic population
clearer than can ELISA and further improve the observation index of
PICS (44). These methods may help
identify PICS early. In clinical practice, the diagnosis of PICS
should be based on laboratory tests and clinical manifestations.
Larger samples and more rigorous prospective studies are required
in the future (11). Therefore,
clinical staff should pay attention to the etiology and severity of
SAP and lymphocyte count measurements (6). In addition, they should strengthen
symptom recognition in patients with SAP to raise awareness of
self-monitoring and avoid worsening of symptoms.
 | Table IClinical determinants of PICS. |
Table I
Clinical determinants of PICS.
| Diagnostic
criteria | Numerical
value | Existing problems
of diagnostic criteria | Suggestion |
|---|
| ICU hospitalization
days | Prolonged
hospitalization >14 days (6,11) | ICU time is not
uniform (40) | Evidence-based
medicine may be used to clarify the issue of ICU length of stay,
such as meta-analysis |
4. Importance of clinical treatment for
PICS
Patients with PICS experience a multi-level and
multi-step cycle of immunosuppression, persistent inflammation and
high metabolic rate, which eventually affects the outcome of
disease, leading to a long hospital stay and poor clinical
prognosis (3). Therefore, it is
necessary to investigate PICS prevention, early identification and
timely intervention (45).
However, a survey on the readiness for discharge of patients with
SAP indicated that the readiness for discharge of patients with SAP
is low (46). Clinical staff
should have an understanding of PICS, know the early warning of
high-risk patients and strengthen health education during
hospitalization, which can improve compliance and help in managing
PICS.
5. Clinical treatments for PICS in patients
with SAP
There is a complex interactive dialogue among
immunosuppression, persistent inflammation and hypercatabolism in
patients with PICS (11). The body
undergoes a cycle of immunosuppression, repeated infection,
persistent inflammation and hypercatabolism, which ultimately
affects the outcome of patients with PICS, leading to a long
hospital stay and poor clinical prognosis (3). It is important to determine how to
carry out early warning, risk stratification and prognosis
assessment for patients with PICS. The Modified Early Warning Score
(MEWS) is an objective indicator tool that can be used for early
warning of patients and rapid assessment of the severity and
potential changes in the condition (47). It evaluates the condition of the
patient by scoring five indicators, including heart rate, systolic
blood pressure, respiratory rate, temperature and consciousness,
and assigns certain scores to physiological indicators within a
certain range (47). The
temperature is scored as 0-2 points and the other items are scored
as 0-3 points and the maximum possible score was 14. The higher the
score, the more serious the condition of the patient. It is of
great significance for identifying potentially critical patients
(48,49). The combination of MEWS and some
related indicators such as serological indicators, inflammatory
factors, acute physiology of intra-abdominal pressure and chronic
health score (APACHE) ⅡScore, sequential organ failure (SOFA)
score, CRP, pro-calcitonin and Bedside Index for Severity, improves
the prediction of the severity and prognosis of patients with PICS
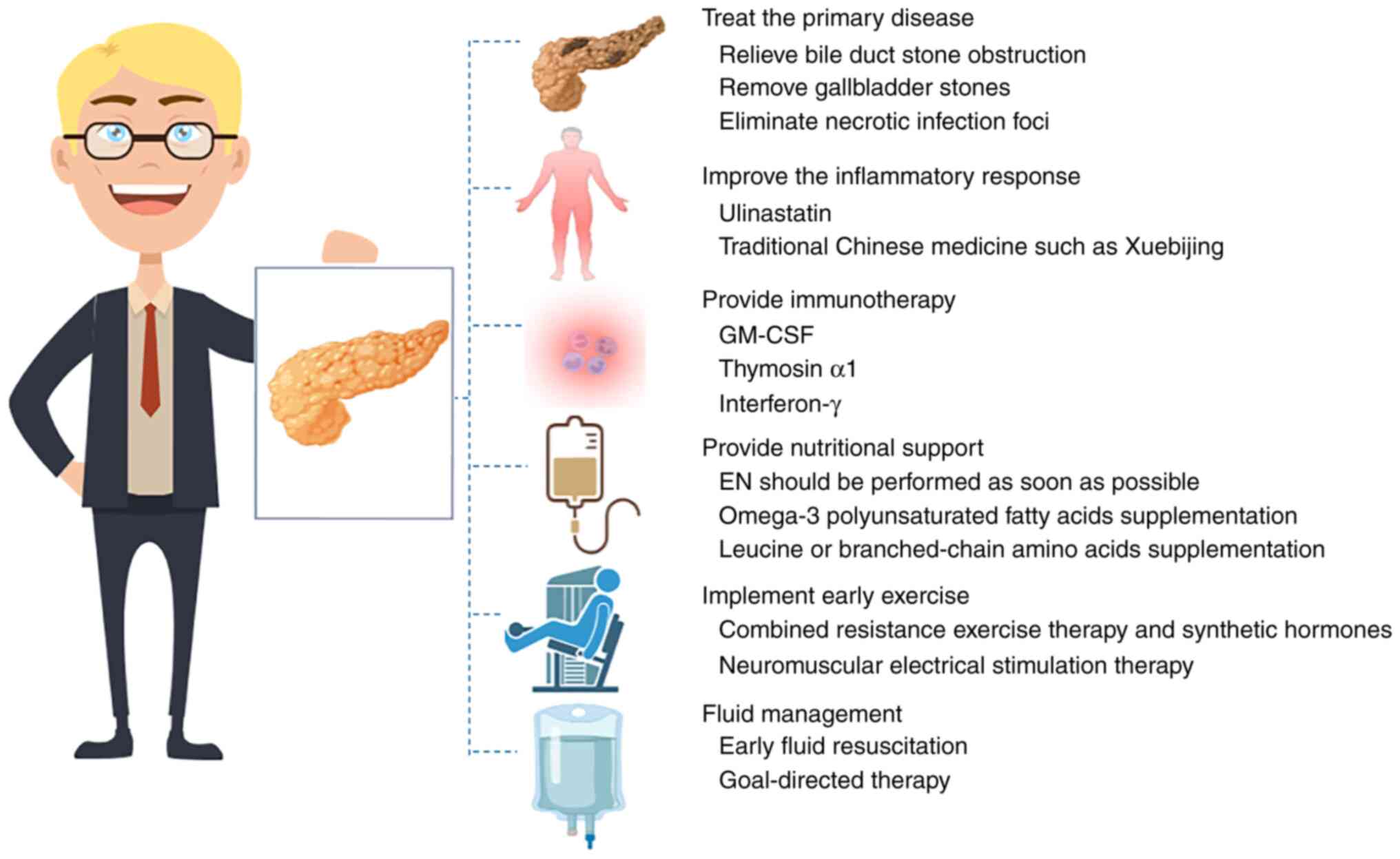
The possible treatment measures targeting the pathogenesis are
shown in Fig. 2 and the possible
pathogenesis and corresponding treatment methods are summarized in
Table SI.
Treat the primary disease
Several studies (50,51)
have demonstrated that the mortality rate of SAP ranges between 10
and 33%. Peripancreatic necrotic infection is the main cause of
death (37), which may occur in
the later stage of the disease and is also a major contributor to
PICS. Active treatment of primary disease should be implemented to
weaken SIRS in patients with SAP, thereby preventing secondary PICS
(37). For patients with bile duct
stone obstruction, the obstruction should promptly be relieved
(37). Individuals with
gallbladder stones should undergo cholecystectomy as soon as
possible once the condition is under control (13). Patients afflicted with necrotizing
acute pancreatitis can be treated with necrotic tissue removal
surgery (13). Individuals
suffering from hyperlipidemic SAP should avoid fat emulsions and
refrain from utilizing medications that may potentially elevate
blood lipids (13). Small dosages
of such drugs are recommended. Hypercalcaemia SAP is frequently
associated with hyperparathyroidism, which requires calcitonin
therapy (37). Symptomatic
treatment should be provided for patients with pancreatic
anatomical and physiological abnormalities, medication-induced
conditions and pancreatic tumors (37). Furthermore, for patients with
co-infections, it is imperative to actively eliminate necrotic
infection foci and enhance drainage care through appropriate
fixation of the drainage tube and improved quality of care
(37). This not only ensures
efficient drainage and mitigates inflammation but also reduces
recurrence rates and enhances the quality of life of patients
(13).
Improve the inflammatory response
At present, there is no effective approach to
mitigate PICS by inhibiting the systemic proinflammatory response
from the source (11). The primary
obstacle lies in the rapidity of innate immune response and the
difficulty of intercepting early-stage release of inflammatory
factors or maintaining optimal levels thereof (52). Nevertheless, certain medications
may be explored as potential remedies. Ulinastatin is a versatile
protease inhibitor that stabilizes lysosomal membranes, antagonizes
oxygen free radicals, regulates the release of inflammatory factors
and promotes recovery of specific immune functions (53). It has been widely used in the
treatment of SAP. A study established a rat SAP model to
investigate the therapeutic efficacy of ulinastatin, and the
results demonstrated that ulinastatin effectively suppressed the
expression of inflammatory factors such as TNF-α, IL-1 and
IL-6,which subsequently mitigated the inflammatory response of the
body and enhanced the survival rate of rats (54). Some traditional Chinese medicine
preparations, such as safflower flavin A in Xuebijing injection
(55), have been demonstrated to
effectively inhibit the activity of the transcription factor κB
signaling pathway and reduce endotoxin levels. This can help
alleviate SAP symptoms by reducing inflammatory factors and
delaying the progression of PICS (55,56).
Provide immunotherapy
Immunomodulatory imbalance in the body is an
important cause of death in patients with SAP with PICS (24). Immunotherapy focuses on restoring
the homeostasis of the immune system (57). PICS is associated with alterations
in immune effector cells, and acquired immune agonists can be
utilized for the treatment of immunosuppression (24). Several studies on immunomodulatory
therapy have demonstrated the beneficial effects of
granulocyte-macrophage colony stimulating factor (GM-CSF), thymosin
α1, IL-7, IL-15, programmed cell death protein 1/programmed
death-ligand 1 antibody, anti-cytotoxic T-lymphocyte-associated
protein 4 antibody, anti-lymphocyte activation gene 3 antibody,
anti-T lymphocyte immunoglobulin mucin 3 antibody and interferon-γ
in enhancing the immune function of patients (19,58).
Thymosin α1 is a bioactive peptide secreted by the thymus gland,
which can induce T lymphocyte differentiation and maturation,
enhance the phagocytic function of monocytes and macrophages,
promote the activation, proliferation and antigen presentation
function of dendritic cells, and regulate immune balance (59). GM-CSF is a powerful immune
stimulator (60). When combined
with its receptor, it can not only promote the proliferation and
differentiation of hematopoietic progenitor cells, and enhance
hematopoietic function, but also regulate the proliferation and
differentiation of monocyte macrophages and dendritic cells to
serve an immunoregulatory role (61). Interferon-γ is an inflammatory
factor, which can induce the expression of major histocompatibility
complex I and II, activate monocyte macrophages and natural killer
cells, and regulate the function of dendritic cells and B
lymphocytes (62). Interferon-γ
combined with granulocyte macrophage colony-stimulating factor can
markedly reduce the serum TNF-α level, and improve monocyte
macrophage dysfunction and the immunosuppression status (58). These results suggest that
immunotherapy may become a key approach for the treatment of
patients with SAP with PICS. It has been found (63,64)
that continuous blood purification (CBP) therapy can rapidly and
effectively improve the condition of patients with SAP, mainly by
correcting acid-base balance disorders, removing excessive
inflammatory factors in the body, and maintaining the homeostasis
of the immune system of the body. However, the timing and effect of
CBP in treating SAP remain contentious (63). Excessive reliance on CBP may even
heighten the risk of infection (65). Therefore, CBP should be used in
moderation according to the condition of the patient. Furthermore,
during the implementation of CBP, it is customary to employ
anticoagulation measures to ensure CBP vascular access patency.
Heparin and sodium citrate solution are the two main tube sealing
solutions in clinical practice. The catheter will be pumped back
before the central venous catheter and the pumping volume is mainly
twice the catheter capacity (66).
The pulse tube flushing technique combined with the positive
pressure tube sealing technique is used to seal the tube to reduce
the blood return of the catheter lumen (67). Through effective anticoagulant
care, the effect of CBP is ensured, which can reduce the
inflammatory response of the patient, enhance the immune function
and improve survival rates (68).
Provide nutritional support
Patients with SAP and with PICS enter a state of
continuous consumption early, and the duration of catabolism is
longer than that of anabolism (11). Based on the pathogenesis of PICS,
nutritional support is an important part of the treatment of PICS
(10). At present, there is no
unified standard for nutritional support plans for patients with
SAP with PICS. Research results have demonstrated that in patients
with severe infections and persistent inflammatory status similar
to SAP pathological changes, administering enteral nutrition (EN)
preparations rich in fat and protein could markedly reduce serum
TNF-α, IL-6 and IL-8 levels, inhibit early inflammatory reactions
and improve immune status (69).
An increasing number of studies have demonstrated that EN can not
only provide essential nutrients but also help improve intestinal
mucosal barrier function, reduce endotoxin and bacterial
translocation, and alleviate the inflammatory response (50,51).
It was hypothesized that EN should be performed as soon as possible
for patients without contraindications, and the transition from
parenteral nutrition to EN should be performed as soon as possible
for patients with contraindications after the body function
recovers because the severity of systemic immunosuppression can be
reduced (70). Patients with high
nutritional risk (The Nutrition Risk in Critically ill score ≥5)
and malnutrition should supplement EN within 2-3 days to achieve
calorie and protein levels >80% of the target value. After 7-10
days, if the energy level does not meet the standard,
supplementation of parenteral nutrition should be considered
(71). A prospective randomized
controlled study (10) has
indicated that early and rational EN (with special attention to
protein intake) markedly reduced the incidence of SAP with PICS.
However, there are differences in the recommended guidelines such
as ESPEN Guidelines and The 2016 American Society for Parenteral
and Enteral Nutrition/Society of Critical Care Medicine
(ASPEN/SCCM) guidelines for protein intake in patients in the ICU
(70,72). A protein summit recommends that
protein supplementation of 1.2-2.5 g/kg/day can reduce the
mortality of critically ill patients (73). It has been previously suggested
that supplementation with higher proteins inhibits endogenous
protein breakdown in a dose-dependent manner (74). Enteral immunomicroecological
nutrition can effectively improve the nutritional status of
patients with PICS, protect their intestinal barrier function and
improve immune function, which is conducive to preventing patient
regression and improving prognosis (7). A meta-analysis has demonstrated that
supplementation of parenteral nutrition with omega-3
polyunsaturated fatty acids reduced the 28-day mortality and length
of ICU stay in patients with sepsis (75). Leucine supplementation can improve
muscle protein synthesis by stimulating the mTOR signaling pathway
in elderly patients (≥60 years) and patients with cancer (24). Therefore, the addition of leucine
or branched-chain amino acids to nutritional support may be
beneficial for patients with SAP with PICS. Furthermore, compounds
derived from natural products with antiviral activity and
antioxidant activity, such as oligosaccharides, can also be used to
attempt adjuvant therapy (76).
However, EN often leads to symptoms of feeding intolerance
(77). Therefore, more attention
should be paid to possible risk factors, such as APACHE and whether
soluble fiber serum albumin is added, while performing effective EN
nursing (78,79). It has also been demonstrated that
there is an association between the nutrition infusion rate and
symptoms of feeding intolerance (80). The guidelines of the American
Society for Parenteral and Enteral Nutrition recommend that when
the intra-abdominal pressure of the patient is 12-15 mmHg, routine
EN should be continued, when the intra-abdominal pressure is 16-20
mmHg or continuously increased, nutritional feeding should be
adopted, and when the intra-abdominal pressure is >20 mmHg, EN
should be suspended (72).
Implement early exercise
In critically ill patients, the quadriceps femoris
cross-sectional area can be reduced after the onset of the disease
(81). Severe muscle decomposition
is accompanied by the decline of body movement and organ function
and is closely related to the increase in mortality (81). Early activity and resistance
exercise are important to prevent the loss of fat-free mass,
strength failure and loss of function in patients with PICS
(82,83). After evaluating the cardiopulmonary
function of patients, they can be encouraged to carry out
appropriate physical recovery exercise, supplemented by
psychological counseling when necessary (84). Combined resistance exercise therapy
and synthetic hormones can effectively inhibit muscle breakdown and
even reverse muscle loss in patients (85). In addition, a study has found that
neuromuscular electrical stimulation therapy was helpful in
stimulating muscle protein synthesis and relieving disuse muscle
atrophy (86). Clinicians should
pay attention to the fact that excessive sedation is often an
important factor for long-term bed-rest and ventilator dependence
in patients with PICS (87). In
clinical practice, it is best to implement continuous intravenous
drip or syringe pump administration managed by bedside nurses to
control the depth of sedation to ensure that patients are in a
light sedation state, and can wake up at any time and carry out
corresponding physical rehabilitation activities (88).
Fluid management should be
emphasized
Fluid resuscitation is the key to the early
treatment of SAP with PICS. Severe blood volume deficiency within
72 h after SAP onset can rapidly activate the
renin-angiotensin-aldosterone system and cause sympathetic nervous
system excitement, thus it is critical that we get the timing right
for fluid resuscitation (89).
Isotonic crystals are the preferred liquid and the rate of fluid
infusion (5-10 ml·kg-1 h-1) should be
strictly controlled (90). Early
fluid resuscitation can use an infusion pump to continuously infuse
at a constant rate, and the nurse should dynamically monitor the
amount of inflow and outflow (91). Early goal-directed therapy
(92) is recommended for initial
fluid management in SAP, which achieves resuscitation goals within
6 h, including central venous pressure of 8-12 mmHg, mean arterial
pressure ≥65 mmHg, hourly urine volume ≥0.5 ml/kg and central
venous oxygen saturation ≥70%. However, there is a debate about how
to perform early resuscitation (93). Patients with early shock begin
infusion at a rate of 150-600 ml/h and appropriate fluid
replacement (130-150 ml/h) is applied for non-dehydrated patients.
However, for patients with organ failure, it is necessary to
evaluate the circulatory capacity before determining the infusion
speed. For most patients, the total infusion volume of 2,500-4,000
ml is enough to reach the recovery goal within the first 24 h.
Effective fluid management can reduce the inflammatory response of
the patient, enhance immune function and improve survival rates
(94). Furthermore,
microcirculatory monitoring can help diagnose occult shock
(95). Compared with standard
hemodynamic guided treatment, patients with septic shock who
undergo resuscitation based on peripheral perfusion indicators have
a lower infusion volume, shorter hospital stay, lower mortality
rate and lower scores of SOFA (95). Corresponding clinical studies have
demonstrated the type of fluid resuscitation, starting time, speed
and target of fluid resuscitation (93,96,97);
however, the specific plan needs to be carried out in clinical
practice combined with monitoring. Fluid resuscitation for SAP with
PICS follows the principle of ‘timely and sufficient but not
excessive’. It is important to carry out multi-center studies to
provide a suitable resuscitation protocol for SAP with PICS.
6. Conclusions
The present review highlights the importance of
clinical treatment for patients with SAP with PICS. Although the
pathophysiological mechanism of PICS has not been elucidated, most
scholars hypothesize that it is preventable and treatable.
Therefore, it is important for medical staff to identify
populations at high risk of PICS and to master the prevention and
treatment of patients with SAP with PICS.
The selection of appropriate patients for rigorously
designed controlled studies, clinical evaluation of existing
diagnostic criteria and screening of novel specific biomarkers are
crucial for accurate early warning and prognostic assessment of
PICS. Ultimately, under the guidance of high-level evidence,
treatment should move from experience to standardization to achieve
goal-oriented precision treatment, and standardized and integrated
measures could be taken at an early stage of SAP to stop its
progression to PICS, which is of great significance for improving
the long-term prognosis of patients with CCI.
Supplementary Material
Possible pathogenesis and
corresponding treatment methods of patients with SAP with
PICS.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the National Natural
Science Foundation of China (grant no. 82072699).
Availability of data and materials
Not applicable.
Authors' contributions
BZ, QX, QM and LH conceived, drafted and directed
the study. BZ, QX and LH designed the study. BZ, QX, QM and LH
wrote the article. BZ, QX, QM and LH critically revised the
manuscript. Data authentication is not applicable. All authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS: Acute Pancreatitis
Classification Working Group. Classification of acute
pancreatitis-2012: Revision of the Atlanta classification and
definitions by international consensus. Gut. 62:102–111.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wilkman E, Kaukonen KM, Pettila V,
Kuitunen A and Varpula M: Early hemodynamic variables and outcome
in severe acute pancreatitis: A retrospective single-center cohort
study. Pancreas. 42:272–278. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hawkins RB, Raymond SL, Stortz JA,
Horiguchi H, Brakenridge SC, Gardner A, Efron PA, Bihorac A, Segal
M, Moore FA and Moldawer LL: Chronic critical Illness and the
persistent inflammation, immunosuppression, and catabolism
Syndrome. Front Immunol. 9(1511)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Rosenthal MD and Moore FA: Persistent
inflammation, immunosuppression, and catabolism: Evolution of
multiple organ dysfunction. Surg Infect (Larchmt). 17:167–172.
2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Osuchowski MF, Craciun F, Weixelbaumer KM,
Duffy ER and Remick DG: Sepsis Chronically in MARS: Systemic
cytokine responses are always mixed regardless of the outcome,
magnitude, or phase of sepsis. J Immunol. 189:4648–4656.
2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gentile LF, Cuenca AG, Efron PA, Ang D,
Bihorac A, McKinley BA, Moldawer LL and Moore FA: Persistent
inflammation and immunosuppression: A common syndrome and new
horizon for surgical intensive care. J Trauma Acute Care Surg.
72:1491–1501. 2012.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rosenthal M, Gabrielli A and Moore F: The
evolution of nutritional support in long term ICU patients: From
multisystem organ failure to persistent inflammation
immunosuppression catabolism syndrome. Minerva Anestesiol.
82:84–96. 2016.PubMed/NCBI
|
|
8
|
Mira JC, Gentile LF, Mathias BJ, Efron PA,
Brakenridge SC, Mohr AM, Moore FA and Moldawer LL: Sepsis
pathophysiology, chronic critical Illness, and persistent
inflammation-immunosuppression and catabolism Syndrome. Crit Care
Med. 45:253–262. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shepherd JM, Cole E and Brohi K:
Contemporary patterns of multiple organ dysfunction in trauma.
Shock. 47:429–435. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Moore FA, Phillips SM, McClain CJ, Patel
JJ and Martindale RG: Nutrition support for persistent
inflammation, immunosuppression, and catabolism Syndrome. Nutr Clin
Pract. 321 (1_suppl):121S–127S. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mira JC, Brakenridge SC, Moldawer LL and
Moore FA: Persistent inflammation, immunosuppression and catabolism
Syndrome. Crit Care Clin. 33:245–258. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Oland GL and Hines OJ: New guidelines for
the treatment of severe acute pancreatitis. Hepatobiliary Surg
Nutr. 11:913–916. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Portelli M and Jones CD: Severe acute
pancreatitis: Pathogenesis, diagnosis and surgical management.
Hepatobiliary Pancreat Dis Int. 16:155–159. 2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Yang N, Li B, Ye B, Ke L, Chen F, Lu G,
Jiang F, Tong Z, Li J and Li W: The long-term quality of life in
patients with persistent inflammation-immunosuppression and
catabolism syndrome after severe acute pancreatitis: A
retrospective cohort study. J Crit Care. 42:101–106.
2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Koksal AR, Boga S, Alkim H, Sen I,
Neijmann ST and Alkim C: Chemerin: A new biomarker to predict
postendoscopic retrograde cholangiopancreatography pancreatitis.
Eur J Gastroenterol Hepatol. 28:714–721. 2016.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li J, Yang J, Huang J, Jiang DL, Zhang F,
Liu MF, Qiang Y and Gu YL: Immunosuppression and the infection
caused by gut mucosal barrier dysfunction in patients with early
severe acute pancreatitis. Front Biosci (Landmark Ed). 18:892–900.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Glaubitz J, Wilden A, Frost F, Ameling S,
Homuth G, Mazloum H, Rühlemann MC, Bang C, Aghdassi AA, Budde C, et
al: Activated regulatory T-cells promote duodenal bacterial
translocation into necrotic areas in severe acute pancreatitis.
Gut. 72:1355–1369. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Glaubitz J, Wilden A, van den Brandt C,
Weiss FU, Bröker BM, Mayerle J, Lerch MM and Sendler M:
Experimental pancreatitis is characterized by rapid T cell
activation, Th2 differentiation that parallels disease severity,
and improvement after CD4+ T cell depletion.
Pancreatology. 20:1637–1647. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Joshi I, Carney WP and Rock EP: Utility of
monocyte HLA-DR and rationale for therapeutic GM-CSF in sepsis
immunoparalysis. Front Immunol. 14(1130214)2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wen W, Zheng H, Jiang Y, Huang L, Li D,
Zhang J and Zhang D: Effect of intestinal epithelial autophagy on
bacterial translocation in severe acute pancreatitis. Clin Res
Hepatol Gastroenterol. 41:703–710. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Liu J, Huang L, Luo M and Xia X: Bacterial
translocation in acute pancreatitis. Crit Rev Microbiol.
45:539–547. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Efron PA, Mohr AM, Bihorac A, Horiguchi H,
Hollen MK, Segal MS, Baker HV, Leeuwenburgh C, Moldawer LL, Moore
FA and Brakenridge SC: Persistent inflammation, immunosuppression,
and catabolism and the development of chronic critical illness
after surgery. Surgery. 164:178–184. 2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Fattahi F and Ward PA: Understanding
immunosuppression after sepsis. Immunity. 47:3–5. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Horiguchi H, Loftus TJ, Hawkins RB,
Raymond SL, Stortz JA, Hollen MK, Weiss BP, Miller ES, Bihorac A,
Larson SD, et al: Innate immunity in the persistent inflammation,
immunosuppression, and catabolism Syndrome and its implications for
therapy. Front Immunol. 9(595)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Veglia F, Perego M and Gabrilovich D:
Myeloid-derived suppressor cells coming of age. Nat Immunol.
19:108–119. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Haimovich B, Zhang Z, Calvano JE, Calvano
SE, Kumar A, Macor MA, Corbett S, Coyle SM and Lowry SF: Cellular
metabolic regulators: Novel indicators of low-grade inflammation in
humans. Ann Surg. 259:999–1006. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Eyenga P, Roussel D, Morel J, Rey B,
Romestaing C, Gueguen-Chaignon V, Sheu SS and Viale JP: Time course
of liver mitochondrial function and intrinsic changes in oxidative
phosphorylation in a rat model of sepsis. Intensive Care Med Exp.
6(31)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Asehnoune K, Hotchkiss RS and Monneret G:
Understanding why clinicians should care about danger-associated
molecular patterns. Intensive Care Med. 42:611–614. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhao H, Kilgas S, Alam A, Eguchi S and Ma
D: The role of extracellular adenosine triphosphate in ischemic
organ injury. Crit Care Med. 44:1000–1012. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kugelberg E: Immunometabolism: Complex
metabolic responses to microbial stimuli. Nat Rev Immunol.
17:78–79. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cheng SC, Scicluna BP, Arts RJ, Gresnigt
MS, Lachmandas E, Giamarellos-Bourboulis EJ, Kox M, Manjeri GR,
Wagenaars JA, Cremer OL, et al: Broad defects in the energy
metabolism of leukocytes underlie immunoparalysis in sepsis. Nat
Immunol. 17:406–413. 2016.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kau AL, Ahern PP, Griffin NW, Goodman AL
and Gordon JI: Human nutrition, the gut microbiome and the immune
system. Nature. 474:327–336. 2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li WQ, Tong ZH, Quan ZF, Zhao RZ, Yu WK,
Ye XH, Wang ZM, Wang XY, Wang ZQ, Ji DX, et al: Treatment
experience of severe acute pancreatitis on 1033 cases. Zhonghua Wai
Ke Za Zhi. 47:1472–1482. 2009.PubMed/NCBI(In Chinese).
|
|
34
|
Kim YJ, Kim DB, Chung WC, Lee JM, Youn GJ,
Jung YD, Choi S and Oh JH: Analysis of factors influencing survival
in patients with severe acute pancreatitis. Scand J Gastroenterol.
52:904–908. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Vanzant EL, Lopez CM, Ozrazgat-Baslanti T,
Ungaro R, Davis R, Cuenca AG, Gentile LF, Nacionales DC, Cuenca AL,
Bihorac A, et al: Persistent inflammation, immunosuppression, and
catabolism syndrome after severe blunt trauma. J Trauma Acute Care
Surg. 76:21–29; discussion 29-30. 2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sadighi Akha AA: Aging and the immune
system: An overview. J Immunol Methods. 463:21–26. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Leppaniemi A, Tolonen M, Tarasconi A,
Segovia-Lohse H, Gamberini E, Kirkpatrick AW, Ball CG, Parry N,
Sartelli M, Wolbrink D, et al: 2019 WSES guidelines for the
management of severe acute pancreatitis. World J Emerg Surg.
14(27)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lane-Fall MB, Kuza CM, Fakhry S and Kaplan
LJ: The lifetime effects of injury: Postintensive care syndrome and
posttraumatic stress disorder. Anesthesiol Clin. 37:135–150.
2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Nakamura K, Ogura K, Nakano H, Naraba H,
Takahashi Y, Sonoo T, Hashimoto H and Morimura N: C-reactive
protein clustering to clarify persistent inflammation,
immunosuppression and catabolism syndrome. Intensive Care Med.
46:437–443. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Hu D, Ren J, Wang G, Gu G, Chen J, Zhou B,
Liu S, Wu X and Li J: Persistent inflammation-immunosuppression
catabolism syndrome, a common manifestation of patients with
enterocutaneous fistula in intensive care unit. J Trauma Acute Care
Surg. 76:725–729. 2014.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ding RY and Ma XC: Persistent inflammatory
response-immunosuppressed catabolic syndrome: A special type of
chronic severe disease. Chin J Gastrointestinal Surgery.
19:734–736. 2016.(In Chinese).
|
|
42
|
Whitfield JB: Genetics and molecular
biology in laboratory medicine, 1963-2013. Clin Chem Lab Med.
51:113–117. 2013.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lai Y, Feldman KL and Clark RS:
Enzyme-linked immunosorbent assays (ELISAs). Crit Care Med. 33 (12
Suppl):S433–S434. 2005.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Manohar SM, Shah P and Nair A: Flow
cytometry: Principles, applications and recent advances.
Bioanalysis. 13:181–198. 2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Xu D and Xun J: Research progress of
severe acute pancreatitis with persistent
inflammatory-immunosuppressive-catabolic syndrome. Chin J Practical
Diagnosis and Therapy. 32:725–728. 2018.(In Chinese).
|
|
46
|
Antonini F, De Minicis S, Macarri G and
Pezzilli R: . Are we ready for early discharge of patients with
mild non-alcoholic acute interstitial pancreatitis? Pancreatology.
16:322–3. 2016.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Subbe CP, Kruger M, Rutherford P and
Gemmel L: Validation of a modified Early Warning Score in medical
admissions. QJM. 94:521–526. 2001.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Alaa AM, Yoon J, Hu S and van der Schaar
M: Personalized risk scoring for critical care prognosis using
mixtures of gaussian processes. IEEE Trans Biomed Eng. 65:207–218.
2018.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kramer AA, Sebat F and Lissauer M: A
review of early warning systems for prompt detection of patients at
risk for clinical decline. J Trauma Acute Care Surg. 87 (1S Suppl
1):S67–S73. 2019.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wolbrink DRJ, Kolwijck E, Ten Oever J,
Horvath KD, Bouwense SAW and Schouten JA: Management of infected
pancreatic necrosis in the intensive care unit: A narrative review.
Clin Microbiol Infect. 26:18–25. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Karjula H, Nordblad Schmidt P, Makela J,
Liisanantti JH, Ohtonen P and Saarela A: Prophylactic pancreatic
duct stenting in severe acute necrotizing pancreatitis: A
prospective randomized study. Endoscopy. 51:1027–1034.
2019.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Su HY, Mo ZX, Chen Z, et al: Severe immune
imbalance in ICU: persistent
inflammatory-immunosuppressive-catabolic syndrome. Chin Critical
Care Emerg Med. 29:760–764. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
53
|
Wang X, Zhuang X, Wei R, Wang C, Xue X and
Mao L: Protective effects of Acanthopanax vs. Ulinastatin against
severe acute pancreatitis-induced brain injury in rats. Int
Immunopharmacol. 24:285–298. 2015.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Pan Y, Fang H, Lu F, Pan M, Chen F, Xiong
P, Yao Y and Huang H: Ulinastatin ameliorates tissue damage of
severe acute pancreatitis through modulating regulatory T cells. J
Inflamm (Lond). 14(7)2017.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhu F, Yin S, Zhou L, Li Z, Yan H, Zhong
Y, Wu X, Luo B, Yang L, Gan D, et al: Chinese herbal medicine
xuebijing injection for acute pancreatitis: An overview of
systematic reviews. Front Pharmacol. 13(883729)2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Li C, Wang P, Zhang L, Li M, Lei X, Liu S,
Feng Z, Yao Y, Chang B, Liu B and Shang H: Efficacy and safety of
Xuebijing injection (a Chinese patent) for sepsis: A meta-analysis
of randomized controlled trials. J Ethnopharmacol. 224:512–521.
2018.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Munir F, Jamshed MB, Shahid N, Hussain HM,
Muhammad SA, Mamun AA and Zhang Q: . Advances in immunomodulatory
therapy for severe acute pancreatitis. Immunol Letters. 217:72–76.
2020.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hotchkiss RS, Monneret G and Payen D:
Immunosuppression in sepsis: A novel understanding of the disorder
and a new Therapeutic approach. Lancet Infect Dis. 13:260–268.
2013.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Wan J, Shan Y, Shan H, Li G, Wang T, Guan
J, Liu X, Chen D, Li W and Lin Z: Thymosin-alpha1 promotes the
apoptosis of regulatory T cells and survival rate in septic mice.
Front Biosci (Landmark Ed). 16:3004–3013. 2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Hamilton JA: GM-CSF in inflammation. J Exp
Med. 217(e20190945)2020.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Lee KMC, Achuthan AA and Hamilton JA:
GM-CSF: A promising target in inflammation and autoimmunity.
Immunotargets Ther. 9:225–240. 2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Fu XZ and Wang Y: Interferon-gamma
regulates immunosuppression in septic mice by promoting the Warburg
effect through the PI3K/AKT/mTOR pathway. Mol Med.
29(95)2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Hu Y, Xiong W, Li C and Cui Y: Continuous
blood purification for severe acute pancreatitis: A systematic
review and meta-analysis. Medicine (Baltimore).
98(e14873)2019.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Gong D, Zhang P, Ji D, Chen Z, Li W, Li J,
Li L and Liu Z: Improvement of immune dysfunction in patients with
severe acute pancreatitis by high-volume hemofiltration: A
preliminary report. Int J Artif Organs. 33:22–29. 2010.PubMed/NCBI
|
|
65
|
Clark E, Molnar AO, Joannes-Boyau O,
Honoré PM, Sikora L and Bagshaw SM: High-volume hemofiltration for
septic acute kidney injury: A systematic review and meta-analysis.
Crit Care. 18(R7)2014.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Almeida BM, Moreno DH, Vasconcelos V and
Cacione DG: Interventions for treating catheter-related bloodstream
infections in people receiving maintenance haemodialysis. Cochrane
Database Syst Rev. 4(CD013554)2022.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Gorski LA, Hadaway L, Hagle ME, Broadhurst
D, Clare S, Kleidon T, Meyer BM, Nickel B, Rowley S, Sharpe E and
Alexander M: Infusion therapy standards of practice, 8th Edition. J
Infus Nurs. 44 (1S Suppl 1):S1–224. 2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Legrand M and Tolwani A: Anticoagulation
strategies in continuous renal replacement therapy. Semin Dial.
34:416–422. 2021.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Lubbers T, Kox M, de Haan JJ, Greve JW,
Pompe JC, Ramakers BP, Pickkers P and Buurman WA: Continuous
administration of enteral lipid- and protein-rich nutrition limits
inflammation in a human endotoxemia model. Crit Care Med.
41:1258–1265. 2013.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Plauth M, Bernal W, Dasarathy S, Merli M,
Plank LD, Schütz T and Bischoff SC: ESPEN guideline on clinical
nutrition in liver disease. Clin Nutr. 38:485–521. 2019.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Wang CY, Huang CT, Chen CH, Chen MF, Ching
SL and Huang YC: Optimal energy delivery, rather than the
implementation of a feeding protocol, may benefit clinical outcomes
in critically Ill patients. Nutrients. 9(527)2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
McClave SA, Taylor BE, Martindale RG,
Warren MM, Johnson DR, Braunschweig C, McCarthy MS, Davanos E, Rice
TW, Cresci GA, et al: Guidelines for the provision and assessment
of nutrition support therapy in the adult critically ill patient:
Society of Critical Care Medicine (SCCM) and American Society for
Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter
Enteral Nutr. 40:159–211. 2016.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Bozzetti F, Arends J, Lundholm K,
Micklewright A, Zurcher G and Muscaritoli M: ESPEN. ESPEN
guidelines on parenteral nutrition: Non-surgical oncology. Clin
Nutr. 28:445–454. 2009.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Wolfe RR: Perspective: Optimal protein
intake in the elderly. J Am Med Dir Assoc. 14:65–66.
2013.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Lu C, Sharma S, McIntyre L, Rhodes A,
Evans L, Almenawer S, Leduc L, Angus DC and Alhazzani W: Omega-3
supplementation in patients with sepsis: A systematic review and
meta-analysis of randomized trials. Ann Intensive Care.
7(58)2017.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Wang M, Veeraperumal S, Zhong S and Cheong
KL: Fucoidan-Derived functional oligosaccharides: Recent
developments, preparation, and potential applications. Foods.
12(878)2023.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Lin J, Lv C, Wu C, Zhang H, Liu Z, Ke L,
Li G, Tong Z, Tu J, et al: Incidence and risk factors of
nasogastric feeding intolerance in moderately-severe to severe
acute pancreatitis. BMC Gastroenterol. 22(327)2022.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Sun JK, Li WQ, Ke L, Tong ZH, Ni HB, Li G,
Zhang LY, Nie Y, Wang XY, et al: Early enteral nutrition prevents
intra-abdominal hypertension and reduces the severity of severe
acute pancreatitis compared with delayed enteral nutrition: a
prospective pilot study. World J Surg. 37:2053–2060.
2013.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Heyland DK, Ortiz A, Stoppe C, Patel JJ,
Yeh DD, Dukes G, Chen YJ, Almansa C and Day AG: Incidence, risk
factors, and clinical consequence of enteral feeding intolerance in
the mechanically ventilated critically Ill: An analysis of a
multicenter, multiyear database. Crit Care Med. 49:49–59.
2021.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Gungabissoon U, Hacquoil K, Bains C,
Irizarry M, Dukes G, Williamson R, Deane AM and Heyland DK:
Prevalence, risk factors, clinical consequences, and treatment of
enteral feed intolerance during critical illness. JPEN J Parenter
Enteral Nutr. 39:441–448. 2015.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Wischmeyer PE, Puthucheary Z, San Millán
I, Butz D and Grocott MPW: Muscle mass and physical recovery in
ICU: Innovations for targeting of nutrition and exercise. Curr Opin
Crit Care. 23:269–278. 2017.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Fuke R, Hifumi T, Kondo Y, Hatakeyama J,
Takei T, Yamakawa K, Inoue S and Nishida O: Early rehabilitation to
prevent postintensive care syndrome in patients with critical
illness: A systematic review and meta-analysis. BMJ Open.
8(e019998)2018.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Patel BK, Pohlman AS, Hall JB and Kress
JP: Impact of early mobilization on glycemic control and
ICU-acquired weakness in critically ill patients who are
mechanically ventilated. Chest. 146:583–589. 2014.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Wang CH, Qin JM and Ben YL: Effect of
early rehabilitation training on ICU acquired myasthenia in
patients with mechanical ventilation. Chin Nurs Manag. 19:457–461.
2019.(In Chinese).
|
|
85
|
Maffiuletti NA, Roig M, Karatzanos E and
Nanas S: Neuromuscular electrical stimulation for preventing
skeletal-muscle weakness and wasting in critically ill patients: A
systematic review. BMC Med. 11(137)2013.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Dirks ML, Hansen D, Van Assche A, Dendale
P and Van Loon LJ: Neuromuscular electrical stimulation prevents
muscle wasting in critically ill comatose patients. Clin Sci
(Lond). 128:357–365. 2015.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Yang YJ, Liu GY, Li RY and Liu H: Research
progress of inspiratory muscle training in ICU patients with
mechanical ventilation. Chin Nurs Manag. 20:1436–1440. 2020.(In
Chinese).
|
|
88
|
Page VJ and McAuley DF: Sedation/drugs
used in intensive care sedation. Curr Opin Anaesthesiol.
28:139–144. 2015.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Tenner S, Baillie J, DeWitt J and Vege SS:
American College of Gastroenterology. American College of
Gastroenterology guideline: Management of acute pancreatitis. Am J
Gastroenterol. 108:1400–1415, 1416. 2013.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Working Group IAP/APA Acute Pancreatitis
Guidelines. IAP/APA evidence-based guidelines for the management of
acute pancreatitis. Pancreatology. 13 (4 Suppl 2):e1–e15.
2013.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Zhu Y, Cui Y, Zhang YC, Miao HJ, Wang F,
Chen RX and Rong QF: Efficacy of continuous blood purification in
treatment of severe acute pancreatitis in children. Zhonghua Er Ke
Za Zhi. 55:338–342. 2017.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
92
|
Crockett SD, Wani S, Gardner TB,
Falck-Ytter Y and Barkun AN: American Gastroenterological
Association Institute Clinical Guidelines Committee. American
gastroenterological association institute guideline on initial
management of acute pancreatitis. Gastroenterology. 154:1096–1101.
2018.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Mouncey PR, Osborn TM, Power GS, Harrison
DA, Sadique MZ, Grieve RD, Jahan R, Harvey SE, Bell D, Bion JF, et
al: Trial of early, goal-directed resuscitation for septic shock. N
Engl J Med. 372:1301–1311. 2015.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Cooper ES and Silverstein DC: Fluid
therapy and the microcirculation in health and critical Illness.
Front Vet Sci. 8(625708)2021.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Kara A, Akin S and Ince C: Monitoring
microcirculation in critical illness. Curr Opin Crit Care.
22:444–452. 2016.PubMed/NCBI View Article : Google Scholar
|
|
96
|
De-Madaria E, Herrera-Marante I,
Gonzalez-Camacho V, Bonjoch L, Quesada-Vázquez N, Almenta-Saavedra
I, Miralles-Maciá C, Acevedo-Piedra NG, Roger-Ibáñez M,
Sánchez-Marin C, et al: Fluid resuscitation with lactated Ringer's
solution vs. normal saline in acute pancreatitis: A triple-blind,
randomized, controlled trial. United European Gastroenterol J.
6:63–72. 2018.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Cordemans C, De Laet I, Van Regenmortel N,
Schoonheydt K, Dits H, Huber W and Malbrain ML: Fluid management in
critically ill patients: The role of extravascular lung water,
abdominal hypertension, capillary leak, and fluid balance. Ann
Intensive Care. 2 (Suppl 1 Diagnosis and management of
intra-abdominal hyperten)(S1)2012.PubMed/NCBI View Article : Google Scholar
|