Introduction
Anthracycline (ANT) is a first-line chemotherapy
drug with high clinical efficacy and a broad antitumor spectrum
(1). Adriamycin, daunorubicin,
epirubicin, mitoxantrone and nordoxorubicin all belong to the ANT
family of agents that are commonly used in clinical practice and
widely used for treating hematological and solid tumors (2). As the clinical application of ANTs
has become more common, clinical side effects on the heart
following ANT treatment are also becoming gradually understood.
Since Lefrak et al (3)
first reported cardiotoxicity induced by ANT in 1973, various
reports have pointed out that its cardiotoxicity is potentially
more harmful to the prognosis of patients with cancer compared with
the cancer recurrence itself (4,5). In
particular, information regarding delayed-onset cardiotoxicity as a
result of ANT treatment remains lacking. Recent studies have found
that subclinical cardiotoxicity caused by low total cumulative
doxorubicin doses can manifest into cardiomyopathy in long-term
cancer survivors (6,7). Chronic cardiotoxicity of chemotherapy
drugs in cancer patients is clinically common, and typically occurs
within 1 year of treatment (6). It
manifests as congestive heart failure and/or cardiomyopathy that
can induce irreversible changes such as Enlargement of the atria
and ventricles or decreased myocardial activity (6). In addition, its clinical onset is
frequently hidden and the mortality rate can reach as high as
30-60% (6). The occurrence of
chronic cardiotoxicity has been documented to be closely associated
with the cumulative dose of ANTs (7).
The present case report describes a 36-year-old
patient with heart failure due to new adjuvant chemotherapy and
osteosarcoma resection based on a combination of Doxorubicin,
Cisplatin, and high-dose Methotrexate (MAP regimen). The expert
group of the hospital held a consultation to assess the situation
of the patient in detail and formulated a diagnosis and treatment
plan. In addition, the existing literature was reviewed to provide
a basis for the diagnosis and treatment of the patient.
Case report
A 36-year-old young male patient presented to the
Cardiology Department of Guiqian International General Hospital
(Guiyang, China) in October 2022 due to ‘difficulty breathing for
>10 days. The patient felt shortness of breath after a little
exercise, following which the symptoms progressively worsened,
accompanied by paroxysmal nocturnal dyspnea and orthopnea. The
electrocardiogram (ECG) (ECG-2306, Shanghai Optoelectronic Medical
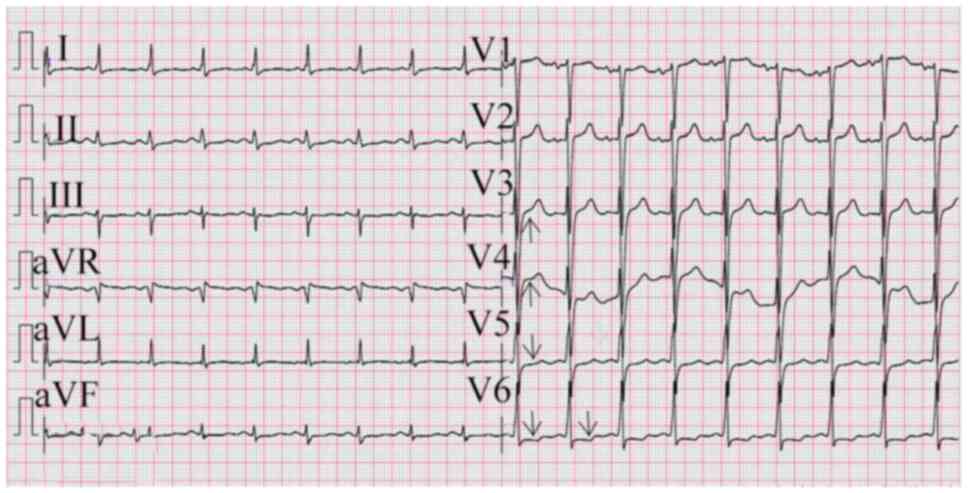
Electronic Instrument Co., Ltd) revealed ‘ST-segment elevation in
leads V1-V4, ST-segment depression in multiple leads, and flat and
inverted T waves (Fig. 1).
Comprehensive evaluation of the chest CT and cardiac
ultrasonography results revealed no anomalous findings.
The patient was diagnosed with osteosarcoma of the
lower left extremity 14 years before presenting to the hospital,
and underwent MAP regimen at Guangzhou Cancer Hospital (Guangzhou,
China). During the following 5 years of regular follow-up, there
was no recurrence of osteosarcoma and no abnormalities were
detected in the cardiac-related examinations. Auxiliary examination
at the time of visit revealed that serum B-type natriuretic peptide
(BNP) level in the patient was 2,146 pg/ml (normal, 0-100 pg/ml)
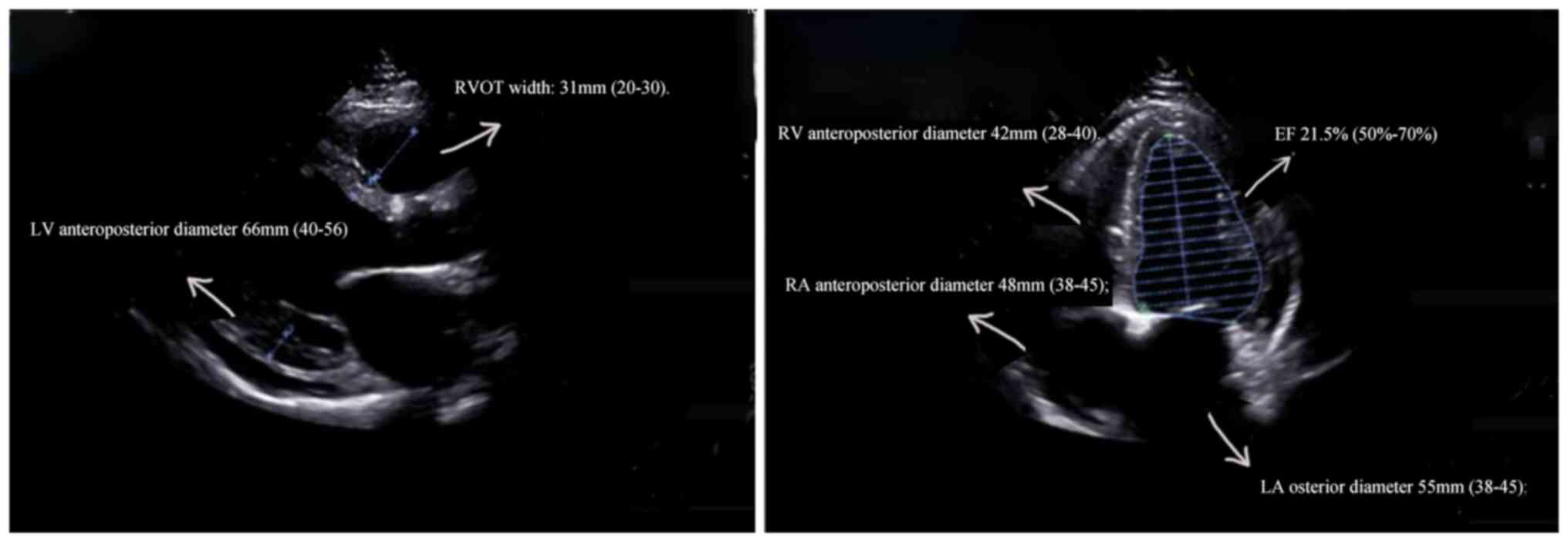
(7). Echocardiography (EPIQ 7C,
Royal Dutch Philips Electronics Ltd.) indicated that the whole
heart was enlarged, and the left ventricular systolic function was
markedly reduced (Fig. 2),
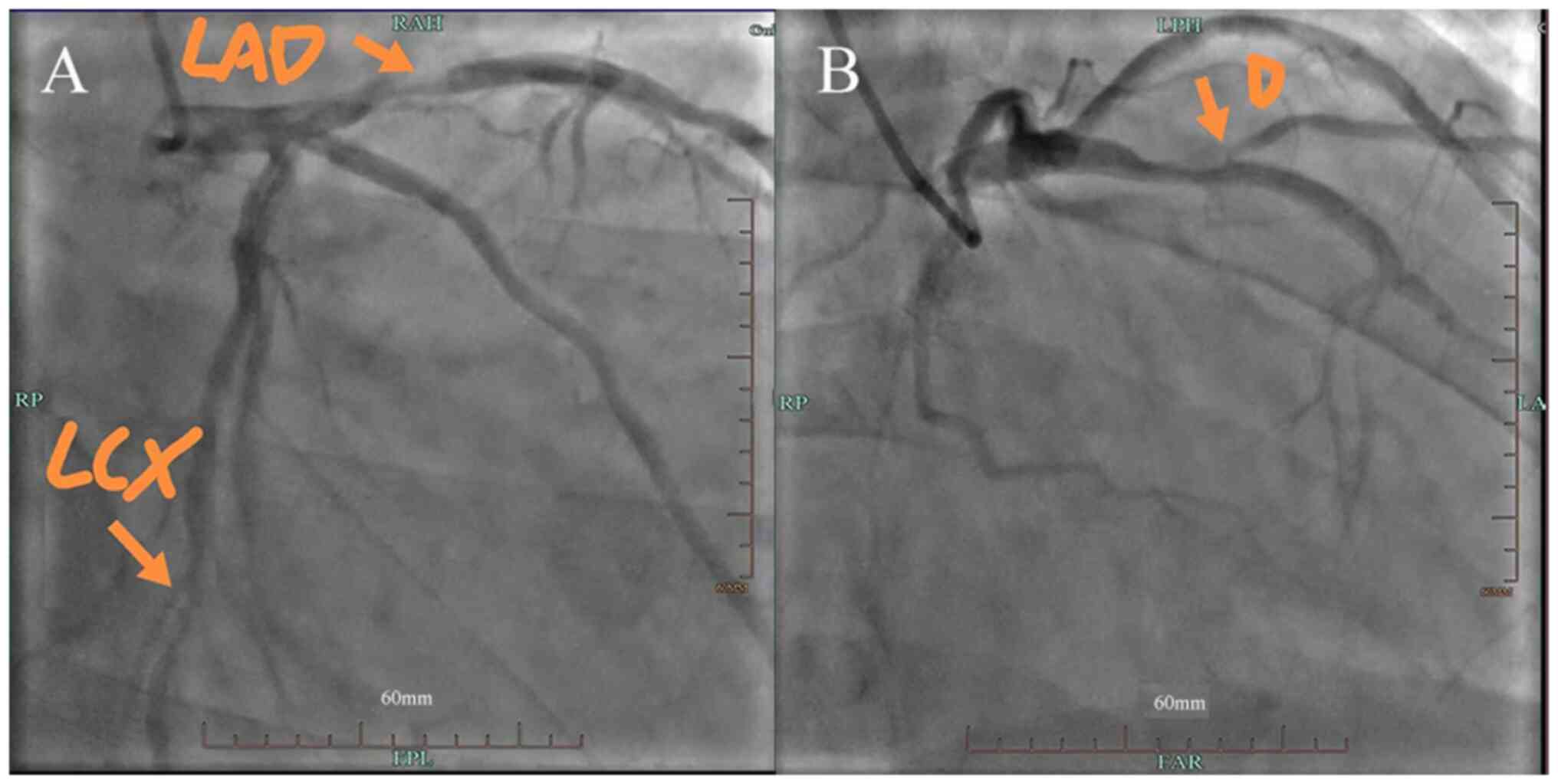
suggesting heart failure. Coronary angiography (Azurion 7 M20;
Royal Dutch Philips Electronics Ltd; contrast agent dosage, 60 ml,
Contrast catheter: TIG 5F, contrast guidewire: 150 cm) revealed
coronary atherosclerotic heart disease, and the most severe
stenosis was observed in the proximal segment of the anterior
descending artery. Specifically, 80% stenosis was found in the
proximal segment of the anterior descending artery; there was 90%
stenosis in the opening of the first diagonal branch and 70%
stenosis in the distal segment of the circumflex artery (Fig. 3). Cardiac MRI indicated no obvious
myocardial diffuse fibrosis, myocardial inflammation, myocardial
infarction or specific cardiomyopathy manifestations. In addition,
urine light chain, serum antibody light chain (κ/λ), immunoglobulin
electrophoresis, antinuclear antibody spectrum were also found to
be normal.
In the present case, it was first considered whether
the clinical symptoms were caused by acute myocardial infarction.
Although the admission ECG yielded multiple leads, such as
ST-segment depression and T wave low flatness, there was no change
in their localizations or any marked increases in troponin levels
after two reexaminations 1 day apart (October 2022). In addition,
the patient had no recent history of sudden chest tightness or
chest pain, which was not consistent with acute myocardial
infarction. In addition, ultrasound showed enlargement of the whole
heart, thickening of the posterior left ventricular wall and
ventricular septum not reaching 15 mm, but the ratio of ventricular
septum to left ventricular posterior wall did not reach 1.3-1.5
(1.18) and was not classed as hypertrophic cardiomyopathy (5,7).
Patients with hypertension with blood pressure ≤160/100 mmHg may
develop hypertensive heart disease without antihypertensive therapy
(4) This may explain the
thickening of ventricular septum in the present case. However, this
was not sufficient to explain the concentricular enlargement
observed. Therefore, it was considered whether the coronary
multivessel lesion in the patient could be due to ischemic
cardiomyopathy. However, if such a severe type of heart failure has
recently been reached, then the clinical history of coronary heart
disease should be long with the relevant symptoms such as changes
in myocardial enzymes and previous exertion-associated chest pain
and tightness (7). Therefore, the
occurrence of sudden illness in the present patient was
inconsistent with the diagnosis of ‘coronary heart disease’. The
patient was diagnosed with chronic heart failure, cardiac function
class IV (New York Heart Association class) (8). However, it was considered uncommon
that a patient of such young age was not found with any
predisposing factors for severe heart failure. After assessing the
medical history, it was revealed that the patient underwent
chemotherapy 14 cycles of treatment 5 years ago, specifically with
ifosfamide (108 g), cisplatin (200 mg) and paclitaxel (1,400 mg),
where the total usage of pirarubicin hydrochloride reached 1,031
mg, exceeding the recommendation (National Comprehensive Cancer
Network. Bone Cancer, Version 2.2021). Finally, following
discussion with the panel, antineoplastic drug-induced heart
failure was considered. Chemotherapy for osteosarcoma typically
consists of three or more chemotherapy drugs. Cisplatin is the
basic chemotherapeutic agent for osteosarcoma and can also cause
cardiotoxicity (6). However, since
there was no evidence of cisplatin overdose in the treatment
history of the patient, cardiac toxicity due to cisplatin appeared
unlikely. Furthermore, since no other drugs reported to cause
marked cardiotoxicity (such as Hydrochloride Tolvaptan, Vitamin C)
(7) were found in the treatment
history of the patient, the symptoms observed in the present report
were proposed to be attributed to delayed cardiotoxicity caused by
ANT. Therefore, subsequent reviews focused on this. Follow-up
patients by phone every six months after discharge to track their
health. The patient reported during the latest phone follow-up in
December 2022, that clinical symptoms had not significantly
worsened over the past months.
Discussion
The present patient was young and onset of
cardiotoxicity was sudden. Acute myocardial infarction was first
considered; however, the admission ECG had multiple leads of
ST-segment depression and T-wave flattening, coupled with no
localization changes or significant increases in troponin levels.
There was also no history of sudden chest tightness, chest pain or
elevated cardiac enzymes. None of these aforementioned observations
conformed to the manifestations of acute myocardial infarction
(9). Subsequent echocardiography
revealed enlargement of the whole heart and thickening of the
posterior wall of the left ventricle. However, the ventricular
septum did not reach 15 mm and the ratio of ventricular septum to
posterior wall of the left ventricle did not reach 1.3-1.5, which
was not in line with hypertrophy cardiomyopathy (10). Therefore, the overall ECG was more
consistent with that of a coronary ischemia ECG. To achieve such a
severe form of heart failure, the medical history should have been
long (11,12).
The patient had an enlarged heart and a history of
chemotherapy. Among the chemotherapy drugs, ANTs have potent
cardiotoxic effects. Cardiotoxicity caused by ANTs can be divided
into three categories, namely acute, chronic and delayed
cardiotoxicity (13). A recent
report estimated that subclinical but pathological
echocardiographic findings of left ventricular tissue and function,
such as increased afterload or decreased systolic function,
typically occur in >50% of patients in 1 years after ANT
administration (14). For the
maximum cumulative injected dose of pirarubicin hydrochloride, the
total limit is recommended to be 700-950 mg/m2 based on
the body surface area (8).
However, the total dose received by the present patient was 1,030
mg. Therefore, global heart enlargement may be considered to be a
characteristic of delayed cardiotoxicity caused by ANT. Although
the notion that such a delayed effect can continue for ~10 years is
difficult to understand, similar case of this have been reported
previously. Tran et al (15) reported a case of delayed and sudden
doxorubicin-associated cardiotoxicity that occurred 7 years after
MAP regimen chemotherapy completion, which provides evidence that
this type of long-term effect is possible.
The present case can be summarized with the
following: i) The patient had a foundation of heart disease and the
left ventricular ejection fraction (LVEF) was low, suggesting that
this patient had a long history of heart disease; ii) although the
coronary artery had lesions and the anterior descending artery had
borderline lesions, it was not sufficient to explain the size of
the heart and the low ejection fraction; iii) based on the recent
heart failure and, combined with the low ejection fraction results,
it can be inferred that cardiac function has been severely
compromised.; and iv) damage of chemotherapy drugs to the heart can
include direct damage to cardiomyocytes, influence on cell
signaling and systemic changes during chemotherapy. The condition
of the present patient was attributable to the destruction of
cardiomyocytes, but it is unclear to what extent the damage caused
by the chemotherapeutic drugs contributed to the symptoms of this
patient. According to these hypotheses, a review of the relevant
literature was conducted.
For the literature search, the following databases
were searched: i) PubMed (ncbi.nlm.nih.gov/); ii) Embase (https://www.embase.com); and iii) Cochrane Library
(https://www.cochranelibrary.com). The
search terms used were: i) ‘heart failure’; ii) ‘chemotherapy’;
iii) ‘cancer’; iv) ‘treatment’; v) ‘side effects’; vi) ‘adverse
events’; vii) ‘management’; viii) ‘prevention’; and ix)
‘interventions’. Subsequently, 2 doctors independently screened the
relevant literature. Searches were limited to studies published in
English and conducted on human individuals. The inclusion criteria
were: i) Studies investigating chemotherapy treatment for cancer;
ii) studies reporting on the side effects or adverse events of
chemotherapy; iii) studies investigating interventions or
management strategies for chemotherapy side effects; and iv)
studies published in English and conducted on human subjects. The
exclusion criteria were: i) Studies conducted on animals or in
vitro; ii) studies not reporting on chemotherapy treatment,
side effects or management strategies; iii) studies published in
non-English languages; and iv) studies published as abstracts or
conference proceedings without full-text articles available.
ANT is one of the most common clinically used drugs
for chemotherapy (16). However,
cumulative low-dose cardiac toxicity has hindered its further
clinical applications. As early as 1979, a clinical study confirmed
the relationship between heart damage caused by ANT and its
cumulative dosage (16).
The Guidelines for the Prevention and Treatment of
Cardiotoxicity of ANT Chemotherapy Drugs (2013 Edition) (17) define cardiotoxicity as having one
or more of the following manifestations, but do not include
subclinical cardiovascular damage occurring early following the use
of chemotherapeutic drugs/targeted drugs: i) Cardiomyopathy with
reduced LVEF, manifested by decreased global function or markedly
diminished interventricular septal motion; ii) symptoms related to
congestive heart failure appear; iii) appearance of signs related
to congestive heart failure, such as S3 gallop rhythm and/or
tachycardia; and iv) If LVEF decreased by ≥5% compared to the
baseline value, and the absolute value is <55%, it should be
accompanied by symptoms or signs of congestive heart failure.
Alternatively, if there is a ≥10% reduction in LVEF with an
absolute value <55%, it can be considered without associated
symptoms or signs.
Depending on the occurrence of heart damage, heart
damage caused by ANT can be divided into acute, chronic and delayed
heart damage (17). Acute heart
damage refers to heart damage occurring within a few hours or days
after medication, which is manifested as internal conduction
disorders and arrhythmia. A proportion of patients can experience
pericarditis and acute left heart failure. Chronic heart damage
refers to heart damage that occurs within 1 year after
chemotherapy, which can be manifested as a left ventricular
dysfunction, and there is a risk of congestive heart failure.
Delayed heart damage refers to cardiac damage that occurs >1 or
several years after treatment ends, which may present in various
forms (17).
There are various proposed mechanisms of
cardiotoxicity caused by ANT use. ANT-generated free radicals are
produced through enzymatic mechanisms, with NADPH oxidase as an
important mediator (18). NADH
dehydrogenase and other reductases react with oxygen to generate
superoxide anion radicals and hydroxyl radicals, resulting in
mitochondria damage and lipid microsome peroxidation, which damages
cardiomyocytes (18). In addition,
the ANT family of chemotherapeutic drugs can enter the myocardium,
which decreases antioxidant enzymes expression in the
cardiomyocytes and leads to the accumulation of free radicals and
superoxides, which can aggravate the damage to the cardiomyocytes
(Fig. 4) (19).
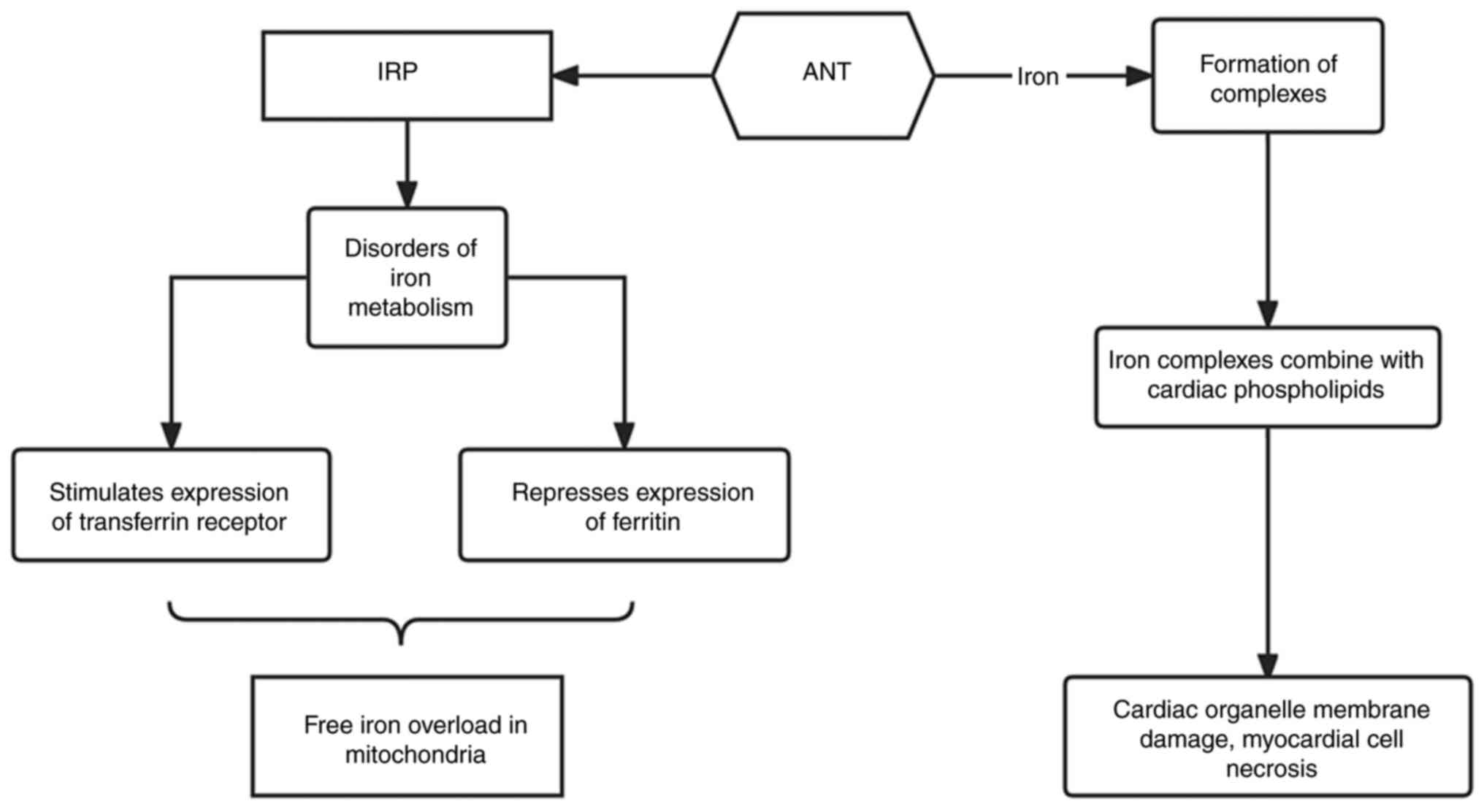
Disruption of iron homeostasis is another key
mechanism that can cause ANT cardiotoxicity. ANT drugs can disrupt
the accumulation of iron ions in cells through iron regulatory
proteins and transferrin receptors (20). They can achieve this by interacting
with iron regulatory proteins, resulting in the promotion of
transferrin receptor expression and inhibition of ferritin
expression. As a result, iron uptake is increased but iron storage
is decreased, leading to free iron overload, especially in
mitochondria (20). In addition,
ANTs are capable of binding to iron to form complexes, which can
then combine with cardiac phospholipids to damage organelle
membranes to induce myocardial cell necrosis (Fig. 5) (21). In addition, calcium overload,
apoptosis, DNA damage response, cardiac inflammation, cardiac
energy stress and the adenylate-activated protein kinase signaling
pathway have also been reported to be associated with progressive
cardiac damage (22,23). However, these mechanisms require
further research.
ECG is the most economical and convenient monitoring
method in clinical application. Cardiotoxicity caused by ANT
chemotherapy can manifest as abnormalities in the cardiac
conduction system, such as non-specific ST or T wave abnormalities,
decreased QRS complex voltage, prolonged QT interval in the cardiac
conduction system (24). In
particular, the QT interval dispersion of patients with breast
cancer after 4 cycles of ANT chemotherapy was markedly increased
according to a previous study, and cardiac damage may occur in the
early stage of ANT chemotherapy (24). This underlines the necessity of ECG
as an inspection method. However, the ECG examines the heart
condition of a patient within a specific time period, but its
specificity and accuracy for diagnosing cardiotoxicity are poor.
This is compounded by the lack of clear guiding significance for
clinical practice.
Cardiac color Doppler ultrasound is another
monitoring method that is commonly used for diagnosing
cardiotoxicity, where LVEF is the most commonly used parameter
(25). Markedly lower LVEF
frequently occurs in the latter stages of chemotherapy-induced
cardiotoxicity, indicating abnormalities in the cardiac structure
and function of the patient (26).
However, in the early stages of chemotherapy, patients typically
present with subclinical cardiotoxicity without any changes in the
cardiac structure and function (13,15).
Therefore, LVEF cannot be used as a parameter to observe whether
early cardiac damage has occurred in patients with tumors during
chemotherapy. Velocity vector imaging technology is an ultrasound
technology used to study the overall and local tissue movement of
the heart, which is based on dimensional grayscale imaging
(26). It avoids the angle
dependence of Doppler technology and can accurately perform
automatic eye tracking of myocardial movement, which provides a
novel method for evaluating ANT myocardial damage (26).
Serum biological indicators of myocardial injury,
including the myocardial enzyme cardiac troponin T (CTnT), are used
as detection indicators for the early diagnosis of cardiac damage
before any permanent and irreversible cardiac damage occurs
(14,21). The clinical significance of CTnT
and N-terminal precursor BNP (NT-pro-BNP) in the diagnosis of
cardiotoxicity after chemotherapy is controversial. Elevated CTnT
indicates myocardial cell damage, whereas elevated NT-pro-BNP
reflects increased myocardial stress. Previous studies have
confirmed its feasibility in predicting cardiotoxicity (21,27).
However, other previous studies have also found that CTnT and
NT-pro-BNP lacked specificity for monitoring early myocardial
damage for diagnosing ANT-induced cardiotoxicity (28,29).
Therefore, the clinical value of CTnT and NT-pro-BNP for monitoring
early cardiac damage remains questionable.
Strategies for preventing cardiotoxicity due to ANT
chemotherapy include limiting the cumulative dose of the drug,
changing the mode of administration and using cardioprotective
drugs. Von Hoff et al (16)
previously found that the effects of ANT chemotherapy drugs on the
heart mainly depend on the cumulative dose of ANT chemotherapy
drugs. Although the development and application of drugs such as
propofol, β-blockers have reduced the occurrence of cardiac damage,
dose-related cardiotoxicity is inevitable. Therefore, the
cumulative dose of the drug should be limited during treatment to
reduce the risk of cardiotoxicity. Changing the method of
administration can also effectively decrease the cardiac toxicity
of ANT. Previous meta-analysis have demonstrated that the
intravenous ANT injection method can reduce the incidence of heart
damage in adults compared with oral administration (30). In addition, lipid ANT drugs are
packed in lipids to protect the drugs from being degraded and lost
in plasma, thereby preventing cardiotoxicity by decreasing drug
uptake (31).
During treatment, cardiotoxicity can be reduced by
using cardioprotective drugs. At present, studies have demonstrated
that the addition of ANTs for patients with breast cancer using the
iron ion chelator dextropropanimine can reduce the cardiac adverse
reactions caused by chemotherapy drugs. However, the exact
mechanism remains unclear (32-34).
Janbabai et al (33)
previously found that for breast cancer patients receiving
chemotherapy with ANTs, oral administration of enalapril 7 days to
6 months before treatment could effectively preserve the systolic
and diastolic functions of the heart. In addition, Chotenimitkhun
et al (34) found that
chronic statin administration may attenuate early
anthracycline-associated declines in left ventricular ejection
function by studying clinical data of 51 patients with breast
cancer. These aforementioned studies therefore provide a basis for
clinical medication.
In conclusion, the treatment plan provided in the
present case was compared with the latest research. The judgment
for the present case was accurate. However, for the condition, only
symptomatic treatment could be provided, which cannot improve the
poor prognosis of the patient due to the significant enlargement of
the heart, it is difficult to completely reverse with drug
treatment. Furthermore, due to loss to follow-up, we are still
unable to ascertain whether the patient is currently alive.
Compared with delayed-onset cardiac toxicity, it is
rarer for patients with cancer to relapse after ~10 years of
long-term disease-free survival. Therefore, discussion remains
valuable for such a rare case. The present case is a reminder that
even if the heart injured by ANT toxicity does not develop clinical
symptoms immediately, they can manifest in the long-term.
Therefore, early monitoring is of great significance for the
diagnosis and treatment of cardiotoxicity. It is hoped that the
diagnosis and treatment of the present case may provide references
for the treatment of such patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XC and JL conceived and participated in the design
of the study. XC and JL confirm the authenticity of all the raw
data. MK wrote the manuscript and made significant contributions to
the conceptualization and design of the work. QP made substantial
contributions to analysis and interpretation of data, participated
in the clinical diagnosis and treatment of the patient and reviewed
the manuscript. YG and XT reviewed the manuscript and interpreted
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The Guiqian International General Hospital (GIGH)
Research Ethics Committee (Guiyang, China) confirmed that no
ethical approval was required.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of their data in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Røssevold AH, Andresen NK, Bjerre CA,
Gilje B, Jakobsen EH, Raj SX, Falk RS, Russnes HG, Jahr T,
Mathiesen RR, et al: Atezolizumab plus anthracycline-based
chemotherapy in metastatic triple-negative breast cancer: The
randomized, double-blind phase 2b ALICE trial. Nat Med.
28:2573–2583. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sun M, Shi W, Wu Y, He Z, Sun J, Cai S and
Luo Q: Immunogenic nanovesicle-tandem-augmented chemoimmunotherapy
via efficient cancer-homing delivery and optimized ordinal-interval
regime. Adv Sci (Weinh). 10(e2205247)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lefrak EA, Pitha J, Rosenheim S and
Gottlieb JA: A clinicopathologic analysis of adriamycin
cardiotoxicity. Cancer. 32:302–314. 1973.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nemmar A, Al-Salam S, Greish YE, Beegam S,
Zaaba NE and Ali BH: Impact of intratracheal administration of
polyethylene glycol-coated silver nanoparticles on the heart of
normotensive and hypertensive mice. Int J Mol Sci.
24(8890)2023.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dent SF, Botros J, Rushton M, Aseyev O,
Levine MN, Parulekar WR, O'Brien P, Burnell M, Pritchard KI, Chen
BE and Shepherd LE: Anthracycline-induced cardiotoxicity in
patients with early-stage breast cancer: The Canadian Cancer Trials
Group (CCTG) MA.21 experience. Breast Cancer Res Treat.
184:733–741. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Desai VG, Vijay V, Han T, Moland CL,
Phanavanh B, Lee T, Davis KJ, Muskhelishvili L, Stine KC and Fuscoe
JC: Doxorubicin-induced delayed-onset subclinical cardiotoxicity in
mice. J Appl Toxicol. 42:778–792. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Zhang Y, Dron JS, Bellows BK, Khera AV,
Liu J, Balte PP, Oelsner EC, Amr SS, Lebo MS, Nagy A, et al:
Association of severe hypercholesterolemia and familial
hypercholesterolemia genotype with risk of coronary heart disease.
Circulation. 147:1556–1559. 2023.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cosiano MF, Vista A, Sun JL, Alhanti B,
Harrington J, Butler J, Starling RC, Mentz RJ and Greene SJ:
Comparing New York Heart Association class and patient-reported
outcomes among patients hospitalized for heart failure. Circ Heart
Fail. 16(e010107)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Koch T, Lahu S, Coughlan JJ, Cassese S,
Voll F, Ndrepepa G, Menichelli M, Valina C, Hemetsberger R,
Witzenbichler B, et al: Association between platelet count and
treatment effect of ticagrelor or prasugrel in patients with acute
coronary Syndromes. Thromb Haemost. 123:464–477. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hwang IC, Choi D, Choi YJ, Ju L, Kim M,
Hong JE, Lee HJ, Yoon YE, Park JB, Lee SP, et al: Differential
diagnosis of common etiologies of left ventricular hypertrophy
using a hybrid CNN-LSTM model. Sci Rep. 12(20998)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Perera D, Clayton T, O'Kane PD, Greenwood
JP, Weerackody R, Ryan M, Morgan HP, Dodd M, Evans R, Canter R, et
al: Percutaneous revascularization for ischemic left ventricular
dysfunction. N Engl J Med. 387:1351–1360. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Abbasi J: Highlights From the American
College of Cardiology's scientific sessions-new heart failure
management guidelines, alirocumab after a myocardial infarction,
and treating mild chronic hypertension in pregnancy. JAMA.
327:1745–1747. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lima MAC, Brito HRA, Mitidieri GG, de
Souza EP, Sobral ACG, Melo HHMA, Vasconcelos GB, de Almeida BBD,
Figueiredo TAD, Filho MAAS, et al: Cardiotoxicity in cancer
patients treated with chemotherapy: A systematic review. Int J
Health Sci (Qassim). 16:39–46. 2022.PubMed/NCBI
|
|
14
|
Balmagambetova S, Tlegenova Z, Zholdin B,
Kurmanalina G, Talipova I, Koyshybaev A, Nurmanova D, Sultanbekova
G, Baspayeva M, Madinova S, et al: Early diagnosis of
chemotherapy-linked cardiotoxicity in breast cancer patients using
conventional biomarker panel: A prospective study protocol.
Diagnostics (Basel). 12(2714)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tran DB, AlAshi AK and Hernandez A:
Delayed onset anthracycline-associated cardiotoxicity presenting as
acute decompensated heart failure seven years after chemotherapy
completion. Cureus. 13(e16920)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Von Hoff DD, Layard MW, Basa P, Davis HL
Jr, Von Hoff AL, Rozencweig M and Muggia FM: Risk factors for
doxorubicin-induced congestive heart failure. Ann Intern Med.
91:710–717. 1979.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ma Jun, Qin Shukui and Shen Zhixiang:
Guidelines for the prevention and treatment of Anthracycline
cardiotoxicity (2013 edition). J Clin Oncol. 18:925–934. 2013.
|
|
18
|
Olson RD and Mushlin PS: Doxorubicin
cardiotoxicity: Analysis of prevailing hypotheses. FASEB J.
4:3076–3086. 1990.PubMed/NCBI
|
|
19
|
Negishi T, Thavendiranathan P, Penicka M,
Lemieux J, Murbraech K, Miyazaki S, Shirazi M, Santoro C, Cho GY,
Popescu BA, et al: Cardioprotection using strain-guided management
of potentially cardiotoxic cancer therapy: 3-year results of the
SUCCOUR Trial. JACC Cardiovasc Imaging. 16:269–278. 2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Stěrba M, Popelová O, Vávrová A, Jirkovský
E, Kovaříková P, Geršl V and Simůnek T: Oxidative stress, redox
signaling, and metal chelation in anthracycline cardiotoxicity and
pharmacological cardioprotection. Antioxid Redox Signal.
18:899–929. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Raj S, Franco VI and Lipshultz SE:
Anthracycline-induced cardiotoxicity: A review of pathophysiology,
diagnosis, and treatment. Curr Treat Options Cardiovasc Med.
16(315)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Awad HH, El-Derany MO, Mantawy EM, Michel
HE, El-Naa MM, Salah El-Din RA, El-Brairy AI and El-Demerdash E:
Comparative study on beneficial effects of vitamins B and D in
attenuating doxorubicin induced cardiotoxicity in rats: Emphasis on
calcium homeostasis. Biomed Pharmacother.
140(111679)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim SW, Ahn BY, Tran TTV, Pyun JH, Kang JS
and Leem YE: PRMT1 suppresses doxorubicin-induced cardiotoxicity by
inhibiting endoplasmic reticulum stress. Cell Signal.
98(110412)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Pokorná Z, Kollárová-Brázdová P,
Lenčová-Popelová O, Jirkovský E, Kubeš J, Mazurová Y, Adamcová M,
Holečková M, Palička V, Šimůnek T and Štěrba M: Primary prevention
of chronic anthracycline cardiotoxicity with ACE inhibitor is
temporarily effective in rabbits, but benefits wane in
post-treatment follow-up. Clin Sci (Lond). 136:139–161.
2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Venturelli F, Masetti R, Fabi M, Rondelli
R, Martoni A, Prete A, Bonvicini M and Pession A: Tissue Doppler
Imaging for anthracycline cardiotoxicity monitoring in pediatric
patients with cancer. Cardiooncology. 4(6)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sourdon J, Lager F, Viel T, Balvay D,
Moorhouse R, Bennana E, Renault G, Tharaux PL, Dhaun N and Tavitian
B: Cardiac metabolic deregulation induced by the tyrosine kinase
receptor inhibitor sunitinib is rescued by endothelin receptor
antagonism. Theranostics. 7:2757–2774. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nageeb MM, Saadawy SF and Attia SH: Breast
milk mesenchymal stem cells abate cisplatin-induced cardiotoxicity
in adult male albino rats via modulating the AMPK pathway. Sci Rep.
12(17554)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ruggiero A, De Rosa G, Rizzo D, Leo A,
Maurizi P, De Nisco A, Vendittelli F, Zuppi C, Mordente A and
Riccardi R: Myocardial performance index and biochemical markers
for early detection of doxorubicin-induced cardiotoxicity in
children with acute lymphoblastic leukaemia. Int J Clin Oncol.
18:927–933. 2013.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mavinkurve-Groothuis AM, Kapusta L, Nir A
and Groot-Loonen J: The role of biomarkers in the early detection
of anthracycline-induced cardiotoxicity in children: A review of
the literature. Pediatr Hematol Oncol. 25:655–664. 2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Guo Linjuan, Yu Jianhua, Li Juxiang, et
al: Meta-analysis of continuous slow intravenous infusion and
intravenous bolus administration reducing the risk of cardiac
toxicity of anthracycline drugs. Journal of Clinical Cardiology.
34–39. 2017.(In Chinese).
|
|
31
|
Fox CA and Ryan RO: Studies of the
cardiolipin interactome. Prog Lipid Res. 88(101195)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang Zhongtao, Fu Dayian and Wu Zhong:
Analysis of cardioprotective effect of low-dose carvedilol combined
with trimetazidine on breast cancer patients receiving
anthracycline-containing chemotherapy. Chin J Cancer Prev Treat.
43–45. 2020.
|
|
33
|
Janbabai G, Nabati M, Faghihinia M, Azizi
S, Borhani S and Yazdani J: Effect of enalapril on preventing
anthracycline-induced cardiomyopathy. Cardiovasc Toxicol.
17:130–139. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chotenimitkhun R, D'Agostino R Jr,
Lawrence JA, Hamilton CA, Jordan JH, Vasu S, Lash TL, Yeboah J,
Herrington DM and Hundley WG: Chronic statin administration may
attenuate early anthracycline-associated declines in left
ventricular ejection function. Can J Cardiol. 31:302–307.
2015.PubMed/NCBI View Article : Google Scholar
|