Introduction
Ovarian cancer (OC) ranks third in incidence among
female reproductive tract malignant tumors and it ranks first in
terms of its mortality rate. The onset of OC is insidious, with no
typical symptoms in the early stage, and most of the patients are
clinically diagnosed in the intermediate and late stages (1). According to global cancer statistics,
there were ~300,000 new cases of OC and >180,000 associated
deaths worldwide in 2020, with a mortality to morbidity ratio of
>0.6(2). At present, for
patients with OC, the main treatment options are total resection
without pathological staging or combined surgery to reduce the
total tumor and adjuvant chemotherapy after surgery. However, most
of these patients relapse after initial treatment and
platinum-sensitive patients may develop platinum-resistant
recurrent OC as the number of courses increases, with a poor
prognosis (3). In addition, there
are numerous types of drugs that may be used as second-line
chemotherapy for OC, but the efficacy of most of the treatments is
poor, and there is no uniform treatment plan for the scenario that
the tumor progresses again after second-line treatment (4). Therefore, it is urgent to find
effective drugs for the prevention and treatment of the metastasis
and drug-resistance of OC.
Nanomaterials are natural or artificial materials in
powder or clumps consisting of one or more basic particles with a
size ranging from 1-110 nm. They have the advantages of good
selectivity, low side effects and long-term stability (5). In recent years, nanomaterial-based
therapy has shown promise as an important strategy for the
treatment of tumors. Zinc oxide nanoparticles (ZnO-NPs) are common
engineering nanomaterials, which have exhibited great potential in
tumor treatment due to their biocompatibility, biodegradability and
unique physicochemical properties. A previous study has
demonstrated that ZnO-NPs may induce apoptosis of breast cancer
cells through a mitochondrial apoptotic pathway (6). In addition, ZnO-NPs have been
evidenced to possess significant antitumor activity in various
types of malignant tumor, including liver cancer, lung cancer,
breast cancer, colon cancer, osteosarcoma and cervical cancer
(7). Padmanabhan et al
(8) have confirmed that ZnO-NPs
may induce oxidative stress and proteotoxic stress in OC cells,
thus promoting the apoptosis of OC cells. Furthermore, the
combination of ZnO-NPs with cisplatin and gemcitabine may
significantly enhance the pro-apoptotic effect of cisplatin and
gemcitabine on non-small cell lung cancer (9). However, the mechanism of ZnO-NPs in
inhibiting the malignant progression and the chemotherapy
resistance of OC has remained to be elucidated.
As an important cell survival pathway, autophagy has
an important role in the occurrence and development of a variety of
diseases, including cancers (10-12).
A large number of studies have confirmed that autophagy has a key
function in controlling the tumor microenvironment and exerting
tumor-inhibitory effects (13,14).
By regulating autophagy, advances in biomaterials tailored for drug
delivery have the potential to overcome the limited selectivity and
side effects frequently associated with traditional therapeutic
agents in tumors (15). In
addition, Liu et al (16)
have confirmed that ZnO-NPs at non-cytotoxic concentrations can
promote autophagy, while ZnO-NPs at cytotoxic concentrations can
inhibit autophagy. However, the exact regulatory roles of ZnO-NPs
in autophagy in OC have remained elusive.
Endoplasmic reticulum stress (ERS) that results from
external stimuli or intracellular damage is often associated with
autophagy, hypoxia signaling or reactive oxygen species (ROS)
responses, and has a close relation with a variety of human
diseases, including malignant tumors (17). The relationship between ERS and
tumorigenesis has also been a hot topic in recent years. ERS may
not only induce apoptosis of tumor cells, but also promote cell
survival and lead to drug resistance of tumor cells (18). Therefore, an in-depth understanding
of the complexity of ERS may contribute to the discovery of novel
drug targets and therapeutic intervention strategies. A previous
study has found that ZnO-NPs have an important role in inducing ERS
(19). However, it has remained
elusive whether the effect of ZnO-NPs on ERS is associated with the
malignant proliferation and chemotherapy resistance of OC.
Therefore, the present study investigated the
mechanism of ZnO-NPs in the regulation of the malignant development
and chemotherapy resistance of OC, intending to provide novel
insight for the clinical use of ZnO-NPs as a treatment for OC.
Materials and methods
Cell culture
Human OC SKOV3 cells (cat. no. YS2383C; YaJi
Biological) and cisplatin (DDP)-resistant SKOV3/DDP cells (cat. no.
YS3657C; YaJi Biological) were cultured in RPMI-1640 medium (Gibco;
Thermo Fisher Scientific, Inc.) containing 10% fetal bovine serum
(FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37˚C with 5%
CO2. DDP (1 µg/ml; MedChemExpress) was added to the
medium of SKOV3/DDP cells to maintain the chemoresistance of cells.
ZnO-NPs were prepared as previously described (20). Different concentrations of ZnO-NPs
(1, 10, 20, 30, 40, 50, 60, 70 and 80 µg/ml) were used to treat
SKOV3 or SKOV3/DDP cells for 24 h. In order to further explore the
effects of ZnO-NPs on ERS and autophagy, ERS inhibitor
4-phenylbutyric acid (4-PBA; 7 mM) was used to pretreat SKOV3 or
SKOV3/DDP cells for 4 h (21) and
autophagy inhibitor 3-methyladenine (3-MA; 200 µM) was used to
pretreat SKOV3 or SKOV3/DDP cells for 1 h (22).
Cell counting kit-8 (CCK8) assay
The viability of cells in all groups was measured
using a CCK-8 kit (Nanjing Jiancheng Bioengineering Institute)
according to the manufacturer's instructions. At the end of the
incubation periods, 10 µl CCK-8 solution was added to each well and
cells were incubated for an additional 2 h. The absorbance was read
at an optical density of 450 nm using a microplate reader. The cell
viability was presented as a percentage of the control.
EdU staining assay
Following the indicated treatments, cells were
incubated with EdU (20 mmol/l) for 2 h. Subsequently, the cells
were fixed with 4% paraformaldehyde for 20 min at room temperature
according to the kit instructions (cat. no. ab219801; Abcam).
Subsequently, the cells were permeated with 0.5% Triton X-100 for
15 min at room temperature. After the addition of added Click
reaction solution, the cells were incubated in the dark for 30 min
at room temperature. Finally, images of the cells were acquired
under a fluorescence microscope.
Colony-formation assay
Following the indicated treatments, cells were
cultured in RPMI-1640 medium at 37˚C for 14 days. Subsequently, the
cells were fixed with 4% paraformaldehyde for 15 min at room
temperature and stained with 0.1% crystal violet for 15 min at room
temperature. The number of colonies (>50 cells) was counted
under a microscope.
Flow cytometric analysis
For apoptosis analysis, the Annexin V-FITC Apoptosis
Detection kit (Biobud Inc.) was used according to the
manufacturer's instructions. The cells were suspended in the
binding buffer and then stained with propidium iodide (PI) for 5
min and FITC-conjugated Annexin V for 15 min in the dark at 4˚C.
For cell cycle analysis, the Cell Cycle Detection Kit (Keygen
Biotech) was used according to the manufacturer's instructions. The
cells were fixed in 70% cold ethanol at 4˚C overnight. Following
centrifugation (450 x g at 4˚C and 5 min) and washing with PBS,
cells were then stained with 500 µl PI RNase at room temperature
for 30 min in the dark. Finally, the analysis of cell apoptosis and
cell cycle was performed with a flow cytometer (BD
LSRFortessa).
Western blot analysis
To obtain the total protein, cells with the
indicated treatments were lysed on ice for 30 min with RIPA lysis
buffer (Beyotime Institute of Biotechnology) and then centrifuged
at 24,080 x g for 10 min at 4˚C. The concentration of proteins was
measured by the BCA method (Bio-Rad Laboratories, Inc.). A total of
30 µg protein per lane was separated by 12% SDS-PAGE and then
transferred to PVDF membranes (iBlot PVDF Regular Stacks;
Invitrogen; Thermo Fisher Scientific, Inc.). After blocking with 5%
BSA (Invitrogen; Thermo Fisher Scientific, Inc.), the membranes
were probed with primary antibodies at 4˚C overnight and
subsequently incubated with secondary antibody (cat. no. ab6721;
1:5,000 dilution; Abcam) for 1 h on the next day. Finally, an
Enhanced ECL Chemiluminescent Substrate Kit (Yeasen Biotechnology
Co., Ltd.) was used to develop the protein bands and the signal
intensity was measured with ImageJ software (v1.53a; National
Institutes of Health). The following antibodies were used:
E-cadherin (1:1,000; cat. no. ab227639; Abcam), N-cadherin
(1:1,000; cat. no. ab76011; Abcam), Snail (1:1,000; cat. no.
ab216347; Abcam), phosphorylated-PKR-like endoplasmic reticulum
kinase (p-PERK; 1:1,000; cat. no. ab79483; Abcam), PERK (1:1,000;
cat. no. ab229912; Abcam), phosphorylated-eukaryotic translation
initiation factor 2α (p-eIF2α; 1:1,000; cat. no. ab32157; Abcam),
eIF2α (1:1,000; cat. no. ab26197; Abcam), activating transcription
factor 4 (ATF4; 1:1,000; cat. no. ab270980; Abcam), C/EBP
homologous protein (CHOP; 1:1,000; cat. no. ab11419; Abcam),
Caspase 12 (1:1,000; cat. no. ab62484; Abcam), LC3 (1:1,000; cat.
no. ab192890; Abcam), Beclin1 (1:1,000; cat. no. ab207612; Abcam),
p62 (1:1,000; cat. no. ab109012; Abcam) and
glyceraldehyde-3-phosphate dehydrogenase (1:1,000; cat. no. ab8245
or ab9485; Abcam).
Wound-healing assay
Cells were inoculated into a six-well plate at a
density of 1x105 cells/well. A pipette was used to
generate a straight line scratch in the center of the plate. After
a rinse with PBS, the cells were cultured in a serum-free medium
for 24 h. The width of the scratch was observed with a microscope
and measured by ImageJ software (v1.53a; National Institutes of
Health).
Transwell assay
The cells were planted in 24-well Transwell cell
culture chambers (8-µm pore size) pre-coated with or without
Matrigelâ basement membrane gel (Corning, Inc.). In the
lower chamber of a 24-well plate, 700 µl of medium containing 15%
FBS was added. After the routine culture of cells for 24 h, the
membranes were collected and then stained with 0.5% crystal violet
for 15 min at room temperature. At last, the cells were
photographed under a microscope and evaluated by ImageJ software
(v1.53a; National Institutes of Health).
Statistical analysis
All experiments were repeated in triplicate and the
data are presented as the mean ± standard deviation. Data of
multiple groups were compared by one-way analysis of variance
followed by Tukey's test with GraphPad Prism software 8.3.0
(GraphPad; Dotmatics).
Results
ZnO-NPs inhibit SKOV3 cell
proliferation, facilitate apoptosis and induce cell cycle
arrest
Following the treatment of SKOV3 cells with
ascending concentrations of ZnO-NPs, the cell viability and IC50
were detected by a CCK8 assay. The results indicated that the cell
activity was significantly decreased with the increase in the
concentration of ZnO-NPs, and the IC50 value of ZnO-NPs was 20.24
µg/ml (Fig. 1A). According to this
IC50 value, ZnO-NPs at concentrations of 10, 20 and 30 µg/ml were
selected for the subsequent experiments. EdU staining and
colony-formation assays were applied for the detection of cell
proliferation and the results showed that the cell proliferation
ability was significantly decreased with increasing concentrations
of ZnO-NPs compared to the control group (Fig. 1B and C). Subsequently, flow cytometry was used
to detect apoptosis and cell cycle, and the results showed that,
compared to the control group, cell apoptosis was significantly
increased and cell cycle arrest was noticed in the ZnO-NPs group
(Fig. 1D and E).
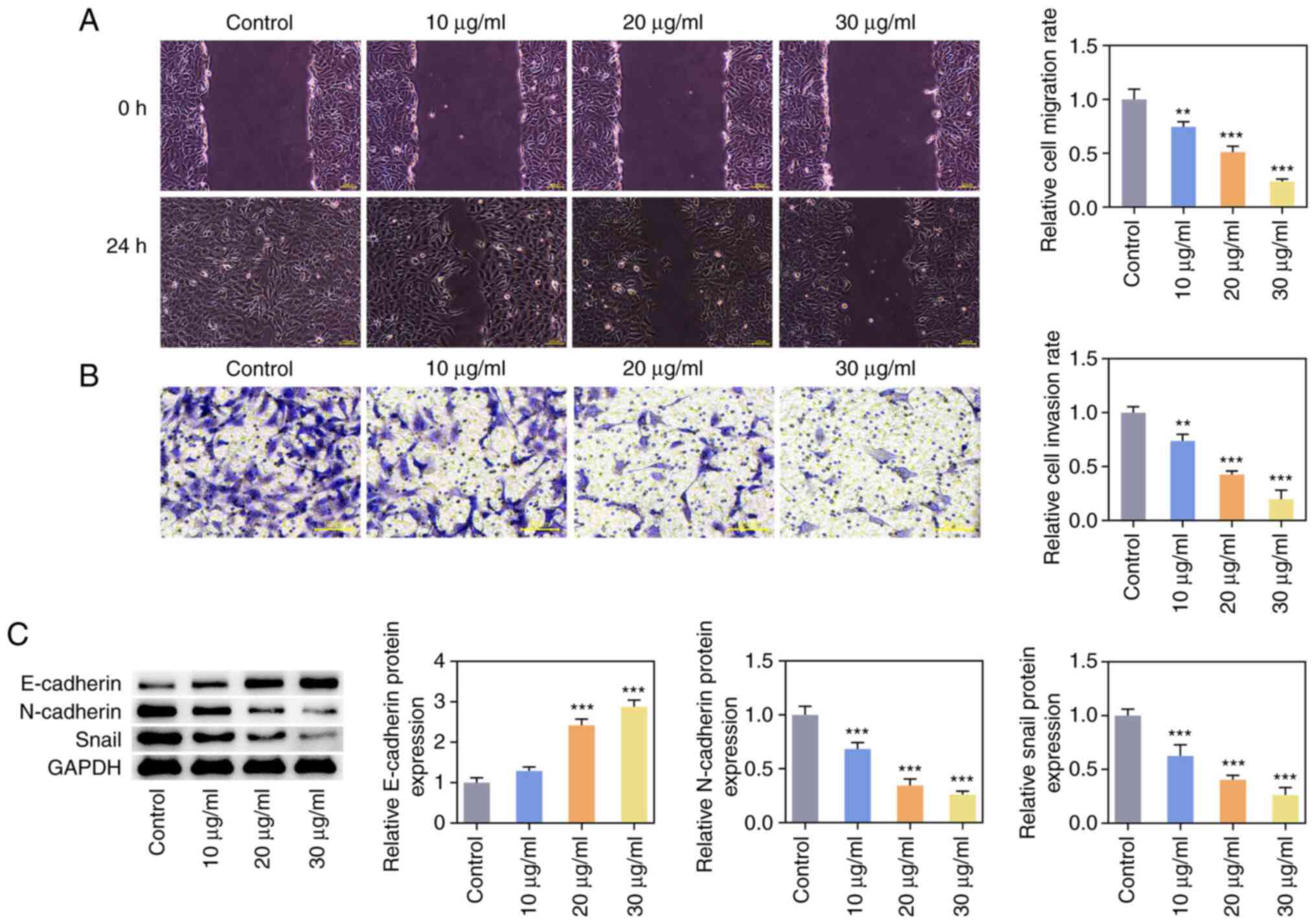
ZnO-NPs inhibit invasion, migration
and epithelial to mesenchymal transition (EMT) of SKOV3 cells
Wound-healing and Transwell assays indicated that
ZnO-NPs inhibited the migration and invasion of OC cells in a
concentration-dependent manner (Fig.
2A and B). Western blot
analysis was used to detect the expression of EMT-related proteins
and it was found that the expression of E-cadherin was increased,
while the expression of N-cadherin and Snail was decreased in the
ZnO-NPs group compared with that in the control group (Fig. 2C).
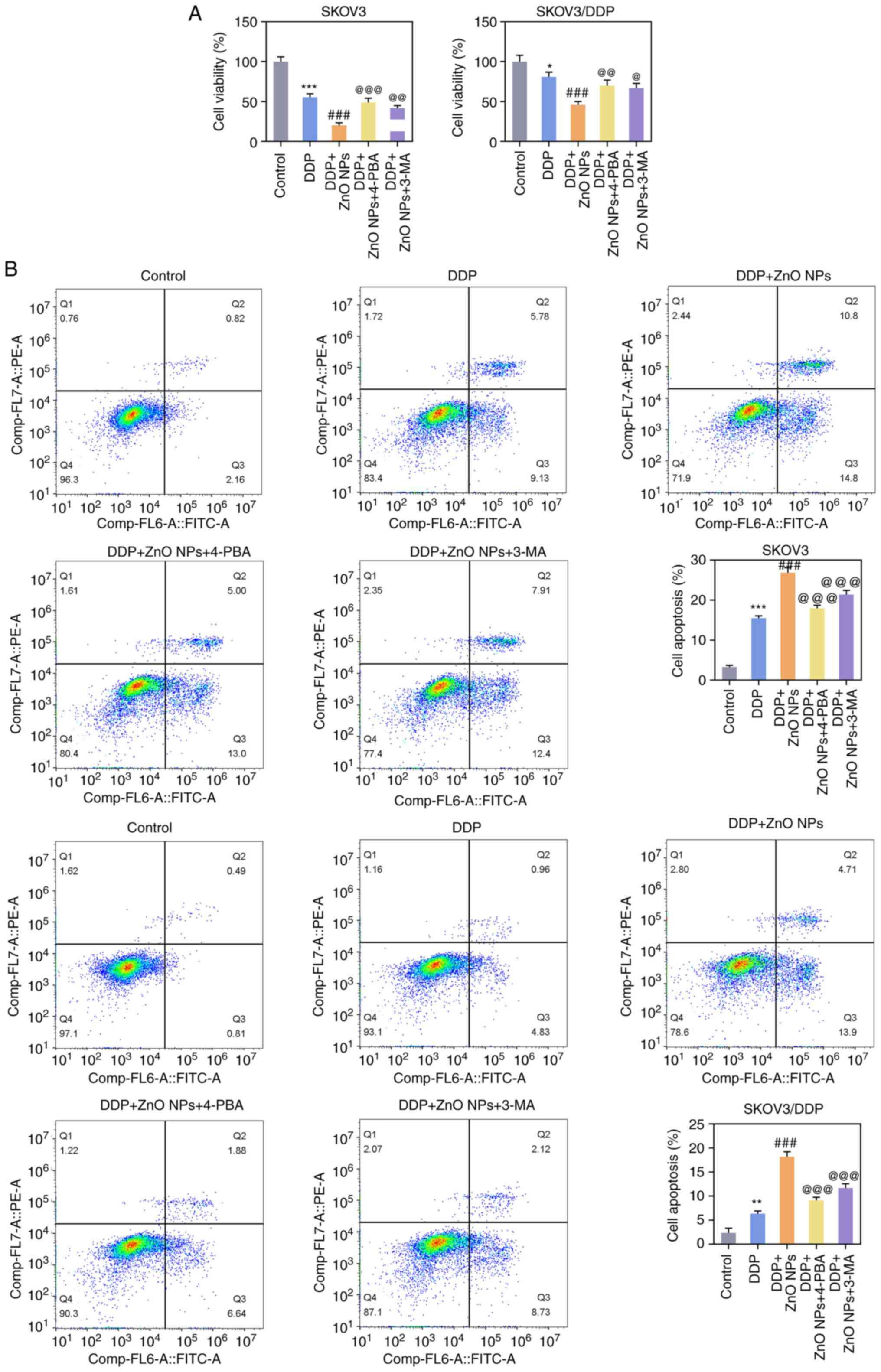
ZnO-NPs inhibit chemotherapy
resistance of SKOV3 cells
A CCK8 assay was used to detect the effect of DDP on
the viability of SKOV3 cells treated by different concentrations of
ZnO-NPs and the IC50 was calculated. The results indicated that the
viability of SKOV3 and SKOV3/DDP cells was significantly decreased
with the increase in the concentration of ZnO-NPs. The IC50 value
of ZnO-NP-treated SKOV3 cells exposed to DDP was 10.27 µM and the
IC50 value of ZnO-NP-treated SKOV3/DDP cells exposed to DDP was
54.57 µM (Fig. 3A). According to
these results, 10 µM DDP was chosen to induce SKOV3 cells and
SKOV3/DDP cells. SKOV3 or SKOV3/DDP cells were divided into
control, DDP, DDP + 10 µg/ml ZnO-NP, DDP + 20 µg/ml ZnO-NP and DDP
+ 30 µg/ml ZnO-NP groups. Cell viability was measured with the CCK8
and the results suggested that SKOV3-cell viability was decreased
to 55% and SKOV3/DDP-cell viability was decreased to 80% after DDP
treatment. Compared with the DDP group, ZnO-NP treatment further
inhibited the viability of SKOV3 and SKOV3/DDP cells (Fig. 3B). The flow cytometry results
showed that the apoptosis of SKOV3 and SKOV3/DDP cells was
significantly increased in the DDP group compared with the control
group. Compared with the DDP group, the apoptosis of SKOV3 and
SKOV3/DDP cells was further increased after the administration of
ZnO-NPs (Fig. 3C). When SKOV3
cells and SKOV3/DDP cells were treated with the same dose of DDP,
the activity of the resistant cell line remained much higher than
that of the native cancer cell line, which demonstrated the drug
resistance of SKOV3/DDP cells (or reduced sensitivity of SKOV3/DDP
cells to DDP). The activity of the SKOV3/DDP resistant cell line
decreased significantly after ZnO-NPs treatment, indicating that
ZnO-NPs reduced the drug resistance of SKOV3/DDP cells (or the
sensitivity of SKOV3/DDP cells to DDP increased after ZnO-NP
treatment).
ZnO-NPs activate ERS and promote
autophagy
Western blot analysis was used to detect the
expression of ERS-related proteins and the results showed that
p-PERK, p-eIF2α, ATF4, CHOP and Caspase 12 expression were
increased by ZnO-NPs treatment in a concentration-dependent manner
compared to the control group (Fig.
4A). Western blot analysis was also employed to detect the
expression of autophagy-related proteins. It was found that ZnO-NPs
concentration-dependently increased LC3II/I and Beclin-1 expression
and decreased P62 expression compared to the control group
(Fig. 4B). Since 30 µg/ml ZnO-NPs
had the most significant effect, this specific concentration was
selected for subsequent experiments.
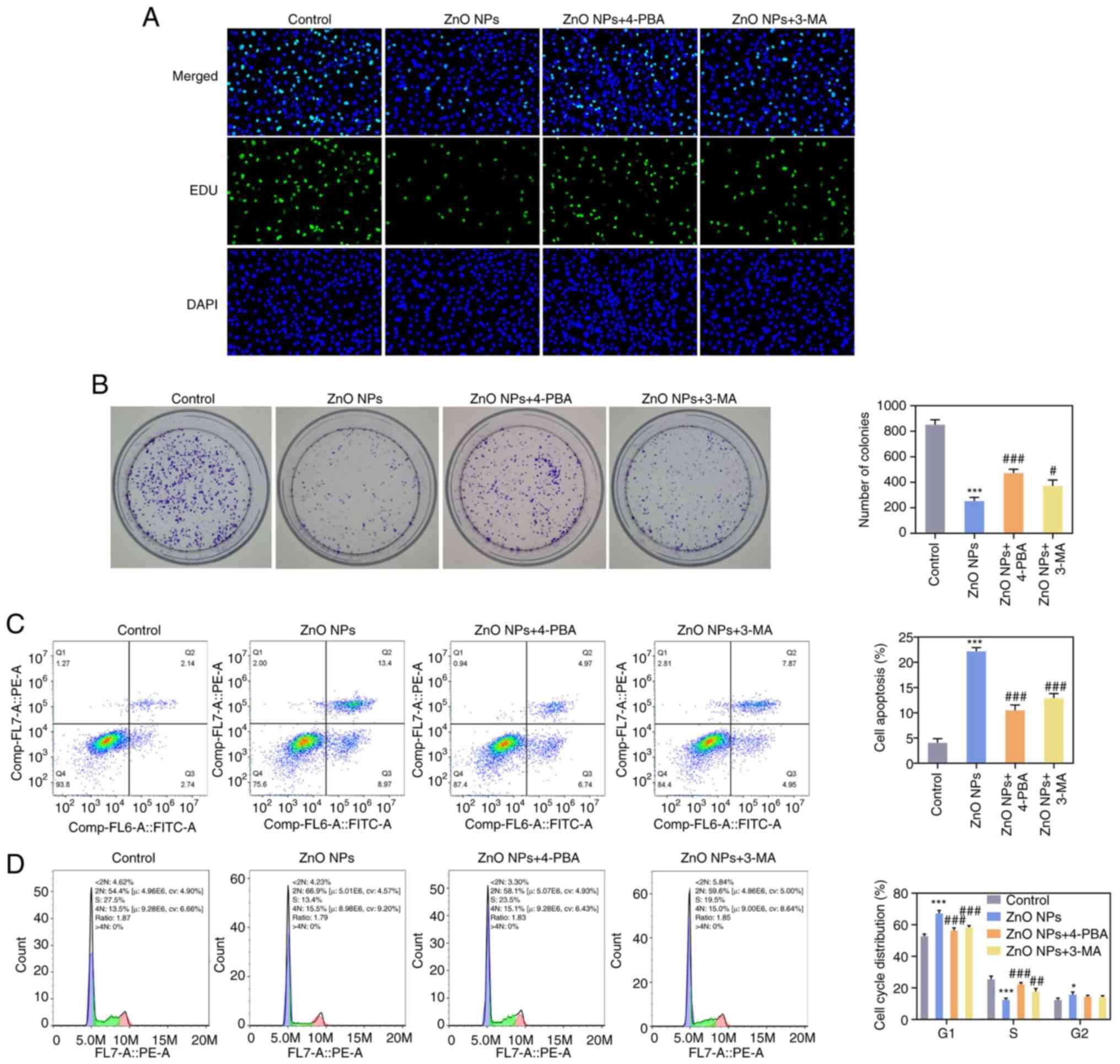
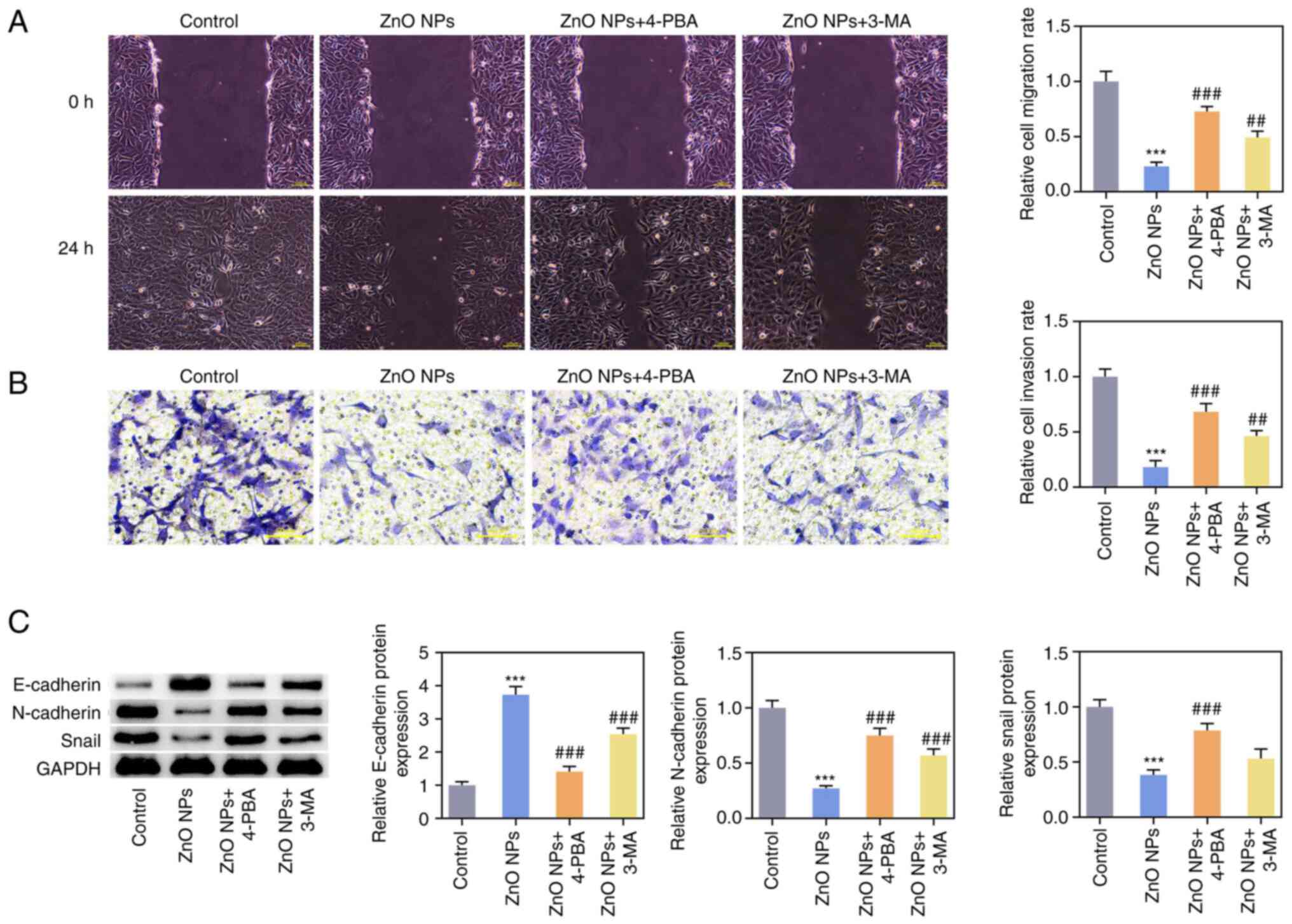
ZnO-NPs inhibit malignant progression
and chemotherapy resistance of SKOV3 cells by activating ERS and
promoting autophagy
Subsequently, in order to further explore the
effects of ZnO-NPs on ERS and autophagy, the ERS inhibitor 4-PBA
and autophagy inhibitor 3-MA were respectively applied to the
cells. The cells were divided into the control, ZnO-NPs, ZnO-NPs +
4-PBA and ZnO-NPs + 3-MA groups. EdU staining and colony-formation
assays indicated that the cell proliferation capacity in the
ZnO-NPs + 4-PBA and ZnO-NPs + 3-MA groups was significantly
increased compared with that in the ZnO-NPs group (Fig. 5A and B). Flow cytometry suggested that,
compared with the ZnO-NPs group, cell apoptosis in the ZnO-NPs +
4-PBA and ZnO-NPs + 3-MA groups was significantly decreased and
cell cycle arrest was reduced (Fig.
5C and D). Results from the
wound-healing and Transwell assays showed that the invasion and
migration abilities in the ZnO-NPs + 4-PBA and ZnO-NPs + 3-MA
groups were significantly increased compared with those in the
ZnO-NPs group (Fig. 6A and
B). Compared with the ZnO-NPs
group, the expression of E-cadherin was decreased and the
expression of N-cadherin and Snail were increased in the ZnO-NPs +
4-PBA and ZnO-NPs + 3-MA groups (Fig.
6C). In terms of chemotherapy resistance, SKOV3 or SKOV3/DDP
cells were divided into the control, DDP, DDP + ZnO-NPs, DDP +
ZnO-NPs + 4-PBA and DDP + ZnO-NPs + 3 MA groups. It was found that,
compared with the DDP + ZnO-NPs group, the cell viability in the
DDP + ZnO-NPs + 4-PBA and DDP + ZnO-NPs + 3-MA groups was
significantly increased and cell apoptosis was significantly
decreased (Fig. 7A and B).
Discussion
Currently, the main treatment methods for OC are
tumor cell reduction and postoperative platinum-based chemotherapy.
Although the clinical remission rate of OC has reached 60-80% due
to the application of initial surgery and chemotherapy, most
patients will relapse and develop drug resistance, which may result
in a high propensity for tumor invasion and metastasis. Patients
with advanced OC often succumb to distant metastasis after platinum
resistance and the 5-year overall survival rate is only ~30%
(23-25).
Therefore, it is urgent to further study the mechanism of
chemotherapy resistance of OC and to find relevant targets or drugs
that may effectively inhibit chemotherapy resistance of OC.
ZnO-NPs as nanomaterials can effectively improve the
stability of clinical drugs and improve patients' drug absorption
capacity. ZnO-NPs have an important antitumor role in a variety of
cancer types. A study has shown that ZnO-NP treatment may lead to
prostate cancer cell apoptosis and death (26). ZnO-NPs stimulate oxidative stress
to induce melanoma-like skin lesions and apoptosis of melanoma
cells in mice with epidermal barrier dysfunction through the
activation of the NF-κB pathway (27,28).
A recent study indicated that ZnO-NPs can promote OC cell death,
thus inhibiting the progression of OC (29). In the present study, it was
confirmed that ZnO-NPs were able to significantly inhibit the
activity of SKOV3 cells, inhibit cell invasion and migration, as
well as induce apoptosis and cell cycle arrest in OC. A study by
Bai et al (30) showed that
ZnO-NPs may induce apoptosis and autophagy of SKOV3 cells,
indicating that ZnO-NPs have a certain inhibitory effect on the
proliferation of OC cells, which is consistent with the
experimental results of the present study. Furthermore, a previous
study by our group has shown that ZnO-NPs are able to inhibit the
proliferation and apoptosis of SKOV3 cells (20). For this reason, SKOV3 cells were
also used in the present study. In addition, ZnO-NPs were indicated
to inhibit the EMT and chemotherapy resistance of SKOV3 cells.
The ER is the main site for lipid synthesis, protein
function and calcium ion metabolism (31). Multiple studies have confirmed that
ERS is involved in the regulation of malignant tumor proliferation
and chemotherapy resistance (17,32).
Saikosaponin A can induce cervical cancer cell apoptosis through
upregulating G-protein coupled receptor 78, CHOP and caspase-12
expression to activate ERS-dependent pathways (33). Shikotin can promote the resistance
of colorectal cancer cells to 5-fluorouracil by activating ERS
(34). It has been reported that
ERS induces apoptosis of OC cells (35,36).
Therefore, exploring the regulation of ERS on OC cells is also a
strategy for the treatment of OC. It has been found that ZnO-NPs
have an important role in inducing ERS (19). However, the regulatory effect of
ZnO-NPs on ERS in OC has not been reported so far, to the best of
our knowledge. The present results showed that ZnO-NPs were able to
induce ERS, thereby inhibiting the malignant progression and
chemotherapy resistance of OC cells. Fang et al (37) found that ERS inhibitor
tauroursodeoxycholic acid can reduce PERK expression and increase
the production of autophagy-related protein LC3-II to restore
autophagy after injury, thus reducing kidney injury in mice.
Lipopolysaccharide stimulation can activate ERS and inhibit
autophagy, while spermidine can reduce acute lung injury and
inflammation induced by lipopolysaccharide by inhibiting ERS and
activating autophagy (38). In
addition, alpha-tomatine can inhibit the proliferation of OC cells
and exert a pro-apoptotic role by inhibiting autophagy (39). Zhou et al (40) confirmed that microRNA-133a is able
to reduce cisplatin resistance of OC cells by downregulating the
expression of Yamaguchi sarcoma viral homolog 1, thereby inhibiting
autophagy. Furthermore, p53 and LC3 expression are upregulated in
ZnO-NP-treated cells, indicating that ZnO-NPs can promote apoptosis
and induce autophagy, and ZnO-NPs induced significant cytotoxicity,
apoptosis and autophagy in human OC cells through the production of
ROS and oxidative stress (41). In
the present study, it was found that ZnO-NPs promoted autophagy and
inhibited malignant progression and chemotherapy resistance of OC
cells by activating ERS.
Of note, the present study has certain limitations.
First, the experiment was conducted in only one OC cell line,
SKOV3, in future subsequent experiments, the present findings will
be verified using other OC cell lines. In addition, the regulatory
relationship between ERS and autophagy under ZnO-NPs regulation in
OC was not further investigated, which will be the focus of future
research by our group.
In conclusion, ZnO-NPs may inhibit malignant
progression and chemotherapy resistance of OC cells by activating
ERS and promoting autophagy.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Ningxia Natural
Science Foundation project (grant no. 2023AAC03534).
Availability of data and materials
The datasets generated and/or analyzed during the
present study are available from the corresponding author on
reasonable request.
Authors' contributions
CY and WG designed and conceived the study and
selected the experiments. WG performed the experiments. WG wrote
the manuscript. CY processed the experimental data. CY and WG
confirm the authenticity of all the raw data. Both authors have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Penny SM: Ovarian Cancer: An Overview.
Radiol Technol. 91:561–575. 2020.PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Orr B and Edwards RP: Diagnosis and
treatment of ovarian cancer. Hematol Oncol Clin North Am.
32:943–964. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ray-Coquard I, Mirza MR, Pignata S,
Walther A, Romero I and du*Bois A: Therapeutic options following
second-line platinum-based chemotherapy in patients with recurrent
ovarian cancer: Comparison of active surveillance and maintenance
treatment. Cancer Treat Rev. 90(102107)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Mabrouk M, Das DB, Salem ZA and Beherei
HH: Nanomaterials for biomedical applications: Production,
characterisations, recent trends and difficulties. Molecules.
26(1077)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yan M and Majd MH: Evaluation of induced
apoptosis by biosynthesized zinc oxide nanoparticles in MCF-7
breast cancer cells using Bak1 and Bclx expression. Dokl Biochem
Biophys. 500:360–367. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Anjum S, Hashim M, Malik SA, Khan M,
Lorenzo JM, Abbasi BH and Hano C: Recent advances in zinc oxide
nanoparticles (ZnO NPs) for cancer diagnosis, target drug delivery,
and treatment. Cancers (Basel). 13(4570)2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Padmanabhan A, Kaushik M, Niranjan R,
Richards JS, Ebright B and Venkatasubbu GD: Zinc Oxide
nanoparticles induce oxidative and proteotoxic stress in ovarian
cancer cells and trigger apoptosis Independent of p53-mutation
status. Appl Surf Sci. 487:807–818. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Hu C and Du W: Zinc oxide nanoparticles
(ZnO NPs) combined with cisplatin and gemcitabine inhibits tumor
activity of NSCLC cells. Aging (Albany NY). 12:25767–25777.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Sehrawat A, Mishra J, Mastana SS, Navik U,
Bhatti GK, Reddy PH and Bhatti JS: Dysregulated autophagy: A key
player in the pathophysiology of type 2 diabetes and its
complications. Biochim Biophys Acta Mol Basis Dis.
1869(166666)2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wilson N, Kataura T, Korsgen ME, Sun C,
Sarkar S and Korolchuk VI: The autophagy-NAD axis in longevity and
disease. Trends Cell Biol. 33:788–802. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Onorati AV, Dyczynski M, Ojha R and
Amaravadi RK: Targeting autophagy in cancer. Cancer. 124:3307–3318.
2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jain V, Singh MP and Amaravadi RK: Recent
advances in targeting autophagy in cancer. Trends Pharmacol Sci.
44:290–302. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Debnath J, Gammoh N and Ryan KM: Autophagy
and autophagy-related pathways in cancer. Nat Rev Mol Cell Biol.
24:560–575. 2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Lopez-Mendez TB, Sánchez-Álvarez M,
Trionfetti F, Pedraz JL, Tripodi M, Cordani M, Strippoli R and
González-Valdivieso J: Nanomedicine for autophagy modulation in
cancer therapy: A clinical perspective. Cell Biosci.
13(44)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu L, Wang J, Zhang J, Huang C, Yang Z
and Cao Y: The cytotoxicity of zinc oxide nanoparticles to 3D brain
organoids results from excessive intracellular zinc ions and
defective autophagy. Cell Biol Toxicol. 39:259–275. 2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Cubillos-Ruiz JR, Bettigole SE and
Glimcher LH: Tumorigenic and immunosuppressive effects of
endoplasmic reticulum stress in cancer. Cell. 168:692–706.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sisinni L, Pietrafesa M, Lepore S,
Maddalena F, Condelli V, Esposito F and Landriscina M: Endoplasmic
reticulum stress and unfolded protein response in breast cancer:
The balance between apoptosis and autophagy and its role in drug
resistance. Int J Mol Sci. 20(857)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Luo Y, Wu C, Liu L, Gong Y, Peng S, Xie Y
and Cao Y: 3-Hydroxyflavone enhances the toxicity of ZnO
nanoparticles in vitro. J Appl Toxicol. 38:1206–1214.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Gu WL, Li H, Niu X and Zhou J: Biological
fabrication of zinc oxide nanoparticles from Nepeta cataria
potentially produces apoptosis through inhibition of proliferative
markers in ovarian cancer. Green Process Synth. 11:316–326.
2022.
|
|
21
|
Ma YY, Di ZM, Cao Q, Xu WS, Bi SX, Yu JS,
Shen YJ, Yu YQ, Shen YX and Feng LJ: Xanthatin induces glioma cell
apoptosis and inhibits tumor growth via activating endoplasmic
reticulum stress-dependent CHOP pathway. Acta Pharmacol Sin.
41:404–414. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang Q, Wang X, Cao S, Sun Y, He X, Jiang
B, Yu Y, Duan J, Qiu F and Kang N: Berberine represses human
gastric cancer cell growth in vitro and in vivo by inducing
cytostatic autophagy via inhibition of MAPK/mTOR/p70S6K and Akt
signaling pathways. Biomed Pharmacother. 128(110245)2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Torre LA, Trabert B, DeSantis CE, Miller
KD, Samimi G, Runowicz CD, Gaudet MM, Jemal A and Siegel RL:
Ovarian cancer statistics, 2018. CA Cancer J Clin. 68:284–296.
2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kathawala RJ, Kudelka A and Rigas B: The
chemoprevention of ovarian cancer: The need and the options. Curr
Pharmacol Rep. 4:250–260. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Giornelli GH: Management of relapsed
ovarian cancer: A review. Springerplus. 5(1197)2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Virych PA, Zadvorniy TV, Borikun TV,
Lykhova OO, Chumachenko VA, Virych PA, Pavlenko VA, Kutsevol NV and
Lukianova NY: Effects of dextran-graft-polyacrylamide/ZnO
nanoparticles on prostate cancer cell lines in vitro. Exp Oncol.
44:217–221. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang P, Hu G, Zhao W, Du J, You M, Xv M,
Yang H, Zhang M, Yan F, Huang M, et al: Continuous ZnO nanoparticle
exposure induces melanoma-like skin lesions in epidermal barrier
dysfunction model mice through anti-apoptotic effects mediated by
the oxidative stress-activated NF-κB pathway. J Nanobiotechnology.
20(111)2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Fan P, Yang C, Wang L, Wang Q, Zhang Y,
Zhou J, Weng J and Feng B: ZnO nanoparticles stimulate oxidative
stress to induce apoptosis of B16F10 melanoma cells:In vitroandin
vivostudies. Biomed Phys Eng Express. 7:2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Khan MS, Altwaijry N, Jabir NR, Alamri AM,
Tarique M and Khan AU: Potential of green-synthesized ZnO-NPs
against human ovarian teratocarcinoma: An in vitro study. Mol Biol
Rep. 50:4447–4457. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bai DP, Zhang XF, Zhang GL, Huang YF and
Gurunathan S: Zinc oxide nanoparticles induce apoptosis and
autophagy in human ovarian cancer cells. Int J Nanomedicine.
12:6521–6535. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Saraswat Ohri S, Mullins A, Hetman M and
Whittemore SR: Activating transcription factor-6α deletion
modulates the endoplasmic reticulum stress response after spinal
cord injury but does not affect locomotor recovery. J Neurotrauma.
35:486–491. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Bahar E, Kim JY and Yoon H: Chemotherapy
resistance explained through endoplasmic reticulum stress-dependent
signaling. Cancers (Basel). 11(338)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Du J, Song D, Cao T, Li Y, Liu J, Li B and
Li L: Saikosaponin-A induces apoptosis of cervical cancer through
mitochondria- and endoplasmic reticulum stress-dependent pathway in
vitro and in vivo: Involvement of PI3K/AKT signaling pathway. Cell
Cycle. 20:2221–2232. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Piao MJ, Han X, Kang KA, Fernando PDSM,
Herath HMUL and Hyun JW: The endoplasmic reticulum stress response
mediates shikonin-induced apoptosis of 5-fluorouracil-resistant
colorectal cancer cells. Biomol Ther (Seoul). 30:265–273.
2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Rezghi Barez S, Movahedian Attar A and
Aghaei M: MicroRNA-30c-2-3p regulates ER stress and induces
apoptosis in ovarian cancer cells underlying ER stress. EXCLI J.
20:922–934. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Xu J, Bi G, Luo Q, Liu Y, Liu T, Li L,
Zeng Q, Wang Q, Wang Y, Yu J and Yi P: PHLDA1 Modulates the
endoplasmic reticulum stress response and is required for
resistance to oxidative stress-induced cell death in human ovarian
cancer cells. J Cancer. 12:5486–5493. 2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Fang L, Zhou Y, Cao H, Wen P, Jiang L, He
W, Dai C and Yang J: Autophagy attenuates diabetic glomerular
damage through protection of hyperglycemia-induced podocyte injury.
PLoS One. 8(e60546)2013.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Baek AR, Hong J, Song KS, Jang AS, Kim DJ,
Chin SS and Park SW: Spermidine attenuates bleomycin-induced lung
fibrosis by inducing autophagy and inhibiting endoplasmic reticulum
stress (ERS)-induced cell death in mice. Exp Mol Med. 52:2034–2045.
2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wu H, Li W, Wang T, Rong Y, He Z, Huang S,
Zhang L, Wu Z and Liu C: α-Tomatine, a novel early-stage autophagy
inhibitor, inhibits autophagy to enhance apoptosis via Beclin-1 in
Skov3 cells. Fitoterapia. 152(104911)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Zhou Y, Wang C, Ding J, Chen Y, Sun Y and
Cheng Z: miR-133a targets YES1 to reduce cisplatin resistance in
ovarian cancer by regulating cell autophagy. Cancer Cell Int.
22(15)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Hollister R: Critical incident stress
debriefing and the community health nurse. J Community Health Nurs.
13:43–49. 1996.PubMed/NCBI View Article : Google Scholar
|