Introduction
Patients with neurosyphilis often present with a
wide range of neurological symptoms. For this reason, neurosyphilis
is commonly referred to as the great imitator. In rare cases,
neurosyphilis can be the primary manifestation of dyskinesia; the
most common dyskinesia is Parkinson's syndrome, with others
existing such as dystonia, ataxia, myoclonus and chorea (1). Orofacial dyskinesia caused by
neurosyphilis and characterized by continuous, slow, and regular
rhythmic contraction of masticatory muscles is referred to as the
‘Candy sign’ (2). Although the
disease is generally rarely reported in literature, neurosyphilis
with oral-facial-lingual dyskinesia is even rarer, leaving
clinicians with insufficient knowledge about it and the potential
for misdiagnosis. The present study reports a case of neurosyphilis
with oral-facial-lingual dyskinesia as the main symptom, therefore
raising awareness of the disease and reducing the risk of
misdiagnosis
Case report
A 52-year-old male patient was admitted to the
Department of Neurology of the Xiangya Hospital of Central South
University (Changsha, China) in May 2020 due to dysarthria for 2
months and severe involuntary oral-facial-lingual movements for one
month. At 2 months prior to admission, the patient developed
dysarthria without other symptoms such as dysphagia, a choking
cough due to drinking water, dizziness, headaches, nausea,
vomiting, limb hemiplegia, or numbness. The patient was diagnosed
with acute cerebral infarction in the First Hospital of Changsha
(Changsha, China) and was prescribed aspirin enteric-coated tablets
100 mg and atorvastatin calcium 20 mg once a night for 1 month.
However, these treatments failed to improve his dysarthria.
Unfortunately, 1 month later, the dysarthria worsened and the
patient experienced persistent orofacial and lingual involuntary
movements, as well as pain in both sides of the lower jaw.
Occasionally, a choking cough occurred when drinking water. The
patient refused to undergo polysomnography procedure, but clinical
observation suggested the recurrence of dyskinesias only during
wakefulness, but not any stage of sleep. There was a prior history
of hypertension and a history of ‘penicillin’ allergy. The patient
had no history of tremors, jerks, intravenous drug abuse, toxin
exposure, or head injury. None of the family members had a known
history of tremors, Parkinsonism, memory disturbance, psychiatric
disorders, and venereal diseases. A physical examination performed
on admission revealed a heart rate of 86 beats/min (normal range,
60-100 beats/min) and a blood pressure of 142/86 mmHg (normal
range, 90-120/60-80 mmHg). Moreover, the patient was conscious,
alert, and had dysarthria. There was no neck rigidity associated
with meningitis, and all the cranial nerves were normal on
examination. The patient had a light and accommodation reflex.
Examination of the motor system revealed involuntary movements in
the form of frequent opening and closing of the mouth, continuous
perioral movement (chewing movements), and protrusion of the
tongue. All the deep tendon reflexes were well stimulated and the
plantar showed flexor responses on both sides. No cerebellar or
sensory deficits were found and his gait was grossly normal.
Laboratory test results
Erythrocyte sedimentation rate of 35 mm/h (normal
range, 0-10 mm/h), fasting blood glucose of 5.1 mmol/l (normal
range, 3.9-6.1 mmol/l), glycosylated hemoglobin of 5.0% (normal
range, 4.0-6.0%), and tumor markers were normal. Acanthocytes, HIV,
hepatitis B, and hepatitis C were negative. The serum
paraneoplastic anti-Hu, anti-Yo, anti-Ri, anti-Ma2/Ta,
anti-CV2/CRMP5, anti-Amphiphysin antibodies, NMDAR, LGl1, GABABR,
CASPR2 antibodies in the blood and CSF were negative using the
indirect immunofluorescence method of cell-based assay (CBA). The
patient had a positive rapid plasma reagin (RPR) test with a serum
titer of 1:16 and a positive Treponema pallidum
hemagglutination assay (TPHA). Moreover, lumbar puncture pressure
was 150 mmH20 (80-180 mmH20), the total number of cells in the CSF
was 6(≤10x106 cells/l), protein concentration was 1.01
g/l (0.15-0.45 g/l), the patient had a positive RPR with a CSF
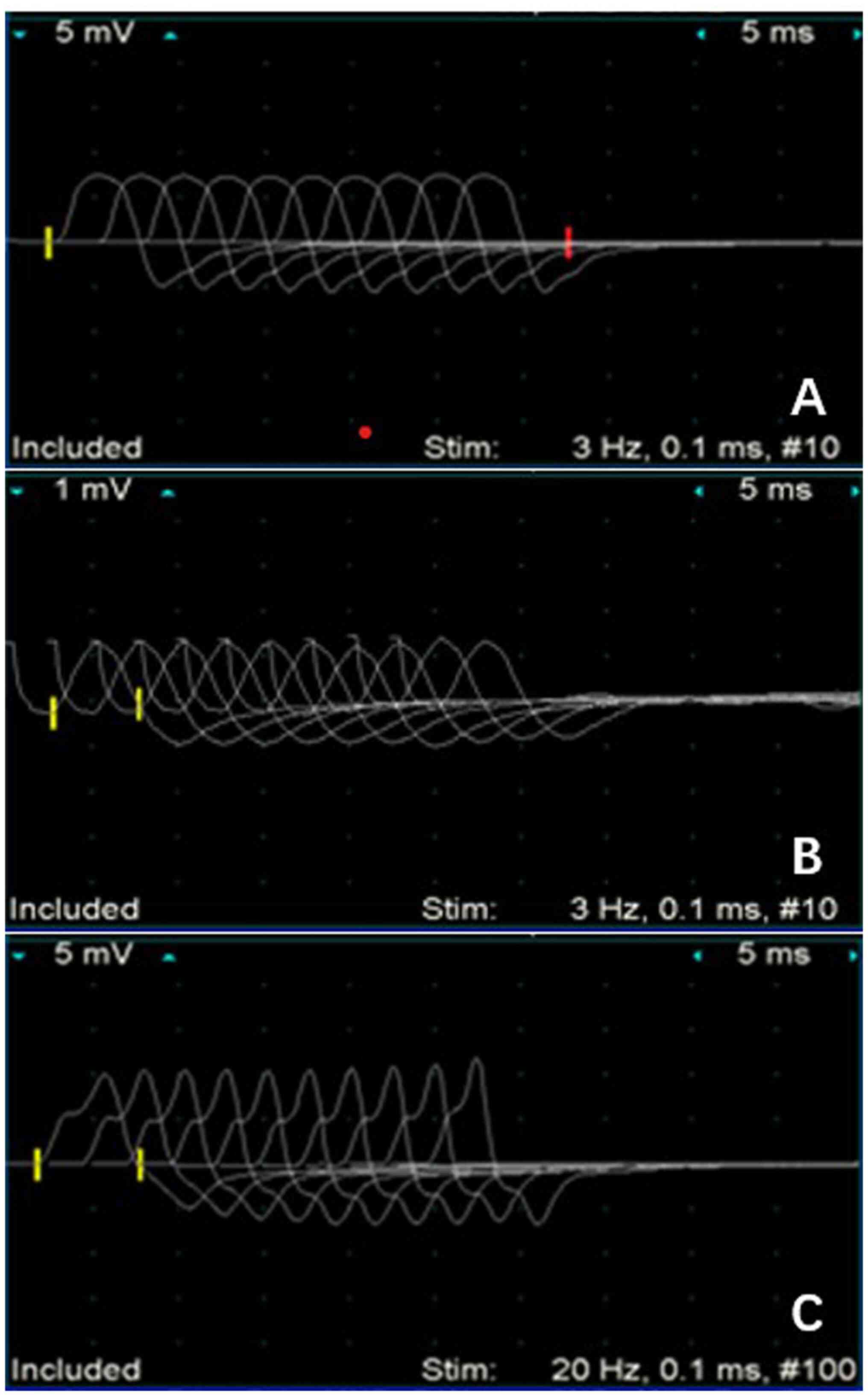
titer of 1:4 and the CSF a positive TPHA (Table I). The findings of electromyogram
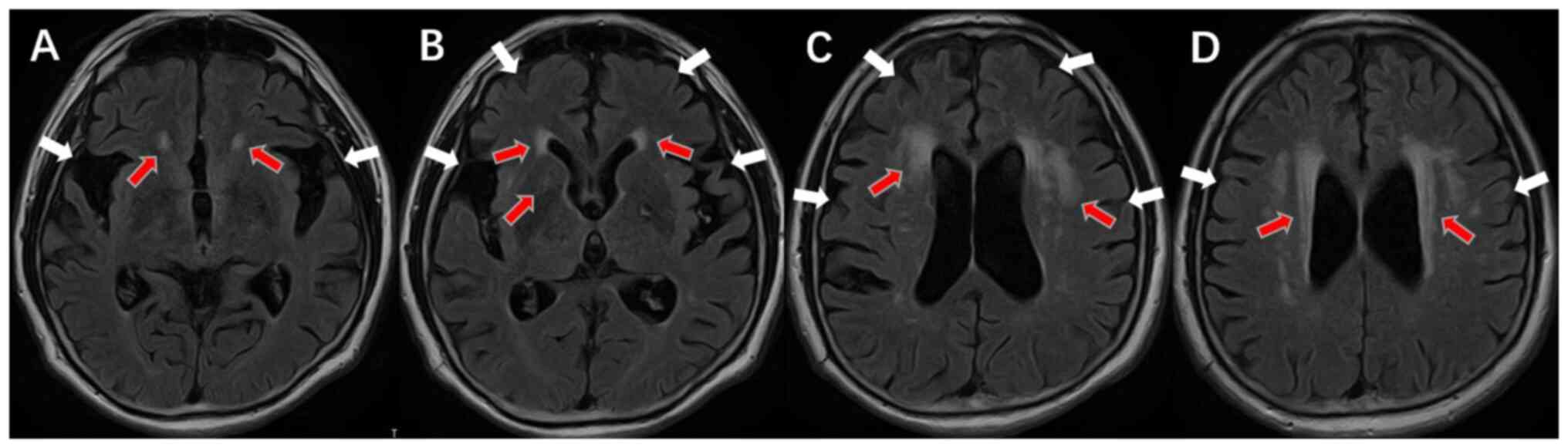
(EMG) (Fig. 1) and brain MRI
scanning showed no abnormalities and enhancement signs such as
brain atrophy and lacunar lesions in the bilateral basal ganglia.
The Fazekas score in the deep brain and near the ventricle was
3(3), indicating increased white
matter content in the brain (Fig.
2).
 | Table ILaboratory findings. |
Table I
Laboratory findings.
| Types | Value | Laboratory range |
|---|
| Blood | | |
|
RPR | 1:16 positive | Negative |
|
TPHA | Positive | Negative |
| CSF | | |
|
RPR | 1:4 positive | Negative |
|
TPHA | Positive | Negative |
|
WBC
(x106cells/l) | 6 | ≤10 |
|
Protein
(g/l) | 1.01 | 0.15-0.45 |
Treatment and follow-up
According to CDC 2015 Guideline for the Diagnosis
and Treatment of Syphilis (4),
doxycycline is an effective alternative for early and late latent
syphilis in cases where penicillin cannot be used. Because the
patient had a history of ‘penicillin’ allergy, intravenous
doxycycline (100 mg, Bid) was administered for 15 days. It was
standard clinical treatment. During the follow-up visit in August
2022, orofacial and lingual dyskinesia were significantly improved,
but his dysarthria was not improved and mild cognitive decline was
observed.
Discussion
The present study describes the case of a
52-year-old male patient who presented with subacute onset
dysarthria and oral-facial-lingual dyskinesia. The patient had
normal blood glucose and echinocytocytes, with no history of drug
abuse. Moreover, autoimmune encephalitis-related antibodies and
paraneoplastic-related antibodies were negative. Based on these
results, the possibility of echinocytosis, anti-NMDA autoimmune
encephalitis, and drug-related movement disorders, among others
were excluded. The TPHA and RPR test results for serum were all
positive. In addition, the lumbar puncture pressure was 150
mmH20, the protein concentration was 1.01 g/l, while
TPHA and RPR tests for CSF were positive. Based on the 2020 German
Guideline on the Diagnosis and Treatment of neurosyphilis (5), a diagnosis of neurosyphilis
(meningeal vascular syphilis) was made. The patient showed a
significant improvement in oral-facial-lingual dyskinesia after
treatment with doxycycline, which further supported the diagnosis
of neurosyphilis. Several oral and facial movement disorders
present with multiple symptoms, such as Neuroacanthocytosis, Meige
syndrome, anti-NMDA autoimmune encephalitis, frontotemporal
dementia, Huntington's disease, neurodegeneration of cerebral iron
deposition, cerebrovascular disease, levodopa-induced dyskinesia in
Parkinson's disease, antipsychotic-induced tardive dyskinesia,
among others. However, orofacial dyskinesia caused by neurosyphilis
has been rarely reported.
Neurosyphilis is a chronic infectious disease caused
by Treponema pallidum which infects the central nervous system. It
can also involve the brain, meninges, spinal cord, or blood vessels
leading to diverse pathological and clinical manifestations. For
this reason, it is known as a great imitator. Orofacial dyskinesia
caused by neurosyphilis is known as the ‘Candy sign’ (2). Some scholars even believe that the
candy sign can be regarded as a specific symptom of neurosyphilis
(6). Lenka et al (7) reported that penicillin treatment
improved the symptoms of neurosyphilis, which presented only with
the candy sign and abnormal vocalization. Martinelli et al
(6) reported that, in addition to
the candy sign, neurosyphilis was accompanied with mental disorders
and memory loss. Notably, anti-syphilis treatment significantly
improved the orofacial movement disorder but did not ameliorate
cognitive disorder. Moreover, the penicillin effectively treated
neurosyphilis with cognitive impairment, accompanied with the candy
sign (8). In another study, it was
found that neurosyphilis was also characterized by Argyll Robertson
pupil, frequent paroxysmal oral-automatism seizures, periodic
lateralized discharges (PLEDs) with triphasic waves, behavioral
changes, and memory decline (9).
Administration of penicillin significantly decreased the severity
of PLED, seizures, behavioral changes, and memory decline. However,
it did not improve rhythmic orofacial involuntary movements and
Argyll Robertson pupil, which indicated an irreversible
characteristic of late-stage neurosyphilis syndromes. In the
present case, the patient presented with tongue dyskinesia and
dysarthria in addition to candy sign. Administration of the
anti-syphilis treatment resulted in a significant reduction in the
oral-facial-lingual dyskinesia, although it did not improve the
dysarthria. Considering that neurosyphilis with oral-facial-lingual
dyskinesia is rare in clinical practice, it is likely to be
misdiagnosed.
Movement disorders in patients with neurosyphilis
can result from ischemic lesions in various areas such as the
midbrain (red nucleus, substantia nigra), cerebellum, and basal
ganglia. Moreover, the presentations of clinical movement disorders
in these cases are generally similar to those seen in cases with
ischemic lesions in the same locations due to other causes
(10). Based on these findings, we
hypothesized that the candy sign may be caused by the following
factors. i) Vascular occlusion in arteritis leading to ischemic
necrosis in the basal ganglia region and decreased number of
neurons in the substantia nigra striatum; ii) dysfunction of
transmitter transmission or metabolism in cortico-basal ganglia
pathway. Among these factors, ischemic necrosis in the basal
ganglia region caused by arteritis vascular occlusion may be the
main mechanism underlying the occurrence of the candy sign.
Neurosyphilis, known for its elusive nature,
manifests with a wide array of complex and diverse clinical
symptoms. It can potentially affect any region of the nervous
system without presenting distinct features, thereby resulting in a
considerable rate of misdiagnosis during the initial stages.
Therefore, if a patient presents with orofacial dyskinesia, a
comprehensive analysis of their medical history, clinical symptoms,
and imaging data should be made, while considering the risk of
neurosyphilis. Fortunately, neurosyphilis is curable; thus, once
diagnosed, regular anti-syphilis treatment should be administered
to improve patient's prognosis.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from the National
Natural Science Foundation of China (grant no. 82160420), the
Guangxi Province Health Committee Self-funded Project (grant no.
Z20210494), The Clinical Climbing Program of the First Affiliated
Hospital of Guangxi Medical University (grant no. YYZS2020022), the
Open Project of Guangxi Key Laboratory of Regenerative Medicine,
Guangxi Medical University (grant no. 202203) and the ‘Medical
Excellence Award’ funded by the Creative Research Development Grant
from the First Affiliated Hospital of Guangxi Medical
University.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YL and HZ contributed to the conceptualization and
design of the study, the collection of clinical information and the
drafting of the manuscript. BL and DO obtained MR images and
analyzed patient data. GL was responsible for formulating the
patient's treatment plan. YL and GL contributed to critical
revisions of the intellectual content and confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The patient provided their written informed consent
to participate in this study. Written informed consent was obtained
from the patient for the publication of any potentially
identifiable images or data included in the article.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of any accompanying images or data
included in this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shah BB and Lang AE: A case of
neurosyphilis presenting with myoclonus, cerebellar ataxia, and
speech disturbance. Mov Disord. 27(794)2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang X, Zhang W, Liu R, Dong Z and Yu S:
Factors that influence Tolosa-Hunt syndrome and the short-term
response to steroid pulse treatment. J Neurol Sci. 341:13–16.
2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fazekas F, Barkhof F, Wahlund LO, Pantoni
L, Erkinjuntti T, Scheltens P and Schmidt R: CT and MRI rating of
white matter lesions. Cerebrovasc Dis. 13 (Suppl 2):31–36.
2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ghanem KG: Management of adult syphilis:
Key questions to inform the 2015 centers for disease control and
prevention sexually transmitted diseases treatment guidelines. Clin
Infect Dis. 61(suppl 8):S818–S836. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Klein M, Angstwurm K, Esser S, Hahn K,
Maschke M, Scheithauer S, Schoefer H, Sturzenegger M, Wildemann B
and Weber J: German guidelines on the diagnosis and treatment of
neurosyphilis. Neurol Res Pract. 2(33)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Martinelli P, Rizzo G, Scaglione C and
Capellari S: Neurosyphilis orofacial dyskinesia: The candy sign.
Mov Disord. 28:246–247. 2013.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lenka A, Thota N, Stezin A, Pal PK and
Yadav R: Orofacial involuntary movements in neurosyphilis: Beyond
the candy sign. Tremor Other Hyperkinet Mov (N Y).
7(507)2017.PubMed/NCBI View
Article : Google Scholar
|
|
8
|
Marto JP, Borbinha C, Lampreia T, Alves L
and Viana-Baptista M: Teaching Video NeuroImages: Candy sign: The
clue to the diagnosis of neurosyphilis. Neurology.
88(e35)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang X, Mu P, Zhang W and Liu Y: Case
Report: Not all neurological symptoms respond well to penicillin in
patients with neurosyphilis. Front Neurol.
12(813829)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shah BB and Lang AE: Acquired
neurosyphilis presenting as movement disorders. Mov Disord.
27:690–695. 2012.PubMed/NCBI View Article : Google Scholar
|