Introduction
Neurodegenerative diseases resulting from the
progressive loss of structure and/or function of neurons contribute
to different paralysis degrees and loss of cognition (1). Progressive neuronal loss is a
prominent pathological feature of neurodegenerative diseases, in
which oxidative stress serves a vital role in neuronal apoptosis,
and is difficult to restore (2).
Neurons contain polyunsaturated fatty acids, which are sensitive to
free radicals. Polyunsaturated fatty acids are easily attacked by
free radicals, and neurons have a low content of antioxidant
enzymes. Therefore, the antioxidant capacity of neurons is reduced
(3). Together, these factors
promote the sensitivity of neurons to oxidative stress injury
(3,4). Methods to effectively reduce
oxidative stress injury in neurons have attracted increasing
attention. PC12 cells, which have the properties of neurosecretory
cells and neurons, along with high stability, homogeneity and a
high degree of differentiation, are currently widely used in the
study of nerve cell function, differentiation, development and
death as a cell model (5).
It is well known that apoptosis is a tightly
regulated process, which involves changes in the expression of a
distinct set of genes (6). Bax and
Bcl-2 are major genes responsible for regulating apoptosis. Bax is
a member of the Bcl-2 family, and promotes apoptosis, while Bcl-2
blocks cell death (7). The
Bax/Bcl-2 ratio is a widely used parameter to determine cell
susceptibility to apoptosis. Caspase-3 is the most important
executing protease in the process of apoptosis (8). Cleaved caspase-3 is the activated
form of caspase-3(9). Previous
studies have confirmed that H2O2 can induce
PC12 cell injury (10) and the
expression and activation of the apoptosis-related gene
caspase-3(11).
Peroxisome proliferator-activated receptor γ (PPARγ)
is a ligand-activated nuclear receptor that regulates glucose and
lipid metabolism, endothelial function and inflammation (12). Thiazolidinediones (TZDs) are
ligands that are known to bind to and activate nuclear PPARγ and
are currently used as insulin sensitizers in type 2 diabetes
(13). Our previous study has
demonstrated that PPARγ agonists TZDs could protect the neuronal
microenvironment and preserve nerve cells in the hippocampi of
spontaneously hypertensive rats (SHRs) via antioxidative and
antiapoptotic pathways (14).
However, the underlying mechanism needs to be further studied. In
the present study, a model of oxidative stress damage was generated
in PC12 cells using H2O2 to observe whether
pioglitazone had a neuroprotective effect and to determine the
underlying mechanism.
Materials and methods
Cell culture and preconditioning
protocols
PC12 cells were obtained from the Institute of
Neurobiology, School of Medicine, Xi'an Jiaotong University (Xi'an,
China). The cells were plated at a density of 3x105
cells/well in 6-well plates and maintained in DMEM/F12 supplemented
with 10% heat-inactivated foetal bovine serum (both Gibco; Thermo
Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin (Thermo Fisher Scientific, Inc.) at 37˚C under an
atmosphere of 5% CO2 and 95% air. The culture medium was
changed three times per week. PC12 cells were preconditioned by 0,
6, 12, 24 or 36 h of exposure to different concentrations (0, 25,
50, 100, 200 and 400 µmol/l) of H2O2 at 37˚C.
Experiments were performed at least three times.
Transfection experiments
A total of three alternative siRNA sequences
targeting PPARγ were used to know down PPARγ protein expression.
The following groups were used: NC group, siRNA-NC (non-targeting)
group, PPARγ-siRNA 1 group, PPARγ-siRNA 2 group and PPARγ-siRNA 3
group. PPARγ-siRNAs and negative control siRNA were designed and
synthesized by Shanghai GenePharma Co., Ltd. First, PC12 cells were
seeded in 24-well plates at an optimized concentration of
1x105 cells/well, 24 h before transfection. On the
following day, when cell confluence had reached 60-70%, they were
transfected with PPARγ-siRNA 1 (10 µM; 10 µl/well), PPARγ-siRNA 2
(10 µM; 10 µl/well), PPARγ-siRNA 3 (10 µM; 10 µl/well) or siRNA-NC
(10 µM; 10 µl/well) using Lipofectamine® 2000 (cat. no.
11668019; Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C for 2
days according to the manufacturer's protocol. The sequence with
the best inhibition rate, as determined by PPARγ protein expression
analysis 48 h after transfection, was selected. The sequences are
shown in Table I. Following 48 h
of transfection, the cells were collected for subsequent
experiments.
 | Table IPPARγ-siRNA sequences. |
Table I
PPARγ-siRNA sequences.
| siRNA | Sequence
(5'-3') |
|---|
| PPARγ-siRNA 1
(sense) |
AGAUAAAGCUUCUGGAUUU |
| PPARγ-siRNA
1(antisense) |
AAAUCCAGAAGCUUUAUCU |
| PPARγ-siRNA 2
(sense) |
AGGAAAGACAACAGACAAA |
| PPARγ-siRNA 2
(antisense) |
UUUGUCUGUUGUCUUUCCU |
| PPARγ-siRNA 3
(sense) |
CCUCCCUGAUGAAUAAAGATT |
| PPARγ-siRNA 3
(antisense) |
UCUUUAUUCAUCAGGGAGGTT |
| Negative control
siRNA (sense) |
UUCUCCGAACGUGUCACGUTT |
| Negative control
siRNA (antisense) |
ACGUGACACGUUCGGAGAATT |
MTT assay. Experiment 1: Effect of
H2O2 on the viability of PC12 cells
The viability of cultured cells was measured using
an MTT assay (15). First, PC12
cells were preconditioned by 0, 6, 12, 24 or 36 h of exposure to
different concentrations (0, 25, 50, 100, 200 and 400 µmol/l) of
H2O2 at 37˚C. Subsequently, cells were
incubated with MTT solution (0.5 mg/ml in PBS) at 37˚C for 4 h. The
MTT solution was removed from the plate and the plate was dried.
DMSO (100 µl) was added to each well to dissolve the formazan
crystals before the optical density was measured at 570 nm. The
results are presented as the percentage of MTT reduction, and the
absorbance of the control cells was set as 100%. Experiments were
performed at least three times.
Experiment 2: Protective effects of pioglitazone
on PC12 cells with H2O2-induced
injury. In this experiment, PC12 cells were divided into five
groups: The control, H2O2 (100 µmol/l
H2O2), low-concentration pioglitazone
(1x10-7 mol/l; cat. no. 111025-46-8; Alexis
Biochemicals; Enzo Life Sciences), medium-concentration
pioglitazone (1x10-6 mol/l) and high-concentration
pioglitazone (1x10-5 mol/l) groups. Pioglitazone at
different concentrations was utilized to precondition PC12 cells
for a 1-h period at 37˚C, whereas the H2O2
group cells were instead treated with 0.9% saline; all the PC12
cells of H2O2 and piglitazone groups were
then treated with 100 µM H2O2 for a 24 h
period at 37˚C. For the control group, PC12 cells were treated with
cell culture medium for 25 h at 37˚C. The viability of the cultured
cells was measured by an MTT assay as aforementioned. Experiments
were performed at least three times.
Experiment 3: Role of PPARγ in the protective
effect of pioglitazone on PC12 cells with
H2O2-induced injury. In
this next experiment, PC12 cells were divided into five groups: The
control, H2O2, pioglitazone +
H2O2, pioglitazone + GW9662 +
H2O2 and pioglitazone + PPARγ-siRNA 3 +
H2O2 groups. In the
H2O2 group, PC12 cells were treated with 100
µM H2O2 for 24 h at 37˚C. PC12 cells in the
control group were treated with cell culture medium at 37˚C for 24
h. For the pioglitazone + H2O2 group,
1x10-5 mol/l pioglitazone was utilized to precondition
PC12 cells for a 1-h period at 37˚C, and then cells were treated
with 100 µM H2O2 for 24 h at 37˚C. The
pioglitazone + GW9662+ H2O2 group was treated
with GW9662 (1x10-6 mol/l; cat. no. M6191;
MilliporeSigma) for 1 h at 37˚C, followed by 1x10-5
mol/l pioglitazone for 1 h at 37˚C and H2O2
(100 µmol/l) for 24 h at 37˚C. For the pioglitazone + PPARγ-siRNA 3
+ H2O2 group, after PPARγ-siRNA transfection,
1x10-5 mol/l pioglitazone was added to the cells for 1 h
at 37˚C prior to treatment with H2O2 (100
µmol/l) for 24 h at 37˚C. The viability of cultured cells was
measured by an MTT assay as aforementioned. Experiments were
performed at least three times.
Measurement of malondialdehyde (MDA)
content, and superoxide dismutase (SOD) and glutathione peroxidase
(GSH-Px) activities
Based on the MTT assay results in Experiment 1, the
desired H2O2 concentration, action time and
cell plating density for modelling oxidative stress injury in PC12
cells were 100 µmol/l, 24 h and 1x105 cells/ml,
respectively. To verify the successful modelling of PC12 cells, the
MDA content and SOD and GSH-Px activity were measured in the
control and H2O2 groups. In the
H2O2 group, PC12 cells were treated with 100
µM H2O2 for 24 h at 37˚C. PC12 cells in the
control group were treated with cell culture medium at 37˚C for 24
h. Cells were collected and rinsed with PBS, and lysis buffer
containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM PMSF and 1%
Triton X-100 was then added for homogenization. The cell
supernatants were collected. The content of MDA (cat. no. A003-1-2)
and the activities of SOD (cat. no. A001-1-2) and GSH-Px (cat. no.
A005-1-2) were all determined according to the instructions
provided by Nanjing Jiancheng Bioengineering Institute as
previously described (16).
Briefly, MDA content was determined using the thiobarbituric acid
method. The wavelength of colorimetry was 532 nm. SOD activity was
determined using the hydroxylamine method and the wavelength of
colorimetry was 550 nm. GSH-Px activity was determined using a
colorimetric method and the wavelength of colorimetry was 412 nm.
The optical density value was read using a microplate reader, and
two parallel samples were used to ensure the accuracy of the
experiment. Experiments were performed at least three times.
Detection of apoptotic cells by flow
cytometry
Cells in the control, H2O2,
pioglitazone + H2O2, pioglitazone +
PPARγ-siRNA 3 + H2O2 and pioglitazone +
GW9662 + H2O2 groups were used to detect
apoptosis by annexin V-FITC and PI staining followed by flow
cytometry (Beckman Coulter, Inc.). The treatment of each group was
performed as described for the MTT assay experiment 3. The
procedure described in the documentation of the annexin V-FITC/PI
detection test kit (cat. no. C1062; Beyotime Institute of
Biotechnology) was followed. Cells were resuspended at a
concentration of 1x106 cells/ml in 400 µl 1X binding
buffer solution. Then, 5 µl annexin V-FITC and 10 µl PI were added
to stain the cells for 15 min at room temperature in the dark.
Then, the cell apoptosis was detected by flow cytometric analysis
(NL-CLC 1L-3L; Cytek NL-CLC Full Spectrum Flow Cytometer; Shanghai
Xiatai Biotechnology Co., Ltd.). The excitation wavelength was 488
nm and the emission wavelength was 530 nm. Green fluorescence of
FITC and red fluorescence of PI were observed after excitation.
Data were analysed using FlowJo (v10; FlowJo LLC). Experiments were
performed at least three times.
Reverse transcription-quantitative PCR
(RT-qPCR) of PPARγ in PC12 cells
RT-qPCR was performed for the NC, siRNA-NC,
PPARγ-siRNA 1, PPARγ-siRNA 2 and PPARγ-siRNA 3 groups after the
transfection experiments. RT-qPCR was then performed to analyse
cells in the control, H2O2, pioglitazone +
H2O2, pioglitazone + PPARγ-siRNA 3 +
H2O2 and pioglitazone + GW9662 +
H2O2 groups. The treatment of each group was
performed as described for the MTT assay experiment 3. Total RNA
was extracted using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). RNA samples were transcribed into cDNA using the
PrimeScript RT Master Mix Kit (Takara Bio, Inc.) according to the
manufacturer's instructions. The SYBR ExScript RT-PCR Kit (Takara
Bio, Inc.) was used for RT-qPCR on the IQ Multicolor Real-Time PCR
Detection System (Bio-Rad Laboratories, Inc.). The primers used to
amplify rat PPARγ (forward, 5'-GGAGCCTAAGTTTGAGTTTGCTGTG-3' and
reverse, 5'-TGCAGCAGGTTGTCTTGGATG-3') and reverse,
5'-ACCACCCTGGTCTTGGATCC-3') and β-actin (forward,
5'-GGAGATTACTGCCCTGGCTCCTA-3' and reverse,
5'-GACTCATCGTACTCCTGCTTGCTG-3') were designed and synthesized by
Takara Biotechnology Co., Ltd. Amplification was carried out with
the following thermal cycling program: 95˚C for 30 sec, followed by
40 cycles of 95˚C for 3 sec and 60˚C for 30 sec. Cycle threshold
values were obtained with Bio-Rad iQ5 2.0 Standard Edition Optical
System software (Bio-Rad Laboratories, Inc.). β-actin was used as
the internal control. Relative quantification was performed by the
comparative cycle threshold (2-ΔΔCq) method (17), and the data are presented as the
mean ± SD of three separate experiments performed with triplicate
samples.
Western blot analysis
PPARγ, Bax, Bcl-2, cleaved caspase-3 and caspase-3
protein expression was examined in the control,
H2O2, pioglitazone +
H2O2, pioglitazone + PPARγ-siRNA 3 +
H2O2 and pioglitazone + GW9662 +
H2O2 groups. The treatment of each group was
performed as described for the MTT assay experiment 3. PC12 cells
were harvested by scraping into ice-cold PBS and centrifuged at
12,000 x g for 8 min at 4˚C. Afterwards, RIPA cell lysis buffer
(P0013B; Beyotime Institute of Biotechnology) was used for
extraction of cellular protein using 1 mM PMSF. A BCA kit (P0010;
Beyotime Institute of Biotechnology) was used to determine protein
concentrations. Equal amounts of protein (20 µg/lane) from
different samples were separated on 12% SDS polyacrylamide gels,
transferred to PVDF membranes and blocked in 10% non-fat milk at
room temperature for 2 h. The membranes were incubated at 4˚C
overnight with rabbit polyclonal antibodies against PPARγ (1:400;
cat. no. ab66343; Abcam), Bax (1:800; cat. no. bs2538; Bioworld
Technology, Inc.), Bcl-2 (1:800; cat. no. bs1511; Bioworld
Technology, Inc.), cleaved caspase-3 (1:1,000; cat. no. ab2302;
Abcam), caspase-3 (1:800; cat. no. bs7004; Bioworld Technology,
Inc.) and mouse monoclonal anti-β-actin (1:1,000; cat. no.
sc-517582; Santa Cruz Biotechnology, Inc.). The membranes were
washed with Tris-buffered saline containing Tween 20 (0.1%) three
times and incubated with HRP-conjugated goat anti-rabbit IgG
(1:5,000; cat. no. BS13278; Bioworld Technology, Inc.) and
HRP-conjugated goat anti-mouse IgG (1:5,000; cat. no. BS12478;
Bioworld Technology, Inc.) at room temperature for 2 h. After
washing, protein bands were detected by incubation with
chemiluminescent HRP substrate (SuperSignal West Pico; Thermo
Fisher Scientific, Inc.) for 5 min at room temperature in the dark
and exposure to X-ray film (FUJIFILM Wako Pure Chemical
Corporation). Quantity One software 4.6.2 (Bio-Rad Laboratories,
Inc.) was used to semi-quantify the band intensity, which was
normalized to that of the loading control β-actin. The data are
presented as the mean ± SD of three separate experiments performed
with triplicate samples.
TUNEL assay
A DeadEnd™ Colorimetric TUNEL System
(cat. no. G7130; Promega Corporation) was used to detect PC12
apoptosis in the control, H2O2, pioglitazone
+ H2O2, pioglitazone + PPARγ-siRNA 3 +
H2O2, pioglitazone + GW9662 +
H2O2 groups. The treatment of each group was
performed as described for the MTT assay experiment 3. The
coverslips were placed at the bottom of a 24-well plate. PC12 cells
were inoculated into the 24-well plate and treated according to the
grouping. Subsequently, the coverslips were fixed with 4%
paraformaldehyde at room temperature (15-25˚C) for 30 min,
incubated with protease K at room temperature for 20 min and washed
three times with PBS. The coverslips were covered with
equilibration buffer for 10 min and with rTdT incubation buffer (50
µl; containing TUNEL reagent) at 37˚C in the dark for 1 h. The
coverslips were immersed in 2X saline-sodium citrate buffer for 15
min and rinsed with PBS. The coverslips were counterstained with
DAPI (1:1,000; MilliporeSigma) for 5 min at room temperature,
washed in PBS and mounted with 50% glycerin (IH0272; Beijing Legen
Biotechnology Co., Ltd.). Fluorescence microscopy was carried out
using an Olympus BX51 microscope (Olympus Corporation) with a
mercury lamp power supply. Neurons with bright green nuclei were
identified as TUNEL-positive neurons. The ratio of TUNEL-positive
cells to DAPI-positive cells was used as the experimental indicator
to calculate the apoptosis rate of PC12 cells. The number of
TUNEL-positive cells was normalized to the number of DAPI-stained
cells. A 20X objective lens was used to count apoptotic cells in
nine random fields (3 samples in each group; three fields in each
sample), and the observer was blinded to the treatment groups.
Statistical analysis
Statistical analysis was carried out using SPSS 16.0
software (SPSS, Inc.). Quantitative data are presented as the mean
± SD and are representative of three independent experiments.
Unpaired Student's t-test and one-way ANOVA followed by Tukey's
post hoc test were used to assess the significance of differences
among groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
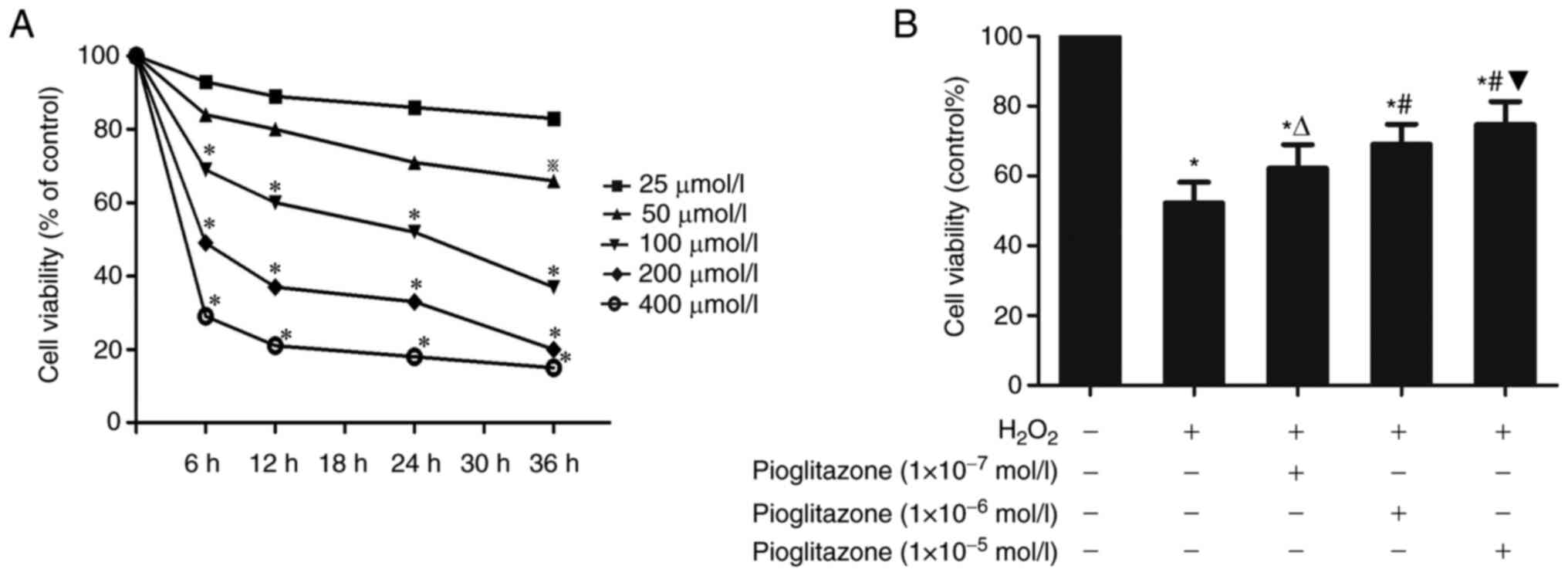
PC12 cell apoptosis is induced by
H2O2
To find the appropriate concentration of
H2O2 for the PC12 cell damage model,
different concentrations of H2O2 (25, 50,
100, 200 and 400 µmol/l) were used. PC12 cell viability was
evaluated using an MTT assay after culture with
H2O2 for 0, 6, 12, 24 and 36 h. The MTT assay
results revealed that with the increase of the
H2O2 concentration and incubation time, the
viability of PC12 cells decreased gradually (Fig. 1A). To simulate the physiological
conditions, the present study aimed to select a treatment method
with slow action and resulting in a specific degree of damage.
Conditions leading to a cell viability rate between 50 and 60% in
the MTT assay were suitable for the apoptosis experiments. In the
present experiment, treatment of PC12 cells with 100 µmol/l
H2O2 for 24 h inhibited PC12 cell viability
to 50-60% of that in the control group. Thus, the desired
H2O2 concentration and action time were 100
µmol/l and 24 h, respectively.
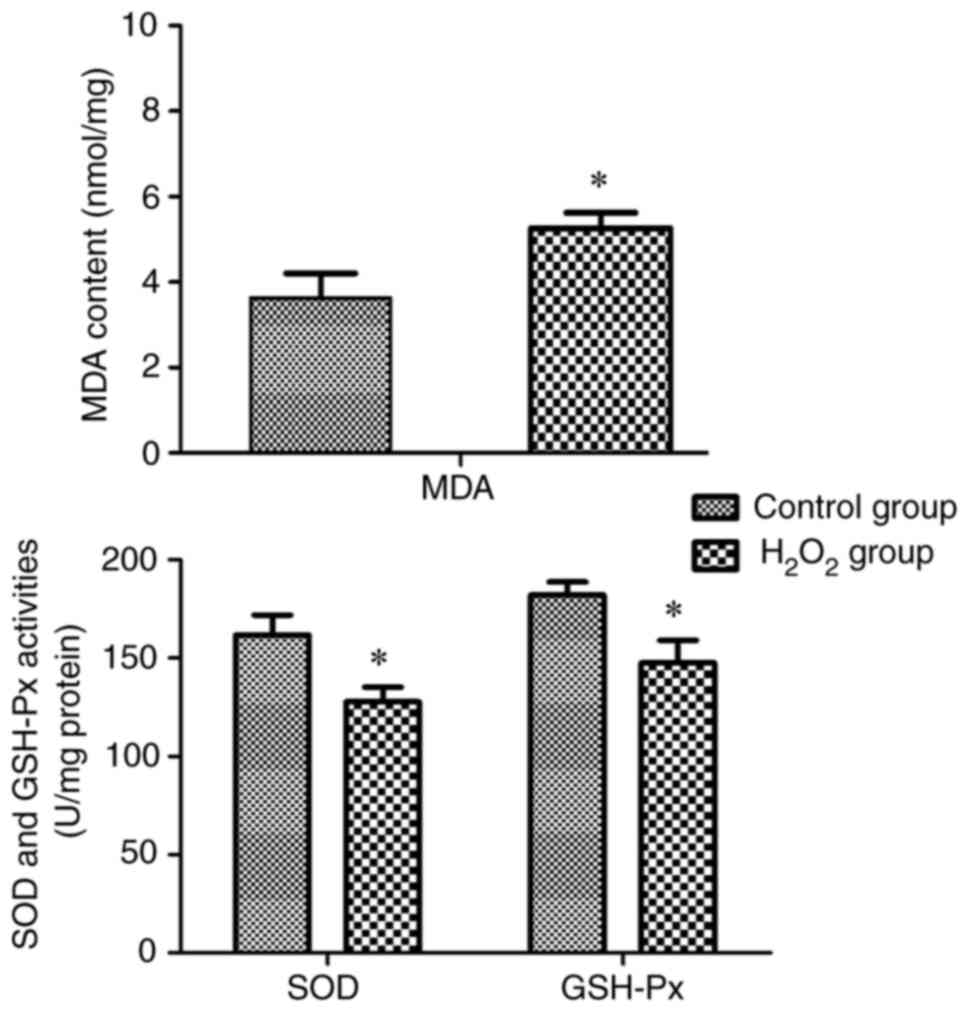
Successful establishment of the PC12
cell model
The MDA content and the activities of SOD and GSH-Px
were measured in the control and H2O2 groups
to verify the successful establishment of the PC12 cell model
(Fig. 2). After treatment with 100
µmol/l H2O2 for 24 h, the content of the
active peroxidation product MDA in the H2O2
group was increased but the activities of SOD and GSH-Px were
decreased compared with those in the control group, indicating that
oxidative stress injury in PC12 cells was successfully
modelled.
Effects of preconditioning with
different concentrations of pioglitazone on PC12 cell viability
reduced by H2O2
PC12 cells in the H2O2 group
were treated with 100 µmol/l H2O2 for 24 h,
while cells in the control group were treated with cell culture
medium. The MTT assay results showed that
H2O2 significantly reduced the viability of
PC12 cells (P<0.01 vs. control group). Pioglitazone served a
protective role in H2O2-treated PC12 cells in
a concentration-dependent manner. The concentration at which
pioglitazone exhibited its maximum effects was 1x10-5
mol/l, and 1x10-4 mol/l pioglitazone did not increased
PC12 viability compared with 1x10-5 mol/l pioglitazone
(data not shown). Therefore, 1x10-5 mol/l was selected
as the concentration for the next experiment.
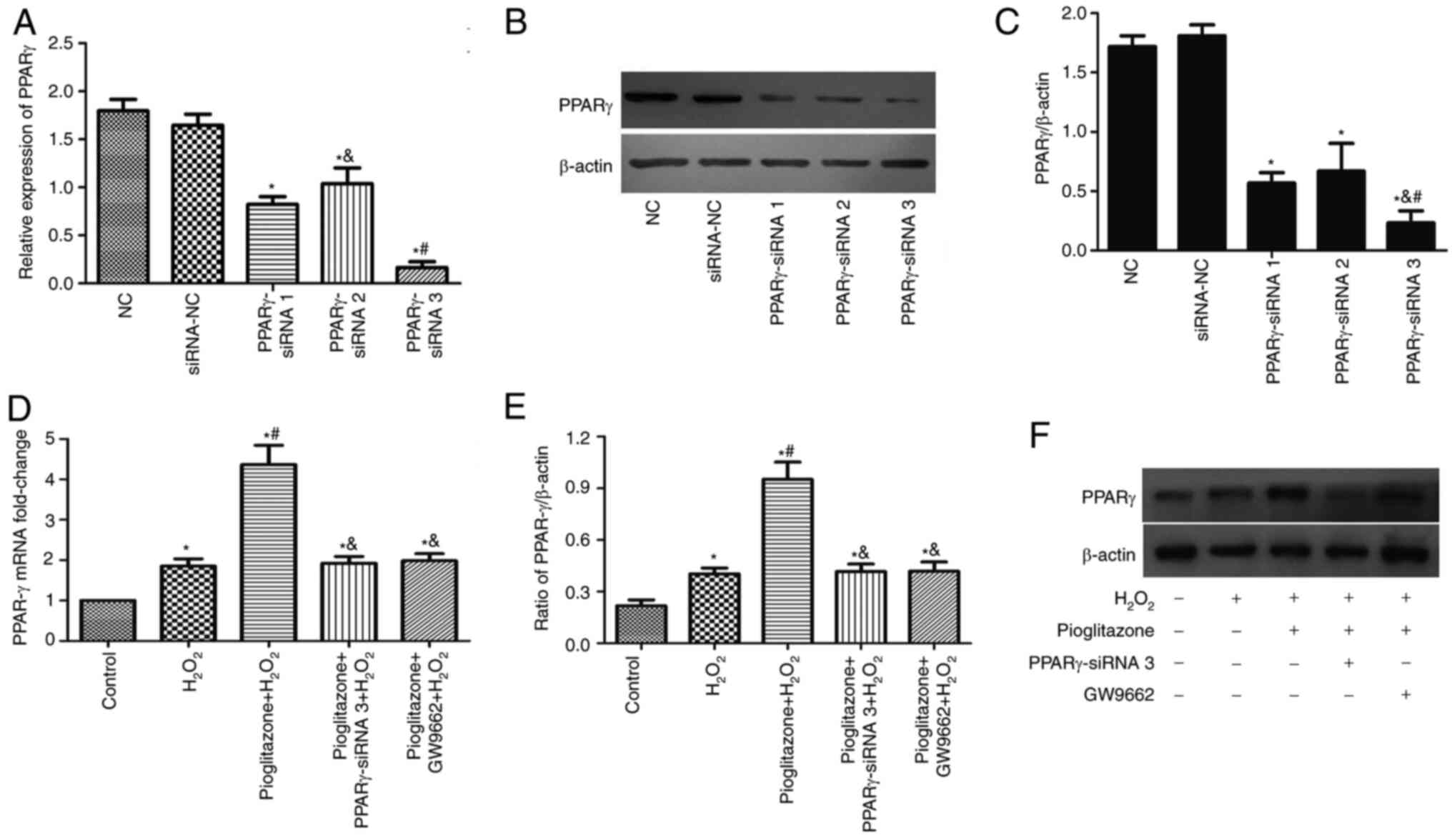
Pioglitazone increases PPARγ mRNA and
protein expression
The RT-qPCR and western blotting results are shown
in Fig. 3. PC12 cells were
transfected with siRNA to knock down PPARγ protein expression. The
following three groups were used: NC group, siRNA-NC group and
PPARγ-siRNA group, divided into PPARγ-siRNA 1, PPARγ-siRNA 2 and
PPARγ-siRNA 3. The gene knockdown rate in all groups was detected
by RT-qPCR (Fig. 3A) and western
blotting (Fig. 3B and C). The results demonstrated that PPARγ
expression was decreased in PC12 cells transfected with PPARγ-siRNA
compared with siRNA-NC, with PPARγ-siRNA 3 showing superior
knockdown efficiency. Therefore, PPARγ-siRNA 3 was selected for
subsequent experiments.
Compared with the control group,
H2O2 induced increases in PPARγ mRNA and
protein expression (P<0.01). Pioglitazone pretreatment
significantly increased PPARγ mRNA and protein expression
(P<0.01 vs. control and H2O2 groups).
Pioglitazone treatment increased PPARγ mRNA and protein expression
4.36- and 4.4-fold, respectively, compared with the control group.
Compared with pioglitazone treatment, treatment with PPARγ-siRNA 3
and the PPARγ antagonist GW9662 significantly reduced PPARγ mRNA
and protein expression (P<0.01).
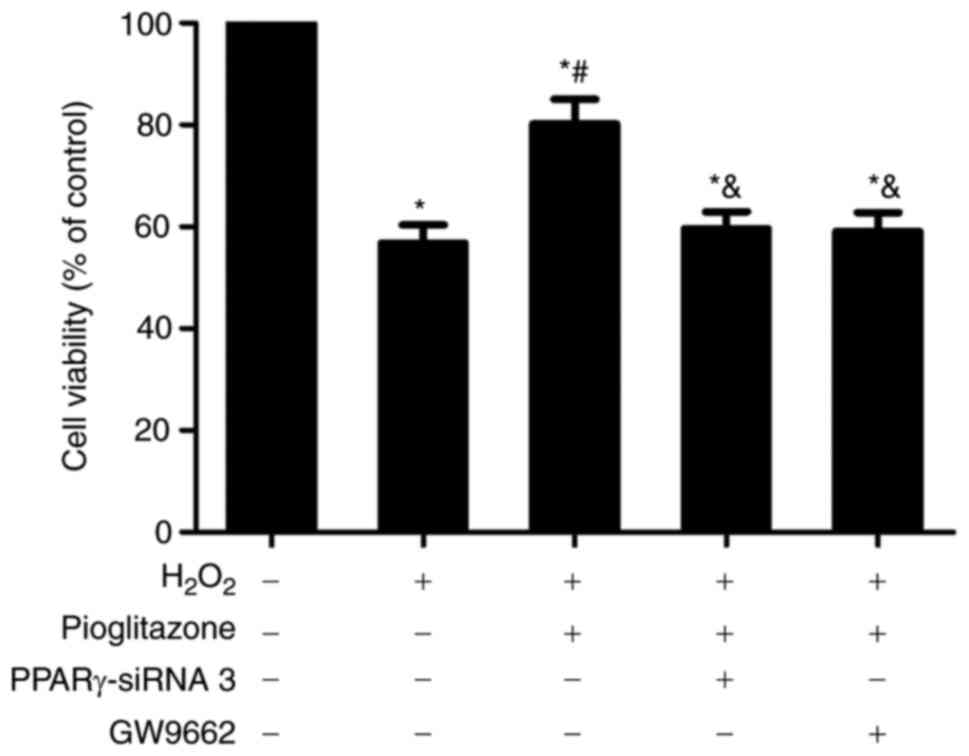
Neuroprotective effect of pioglitazone
on H2O2-treated PC12 cells
To investigate the protective effect of PPARγ on
H2O2-treated PC12 cells, an MTT assay, a
TUNEL assay and flow cytometry were used to observe the
neuroprotective effect of pioglitazone.
MTT assay results. As shown in Fig. 4, treatment with 100 µmol/l
H2O2 significantly reduced PC12 cell
viability to 56.8% of the value in the control group (P<0.01).
Treatment with 1x10-5 mol/l pioglitazone increased PC12
cell viability to 80.2% of that in the control group, and there was
a significant difference between the pioglitazone +
H2O2 and H2O2 groups
(P<0.01), suggesting piglitazone may exert a protective effect
on oxidative stress-injured PC12 cells. However, the viability of
cells in the pioglitazone + H2O2 group also
differed from that of cells in the control group (P<0.01),
suggesting that the protective effect of pioglitazone is
insufficient to completely protect PC12 cells from oxidative
damage. Pretreatment with either PPARγ-siRNA 3 or the PPARγ
antagonist GW9662 reversed the neuroprotective effects of
pioglitazone to similar degrees. The viability of PC12 cells
treated with PPARγ-siRNA and GW9662 decreased to 59.6 and 59.1% of
that in the pioglitazone +H2O2 group,
respectively, with both showing a significant difference compared
with the pioglitazone + H2O2 group
(P<0.01), suggesting that pioglitazone exerts its
neuroprotective effect through PPARγ activation.
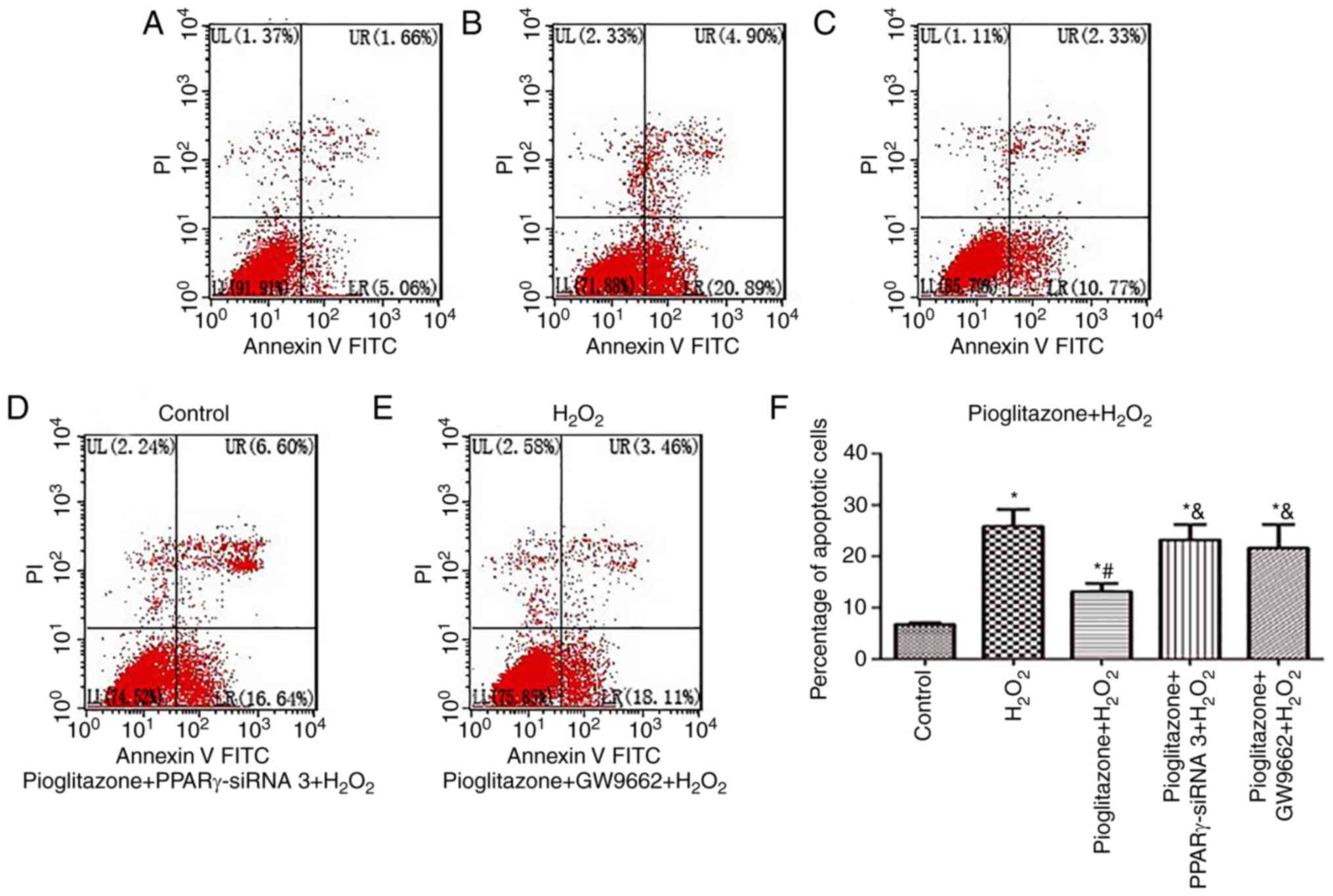
Flow cytometry results. As shown in Fig. 5, PC12 cell apoptosis in the control
group was 6.76% (Fig. 5A). The
percentages in Fig. 5F are the
averages for all three experimental repeats. After
H2O2 treatment, the mean apoptosis increased
significantly to 25.48% (P<0.01 vs. control group; Fig. 5B). Pioglitazone decreased the
apoptosis of PC12 cells to 12.93% (Fig. 5C), which was significantly
different from that in the H2O2 group and the
control group (P<0.01 and P<0.05, respectively), suggesting
that the neuroprotective effect of pioglitazone was insufficient to
completely reverse the oxidative damage caused by
H2O2. Pretreatment with PPARγ-siRNA 3
(Fig. 5D) and the PPARγ antagonist
GW9662 (Fig. 5E) increased the
apoptosis rates of PC12 cells to 23.14 and 21.51%, respectively,
which both showing a significant difference compared with the
pioglitazone + H2O2 group (P<0.01). This
result suggested that all types of PPARγ inhibition reversed the
neuroprotective effect of pioglitazone to different degrees and
that the neuroprotective effect of pioglitazone was mediated
through the PPARγ activation pathway.
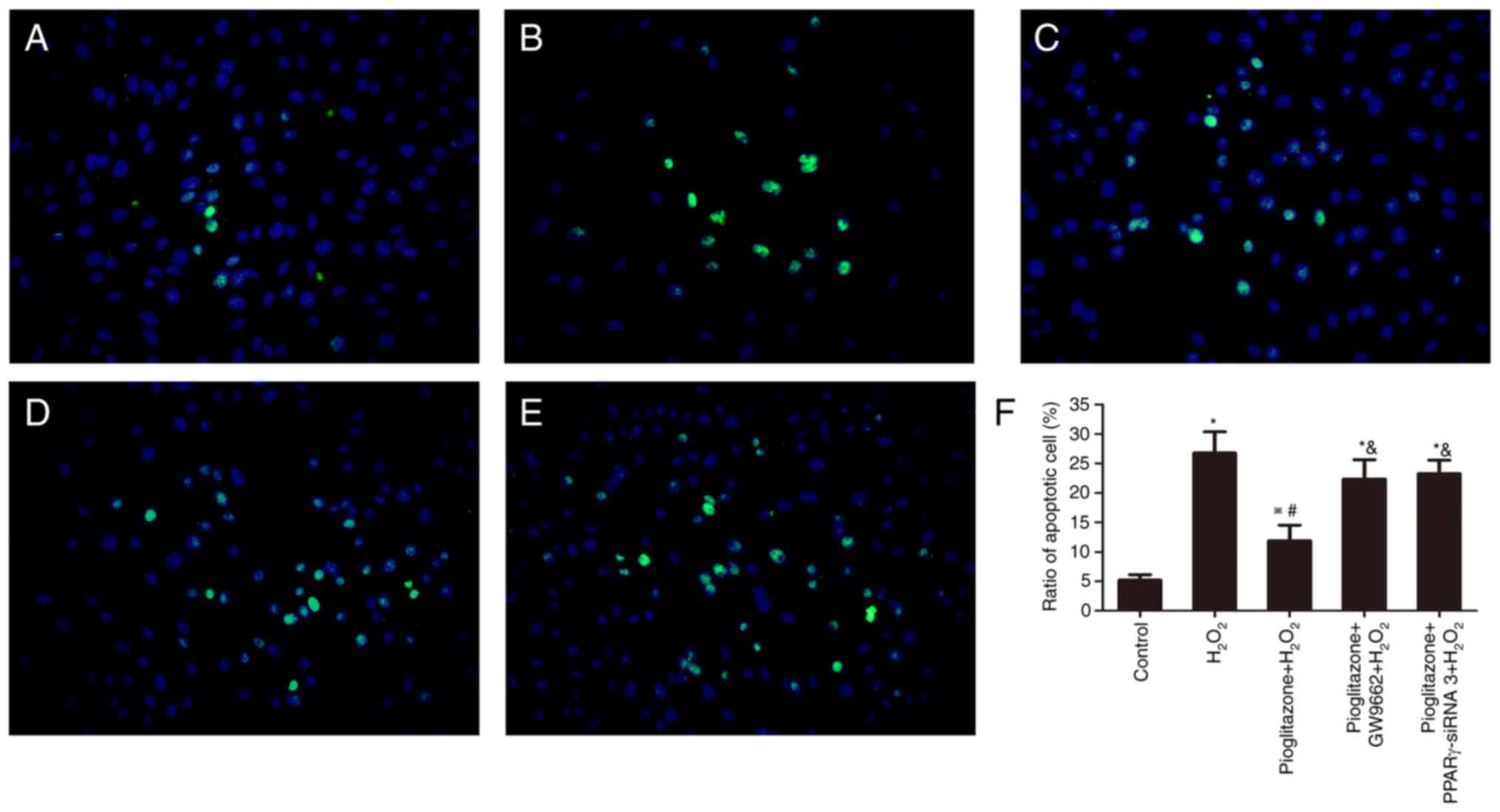
TUNEL assay results. As Fig. 6 shows, the control group showed an
intact cell morphology, a low fluorescence intensity, larger
nuclei, more DAPI-positive cells and fewer TUNEL-positive cells.
The TUNEL/DAPI ratio was 5.20% (Fig.
6A). After treatment with H2O2 for 24 h,
the number of DAPI-positive nuclei decreased, and high-intensity
concentrated fluorescence appeared in the nuclei. The nuclei
decreased in size and became condensed and fragmented, showing the
characteristics of apoptotic cells (Fig. 6B). The mean TUNEL/DAPI ratio
increased to 26.77% (P<0.01 vs. control group; Fig. 6F), suggesting that
H2O2 promoted apoptosis in PC12 cells.
Pioglitazone decreased the mean TUNEL/DAPI ratio to 11.90%
(Fig. 6C), showing differences
compared with the H2O2 group and the control
group (P<0.01 and P<0.05, respectively), suggesting that
pioglitazone could protect PC12 cells from
H2O2-induced oxidative damage. However, the
protective effect was insufficient to completely reverse the
oxidative damage. Pretreatment with the PPARγ antagonist GW9662 and
PPARγ-siRNA increased the mean TUNEL/DAPI ratio to 22.34% (Fig. 6D) and 23.27% (Fig. 6E), respectively, with significant
differences compared with the pioglitazone +
H2O2 group (P<0.01). This result suggested
that pioglitazone exerts a neuroprotective effect through PPARγ
activation.
Pioglitazone decreases the
H2O2-induced increases in the Bax/Bcl-2 ratio
and cleaved caspase-3/caspase-3 ratio
As shown in Fig.
7, after H2O2 treatment, the Bax and
cleaved caspase-3/caspase-3 ratio increased by 3.95- and 3.11-fold,
respectively (both P<0.01 vs. control group; Fig. 7A and D), while Bcl-2 protein expression was
significantly reduced (P<0.01 vs. control group; Fig. 7B). The Bax/Bcl-2 ratio in the
H2O2 group was significantly higher than that
in the control group (P<0.01; Fig.
7C). Pioglitazone reduced the mean protein levels of Bax and
cleaved caspase-3/caspase-3 ratio to 46.91 and 59.38% of the value
in the H2O2 group, respectively (P<0.01),
and increased the mean expression levels of Bcl-2 by 1.53-fold
(P<0.01) compared with those in the H2O2
group, thus significantly reducing the Bax/Bcl-2 ratio (P<0.01).
Compared with the pioglitazone + H2O2 group,
the pioglitazone + GW9662 + H2O2 and
pioglitazone + PPARγ-siRNA 3 + H2O2 groups
showed increased Bax protein levels and cleaved caspase-3/caspase-3
ratio levels, reduced Bcl-2 expression and an increased Bax/Bcl-2
ratio (P<0.01). However, no significant differences between the
pioglitazone + GW9662 + H2O2 and pioglitazone
+ PPARγ-siRNA 3 + H2O2 groups were observed.
This result suggested that the PPARγ agonist pioglitazone can
increase the expression levels of the antiapoptotic protein Bcl-2
and decrease the expression levels of the proapoptotic proteins Bax
and the cleaved caspase-3/caspase-3 ratio, thus protecting PC12
cells from H2O2-induced oxidative damage.
Pioglitazone mainly exerts its antiapoptotic effect through the
PPARγ pathway (18).
 | Figure 7Effect of pioglitazone on the protein
expression levels of Bax, Bcl-2 and cleaved caspase-3/caspase-3
ratio in PC12 cells. (A) Effects of H2O2,
pioglitazone, GW9662 and PPARγ-siRNA 3 on Bax protein expression.
(B) Effects of H2O2, piogitazone, GW9662 and
PPARγ-siRNA 3 on Bcl-2 protein expression. (C) Effects of
H2O2, piogitazone, GW9662 and PPARγ-siRNA 3
on the Bax/Bcl-2 ratio in PC12 cells. (D) Effects of
H2O2, pioglitazone, GW9662 and PPARγ-siRNA 3
on the ratio of cleaved caspase-3/caspase-3. (E) Bax, Bcl-2,
cleaved caspase-3 and caspase-3 expression in different groups
detected by western blotting *P<0.01 vs. control
group; #P<0.01 vs. H2O2 group;
&P<0.01 vs. pioglitazone +
H2O2 group. PPARγ, peroxisome
proliferator-activated receptor γ; siRNA, small interfering
RNA. |
Discussion
Our previous study demonstrated that SHRs exhibited
an age-dependent increase in TUNEL-positive cells in the CA1
subfield of the hippocampus, which was accompanied by increased
expression of oxidative stress markers and reduced mRNA and protein
expression levels of PPARγ (19).
PPARγ agonist rosiglitazone can exert neuroprotective effects
through antioxidative and antiapoptotic pathways independent of
blood pressure control (14).
However, the limitation of our previous study was that PPARγ was
not inhibited in animal experiments (14). In the present study, to verify the
role of PPARγ activation in protection against oxidative stress
injury, PC12 cells were treated with H2O2 as
a cellular model of oxidative stress injury. The PPARγ antagonist
GW9662 and PPARγ-siRNA were used to block PPARγ expression in PC12
cells. Pioglitazone exerted an antiapoptotic effect and promoted
the survival of PC12 cells with oxidative stress injury. This
effect was mediated through PPARγ activation.
PC12 is a tumour cell line isolated from a rat
adrenal pheochromocytoma. Differentiated PC12 cells have typical
neuronal characteristics in terms of morphology and function and
are widely used as a cell model to study the apoptosis and
differentiation of nerve cells (20). H2O2 is
considered to be the major precursor of reactive oxygen species
(ROS) and is widely used to induce oxidative stress injury
(5). Exogenous
H2O2 easily enter cells through the cell
membrane, resulting in the production of large amounts of ROS
(21). Thus,
H2O2 is commonly used to simulate cellular
peroxidative damage in vitro (22). There have been a number of studies
on PC12 cell apoptosis induced by H2O2, and
the conclusions were consistent (23,24).
However, the effective concentrations and action times were
different. In general, low and moderate concentrations (50-500
µmol/l) of H2O2 could induce oxidative
stress, while high concentrations could rapidly cause cell
necrosis. To determine the appropriate concentration and working
time of H2O2, viability of PC12 cells was
analysed using an MTT assay. The results showed that cell viability
was decreased in PC12 cells following treatment with 100 µmol/l
H2O2 for 24 h.
ROS act on the unsaturated lipids in cell
membranes, causing peroxidation of membrane lipids and leading to
cell damage and the formation of lipid peroxidation products
(25). MDA is an important ROS
metabolite in cells and is a good indicator of the degree of tissue
peroxidation (26). SOD and GSH-Px
are two important antioxidant enzymes in living organisms (27). In the present study,
H2O2 induced an increase in the content of
the active peroxidation product MDA and decreases in the SOD and
GSH-Px activities. This result indicated the successful
establishment of the oxidative stress injury model in PC12
cells.
The pathways by which H2O2
induces apoptosis differ among cell types. Dumont et al
(28) reported that
H2O2-induced apoptosis in T cells mainly
depended on mitochondrial ROS and NF-κB activation.
H2O2 promoted apoptosis by activating
caspase-3 in HL-60 cells (29).
Kitamura et al (30) found
that Bcl-2 and Bax expression did not increase significantly after
treatment with H2O2 but mediated apoptosis by
increasing P53 expression. H2O2 induced
apoptosis in hepatoblastoma cells not only by upregulating the
expression of P53 but also by decreasing the protein levels of
Bcl-2 and Bax (31). In the
present study, H2O2 upregulated the
expression levels of Bax and caspase-3 and downregulated the
expression levels of Bcl-2. It was suggested that the mechanism by
which H2O2 induced apoptosis in PC12 cells
may involve increasing the levels of proapoptotic proteins and
decreasing those of antiapoptotic proteins, thus changing the
apoptotic environment in PC12 cells. This is consistent with recent
research showing that H2O2 could increase the
Bax and cleaved caspase-3 protein levels in PC12 cells (32).
The process of apoptosis in neurons is similar to
that in other cells. After recognition of death signals,
apoptosis-promoting proteins such as Bax and BH3 interacting domain
death agonist are translocated to the outer mitochondrial membrane
and interact with antiapoptotic proteins such as Bcl-2, which
abolishes the apoptosis-inhibiting effect of antiapoptotic
proteins, increases the permeability of the mitochondrial membrane,
releases cytochrome c into the cytoplasm and activates caspase-3,
which eventually leads to apoptosis (33). The Bax/Bcl-2 ratio serves a
decisive role in determining whether apoptosis is initiated in
cells; thus, the Bax/Bcl-2 expression ratio is often used to
evaluate the degree of apoptosis (34). The present results showed that
H2O2 increased the protein levels of the
proapoptotic factors Bax and cleaved caspase-3 and reduced the
expression levels of the antiapoptotic protein Bcl-2, thus
increasing the Bax/Bcl-2 ratio, which activated caspase-3 and
induced apoptosis. The results of the TUNEL assay also confirmed
these findings.
PPARγ is a ligand-activated nuclear transcription
factor. PPARγ, when activated by its ligands, can bind to specific
DNA response elements and regulate gene transcription and
expression (35). Our previous
study demonstrated that the PPARγ agonist rosiglitazone could
upregulate PPARγ mRNA and protein expression in aged SHRs, while it
reduced the expression of oxidative stress markers (inducible
nitric oxide synthase and NADPH oxidase subunit
gp47phox) and proapoptotic markers (Bax and caspase-3)
(14). Fuenzalida et al
(36) demonstrated that
rosiglitazone upregulated Bcl-2 protein expression in neurons,
induced mitochondrial stabilization, and prevented oxidative stress
and apoptosis. These results indicated that the PPARγ agonist
rosiglitazone may exert neuroprotective effects through
antioxidative and antiapoptotic mechanisms. To confirm the
protective effect of PPARγ agonists on PC12 cells, PC12 cells were
incubated with different concentrations of pioglitazone before
exposure to H2O2 for 1 h. The MTT assay
results showed that pioglitazone concentration-dependently
increased the survival rate of PC12 cells. The results of flow
cytometric analysis and the TUNEL assay also confirmed the
conclusion of the MTT assay. There were fewer early and late
apoptotic cells in the pioglitazone + H2O2
group than in the H2O2 group, and the
apoptosis rate was considerably lower in the pioglitazone +
H2O2 group. RT-qPCR and western blot analyses
confirmed that pioglitazone significantly increased PPARγ
expression in PC12 cells to a level 4.4-fold higher than that in
the control group. This confirmed a previous report that
neuroprotective concentrations of pioglitazone can induce a 5-fold
increase in PPARγ expression, thereby maintaining responsiveness of
cortical neurons by increasing the expression of its receptors
(37).
In addition, H2O2 induced an
increase in PPARγ expression, which may be a compensatory
protective mechanism for the cells against oxidative stress injury
(38). Western blotting was used
to evaluate the protein expression levels of Bax, Bcl-2 and cleaved
caspase-3/caspase-3 ratio. The results demonstrated that 100 µmol/l
H2O2 increased the protein expression levels
of Bax and cleaved caspase-3/caspase-3 ratio, decreased Bcl-2
protein expression and increased the ratio of Bax to Bcl-2.
Pioglitazone downregulated the protein expression levels of Bax and
cleaved caspase-3/caspase-3 ratio, upregulated Bcl-2 protein
expression, thus reducing the ratio of Bax to Bcl-2. These results
suggested that pioglitazone could attenuate the
H2O2-induced proapoptotic environment in PC12
cells. To further explore whether rosiglitazone can serve a role in
the activation of PPARγ, the PPARγ antagonist GW9662 and
PPARγ-siRNA were used to block PPARγ expression in PC12 cells. The
results demonstrated that pretreatment with GW9662 significantly
increased the Bax/Bcl-2 ratio and cleaved caspase-3/caspase-3 ratio
in PC12 cells compared with those in the pioglitazone +
H2O2 group. PPARγ-siRNA had the same effects.
Therefore, GW9662 and PPARγ-siRNA could offset the protective
effect of pioglitazone on PC12 cells with
H2O2-induced injury.
In conclusion, in the present study, a model of
neuronal apoptosis induced by oxidative stress was established
in vitro, and the neuroprotective effect of pioglitazone was
studied. The results showed that pioglitazone increased antioxidant
activity in PC12 cells with H2O2-induced
injury, increased the expression levels of Bcl-2, and decreased the
protein levels of Bax and cleaved caspase-3, thus ameliorating the
proapoptotic environment and reducing the apoptosis rate of PC12
cells. Treatment with a PPARγ antagonist or PPARγ-siRNA inhibited
the protective effect of pioglitazone on PC12 cells with
H2O2-induced injury, suggesting that
pioglitazone could protect PC12 cells against oxidative stress
injury through PPARγ activation. In conclusion, pioglitazone
exerted an antiapoptotic effect and promoted the survival of PC12
cells in the presence of oxidative stress injury. This effect
occurred through PPARγ activation. Therefore, the present study
suggested that PPARγ activation might have intervention potential
in neurodegenerative disorders. The limitation of the present study
was that primary neuronal cultures, which represent neuronal
properties better than PC12 cells, were not used. In addition, ROS
were not directly detected before and after
H2O2 treatment, which would be an improved
approach to support the successful establishment of the PC12 cell
model.
Acknowledgements
The authors would like to thank Professor Yi Zhu
(Department of Physiology and Pathophysiology, Tian Jin Medical
University, Tianjin, China), Professor Dengfeng Gao (Department of
Cardiovascular Medicine, Xi'an Jiaotong University, Xi'an, China),
Dr Jianbo Yu (Department of Rehabilitation, Weihai Municipal
Hospital, Weihai, China) and Dr Yinchun Jiao (Department of
Rehabilitation, Rushan Hospital of Traditional Chinese Medicine,
Weihai, China) for their excellent technical assistance.
Funding
Funding: The current study was supported by the Doctoral
Research Fund of the Affiliated Hospital of Weifang Medical
University (grant no. 2022BSQD11), the Natural Science Foundation
of Shandong Province (grant no. ZR202102240754), and the Shandong
Medicine and Health Science and Technology Development Plan Project
(grant no. 202003070101).
Availability of data and materials
The datasets used and/or analysed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
YalL, LX and XN conceived and designed the
experiments. LL, BH, LX and JL performed all experiments. YanL, BH,
JL and ZY collected experimental data and performed the statistical
analysis. YalL and LX wrote the paper, which was revised and
polished by XN. JL and YanL confirmed the authenticity of all the
raw data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gitler AD, Dhillon P and Shorter J:
Neurodegenerative disease: Models, mechanisms, and a new hope. Dis
Model Mech. 10:499–502. 2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Huang H, Chen L, Mao G, Bach J, Xue Q, Han
F, Guo X, Otom A, Chernykh E, Alvarez E, et al: The 2019 yearbook
of neurorestoratology. J Neurorestoratology. 8:1–11.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tang XQ, Feng JQ, Chen J, Chen PX, Zhi JL,
Cui Y, Guo RX and Yu HM: Protection of oxidative preconditioning
against apoptosis induced by H2O2 in PC12
cells: Mechanisms via MMP, ROS, and Bcl-2. Brain Res. 1057:57–64.
2005.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Shao A, Lin D, Wang L, Tu S, lenahan C and
Zhang J: Oxidative stress at the crossroads of aging, stroke and
depression. Aging Dis. 11:1537–1566. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wiatrak B, Kubis-Kubiak A, Piwowar A and
Barg E: PC12 cell line: Cell types, coating of culture vessels,
differentiation and other culture conditions. Cells.
9(958)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
D'Arcy MS: Cell death: A review of the
major forms of apoptosis, necrosis and autophagy. Cell Biol Int.
43:582–592. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jensen K, WuWong DJ, Wong S, Matsuyama M
and Matsuyama S: Pharmacological inhibition of Bax-induced cell
death: Bax-inhibiting peptides and small compounds inhibiting Bax.
Exp Biol Med(Maywood). 244:621–629. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhou Z, Meng M and Ni H: Chemosensitizing
effect of astragalus polysaccharides on nasopharyngeal carcinoma
cells by inducing apoptosis and modulating expression of Bax/Bcl-2
ratio and caspases. Med Sci Monit. 23:462–469. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jiang H, Zhao PJ, Su D, Feng J and Ma SL:
Paris saponin I induces apoptosis via increasing the Bax/Bcl-2
ratio and caspase-3 expression in gefitinib-resistant non-small
cell lung cancer in vitro and in vivo. Mol Med Rep.
9:2265–2272. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Jang JH and Surh YJ: Protective effects of
resveratrol on hydrogen peroxide-induced apoptosis in rat
pheochromocytoma (PC12) cells. Mutat Res. 496:181–190.
2001.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Del Rio MJ and Velez-Pardo C: Monoamine
neurotoxins-induced apoptosis in lymphocytes by a common oxidative
stress mechanism: Involvement of hydrogen peroxide
(H(2)O(2)), caspase-3, and nuclear factor
kappa-B (NF-kappaB), p53, c-Jun transcription factors. Biochem
Pharmacol. 63:677–688. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stump M, Mukohda M, Hu C and Sigmund CD:
PPARγ regulation in hypertension and metabolic syndrome. Curr
Hypertens Rep. 17(89)2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang SB, Dougherty EJ and Danner RL: PPARγ
signaling and emerging opportunities for improved therapeutics.
Pharmacol Res. 111:76–85. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Li Y, Yu G, Liu L, Long J, Su S, Zhao T,
Liu W, Shen S and Niu X: Rosiglitazone attenuates cell apoptosis
through antioxidative and anti-apoptotic pathways in the hippocampi
of spontaneously hypertensive rats. Int J Mol Med. 43:693–700.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kumar P, Nagarajan A and Uchil PD:
Analysis of cell viability by the MTT assay. Cold Spring Harb
Protoc 2018, 2018.
|
|
16
|
Xiao Z, Yu X, Zhang S and Liang A: The
expression levels and significance of GSH, MDA, SOD, and 8-OHdG in
osteochondral defects of rabbit knee joints. Biomed Res Int.
2022(6916179)2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Refaie MMM and El-Hussieny M: Protective
effect of pioglitazone on ovarian ischemia reperfusion injury of
female rats via modulation of peroxisome proliferator activated
receptor gamma and heme-oxygenase 1. Int Immunopharmacol. 62:7–14.
2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Y, Liu J, Gao D, Wei J, Yuan H, Niu X
and Zhang Q: Age-related changes in hypertensive brain damage in
the hippocampi of spontaneously hypertensive rats. Mol Med Rep.
13:2552–2560. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lahiani A, Brand-Yavin A, Yavin E and
Lazarovici P: Neuroprotective effects of bioactive compounds and
MAPK pathway modulation in ‘ischemia’-stressed PC12
pheochromocytoma cells. Brain Sci. 8(32)2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Satoh T, Sakai N, Enokido Y, Uchiyama Y
and Hatanaka H: Free radical-independent protection by nerve growth
factor and Bcl-2 of PC12 cells from hydrogen peroxide-triggered
apoptosis. J Biochem. 120:540–546. 1996.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guo Z, Huang M, Yuan Y, Guo Y, Song C,
Wang H and Dang X: Nischarin downregulation attenuates cell injury
induced by oxidative stress via Wnt signaling. Neuroreport.
31:1199–1207. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Nakayama H, Nakahara M, Matsugi E, Soda M,
Hattori T, Hara K, Usami A, Kusumoto C, Higashiyama S and Kitaichi
K: Protective effect of ferulic acid against hydrogen peroxide
induced apoptosis in PC12 cells. Molecules. 26(90)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xiao L, Liao F, Ide R, Horie T, Fan Y,
Saiki C and Miwa N: Enzyme-digested Colla Corii Asini (E'jiao)
prevents hydrogen peroxide-induced cell death and accelerates
amyloid beta clearance in neuronal-like PC12 cells. Neural Regen
Res. 15:2270–2272. 2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Islam MT: Oxidative stress and
mitochondrial dysfunction-linked neurodegenerative disorders.
Neurol Res. 39:73–82. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
de Oliveira Ulbrecht MO, Gonçalves DA,
Zanoni LZG and do Nascimento VA: Association between selenium and
malondialdehyde as an efficient biomarker of oxidative stress in
infantile cardiac surgery. Biol Trace Elem Res. 187:74–79.
2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wang XF, Huang YF, Wang L, Xu LQ, Yu XT,
Liu YH, Li CL, Zhan JY, Su ZR, Chen JN and Zeng HF:
Photo-protective activity of pogostone against UV-induced skin
premature aging in mice. Exp Gerontol. 77:76–86. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dumont A, Hehner SP, Hofmann TG, Ueffing
M, Dröge W and Schmitz ML: Hydrogen peroxide-induced apoptosis is
CD95-independent, requires the release of mitochondria-derived
reactive oxygen species and the activation of NF-kappaB. Oncogene.
18:747–757. 1999.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Matsura T, Kai M, Fujii Y, Ito H and
Yamada K: Hydrogen peroxide-induced apoptosis in HL-60 cells
requires caspase-3 activation. Free Radic Res. 30:73–83.
1999.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Kitamura Y, Ota T, Matsuoka Y, Tooyama I,
Kimura H, Shimohama S, Nomura Y, Gebicke-Haerter PJ and Taniguchi
T: Hydrogen peroxide-induced apoptosis mediated by p53 protein in
glial cells. Glia. 25:154–164. 1999.PubMed/NCBI
|
|
31
|
Jiang MC, Liang HJ, Liao CF and Lu FJ:
Methyl methanesulfonate and hydrogen peroxide differentially
regulate p53 accumulation in hepatoblastoma cells. Toxicol Lett.
106:201–208. 1999.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Li RL, Zhang Q, Liu J, Sun JY, He LY, Duan
HX, Peng W and Wu CJ: Hydroxy-α-sanshool possesses protective
potentials on H2O2-stimulated PC12 cells by
suppression of oxidative stress-induced apoptosis through
regulation of PI3K/Akt signal pathway. Oxid Med Cell Longev.
2020(3481758)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Senichkin VV, Pervushin NV, Zuev AP,
Zhivotovsky B and Kopeina GS: Targeting Bcl-2 family proteins:
What, where, when? Biochemistry (Mosc). 85:1210–1226.
2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619.
1993.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ding YP, Kang J, Liu S, Xu Y and Shao B:
The protective effects of peroxisome proliferator-activated
receptor gamma in cerebral ischemia-reperfusion injury. Front
Neurol. 11(588516)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Fuenzalida K, Quintanilla R, Ramos P,
Piderit D, Fuentealba RA, Martinez G, Inestrosa NC and Bronfman M:
Peroxisome proliferator-activated receptor gamma up-regulates the
Bcl-2 anti-apoptotic protein in neurons and induces mitochondrial
stabilization and protection against oxidative stress and
apoptosis. J Biol Chem. 282:37006–37015. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Cimini A, Benedetti E, Cristiano L,
Sebastiani P, D'Amico MA, D'Angelo B and Di Loreto S: Expression of
peroxisome proliferator-activated receptors (PPARs) and retinoic
acid receptors (RXRs) in rat cortical neurons. Neuroscience.
130:325–337. 2005.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wang N, Han J, Xu Y, Liang H, Cheng Y and
Sun L: Hydrogen peroxide-induced changes of PPARγ in primary
cultured cortical neurons. Zhonghua Yi Xue Za Zhi. 95:1686–1690.
2015.PubMed/NCBI(In Chinese).
|