Introduction
Percutaneous coronary intervention (PCI) is an
interventional technology used for the treatment of acute coronary
syndrome worldwide; it is able to quickly restore the patency of
the occluded blood vessels and recover ischemic myocardial
perfusion (1-3).
Despite the fact that PCI has been shown to markedly decrease the
mortality rate of patients with acute coronary syndrome, the
incidence of major adverse cardiovascular events (MACE) and
dysregulated cardiac function following the PCI procedure is a
severe challenge affecting patient prognosis (4,5).
Thus, it is crucial to explore strategies to reduce the incidence
of these events after PCI (6).
Rosuvastatin is a representative statin mainly used
for treating dyslipidemia, while ticagrelor is a platelet
aggregation inhibitor (7,8). According to the guidelines of the
American Heart Association (AHA), for most patients with acute
coronary syndrome, only ticagrelor is recommended in the
perioperative period of PCI (9).
Although previous studies have reported the application of
rosuvastatin in patients undergoing PCI, there is no consensus on
the benefit of combining rosuvastatin and ticagrelor. For instance,
it has been shown that ticagrelor decreases the rate of target
vessel revascularization compared with prasugrel during the 1-year
of follow-up in patients undergoing PCI (10). In addition, a previous study also
reported that rosuvastatin enhances the left ventricular ejection
fraction (LVEF) and reduces myocardial injury and inflammatory
reaction caused by PCI (11). Of
note, several studies have previously compared the efficacy between
rosuvastatin plus ticagrelor and ticagrelor monotherapy among
patients receiving PCI and the majority of these studies reported
that rosuvastatin plus ticagrelor reduces the incidence of MACE and
recovers myocardial function indices compared with ticagrelor alone
(12-17);
however, another study reported that rosuvastatin plus ticagrelor
could not achieve these beneficial effects (18). Meanwhile, the sample sizes of these
studies are relatively small, as most included <80 participants
in each arm and may thus not lead to confident outcomes taken
alone. Therefore, it is important to conduct a meta-analysis, which
may combine the data of these smaller studies and lead to a
relatively more confident conclusion.
Therefore, the present meta-analysis intended to
comprehensively compare the efficacy between rosuvastatin plus
ticagrelor and ticagrelor monotherapy among patients receiving PCI,
which may provide more solid evidence to facilitate the application
of rosuvastatin plus ticagrelor in these patients in the
future.
Materials and methods
Study search
The present study was performed according to the
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
guidelines (19). Studies that
compared cardiac function or MACE occurrence in patients who
received rosuvastatin plus ticagrelor or ticagrelor monotherapy
following PCI were searched in the following databases: China
National Knowledge Infrastructure (CNKI; https://www.cnki.net/), Wanfang (https://www.wanfangdata.com.cn/index.html), CQVIP
(http://www.cqvip.com/), EMBASE (https://www.embase.com), Cochrane (https://www.cochrane.org/) and PubMed (https://pubmed.ncbi.nlm.nih.gov/) until January
2023. The following key words and the associated Medical Subject
Heading terms were used: ‘Ticagrelor’, ‘Tic’, ‘Brilique’,
‘Brilinta’, ‘rosuvastatin’, ‘Ros’, ‘Crestor’, ‘percutaneous
coronary intervention’, ‘PCI’ and ‘percutaneous coronary
revascularization’.
Eligibility criteria
The studies were eligible if: i) They compared
rosuvastatin plus ticagrelor with ticagrelor monotherapy in
patients receiving PCI; and ii) they reported at least one clinical
outcome that was of interest to the present study, including MACE,
LV end-systolic diameter (LVESD), LV end-diastolic diameter
(LVEDD), LVEF and N-terminal pro-B-type natriuretic peptide
(NT-proBNP) as the endpoint through the follow-up period.
The studies were excluded if: i) They compared
another treatment regimen with rosuvastatin plus ticagrelor, or a
different dose of rosuvastatin plus ticagrelor; ii) they included
the patients without PCI therapy; iii) they reported data that were
not extractable and could not be analyzed in the present study; and
iv) they were case reports, reviews, meta-analyses or animal
studies.
Data extraction
Two investigators independently searched the
studies, reviewed the data and evaluated the risk of bias.
Consensus was reached between the two aforementioned investigators
in case of disagreements. The investigators screened the titles and
abstracts of studies that were considered relevant to the present
study and eligible studies were then identified through full-text
evaluation based on the aforementioned inclusion and exclusion
criteria. Reference lists of eligible studies were also screened.
Following study selection, the data were extracted, which included
author, publication year, study type, patient type, follow-up
duration, sample size, patient age, patient gender, treatment and
outcomes. For risk of bias, the Cochrane Collaboration's tool and
Newcastle-Ottawa Scale criteria were adopted for randomized
controlled trials (RCTs) and cohort studies, respectively (20,21).
Statistical analysis
Meta-analysis was carried out through the use of
Stata (version 14.0; Stata Corp LP). Relative risk (RR) and mean
difference (MD) with 95% confidence intervals (CI) were selected
for binary variable assessment and continuous variable assessment,
respectively. Random-effects models were utilized. Heterogeneity
was assessed using I2 statistics, with
I2≤50.0% indicating low heterogeneity and
I2>50.0% indicating high heterogeneity. The
sensitivity was assessed via a ‘leave-one-out’ method (omitting
each study and repeating the analysis). Publication bias was
examined through Begg's and Egger's tests. If publication bias
existed, the trim-and-fill method was adopted for further
assessment and adjustment (22).
P<0.05 was considered to indicate a statistically significant
difference.
Results
Study selection procedure
A total of 141 records were retrieved in the initial
search (including 65 from CNKI, 29 from Wanfang, 6 from CQVIP, 34
from EMBASE, 6 from Cochrane and 1 from PubMed) and 73 records were
excluded due to duplication (Fig.
1). Next, the remaining 68 records were screened by titles and
abstracts, of which 59 records were excluded (including 23 studies
for other treatment regimens, 17 papers for patients not receiving
PCI, 12 case reports, 5 reviews or meta-analyses and 2 animal
studies). Subsequently, 9 studies were obtained as a full-text
version, of which 2 were excluded for no extractable data. Finally,
7 studies containing 850 patients were included in the
meta-analysis.
Features of the included studies
The included studies contained 3 cohort studies and
4 RCTs (Table I) (12-18).
Briefly, 2 cohort studies were conducted in 2018 (12,13),
while the remaining cohort study and 4 RCTs were conducted after
2019 (14-18).
In total, 852 patients were included in the present meta-analysis,
among which 426 received rosuvastatin plus ticagrelor and 424
patients received ticagrelor monotherapy.
 | Table IDetails of the included studies. |
Table I
Details of the included studies.
| | Sample size, n | Age, years (mean ±
standard deviation) | Sex, n
(male/female) | Treatment | |
|---|
| First author,
year | Study type | Patient type | Follow-up duration,
months | Rosuvastatin plus
ticagrelor | Ticagrelor | Rosuvastatin plus
ticagrelor | Ticagrelor | Rosuvastatin plus
ticagrelor | Ticagrelor | Rosuvastatin plus
ticagrelor | Ticagrelor | Patient
outcomes | (Refs.) |
|---|
| Li et al,
2018 | Cohort study | ACS | 1 | 47 | 47 | 57.5±5.3 | 57.6±5.1 | 26/21 | 25/22 | Rosuvastatin 10 mg
daily; ticagrelor 90 mg twice daily | Ticagrelor 90 mg
twice daily | MACE, LVESD, LVEDD,
LVEF, NT-proBNP | (12) |
| Wang, 2018 | Cohort study | ACS | 12 | 61 | 61 | 61.6±5.2 | 62.3±5.1 | 28/33 | 27/34 | Rosuvastatin 20 mg
daily, ticagrelor 180 mg for the first day, then 90 mg daily | Ticagrelor 180 mg
for the first day, then 90 mg daily | MACE | (13) |
| Li, 2019 | RCT | CHD | 2 | 30 | 30 | 44.3±10.3 | 42.8±11.4 | 15/15 | 16/14 | Rosuvastatin 20 mg
daily, ticagrelor 180 mg for the first day, then 90 mg daily | Ticagrelor 180 mg
for the first day, then 90 mg daily | MACE | (14) |
| Liu et al,
2020 | Cohort study | AMI | 12 | 132 | 130 | 72.7±4.2 | 72.6±4.2 | 76/56 | 68/62 | Rosuvastatin 10 mg
daily; ticagrelor 90 mg twice daily | Ticagrelor 90 mg
twice daily | LVESD, LVEDD, LVEF,
NT-proBNP | (15) |
| Zhang et al,
2020 | RCT | AMI | 3 | 75 | 75 | 63.1±8.4 | 62.4±8.3 | 43/32 | 41/34 | Rosuvastatin 10 mg
daily; ticagrelor 90 mg daily | Ticagrelor 90 mg
daily | MACE, LVESD, LVEDD,
NT-proBNP | (16) |
| Yong and Lei,
2021 | RCT | CHD | 6 | 51 | 51 | 60.3±4.5 | 60.3±4.6 | 28/23 | 27/24 | Rosuvastatin 10 mg
daily; ticagrelor 90 mg twice daily | Ticagrelor 90 mg
twice daily | MACE | (17) |
| Hongjiang,
2021 | RCT | AMI | 1 | 30 | 30 | 57.1±4.2 | 56.9±4.5 | 16/14 | 17/13 | Rosuvastatin 10 mg
daily; ticagrelor 90 mg twice daily | Ticagrelor 90 mg
twice daily | MACE, LVEDD,
LVEFS | (18) |
MACE occurrence
In total, 6 studies (2 cohort studies and 4 RCTs)
compared MACE occurrence between rosuvastatin plus ticagrelor and
ticagrelor alone, while no significant heterogeneity was found
among them (I2=0.0%; P=0.958; Fig. 2). Meta-analysis with the
random-effects model demonstrated that rosuvastatin plus ticagrelor
significantly decreased MACE occurrence compared with ticagrelor
monotherapy (RR, 0.29; 95% CI, 0.18-0.47; P<0.001).
Subgroup analyses were carried out based on
follow-up duration and rosuvastatin dose. Among studies with a
follow-up duration of <6 months, pooled analysis demonstrated
that rosuvastatin plus ticagrelor significantly decreased MACE
occurrence compared with ticagrelor monotherapy (RR, 0.24; 95% CI,
0.13-0.47; P<0.001), while no significant heterogeneity was
discovered (I2=0.0%; P=0.947). In studies with a
follow-up duration of ≥6 months, pooled analysis revealed that
rosuvastatin plus ticagrelor significantly decreased MACE
occurrence compared with ticagrelor monotherapy (RR, 0.36; 95% CI,
0.18-0.70; P=0.003), while no significant heterogeneity was
identified (I2=0.0%; P=0.866; Fig. 3A). Furthermore, in the study with a
rosuvastatin dose of 10 mg, rosuvastatin plus ticagrelor
significantly decreased MACE occurrence compared with ticagrelor
monotherapy (RR, 0.27; 95% CI, 0.15-0.50; P<0.001), while no
significant heterogeneity was identified (I2=0.0%;
P=0.907). Regarding the studies with a rosuvastatin dose of 20 mg,
rosuvastatin plus ticagrelor significantly decreased MACE
occurrence compared with ticagrelor monotherapy (RR, 0.33; 95% CI,
0.16-0.69; P=0.003) without significant heterogeneity between the
two studies (I2=0.0%; P=0.543; Fig. 3B).
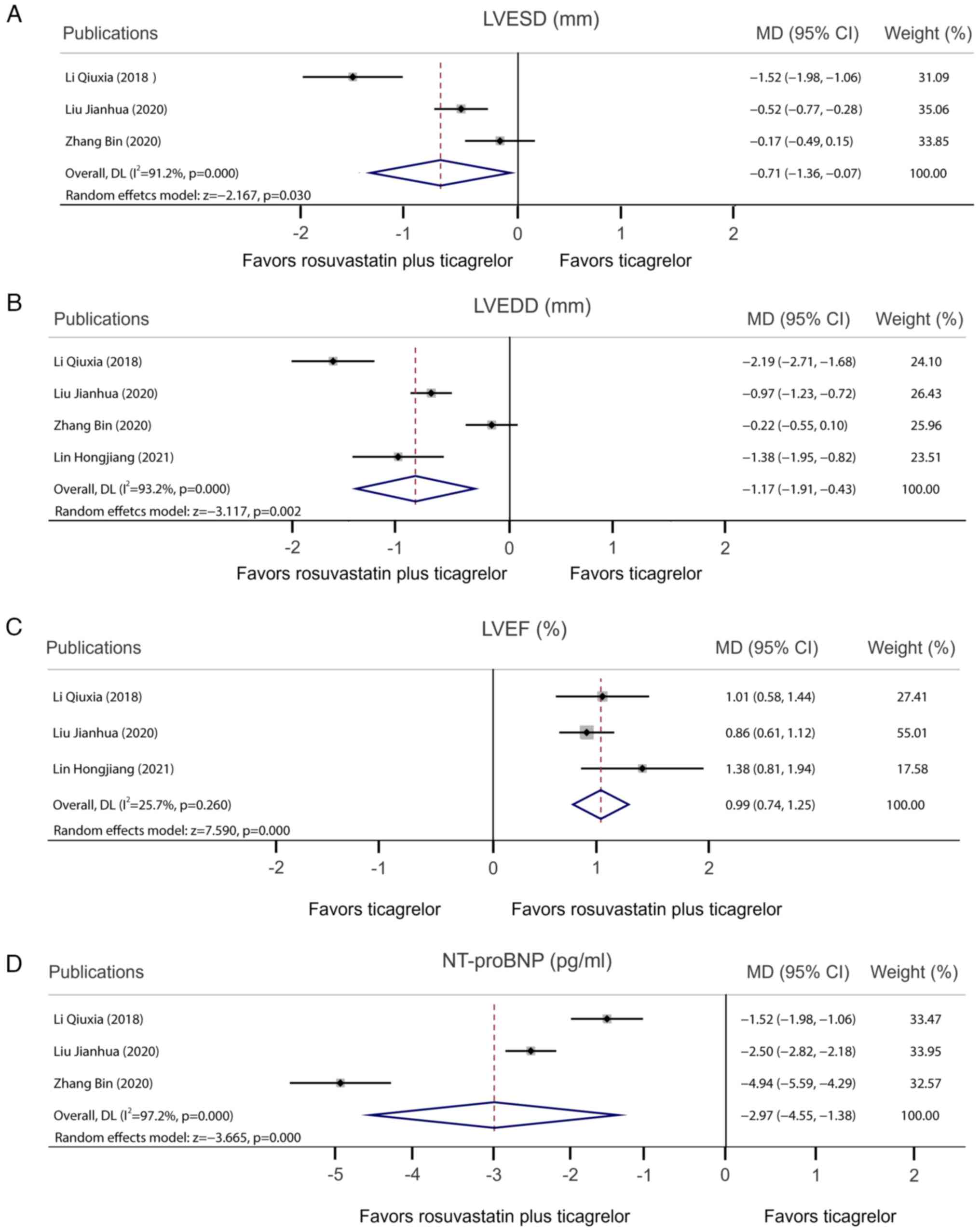
Cardiac function
A total of 3 studies compared LVESD following
rosuvastatin plus ticagrelor vs. ticagrelor alone. Pooled analysis
demonstrated that rosuvastatin plus ticagrelor decreased the LVESD
compared with ticagrelor monotherapy [MD, -0.71; 95% CI,
-(1.36-0.07); P=0.030] with heterogeneity observed among studies
(I2=91.2%; P<0.001; Fig.
4A). Furthermore, 4 studies reported the LVEDD and pooled
analysis demonstrated that, compared with ticagrelor monotherapy,
rosuvastatin plus ticagrelor decreased the LVEDD [MD, -1.17; 95%
CI, -(1.91-0.43); P=0.002]. Meanwhile, heterogeneity was observed
among studies (I2=93.2%; P<0.001; Fig. 4B). In addition, pooled analysis
demonstrated that LVEF rosuvastatin plus ticagrelor increased the
LVEF compared with ticagrelor monotherapy (MD, 0.99; 95% CI,
0.74-1.25; P<0.001) without any heterogeneity observed among the
three studies (I2=25.7%; P=0.260; Fig. 4C). Furthermore, pooled analysis
demonstrated that rosuvastatin plus ticagrelor decreased NT-proBNP
compared with ticagrelor monotherapy [MD, -2.97; 95% CI,
-(4.55-1.38); P<0.001], with heterogeneity observed among the
three studies (I2=97.2%; P<0.001; Fig. 4D).
Quality assessment of included
studies
The risk of bias in RCTs was assessed with the
Cochrane Collaboration's tool, which demonstrated that the overall
risk of bias was low (low risk of sequence generation, completeness
of outcome data and free from selective reporting), while
concealment of allocation and blinded adjudication were unclear
among four RCTs; meanwhile, free from other bias of Zhang et
al (16) was unclear (Table II). Furthermore, the risk of bias
of cohort studies was assessed using the Newcastle-Ottawa Scale
criteria, which demonstrated that the total score of these studies
ranged from 8-9, suggesting low risk of bias (Table III).
 | Table IIAssessment of the risk of bias in
randomized controlled trials by Cochrane Collaboration's tool. |
Table II
Assessment of the risk of bias in
randomized controlled trials by Cochrane Collaboration's tool.
| First author,
year | Sequence
generation | Concealment of
allocation | Blinded
adjudication | Completeness of
outcome data | Free from selective
reporting | Free from other
bias | (Refs.) |
|---|
| Li, 2019 | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk | (14) |
| Zhang et al,
2020 | Low risk | Unclear | Unclear | Low risk | Low risk | Unclear | (16) |
| Yong and Lei,
2021 | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk | (17) |
| Hongjiang,
2021 | Low risk | Unclear | Unclear | Low risk | Low risk | Low risk | (18) |
 | Table IIIAssessment of the risk of bias in
cohort studies by Newcastle-Ottawa Scale criteria. |
Table III
Assessment of the risk of bias in
cohort studies by Newcastle-Ottawa Scale criteria.
| First author,
year | Selection | Comparability | Outcome | Total score | (Refs.) |
|---|
| Li et al,
2018 | 4 | 2 | 2 | 8 | (12) |
| Wang, 2018 | 4 | 2 | 2 | 8 | (13) |
| Liu, 2020 | 4 | 2 | 3 | 9 | (15) |
Sensitivity analysis and publication
bias
Sensitivity analysis demonstrated that omitting the
study by Zhang et al (16)
changed the statistical significance of LVESD and NT-proBNP, while
MACE occurrence, LVEDD and LVEF were not significantly changed by
omitting any single study, which suggested that the results of the
present meta-analysis were stable (Table IV).
 | Table IVSensitivity analysis. |
Table IV
Sensitivity analysis.
| A, MACE
occurrencea |
|---|
| | 95% CI | |
|---|
| First author,
year | Relative risk or
mean difference | Lower | Upper | (Refs.) |
|---|
| Li et al,
2018 | 0.29 | 0.18 | 0.49 | (12) |
| Wang, 2018 | 0.26 | 0.15 | 0.45 | (13) |
| Li, 2019 | 0.30 | 0.18 | 0.48 | (14) |
| Zhang et al,
2020 | 0.29 | 0.17 | 0.48 | (16) |
| Yong and Lei,
2021 | 0.28 | 0.16 | 0.47 | (17) |
| Hongjiang,
2021 | 0.30 | 0.19 | 0.49 | (18) |
| B, LVESD,
mmb |
| | 95% CI | |
| First author,
year | Relative risk or
mean difference | Lower | Upper | (Refs.) |
| Li et al,
2018 | -0.36 | -0.71 | -0.01 | (12) |
| Liu et al,
2020 | -0.83 | -2.16 | 0.50 | (15) |
| Zhang et al,
2020 | -1.00 | -1.98 | -0.03 | (16) |
| C, LVEDD,
mmb |
| | 95% CI | |
| First author,
year | Relative risk or
mean difference | Lower | Upper | (Refs.) |
| Li et al,
2018 | -0.83 | -1.45 | -0.21 | (12) |
| Liu et al,
2020 | -1.25 | -2.50 | -0.01 | (15) |
| Zhang et al,
2020 | -1.50 | -2.24 | -0.75 | (16) |
| Hongjiang,
2021 | -1.11 | -2.02 | -0.20 | (18) |
| D, LVEF,
%b |
| | 95% CI | |
| First author,
year | Relative risk or
mean difference | Lower | Upper | (Refs.) |
| Li et al,
2018 | 0.95 | 0.72 | 1.18 | (12) |
| Liu et al,
2020 | 1.14 | 0.80 | 1.49 | (15) |
| Hongjiang,
2021 | 0.90 | 0.68 | 1.12 | (18) |
| E, NT-proBNP,
pg/mlb |
| | 95% CI | |
| First author,
year | Relative risk or
mean difference | Lower | Upper | (Refs.) |
| Li et al,
2018 | -3.70 | -6.09 | -1.31 | (12) |
| Liu et al,
2020 | -3.22 | -6.57 | 0.12 | (15) |
| Zhang et al,
2020 | -2.03 | -2.98 | -1.07 | (16) |
In addition, Begg's and Egger's tests were conducted
to estimate the potential publication bias and it was demonstrated
that publication bias existed with regard to MACE (P<0.05).
Meanwhile, according to the trim-and-fill method, there was no
difference between the combined and the original results (no new
studies added). Regarding LVESD, LVEDD, LVEF and NT-proBNP, no
obvious publication bias was found (P>0.05; Table V). The funnel plots of MACE, LVESD,
LVEDD, LVEF and NT-proBNP are shown in Fig. S1.
 | Table VPublication bias. |
Table V
Publication bias.
| Item | Begg's test | Egger's test |
|---|
| MACE
occurrence | 0.009 | 0.002 |
| LVESD, mm | 1.000 | 0.529 |
| LVEDD, mm | 0.734 | 0.418 |
| LVEF, % | 0.296 | 0.206 |
| NT-proBNP,
pg/ml | 1.000 | 0.612 |
Discussion
Previous studies have reported that the incidence of
MACE is 10.9-30.5% if there is no related treatment among patients
receiving PCI (23-25).
Several therapies are recommended to prevent the incidence of MACE
during the perioperative period of PCI according to the American
College of Cardiology/AHA guidelines, among which statins serve a
crucial role (26,27). Currently, a number of clinical
trials reported that atorvastatin, simvastatin and rosuvastatin
administered prior to PCI reduced the incidence of MACE (28-31).
Among these drugs, rosuvastatin is able to regulate blood lipid
levels by inhibiting cholesterol synthesis and consequently
suppressing the formation of atherosclerosis; furthermore, its
half-life period is relatively longer and its lipid-lowering effect
is relatively better compared with other statins (32). According to the guidelines of the
AHA for patients with acute coronary syndromes, ticagrelor is
recommended for the perioperative period of PCI, while the
application of rosuvastatin is rarely reported (9). Rosuvastatin and ticagrelor, when used
together, may exert different effects in managing cardiovascular
conditions. Rosuvastatin inhibits
3-hydroxy-3-methyl-glutaryl-coenzyme A reductase and consequently
reduces cholesterol production (7). Ticagrelor, a P2Y12 receptor
antagonist, prevents blood clot formation by inhibiting platelet
activation and aggregation (8).
Several clinical studies reported that rosuvastatin plus ticagrelor
reduces the incidence of MACE and recovers myocardial function
indices compared with ticagrelor alone (12-17);
however, another previous study suggested that rosuvastatin plus
ticagrelor could not achieve these benefits (18). Thus, a future study with a
comprehensive assessment to confirm the effect of rosuvastatin plus
ticagrelor in patients receiving PCI is needed.
In the present study, 7 studies (including 3 cohort
studies and 4 RCTs) including 852 patients receiving rosuvastatin
plus ticagrelor or ticagrelor monotherapy were reviewed. The
subsequent meta-analysis demonstrated that rosuvastatin plus
ticagrelor decreased the incidence of MACE compared with ticagrelor
in patients undergoing PCI. Possible explanations for this may be
the following: i) Rosuvastatin may modify vascular endothelial
function, enhance immune function, accelerate plaque stability and
prevent thrombosis formation, which consequently decreases MACE and
protect the cardiovascular system (7,32);
or ii) rosuvastatin may suppress T-cell-activated inflammation by
inhibiting miRNA (miR)-155 and proinflammatory cytokines, such as
IFN-γ, TNF-α and IL-6, while increasing Src homology 2-containing
inositol phosphatase-1 (SHIP-1) and consequently decreasing MACE
(33). Of note, the subgroup
analysis further confirmed that the effect of rosuvastatin plus
ticagrelor in decreasing the incidence of MACE in patients
undergoing PCI was independent of follow-up duration and
rosuvastatin dose. To the best of our knowledge, this is the first
meta-analysis to explore the efficacy of rosuvastatin plus
ticagrelor vs. ticagrelor monotherapy in patients undergoing PCI,
which may provide solid evidence for the application of
rosuvastatin plus ticagrelor in such patients. Of note, a previous
study reported that rosuvastatin plus ticagrelor increases
myocardial adenosine, reduces infarct size and inhibits
inflammation in rats, which may also explain the findings of the
current meta-analysis (34).
Dysregulated cardiac function is also a severe
complication of PCI (35). Of
note, it has been reported that rosuvastatin treatment may restore
ventricular remodeling and enhance LV systolic function in patients
undergoing PCI (36). The present
meta-analysis compared the effects of rosuvastatin plus ticagrelor
and ticagrelor monotherapy on cardiac function in patients
receiving PCI. The pooled analysis demonstrated that rosuvastatin
plus ticagrelor decreased LVESD, LVEDD and NT-proBNP, but increased
LEVF compared with ticagrelor monotherapy in patients receiving
PCI. It may be suggested that rosuvastatin is able to relieve
cardiac damage by decreasing inflammation and downregulating blood
lipids through several pathways, such as the nod-like receptor
protein 3/toll-like receptor and miR-155/SHIP-1 pathways, which may
attenuate cardiac injury (33,37,38).
However, heterogeneity existed among the analyzed studies reporting
LVESD, NT-proBNP and LVEDD. Meanwhile, the sensitivity analysis
demonstrated that omitting the study by Liu et al (15) changed the significance of LVESD and
NT-proBNP, indicating that further investigation is required.
There were several limitations to the present study.
First, the results were considered robust despite the existence of
publication bias and bias may be due to the small number of
included studies. Furthermore, the type of patient varied among
studies. For instance, some studies only included patients with AMI
(15,16,18),
while other studies included patients with ACS or CHD (12-14,17),
which may lead to bias in the findings. In addition, all included
studies were conducted in China, which may result in regional bias.
The possible reasons for this phenomenon were as follows: i) The
clinicians' prescription preference and decisions may lead to the
prevalent application of rosuvastatin plus ticagrelor; ii) both
rosuvastatin and ticagrelor are covered by the medical insurance of
all patients in China, which may influence the high accessibility
of these drugs to patients; and iii) the efficacy of rosuvastatin
plus ticagrelor may vary among different patient populations;
however, there are currently no studies that have investigated
this. Therefore, the efficacy of rosuvastatin plus ticagrelor
should be verified in patients undergoing PCI from different
populations. As another limitation, the original studies did not
provide the time-line of the clinical course. Hence, it was
difficult to obtain related data for further analysis of the
relationship between the time-points of the clinical course and
concurrent medications. Furthermore, the clinical studies included
in the present meta-analysis were mostly small studies and the
follow-up duration varied among studies, which may limit the
possibility to conclude with confident outcomes. In addition, the
included studies did not present the data of the low-density
lipoprotein-cholesterol (LDL-C) level in both treatment groups,
which is an important individual risk factor of outcome in patients
receiving PCI (39). Therefore,
further studies should verify the impact of LDL-C levels on the
outcomes for patients undergoing PCI who receive rosuvastatin plus
ticagrelor or ticagrelor monotherapy.
In conclusion, the present meta-analysis
demonstrated that rosuvastatin plus ticagrelor decreased the
occurrence of MACE and elevated cardiac function compared with
ticagrelor monotherapy among Chinese patients receiving PCI,
indicating rosuvastatin plus ticagrelor may be a superior treatment
choice for these patients, while its application in other patient
populations requires further exploration.
Supplementary Material
Publication bias by funnel plots.
Funnel plots of (A) MACE occurrence, (B) LVESD, (C) LVEDD, (D) LVEF
and (E) NT-proBNP. MACE, major adverse cardiovascular events;
LVESD, LV end-systolic diameter; LVEDD, LV end-diastolic diameter;
LVEF, left ventricular ejection; NT-proBNP, N-terminal pro-B-type
natriuretic peptide.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HY supervised the study. JS and XJ designed and
conceived the study. JS, XJ and HS participated in the literature
search/selection and data collection. JS and XJ performed the data
analysis and wrote the manuscript. JS, HS, LZ and HY contributed to
the analysis of the results and revised the manuscript. JS and LZ
contributed critically to the intellectual content during the
revision stage. HS and HY confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hoole SP and Bambrough P: Recent advances
in percutaneous coronary intervention. Heart. 106:1380–1386.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Akbari T and Al-Lamee R: Percutaneous
coronary intervention in multi-vessel disease. Cardiovasc Revasc
Med. 44:80–91. 2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hirai T and Grantham JA: Indications and
patient selection for percutaneous coronary intervention of chronic
total occlusions. Interv Cardiol Clin. 10:1–5. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ybarra LF and Rinfret S: Access selection
for chronic total occlusion percutaneous coronary intervention and
complication management. Interv Cardiol Clin. 10:109–120.
2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Simsek B, Kostantinis S, Karacsonyi J,
Hall A, Rangan BV, Croce KJ, Azzalini L, McEntegart M, Shishehbor
M, Egred M, et al: International percutaneous coronary intervention
complication survey. Catheter Cardiovasc Interv. 99:1733–1740.
2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lin YJ, Jiao KL, Liu B, Fang L and Meng S:
Antiplatelet and myocardial protective effect of Shexiang Tongxin
dropping pill in patients undergoing percutaneous coronary
intervention: A randomized controlled trial. J Integr Med.
20:126–134. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lamb YN: Rosuvastatin/Ezetimibe: A review
in hypercholesterolemia. Am J Cardiovasc Drugs. 20:381–392.
2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kabil MF, Dena AS and El-Sherbiny IM:
Ticagrelor. Profiles Drug Subst Excip Relat Methodol. 47:91–111.
2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wallentin L, Becker RC, Budaj A, Cannon
CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, et
al: Ticagrelor versus clopidogrel in patients with acute coronary
syndromes. N Engl J Med. 361:1045–1057. 2009.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Koshy AN, Giustino G, Sartori S, Kyaw H,
Yadav M, Zhang Z, Hooda A, Farooq A, Krishnamoorthy P, Sweeny JM,
et al: Ticagrelor vs prasugrel in a contemporary real-world cohort
undergoing percutaneous coronary intervention. JACC Cardiovasc
Interv. 15:2270–2280. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jiao Y, Hu F, Zhang Z, Gong K, Sun X, Li A
and Liu N: Efficacy and safety of loading-dose rosuvastatin therapy
in elderly patients with acute coronary syndromes undergoing
elective percutaneous coronary intervention. Clin Drug Investig.
35:777–784. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li Q, Sun Y and Mu C: Effect of
Rosuvastatin combined with Ticagrelor in the treatment of acute
coronary syndrome. Henan Med Res. 27:4505–4506. 2018.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
13
|
Wang Y: Effect of rosuvastatin combined
with tegretol on lipid levels and incidence of MACE after PCI in
patients with acute coronary syndrome. J Math Medi. 31:1847–1848.
2018.(In Chinese).
|
|
14
|
Li J: Effect of Rosuvastatin combined with
Tegretol on percutaneous coronary intervention in patients with
coronary artery disease. Strait Pharm J. 31:193–195. 2019.(In
Chinese).
|
|
15
|
Liu J, Jiang R, Zhou J, Yang W, Guo Y, Dai
W and Lv J: Effect of Rosuvastatin combined with Tegretol on
myocardial fibrosis in elderly patients with acute myocardial
infarction. Chin J Gerontol. 40:4496–4499. 2020.(In Chinese).
|
|
16
|
Zhang B, Sun M, Duan C and Shi Y: Effects
of ticagrelor combined with rosuvastatin on cardiac function and
serological indicators in patients with acute ST elevation
myocardial infarction. Drug Evaluation Res. 43:1805–1808. 2020.(In
Chinese).
|
|
17
|
Yong L and Lei J: Effect of rosuvastatin
calcium + tegretol on carotid plaque area, intima-media thickness
and MACE incidence after PCI in patients with coronary artery
disease. Clin Res. 29:83–84. 2021.(In Chinese).
|
|
18
|
Hongjiang L: Effect of rosuvastatin
combined with ticagrelor in patients undergoing percutaneous
coronary intervention for acute myocardial infarction. Chinese and
Foreign Medical Research. 19:133–135. 2021.(In Chinese).
|
|
19
|
Hutton B, Salanti G, Caldwell DM, Chaimani
A, Schmid CH, Cameron C, Ioannidis JP, Straus S, Thorlund K, Jansen
JP, et al: The PRISMA extension statement for reporting of
systematic reviews incorporating network meta-analyses of health
care interventions: checklist and explanations. Ann Intern Med.
162:777–784. 2015.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Higgins JP, Altman DG, Gotzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The cochrane collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Stang A: Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of
nonrandomized studies in meta-analyses. Eur J Epidemiol.
25:603–605. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Luo C, Marks-Anglin A, Duan R, Lin L, Hong
C, Chu H and Chen Y: Accounting for publication bias using a
bivariate trim and fill meta-analysis procedure. Stat Med.
41:3466–3478. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Matsumoto I, Moriya S, Kurozumi M, Namba T
and Takagi Y: Relationship between serum uric acid levels and the
incidence of cardiovascular events after percutaneous coronary
intervention. J Cardiol. 78:550–557. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Savic L, Mrdovic I, Asanin M, Stankovic S,
Krljanac G and Lasica R: Using the RISK-PCI score in the long-term
prediction of major adverse cardiovascular events and mortality
after primary percutaneous coronary intervention. J Interv Cardiol.
2019(2679791)2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang Y and Huang Y: Association between
serum hemoglobin and major cardiovascular adverse event in Chinese
patients with ST-segment elevation myocardial infarction after
percutaneous coronary intervention. J Clin Lab Anal.
36(e24126)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Levine GN, Bates ER, Bittl JA, Brindis RG,
Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, et
al: 2016 ACC/AHA guideline focused update on duration of dual
antiplatelet therapy in patients with coronary artery disease: A
report of the American college of cardiology/American heart
association task force on clinical practice guidelines: An update
of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary
intervention, 2011 ACCF/AHA guideline for coronary artery bypass
graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for
the diagnosis and management of patients with stable ischemic heart
disease, 2013 ACCF/AHA guideline for the management of ST-elevation
myocardial infarction, 2014 AHA/ACC guideline for the management of
patients with non-ST-elevation acute coronary syndromes, and 2014
ACC/AHA guideline on perioperative cardiovascular evaluation and
management of patients undergoing noncardiac surgery. Circulation.
134:e123–e155. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ge Z, Baber U, Claessen BE, Farhan S,
Chandrasekhar J, Li SX, Sartori S, Kini AS, Rao SV, Weiss S, et al:
The prevalence, predictors and outcomes of guideline-directed
medical therapy in patients with acute myocardial infarction
undergoing PCI, an analysis from the PROMETHEUS registry. Catheter
Cardiovasc Interv. 93:E112–E119. 2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Berwanger O, Santucci EV, de Barros ESPGM,
Jesuíno IA, Damiani LP, Barbosa LM, Santos RHN, Laranjeira LN,
Egydio FM, de Oliveira JA, et al: Effect of loading dose of
atorvastatin prior to planned percutaneous coronary intervention on
major adverse cardiovascular events in acute coronary syndrome: The
SECURE-PCI randomized clinical trial. JAMA. 319:1331–1340.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
He W, Cao M and Li Z: Effects of different
doses of atorvastatin, rosuvastatin, and simvastatin on elderly
patients with ST-elevation acute myocardial infarction (AMI) after
percutaneous coronary intervention (PCI). Drug Dev Res. 81:551–556.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Merla R, Daher IN, Ye Y, Uretsky BF and
Birnbaum Y: Pretreatment with statins may reduce cardiovascular
morbidity and mortality after elective surgery and percutaneous
coronary intervention: Clinical evidence and possible underlying
mechanisms. Am Heart J. 154:391–402. 2007.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Chen W, Fan Z, Huang C, Han Z and Liu J:
Enhanced-dose statins for ST-segment elevation myocardial
infarction patients after emergency percutaneous coronary
intervention. Dis Markers. 2022(2751750)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cortese F, Gesualdo M, Cortese A,
Carbonara S, Devito F, Zito A, Ricci G, Scicchitano P and Ciccone
MM: Rosuvastatin: Beyond the cholesterol-lowering effect. Pharmacol
Res. 107:1–18. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xie W, Li P, Wang Z, Chen J, Lin Z, Liang
X and Mo Y: Rosuvastatin may reduce the incidence of cardiovascular
events in patients with acute coronary syndromes receiving
percutaneous coronary intervention by suppressing miR-155/SHIP-1
signaling pathway. Cardiovasc Ther. 32:276–282. 2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Birnbaum Y, Birnbaum GD, Birnbaum I,
Nylander S and Ye Y: Ticagrelor and rosuvastatin have additive
cardioprotective effects via adenosine. Cardiovasc Drugs Ther.
30:539–550. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen C, Wu X, Li Y and Peng Y: Study on
the application effect of bisoprolol combined with sacubitril
valsartan sodium tablets in the cardiac rehabilitation of patients
with acute myocardial infarction combined with left heart failure
after percutaneous coronary intervention (PCI). Ann Palliat Med.
10:5455–5461. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Guo J, Zhang WZ, Zhao Q, Wo JS and Cai SL:
Study on the effect of different doses of rosuvastatin on
ventricular remodeling in patients with acute coronary syndrome
after emergency percutaneous coronary intervention. Eur Rev Med
Pharmacol Sci. 21:4457–4463. 2017.PubMed/NCBI
|
|
37
|
Lee SA, Kim W, Hong TJ, Ahn Y, Kim MH,
Hong SJ, Kim BS, Kim SY, Chae IH, Kim BJ, et al: Effects of
fixed-dose combination of low-intensity rosuvastatin and ezetimibe
versus moderate-intensity rosuvastatin monotherapy on lipid
profiles in patients with hypercholesterolemia: A randomized,
double-blind, multicenter, phase III study. Clin Ther.
43:1573–1589. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ren G, Zhou Q, Lu M and Wang H:
Rosuvastatin corrects oxidative stress and inflammation induced by
LPS to attenuate cardiac injury by inhibiting the NLRP3/TLR4
pathway. Can J Physiol Pharmacol. 99:964–973. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Zhong Z, Hou J, Zhang Q, Zhong W, Li B, Li
C, Liu Z, Yang M and Zhao P: Assessment of the LDL-C/HDL-C ratio as
a predictor of one year clinical outcomes in patients with acute
coronary syndromes after percutaneous coronary intervention and
drug-eluting stent implantation. Lipids Health Dis.
18(40)2019.PubMed/NCBI View Article : Google Scholar
|