Introduction
Sodium-glucose cotransporter 2 inhibitors (SGLT2i)
are a type of medication developed to control diabetic
hyperglycemia that works by reducing renal glucose reabsorption and
inducing glycosuria (1,2). Recent clinical trials have shown that
SGLT2i is effective in reducing mortality and hospitalization due
to heart failure (HF) in patients with type 2 diabetes, regardless
of whether they have HF (3,4). In
patients with diabetes, SGLT2i reduced the risk of all-cause death
and hospitalization for HF by 23% (5).
Studies have also shown that SGLT2i has a positive
effect on renal function, urinary sodium excretion, myocardial
metabolism, and vascular function, making it beneficial for
patients with heart disease (6,7).
Preliminary randomized clinical trials (RCTs) authorized by the US
Food and Drug Administration have also demonstrated the
cardiovascular safety of SGLT2i. Empagliflozin, a commonly used
SGLT2i, has been shown to reduce hospitalization rates,
cardiovascular death, and biomarkers in patients with HF (8). RCTs have evaluated the effects of
SGLT2i in patients with HF for improving symptoms, mortality,
hospitalization, and the levels of relevant biomarkers (9,10).
Based on data from mechanistic studies and preliminary clinical
trials, larger clinical trials with SGLT2i are currently
investigating the potential use of SGLT2i in patients with HF with
and without diabetes mellitus type 2 (T2D) (11). In previous large sample trials,
empagliflozin also showed different outcomes in combination with
cardiovascular endpoints (cardiovascular death, non-fatal
myocardial infarction, or non-fatal stroke); it was found that the
incidence of primary composite cardiovascular outcomes and of death
from any cause was lower after empagliflozin treatment (12). Subjects with diabetes and
atherosclerosis were at greater risk of hospitalization for HF and
vascular disease (13). Therefore,
there is a need for more effective and safer drug treatments.
According to the existing RCT studies, the present meta-analysis
aimed to further elucidate the role of SGLT2i in patients with
preexisting HF with a reduced ejection fraction with or without
diabetes.
Methods
Search strategy. The present meta-analysis
was performed in accordance with the established Preferred
Reporting Items for Systematic Reviews and Meta-analysis (PRISMA)
guidelines (14). The relevant
literature published from conception of a database to July 2021 was
comprehensively and systematically searched across multiple
databases, including PubMed, Embase, Web of Science, and other
databases without language limitations. A range of relevant
keywords were used, including ‘Sodium-glucose cotransporter-2
inhibitors’ or ‘SGLT2i’ or ‘canagliflozin’ or ‘dapagliflozin’ or
‘empagliflozin’ or ‘ertugliflozin’ and ‘HF’ or ‘HF with reduced
ejection fraction’ and ‘randomized clinical trials’ or ‘RCTs’.
Inclusion and exclusion criteria
The following were the criteria for inclusion of
studies: i) RCTs; ii) study population consisted of patients with
HF and a low ejection fraction with or without diabetes [left
ventricular ejection fraction (LVEF) <40%]; iii) treatment
measures had to be SGLT2i and placebo-controlled; iv) the primary
outcome indicators were hospitalization for HF/cardiovascular
death, cardiovascular death, hospitalization for HF and all causes
mortality. The exclusion criteria were: i) Duplicate articles; ii)
conference abstracts, comments, letters, systematic reviews, and
meta-analysis; iii) non-RCTs; and iv) studies where major research
indicators were not reported.
Data extraction
The researchers independently searched the studies
and extracted the data, including trial design, patient baseline
data statistics, and clinical results.
Quality assessment
The literature included underwent a risk bias
assessment using the RCT bias risk assessment tool provided by the
Cochrane Collaboration (15). The
bias assessment included various domains, such as random sequence
generation and allocation concealment, blinding of patients and
investigators, blinding of outcome assessors, flawed outcome data,
and selective reporting.
Statistical analysis
Statistical analyses were undertaken using Stata
statistical software (version 15.0; StataCorp, LLC). Review Manager
version 5.3 (Cochrane Collaboration) was used to assess the risk of
bias. The combined effect was estimated using the odds ratio (OR)
and 95% confidence interval (CI). Based on the heterogeneity test,
random or fixed effects models were selected to estimate the pooled
effects. The Q test and I2 test were used to estimate
inter-study heterogeneity. When P>0.1 and I2≤50%, the
fixed effects model was adopted, whereas when P<0.1 and
I2≥0%, the random effects model was adopted. Each study
was gradually removed for sensitivity analysis, which evaluated the
stability of the results. Due to the small number of studies
included in the present study, funnel plots and Egger's test were
not used for publication bias analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Search results
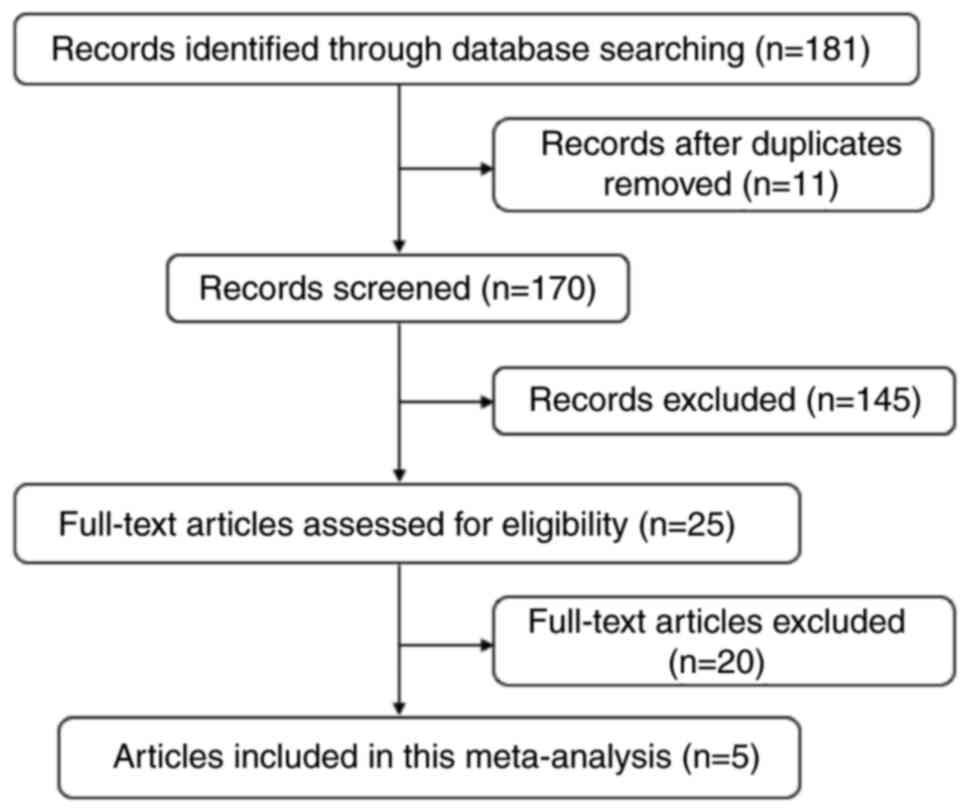
A total of 181 studies were retrieved and
identified, and 11 duplicate studies were eliminated. The titles
and abstracts were read, after which 145 irrelevant studies were
excluded, and 25 full papers were read. According to the inclusion
and exclusion criteria, 20 studies were excluded, and 5 studies
were included in the present meta-analysis. The flow chart of the
study selection process is shown in Fig. 1. Information on the included
studies is presented in Table I.
The mean age of patients was >60 years. The types of SGLT2i used
in the included studies were empagliflozin and dapagliflozin, with
the same intervention dose of 10 mg. The mean LVEF% was <40%.
The intervention time was >12 months.
 | Table IBaseline characteristics of the
included studies. |
Table I
Baseline characteristics of the
included studies.
| First author,
year | Study type | Diabetes status | HFrEF sample, n
I/C | Intervention | Control | Age, years, I/C | Mean LVEF%, I/C | Duration of
treatment | (Refs.) |
|---|
| Packer et al,
2020 | Random double-blind
trail | No diabetes | 1,863/1,867 | Empagliflozin 10 mg
daily | Placebo |
67.2±10.8/66.5±11.2 |
27.7±6.0/27.2±6.1 | 16 months | (8) |
| Kato et al,
2019 | Randomized
double-blind multinational, phase IIIb study | Diabetes | 318/353 | Dapagliflozin 10
mg | Placebo | 63(58,68)/- | 38(30,40)/- | 11.8±7.8 years | (32) |
| Petrie et al,
2020 | Double-blind,
randomized, parallel-group study | No diabetes
Diabetes | 1,298/1307
1,075/1064 | Dapagliflozin 10
mg | Placebo | 66.0±11.8/66.4±11.5
66.3±9.9/66.7±9.8 | 31.0±6.8/30.8±6.9
31.4±6.6/31.0±6.8 | 30 months | (33) |
| Anker et al,
2021 | Randomized, double
blind, parallel-group, placebo-controlled | No diabetes
Diabetes | 936/938
927/929 | Empagliflozin 10
mg | Placebo | 67.6±11.6/66.3±10.0
66.8±10.0/66.6±10.3 | 27.9±6.0/27.2±6.0
27.6±6.0/27.2±6.1 | - | (34) |
| McMurray et
al, 2019 | Phase III, random,
placebo-controlled trial | No diabetes | 2,373/2,371 | Dapagliflozin 10
mg | Placebo |
66.2±11.0/66.5±10.8 |
31.2±6.7/30.9±6.9 | 18.2 months | (35) |
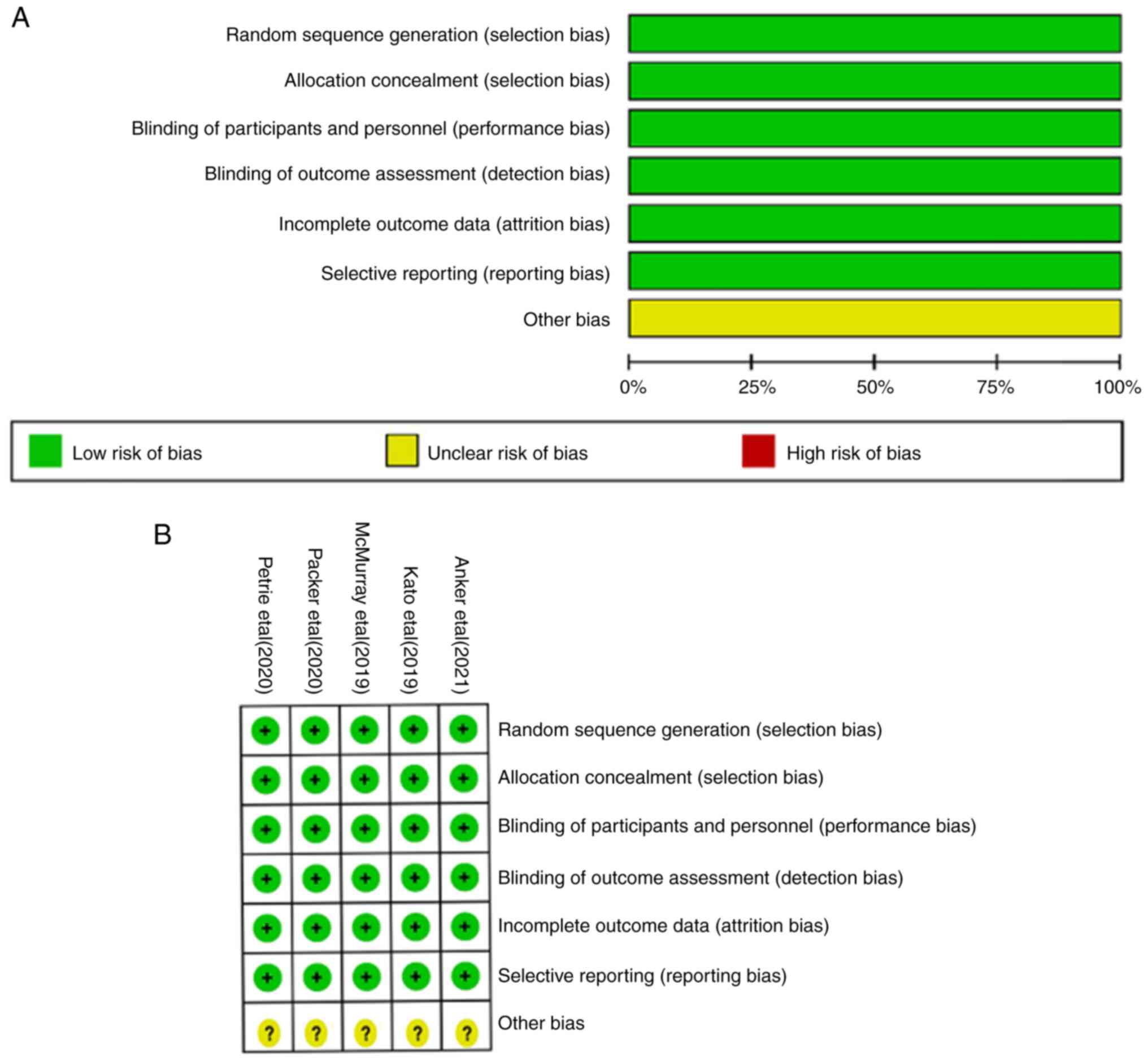
Study characteristics and risk of
bias
The results of the risk bias evaluation in studies
are shown in Fig. 2. The 5 studies
included were all RCTs performed with a clear randomization method.
Stratified seclusion, implementation of blinding, data integrity
without loss, and other risks were unknown. The included studies
were all low-risk and of high quality.
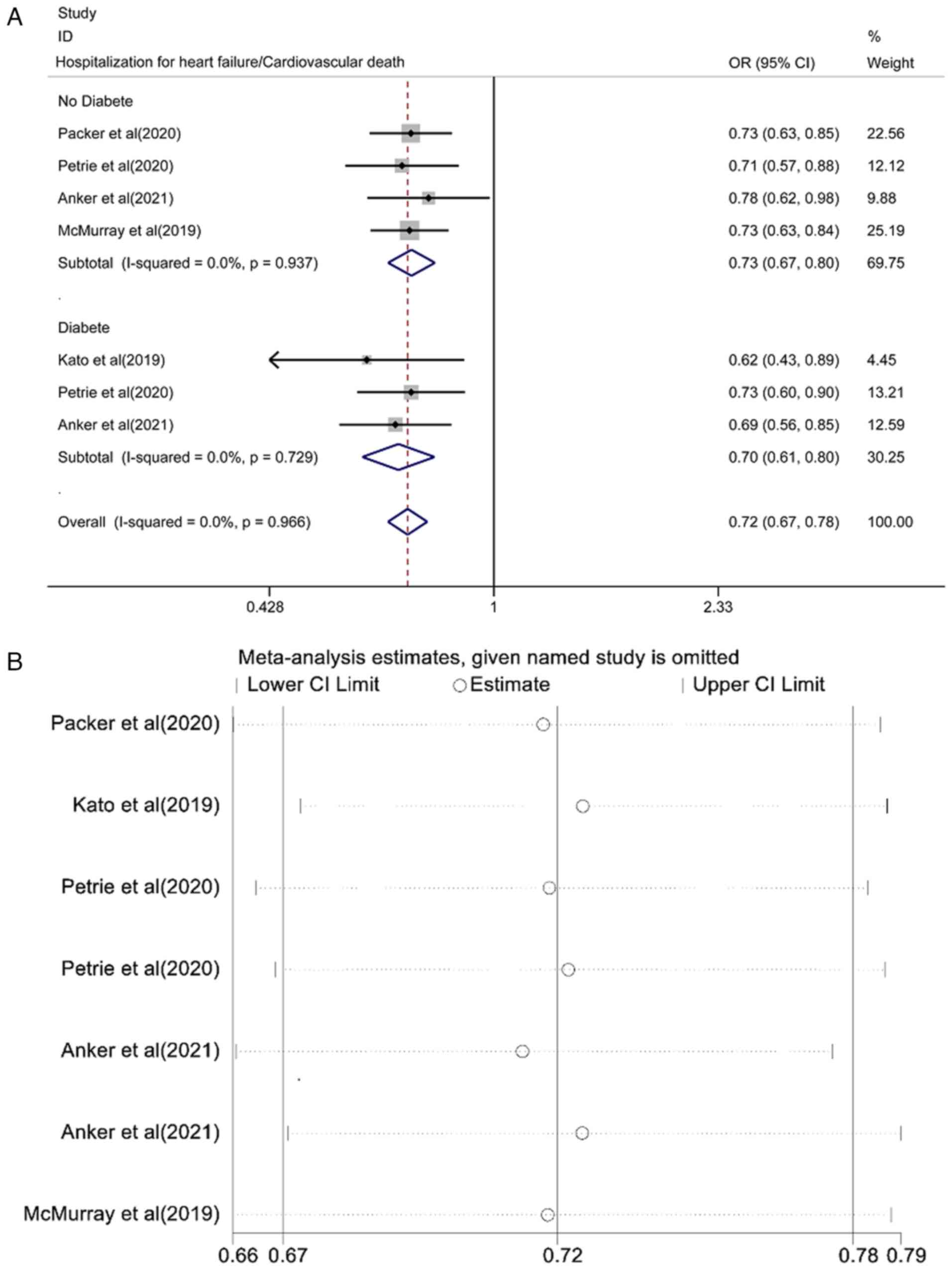
Pooled effect estimation of the risk
of hospitalization for HF/cardiovascular death
The pooled effect estimation of the risk of
hospitalization for HF/cardiovascular death is shown in Fig. 3A. The fixed-effect model showed
that the risk of hospitalization for HF/cardiovascular death in the
SGLT2i group was lower than that in the placebo control group and
the differences were statistically significant [OR=0.72, 95% CI
(0.67-0.78), P<0.0001; I2=0.0%, P=0.966]. There was
no heterogeneity among the studies. A subgroup analysis of the
patients with or without diabetes found that the risk of
hospitalization for HF/cardiovascular death was lower than that of
the placebo control group and the difference was statistically
significant [OR=0.73, 95% CI (0.67-0.80), P<0.0001;
I2=0.0%, P=0.937; and OR=0.70, 95% CI (0.61-0.80),
P<0.0001; I2=0.0%, P=0.729]. Analysis of sensitivity
results is shown in Fig. 3B. Each
study was gradually removed, and the pooled effect value was within
the range of the 95% CI (0.67-0.78) and the results of the study
were stable.
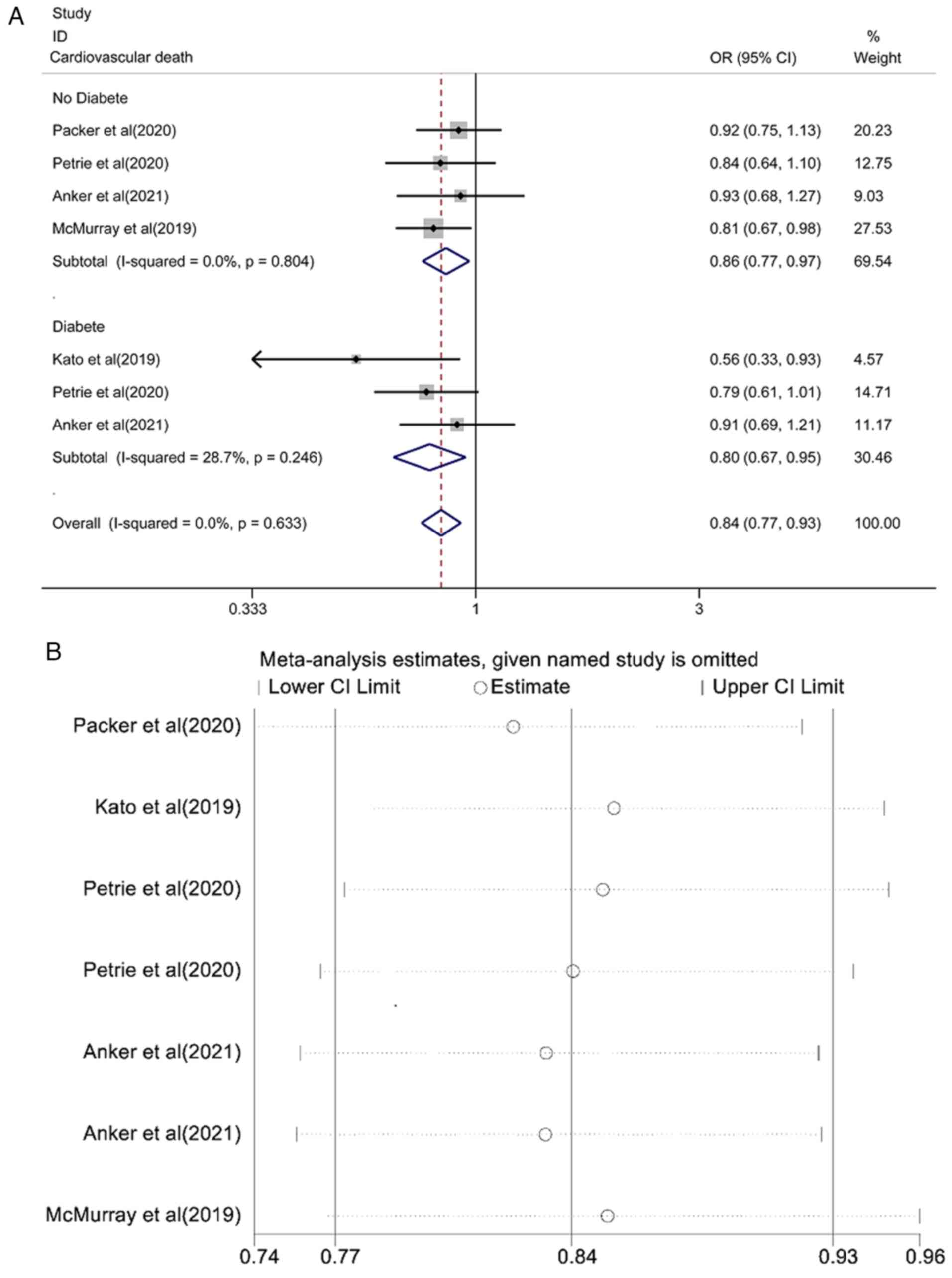
Pooled effect estimation of the risk
of cardiovascular death
The pooled effect estimation of the risk of
cardiovascular death is shown in Fig.
4A. The fixed-effect model showed that the risk of
cardiovascular death in the SGLT2i group was lower than that in the
placebo control group and the difference was statistically
significant [OR=0.84, 95% CI (0.77-0.93), P<0.0001;
I2=0.0%, P=0.633]. There was no heterogeneity among the
studies. A subgroup analysis of patients with or without diabetes
found that the risk of cardiovascular death was lower than that of
placebo control and the difference was statistically significant
[OR=0.86, 95% CI (0.77-0.97), P=0.013; I2=0.0%, P=0.804;
and OR=0.80, 95% CI (0.67-0.95), P<0.0001; I2=28.7%,
P=0.013]. The results of the sensitivity analysis are shown in
Fig. 4B. Each study was gradually
removed, and the pooled effect value was within the range of the
95% CI (0.77-0.93), and the results of the study were stable.
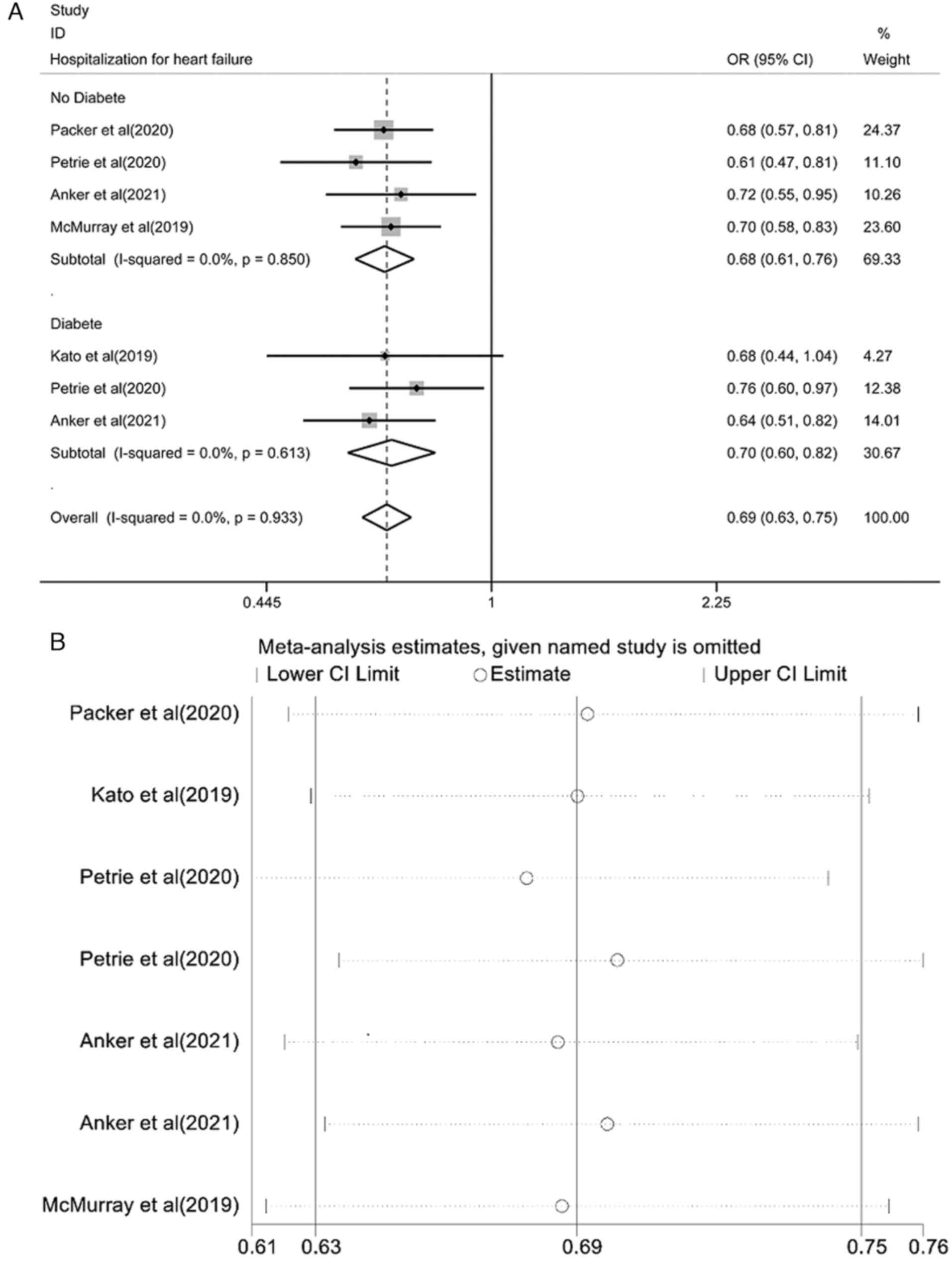
Pooled effect estimation of the risk
of hospitalization for HF
The pooled effect estimation of the risk of
hospitalization for HF is shown in Fig. 5A. The fixed-effect model showed
that the risk of hospitalization for HF in the SGLT2i group was
lower than that in the placebo control group and the differences
were statistically significant [OR=0.69, 95% CI (0.63-0.75),
P<0.0001; I2=0.0%, P=0.933]. There was no
heterogeneity among the studies. A subgroup analysis of patients
with or without diabetes found that the risk of hospitalization for
HF was lower than that of the placebo controls and the differences
were statistically significant [OR=0.68, 95% CI (0.61-0.76),
P<0.0001; I2=0.0%, P=0.850; and OR=0.70, 95% CI
(0.60-0.82), P<0.0001; I2=0.0%, P=0.613]. The results
of the sensitivity analysis are shown in Fig. 5B. Each study was gradually removed,
and the pooled effect value was within the range of the 95% CI
(0.63-0.75), and the results of the study were stable.
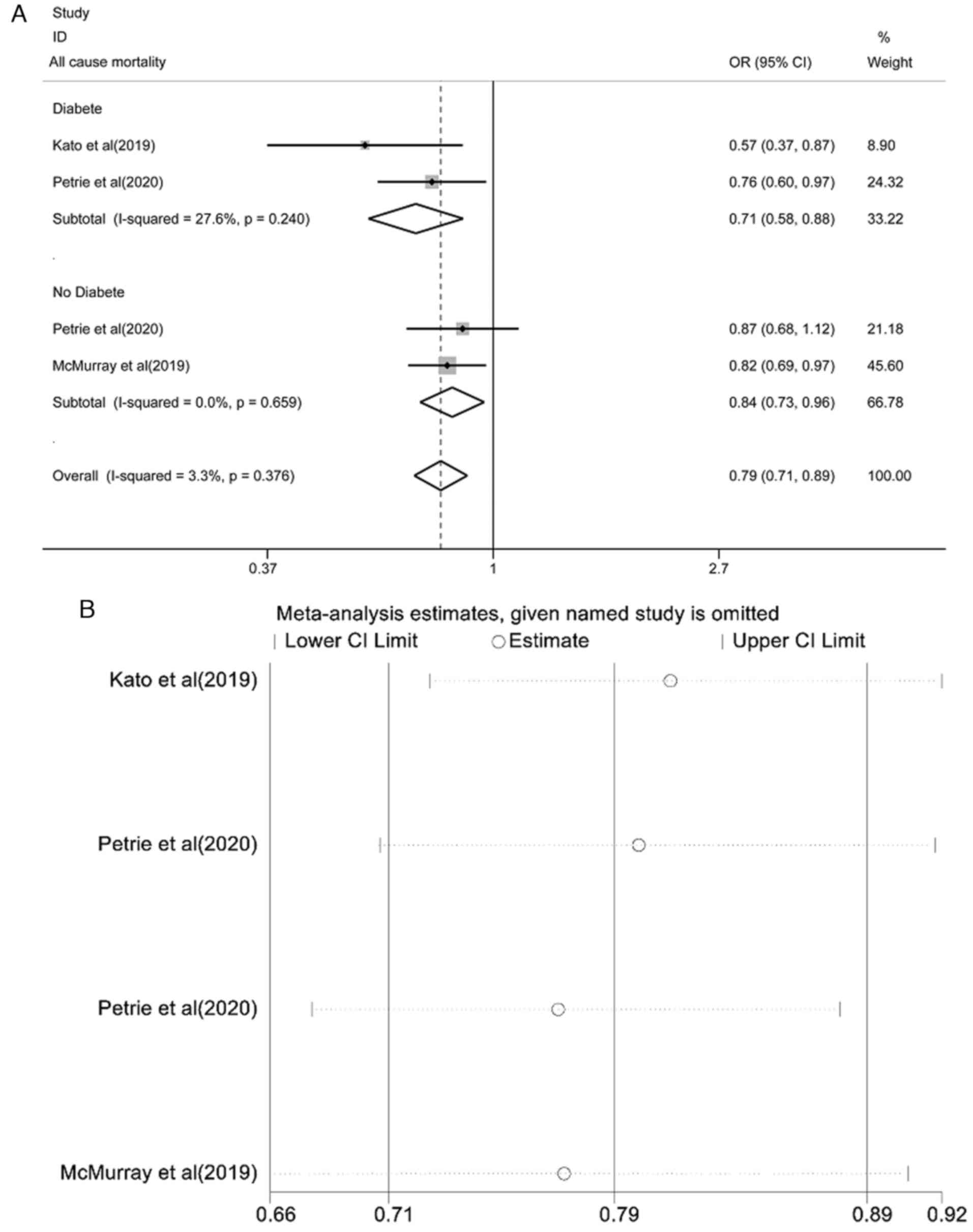
Pooled effect estimation of all-cause
mortality
The pooled effect estimate of all-cause mortality is
shown in Fig. 6A. The fixed-effect
model showed that the risk of all-cause mortality in the SGLT2i
group was lower than that in the placebo control group and the
difference was statistically significant [OR=0.79, 95% CI
(0.71-0.89), P<0.0001; I2=3.3%, P=0.376]. There was
no heterogeneity among the studies. A subgroup analysis of patients
with or without diabetes found that all-cause mortality was lower
than that of placebo controls and the difference was statistically
significant [OR=0.71, 95% CI (0.58-0.88), P<0.0001;
I2=27.6%, P=0.240; and OR=0.84, 95% CI (0.73-0.96),
P=0.012; I2=0.0%, P=0.659]. The results of the
sensitivity analysis are shown in Fig.
6B. Each study was gradually removed, and the pooled effect
value was within the range of the 95% CI (0.71-0.89) and the
results of the study were stable.
Discussion
Studies have shown that SGLT2 is present in early
proximal brush like margin tubules and functions by reabsorbing
almost all filtered glucose (16,17).
SGLT2i blocks glucose reabsorption of proximal convoluted tubules,
further leading to secondary osmosis and then sodium diuretic and
diuretic effects (16). The SGLT2i
dagliflozin not only lowers blood glucose but has also shown a
positive effect on patients with HF in recent studies (18). Blood sugar levels drop due to the
increased glucose excretion. In the case of hypoglycemia, a
reduction in mortality from HF was observed in cohort studies
(19). Given the low levels of
SGLT2 in cardiomyocytes, there is evidence that SGLT2-independent
effects are likely to be achieved by the off-target effect of SGLT2
in the myocardium (20). It is
well established that individuals with diabetes have a higher risk
of cardiovascular complications, and appropriate diabetes control
helps to minimize these complications. SGLT2i has been shown to be
an effective treatment for diabetes with favorable renal side
effects and improved cardiovascular outcomes (5,21,22).
The present meta-analysis focused on treating patients with known
HF and showed a significant reduction in cardiovascular death or
hospitalization for HF compared with placebo groups.
The role of cardiologists and HF specialists in
diabetes management is changing with the introduction of SGLT2i and
its improvement in cardiovascular outcomes in both diabetic and
non-diabetic patients. Studies have shown equivalent efficacy with
SGLT2i therapy in patients without diabetes or with glycated
hemoglobin levels ≥5.7% and <5.7% (23,24).
Consistent with the above research results, the results of the
present meta-analysis showed that for patients with a reduced
ejection fraction, regardless of whether they had diabetes or not,
SGLT2i had a better effect on the treatment of HF. Salah et
al (25) showed that the use
of SGLT2i in AHF patients reduced the risk of HF readmission by 48%
and improved Kansas City Cardiomyopathy Questionnaire (KCCQ) scale
scores. Spertus et al (26)
showed that SGLT2i in HF patients could alleviate HF symptoms
regardless of whether patients had diabetes as was observed based
on the increased KCCQ Total Symptom Score. The present
meta-analysis included patients with HF with or without diabetes
mellitus and an ejection fraction of <40%, and it was concluded
that cardiovascular death or hospitalization for HF was
significantly lower in patients treated with SGLT2i. In this
systematic review and meta-analysis, 5 RCTs with a total of 34,108
participants were included. The fixed-effect model showed that
hospitalization/cardiovascular death, cardiovascular death,
hospitalization for HF, and death from all causes in patients
treated with SGLT2i were significantly lower than those treated
with placebo. That is, the SGLT2i group exhibited significantly
better outcomes compared with the placebo group. In addition,
dapagliflozin reduced the risk of death and worsening of HF and
alleviated the symptoms of the disease in patients of different
ages (27).
Treatment with SGLT2i in patients with T2D has been
shown to reduce the risk of hospitalization for HF and slow the
progression of kidney diseases (5). There is a link between diabetes and
HF. However, no approved drugs reduce the risk of HF in diabetics.
Similar to the findings of the present analysis, a systematic
review and meta-analysis in 2021 showed that patients in the
empagliflozin group had lower rates of cardiovascular mortality and
hospitalization for worsening HF than those in the placebo group
(28). The present study more
comprehensively included the SGLT2i studies and incorporated the
latest research. The present study excluded the effect of diabetes
and found that SGLT2i could improve the risk of HF in terms of HF
hospitalization, cardiovascular death, and all-cause mortality. One
study found that compared with placebo, SGLT2i primarily improved
mortality, HF hospitalization rate, HF emergency department visits,
and reduced serious adverse events (29). A recent study found that SGLT2i
reduced the risk of cardiovascular death and hospitalization for HF
in patients with diabetes (30).
Gager et al (31) found
that empagliflozin did not achieve statistical significance in
reducing cardiovascular and all-cause mortality. The studies
included in the present analysis found that empagliflozin
significantly reduced all-cause mortality in patients with a
reduced ejection fraction and HF. One explanation for the
difference may be due to differences in the range of patients
included. Studies have shown that SGLT2i significantly reduced the
risk of cardiovascular mortality or HF in patients with a lower
ejection fraction (32).
Therefore, all patients with HF included in the present study had
an ejection fraction <40%.
The present analysis has several limitations.
Although there are several SGLT2i, studies using only two SGLT2i
(dapagliflozin and empagliflozin) were included to compare with
placebo. The number of RCTs included in the present analysis was
too small; thus, subgroup analysis of two different SGLT2icould not
be performed. Additional high-quality articles are required to
improve our understanding of the benefits of SGLT2i for the
management of patients with HF with a reduced ejection
fraction.
In conclusion, SGLT2i can reduce the risk of HF
hospitalization, cardiovascular death, and all-cause mortality in
patients with HF and a reduced ejection fraction, regardless of the
presence or absence of diabetes.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by the Medical Research
Project of Sichuan Province (grant no. S22091) and the Medical
Research Project of Sichuan Province (grant no. S21111).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
KZ and QY contributed to the conception and design
of the study, preparation of the material, collection of the data,
and analysis of the data. QY wrote the first draft. KZ and QY both
contributed to the revision of the manuscript. KZ and QY have read
and approved the final manuscript. KZ and QY confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wright EM, Loo DD and Hirayama BA: Biology
of human sodium glucose transporters. Physiol Rev. 91:733–794.
2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nauck MA: Update on developments with
SGLT2 inhibitors in the management of type 2 diabetes. Drug Des
Devel Ther. 8:1335–1380. 2014.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Täger T, Atar D, Agewall S, Katus HA,
Grundtvig M, Cleland JGF, Clark AL, Fröhlich H and Frankenstein L:
Comparative efficacy of sodium-glucose cotransporter-2 inhibitors
(SGLT2i) for cardiovascular outcomes in type 2 diabetes: A
systematic review and network meta-analysis of randomised
controlled trials. Heart Fail Rev. 26:1421–1435. 2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Fernandes GC, Fernandes A, Cardoso R,
Penalver J, Knijnik L, Mitrani RD, Myerburg RJ and Goldberger JJ:
Association of SGLT2 inhibitors with arrhythmias and sudden cardiac
death in patients with type 2 diabetes or heart failure: A
meta-analysis of 34 randomized controlled trials. Heart Rhythm.
18:1098–1105. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zelniker TA, Wiviott SD, Raz I, Im K,
Goodrich EL, Bonaca MP, Mosenzon O, Kato ET, Cahn A, Furtado RHM,
et al: SGLT2 inhibitors for primary and secondary prevention of
cardiovascular and renal outcomes in type 2 diabetes: A systematic
review and meta-analysis of cardiovascular outcome trials. Lancet.
393:31–39. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ujjawal A, Schreiber B and Verma A:
Sodium-glucose cotransporter-2 inhibitors (SGLT2i) in kidney
transplant recipients: What is the evidence? Ther Adv Endocrinol
Metab. 13(20420188221090001)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen S, Coronel R, Hollmann MW, Weber NC
and Zuurbier CJ: Direct cardiac effects of SGLT2 inhibitors.
Cardiovasc Diabetol. 21(45)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Packer M, Anker SD, Butler J, Filippatos
G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann
M, et al: Cardiovascular and renal outcomes with empagliflozin in
heart failure. N Engl J Med. 383:1413–1424. 2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
De Marzo V, Savarese G, Porto I, Metra M
and Ameri P: Efficacy of SGLT2-inhibitors across different
definitions of heart failure with preserved ejection fraction. J
Cardiovasc Med (Hagerstown). 24:537–543. 2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Younes AM, Salem M, Maraey A, Nomigolzar
S, Sewell K, Khalil M, Elzanaty A, Saeyeldin A and Dar M: Safety
outcomes of SGLT2i in the heart failure trials: A systematic review
and meta-analysis. Int J Cardiol. 366:51–56. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Li X, Wu H, Peng H and Jiang H: Comparison
the effects of finerenone and SGLT2i on cardiovascular and renal
outcomes in patients with type 2 diabetes mellitus: A network
meta-analysis. Front Endocrinol (Lausanne).
13(1078686)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wiviott SD, Raz I, Bonaca MP, Mosenzon O,
Kato ET, Cahn A, Silverman MG, Zelniker TA, Kuder JF, Murphy SA, et
al: Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N
Engl J Med. 380:347–357. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zinman B, Wanner C, Lachin JM, Fitchett D,
Bluhmki E, Hantel S, Mattheus M, Devins T, Johansen OE, Woerle HJ,
et al: Empagliflozin, cardiovascular outcomes, and mortality in
type 2 diabetes. N Engl J Med. 373:2117–2128. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Moher D, Liberati A, Tetzlaff J and Altman
DG: Prisma group. Preferred reporting items for systematic reviews
and meta-analyses: The PRISMA statement. PLoS Med. 151:264–269.
2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Tromp J, Ouwerkerk W, van Veldhuisen DJ,
Hillege HL, Richards AM, van der Meer P, Anand IS, Lam CS and Voors
AAJHF: A systematic review and network meta-analysis of
pharmacological treatment of heart failure with reduced ejection
fraction. JACC Heart Fail. 10:73–84. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Shentu Y, Li Y, Xie S, Jiang H, Sun S, Lin
R, Chen C, Bai Y, Zhang Y, Zheng C and Zhou Y: Empagliflozin, a
sodium glucose cotransporter-2 inhibitor, ameliorates peritoneal
fibrosis via suppressing TGF-β/Smad signaling. Int Immunopharmacol.
93(107374)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dharmalingam M, Aravind SR, Thacker H,
Paramesh S, Mohan B, Chawla M, Asirvatham A, Goyal R, Shembalkar J,
Balamurugan R, et al: Efficacy and safety of remogliflozin
etabonate, a new sodium glucose co-transporter-2 inhibitor, in
patients with type 2 diabetes mellitus: A 24-week, randomized,
double-blind, active-controlled trial. Drugs. 80:587–600.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Cao Y, Li P, Li Y and Han Y:
Sodium-glucose cotransporter-2 inhibitors in heart failure: An
updated meta-analysis. ESC Heart Fail. 9:1942–1953. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kaze AD, Zhuo M, Kim SC, Patorno E and
Paik JM: Association of SGLT2 inhibitors with cardiovascular,
kidney, and safety outcomes among patients with diabetic kidney
disease: A meta-analysis. Cardiovasc Diabetol.
21(47)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Koyani CN, Plastira I, Sourij H, Hallström
S, Schmidt A, Rainer PP, Bugger H, Frank S, Malle E and von
Lewinski D: Empagliflozin protects heart from inflammation and
energy depletion via AMPK activation. Pharmacol Res.
158(104870)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Usman MS, Siddiqi TJ, Memon MM, Khan MS,
Rawasia WF, Ayub MT, Sreenivasan J and Golzar Y: Sodium-glucose
co-transporter 2 inhibitors and cardiovascular outcomes: A
systematic review and meta-analysis. Eur J Prev Cardiol.
25:495–502. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zelniker TA, Wiviott SD, Raz I, Im K,
Goodrich EL, Furtado RHM, Bonaca MP, Mosenzon O, Kato ET, Cahn A,
et al: Comparison of the effects of glucagon-like peptide receptor
agonists and sodium-glucose cotransporter 2 inhibitors for
prevention of major adverse cardiovascular and renal outcomes in
type 2 diabetes mellitus. Circulation. 139:2022–2031.
2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
American Diabetes Association. 2.
Classification and diagnosis of diabetes: standards of medical care
in diabetes-2019. Diabetes Care. 42:S13–S28. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chatterton H, Younger T, Fischer A and
Khunti K: Programme Development Group. Risk identification and
interventions to prevent type 2 diabetes in adults at high risk:
Summary of NICE guidance. BMJ. 345(e4624)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Salah HM and Fudim M: Sodium-glucose
cotransporter 2 inhibitors and nonalcoholic fatty liver disease.
Heart Fail Clin. 18:625–634. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Spertus JA, Birmingham MC, Nassif M,
Damaraju C, Abbate A, Butler J, Lanfear DE, Lingvay I, Kosiborod MN
and Januzzi JL: The SGLT2 inhibitor canagliflozin in heart failure:
The CHIEF-HF remote, patient-centered randomized trial. Nat Med.
28:809–813. 2022.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Martinez FA, Serenelli M, Nicolau JC,
Petrie MC, Chiang CE, Tereshchenko S, Solomon SD, Inzucchi SE,
Køber L, Kosiborod MN, et al: Efficacy and safety of dapagliflozin
in heart failure with reduced ejection fraction according to age:
Insights from DAPA-HF. Circulation. 141:100–111. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Pan D, Xu L, Chen P, Jiang H, Shi D and
Guo M: Empagliflozin in patients with heart failure: A systematic
review and meta-analysis of randomized controlled trials. Front
Cardiovasc Med. 8(683281)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Chambergo-Michilot D, Tauma-Arrué A and
Loli-Guevara SJIH: Effects and safety of SGLT2 inhibitors compared
to placebo in patients with heart failure: A systematic review and
meta-analysis. Int J Cardiol Heart Vasc. 32(100690)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yan Y, Liu B, Du J, Wang J, Jing X, Liu Y,
Deng S, Du J and She Q: SGLT2i versus ARNI in heart failure with
reduced ejection fraction: A systematic review and meta-analysis.
ESC Heart Fail. 8:2210–2219. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Gager GM, von Lewinski D, Sourij H, Jilma
B, Eyileten C, Filipiak K, Huelsmann M, Kubica J, Postula M and
Siller-Matula JM: Effects of SGLT2 inhibitors on ion homeostasis
and oxidative stress associated mechanisms in heart failure. Biomed
Pharmacother. 143(112169)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Kato ET, Silverman MG, Mosenzon O,
Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL,
Leiter LA, et al: Effect of Dapagliflozin on heart failure and
mortality in type 2 diabetes mellitus. Circulation. 139:2528–2536.
2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Petrie MC, Verma S, Docherty KF, Inzucchi
SE, Anand I, Bělohlávek J, Böhm M, Chiang CE, Chopra VK, de Boer
RA, et al: Effect of dapagliflozin on worsening heart failure and
cardiovascular death in patients with heart failure with and
without diabetes. JAMA. 323:1353–1368. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Anker SD, Butler J, Filippatos G, Khan MS,
Marx N, Lam CS, Schnaidt S, Ofstad AP, Brueckmann M, Jamal W, et
al: Effect of empagliflozin on cardiovascular and renal outcomes in
patients with heart failure by baseline diabetes status: Results
from the EMPEROR-reduced trial. Circulation. 143:337–349.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
McMurray JJ, Solomon SD, Inzucchi SE,
Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS,
Anand IS, Bělohlávek J, et al: Dapagliflozin in patients with heart
failure and reduced ejection fraction. N Engl J Med. 381:1995–2008.
2019.PubMed/NCBI View Article : Google Scholar
|