Introduction
Gastrointestinal (GI) perforation is one of the most common acute abdominal diseases encountered in emergency departments. Although progress has been made in the treatment of this condition, the associated mortality has not markedly decreased (1,2). The clinical manifestations of GI perforation include sudden abdominal pain accompanied by nausea and vomiting, with a rapid onset and aggressive nature. If GI perforation is not diagnosed and treated promptly and effectively, the leakage of GI contents into the peritoneal cavity can occur, causing peritonitis and septic shock, and in severe cases, endangering the life of the patient (3). The most frequent causes of GI perforation comprise peptic ulcers, GI tumors, inflammatory bowel disease, trauma and iatrogenic injury (4). The prevalence of GI perforation associated with GI ulcers has appreciably diminished owing to extensive advancements in Helicobacter pylori therapy (5). Currently, conservative medical treatment, endoscopic repair and surgical treatment are viable options for the management of GI perforation. The selection of which treatment modality to use greatly influences the survival and prognosis of patients.
As present, there is no consensus on whether patients with GI perforation benefit from emergency laparotomy (4,6,7). To improve patient outcomes following emergency laparotomy, the establishment of dependable selection criteria for surgical candidates in developing nations is particularly crucial due to the substantial costs associated with perioperative care and management in an intensive care unit. Therefore, the present study retrospectively examined 529 cases of GI perforation to assess the influence of preoperative clinical characteristics, laboratory test results and other pertinent indicators on patient survival and prognosis.
Materials and methods
Study design
Demographic and clinical variables were retrospectively collected from patients who had surgically confirmed GI perforation and were admitted to the First Affiliated Hospital of Fujian Medical University (Fuzhou, China) between January 2012 and June 2022.
Exclusion and inclusion criteria
Only patients who had undergone surgery were included in the present study. Patients were first screened for eligibility based on the following criteria: i) Age ≥18 years and ii) underwent emergency abdominal exploratory surgery. Exclusion criteria were: i) Patients with insufficient information (n=179) or duplicate records (n=49) and ii) patients with other systemic surgical indications, including thoracic-abdominal joint injury and abdominal large blood vessel injury (n=27) (Fig. 1).
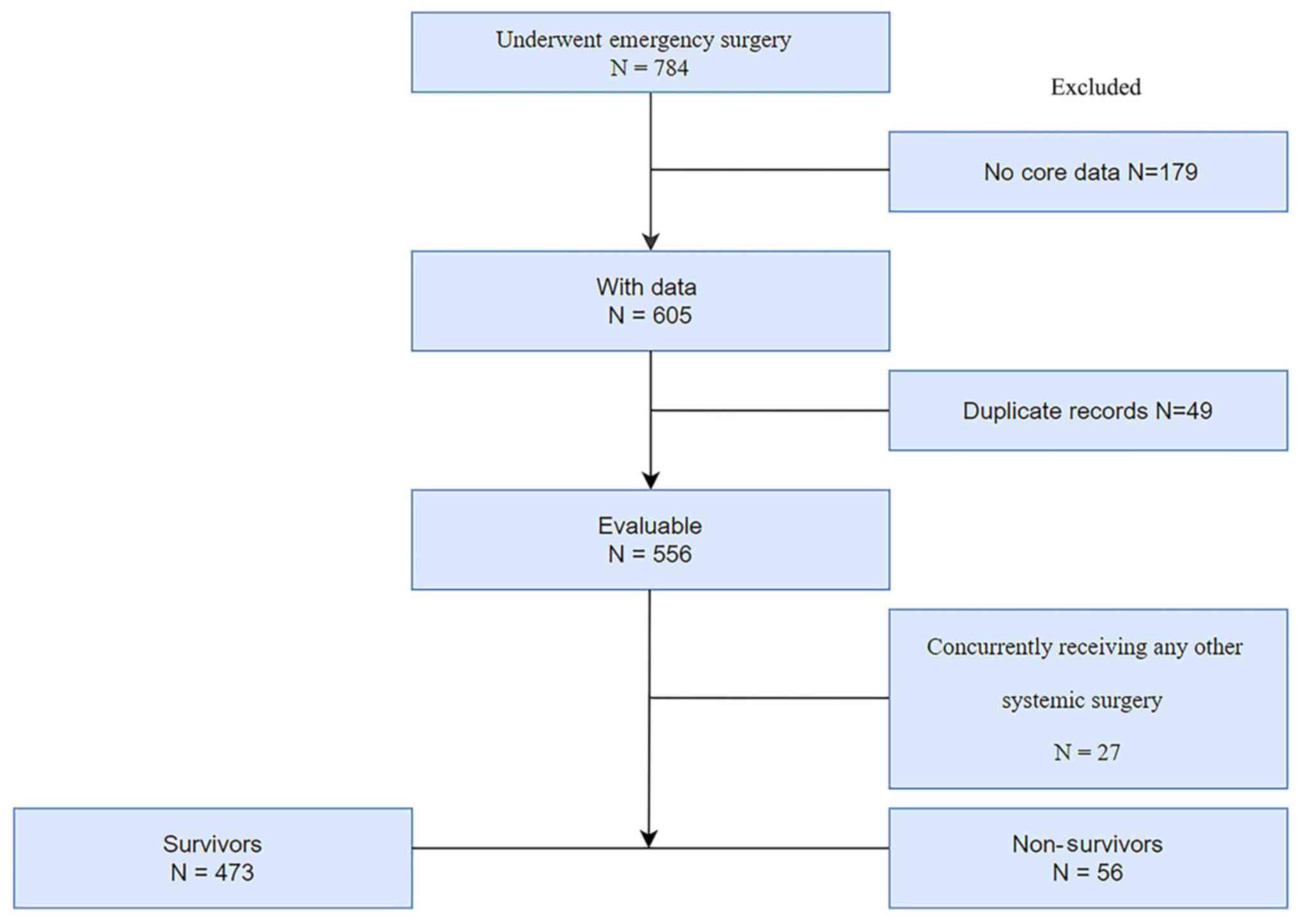 |
Figure 1
Study flow diagram.
|
Data collection
Baseline clinical data and biological characteristics were retrospectively collected, including: Demographic information such as age and sex; admission vital signs, including heart rate, body temperature and blood pressure; pre-hospital symptom duration; laboratory test results on admission; computed tomography; and etiology, categorized as iatrogenic, ulceration, tumor, inflammatory or foreign body. Notably, the duration between hospitalization and treatment/diagnosis was ≤30 min for each patient. The in-hospital mortality after surgery was also recorded for all patients.
Regarding the treatment of the patients, those with neoplasms or inflammatory bowel disease frequently underwent enterostomy, with radical excision for individuals with early-stage neoplasms. Patients presenting with ulcerative, foreign body-induced or iatrogenic perforation typically underwent perforation repair (8,9).
Hypertension was defined in accordance with the guidelines provided by the American Heart Association, wherein high blood pressure stage 1 is characterized by a systolic reading of ≥140 mmHg or a diastolic reading of ≥90 mmHg (10). The diagnostic criteria for septic shock were consistent with the Sepsis-3 definitions (11). The upper and lower alimentary tracts were defined based on their location relative to the Treitz ligament. Severe heart disease was defined as cardiomyopathy and heart failure, rheumatic heart disease, bacterial endocarditis, coronary heart disease and acute coronary syndrome. Plasma potassium was detected by the ion-selective electrode method (12), and hyperkalemia was defined as a plasma potassium level >5.5 mmol/l. C-reactive protein was detected by immunoturbidimetry, and serum creatinine (sCr) was assayed by an enzymatic method using a cobas® 8000 Modular Analyzer (Roche Diagnostics).
Statistical analysis
Data were analyzed using SPSS 23.0 statistical software (IBM Corp.). Unpaired Student's t-test was used to compare normally distributed variables. Categorical variables were compared using Chi-square or Fisher's exact tests. Receiver operating characteristic (ROC) curves were used to analyze continuous indicators to determine the cutoff value. The independent variables were screened by forward stepwise regression, and the independent risk factors of mortality in the patients were determined by multivariable logistic regression analysis. Relative risk was expressed as the odds ratio (OR) and 95% confidence interval (95% CI). The diagnostic efficiency of the predictive model was evaluated by determining the area under the ROC curve (AUC). The AUC ranged from 0.5 to 1.0, with <0.7 indicating low diagnostic value, 0.7-0.9 indicating moderate diagnostic value and >0.9 indicating high diagnostic value. Two-tailed P<0.05 was considered to indicate a statistically significant result.
Results
Characteristics of patients
A total of 784 patients with GI perforation were initially retrieved by screening. Following the exclusion of 179 patients due to incomplete information, 49 duplicate records and 27 patients who had undergone concurrent surgery involving other systems. 529 patients were eventually included in the study (Fig. 1). There were 394 male patients and 135 female patients. The median age of the patients was 60 years (interquartile range, 44-72 years). The in-hospital mortality rate after surgery was 10.59% (56 patients). Table I presents the clinical characteristics of the 529 patients. The patients were divided into survivor and non-survivor groups based on in-hospital mortality.
 |
Table I
Demography and clinical presentation of the patients.
|
Table I
Demography and clinical presentation of the patients.
| Variables |
Total (n=529) |
Non-survivors (n=56) |
Survivors (n=473) |
P-value |
| Male sex, n (%) |
394 (74.5) |
39 (69.6) |
355 (75.1) |
0.380 |
| Age, years, median IQR (1/4) |
60 (44-72) |
73.5 (63-79) |
58 (40-70) |
<0.001 |
| Smoking history, n (%) |
122 (23.1) |
10 (17.9) |
112 (23.7) |
0.328 |
| Drinking history, n (%) |
67 (12.7) |
7 (12.5) |
60 (12.7) |
0.964 |
| HR, beats/min, mean ± SD |
88.75±18.92 |
100.64±22.98 |
87.34±17.90 |
<0.001 |
| HR >90 beats/min, n (%) |
200 (37.8) |
37 (66.1) |
163 (34.5) |
<0.001 |
| Fevera, n (%) |
92 (17.4) |
7 (12.5) |
85 (18.0) |
0.307 |
| Body temperatureb, ˚C, mean ± SD |
36.826±0.566 |
36.696±0.564 |
36.837±0.563 |
0.078 |
| Pre-hospitalization symptom duration, h, mean ± SD |
40.38±38.68 |
48.86±38.62 |
39.37±38.60 |
0.083 |
| Shock, n (%) |
79 (14.9) |
20 (35.7) |
59 (12.5) |
<0.001 |
| Etiology, n (%) |
|
|
|
0.192 |
| Tumor |
107 (20.2) |
17 (30.4) |
90 (17.0) |
|
| Ulceration |
200 (35.9) |
15 (21.4) |
185 (37.6) |
|
| Inflammatory |
174 (32.9) |
20 (35.7) |
154 (32.6) |
|
| Foreign body |
27 (5.1) |
3 (5.4) |
24 (5.1) |
|
| Iatrogenic |
21 (4.0) |
1 (1.8) |
20 (4.2) |
|
| Site, n (%) |
|
|
|
0.553 |
| Upper |
256 (48.4) |
25 (4.7) |
231 (43.7) |
|
| Lower |
273 (51.6) |
31 (5.9) |
242 (45.7) |
|
| Hypertension, n (%) |
103 (19.5) |
18 (32.1) |
85 (18.0) |
0.011 |
| Diabetes, n (%) |
40 (7.6) |
7 (12.5) |
33 (7.0) |
0.226 |
| Severe heart disease, n (%) |
62 (11.7) |
15 (26.8) |
47 (9.9) |
<0.001 |
Patients in the non-survivor group were significantly older than those in the survivor group (median age, 73.5 vs. 58 years; P<0.001), and had a significantly higher HR (100.64 vs. 87.34 beats/min; P<0.001). In addition, in the non-survivor group, the proportion of patients with shock on admission, a HR >90 beats/min, hypertension and severe heart disease was significantly higher compared with that in the survivor group (P<0.05).
The laboratory findings of the patients are displayed in Table II. The proportion of patients with a white blood cell (WBC) count <3.5x109 or >20x109 cells/l, the proportion of patients with hyperkalemia, and the sCr and CRP values were significantly higher in the non-survivor group compared with the survivor group (P<0.05).
 |
Table II
Laboratory findings of the patients.
|
Table II
Laboratory findings of the patients.
| Variables |
Total (n=529) |
Non-survivors (n=56) |
Survivors (n=473) |
P-value |
| WBC, x109 cells/l, mean ± SD |
10.54±5.37 |
11.07±7.23 |
10.48±5.11 |
0.555 |
| WBC count <3.5x109 or >20x109 cells/l, n (%) |
129 (24.4) |
23 (41.1) |
106 (22.4) |
0.002 |
| CRP, mg/l, mean ± SD |
75.72±63.57 |
92.99±73.18 |
73.67±62.11 |
0.031 |
| sCr, µmol/l, mean ± SD |
87.34±76.33 |
147.60±126.55 |
80.47±65.07 |
<0.001 |
| Hyperkalemiaa, n (%) |
23 (4.3) |
8 (14.3) |
15 (3.2) |
<0.001 |
Univariate logistic regression analysis
Univariate regression analysis indicated that age, HR, HR >90 beats/min, shock on admission, hypertension, severe heart disease, CRP, hyperkalemia, elevated sCr and a WBC count <3.5x109 or >20x109 cells/l were associated with mortality (Table III).
 |
Table III
Results of multivariate and univariate logistic analysis of risk factors for mortality.
|
Table III
Results of multivariate and univariate logistic analysis of risk factors for mortality.
| |
95% CI |
| Variables |
Univariate P-value |
Multivariate P-value |
Adjusted OR |
Low |
High |
| Age |
<0.001 |
<0.001 |
1.052 |
1.027 |
1.078 |
| HR |
<0.001 |
0.740 |
1.004 |
0.980 |
1.028 |
| HR >90 beats/min |
<0.001 |
0.149 |
2.071 |
0.771 |
5.562 |
| Shock |
<0.001 |
0.022 |
2.623 |
1.149 |
5.990 |
| Hypertension |
0.013 |
0.896 |
0.949 |
0.431 |
2.087 |
| Severe heart disease |
<0.001 |
0.078 |
2.105 |
0.919 |
4.823 |
| Hyperkalemiaa |
<0.001 |
0.365 |
1.655 |
0.556 |
4.927 |
| sCr |
<0.001 |
0.012 |
1.004 |
1.001 |
1.006 |
| WBC count <3.5x109 or >20x109cells/l |
0.003 |
0.007 |
2.634 |
1.300 |
5.337 |
| CRP |
0.035 |
0.876 |
1.000 |
0.995 |
1.004 |
Multivariate logistic regression analysis
To eliminate the influence of confounding factors, variables with statistically significant differences in the univariate analysis were included in the multivariable logistic regression analysis. The results showed that age (OR=1.052; 95%CI=1.027-1.078; P<0.001), shock (OR=2.623, 95%CI=1.149-5.990; P=0.022), elevated sCr (OR=1.004, 95%CI=1.001-1.006; P=0.012) and WBC count <3.5x109 or >20x109 cells/l (OR=2.634, 95%CI=1.300-5.337; P=0.007) were independent risk factors for in-hospital mortality (Table III). The logistic model was found to fit the observations well (P=0.674). Fig. 2 showed a forest plot of the risk factors for mortality.
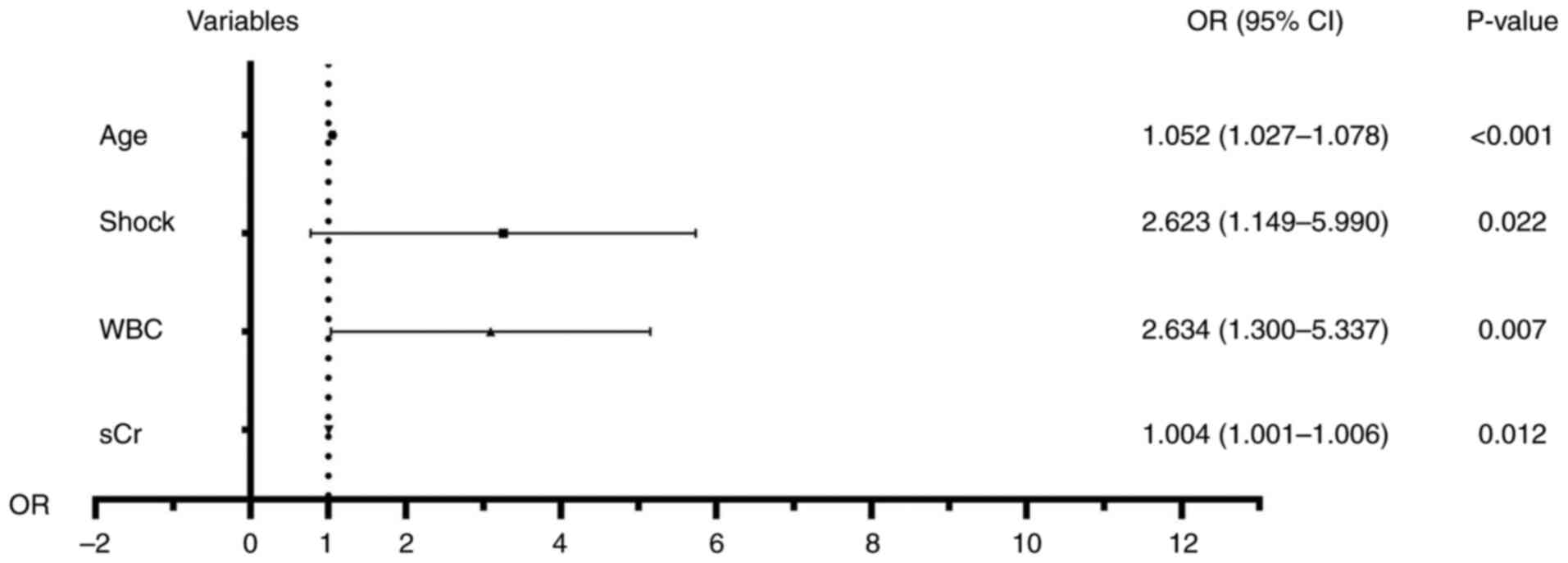 |
Figure 2
Forest plot of the risk factors for mortality. WBC, white blood cell; sCr, serum creatinine; OR, odds ratio; 95%CI, 95% confidence interval.
|
In the ROC analysis, the AUC of the logistic predictive model was 0.854, the Youden index was 0.585, the sensitivity was 0.804, the specificity was 0.781 and the standard error was 0.026 (P<0.001; 95%CI=0.802-0.905). Regarding the sCr value, the AUC was 0.712, the Youden index was 0.430, the sensitivity was 0.569, the specificity was 0.861 and the standard error was 0.047 (P<0.001; 95%CI=0.620-0.804), with a cut-off value of 99.85 µmol/l. The AUC for age was 0.737, with a Youden index of 0.396, sensitivity of 0.824, specificity of 0.573 and standard error of 0.035 (P<0.001; 95%CI=0.669-0.805). The cut-off value for age was 62 years (Fig. 3, Table IV).
 |
Figure 3
Comparison of ROC curves for age, sCr and predictive model. The area under the ROC curve for the logistic predictive model was 0.854, for age was 0.737 and for sCr was 0.712. ROC, receiver operating characteristic curve; sCr, serum creatinine.
|
 |
Table IV
Comparison of mortality risk indicators.
|
Table IV
Comparison of mortality risk indicators.
| Indicator |
AUC |
SE |
P-value |
95% CI |
Youden index |
Sensitivity |
Specificity |
| sCr |
0.712 |
0.047 |
<0.001 |
0.620-0.804 |
0.430 |
0.569 |
0.861 |
| Age |
0.737 |
0.035 |
<0.001 |
0.669-0.805 |
0.396 |
0.824 |
0.573 |
| Predictive model |
0.854 |
0.026 |
<0.001 |
0.802-0.905 |
0.585 |
0.804 |
0.781 |
Subanalysis of upper GI perforation
A subanalysis of all patients with GI perforation was performed to distinguish between those with upper and lower GI perforations. In the subanalysis of upper GI perforation (Table V), several significant differences between the survivor and non-survivor groups were identified, including age, smoking history, HR, presence of shock upon admission, hyperkalemia, severe heart disease, hypertension and sCr levels (P<0.05).
 |
Table V
Demography and clinical presentation of patients with upper gastrointestinal perforation.
|
Table V
Demography and clinical presentation of patients with upper gastrointestinal perforation.
| Variables |
Total (n=256) |
Non-survivors (n=25) |
Survivors (n=231) |
P-value |
| Male sex, n (%) |
204 (79.7) |
17 (68.0) |
187 (73.0) |
0.126 |
| Age, years, median IQR (1/4) |
56.5 (37-70) |
75 (63-84) |
57 (37-72) |
<0.001 |
| Smoking history, n (%) |
63 (24.6) |
1 (4.0) |
62 (26.8) |
0.012 |
| Drinking history, n (%) |
35 (13.7) |
1 (4.0) |
34 (13.3) |
0.240 |
| HR, beats/min, mean ± SD |
88.75±19.17 |
105.40±20.69 |
86.95±18.45 |
<0.001 |
| HR >90 beats/min, n (%) |
97 (37.9) |
20 (80.0) |
77 (33.3) |
<0.001 |
| Fevera, n (%) |
47 (18.4) |
4 (16.0) |
43 (18.6) |
0.961 |
| Body temperatureb, ˚C, mean ± SD |
36.84±0.56 |
36.76±0.63 |
36.84±0.55 |
0.479 |
| Pre-hospitalization symptom duration, h, mean ± SD |
40.09±40.86 |
61.20±61.50 |
37.81±37.45 |
0.074 |
| Shock, n (%) |
37 (14.5) |
9 (36.0) |
28 (12.1) |
0.003 |
| Etiology, n (%) |
|
|
|
0.059 |
| Tumor |
20 (7.8) |
5 (20.0) |
15 (6.5) |
|
| Ulceration |
190 (74.2) |
13 (52.0) |
177 (37.6) |
|
| Inflammatory |
36 (14.1) |
5 (20.0) |
31 (13.4) |
|
| Foreign body |
10 (3.9) |
2 (8.0) |
8 (3.5) |
|
| Iatrogenic |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
| Hyperkalemiac, n (%) |
13 (5.1) |
4 (16.0) |
9 (3.9) |
0.032 |
| Diabetes, n (%) |
20 (7.8) |
4 (16.0) |
16 (6.9) |
0.225 |
| Severe heart disease, n (%) |
33 (12.9) |
11 (44.0) |
22 (9.5) |
<0.001 |
| Hypertension, n (%) |
40 (15.6) |
9 (36.0) |
31 (13.4) |
0.008 |
| WBC count <3.5x109 or >20x109 cells/l, n (%) |
62 (24.2) |
8 (32.0) |
54 (23.4) |
0.339 |
| CRP, mg/l, mean ± SD |
73.27±66.34 |
92.94±69.37 |
71.09±65.80 |
0.118 |
| sCr, µmol/l, mean ± SD |
93.96±88.31 |
173.06±117.52 |
80.47±65.07 |
0.002 |
Subanalysis of lower GI perforation
In the subanalysis of patients with lower GI perforation, significant differences between the two groups were observed in terms of age, presence of shock upon admission, hyperkalemia and WBC count (P<0.05). In addition, the differences in HR and sCr levels (both P=0.052) approached statistical significance (Table VI).
 |
Table VI
Demography and clinical presentation of patients with lower gastrointestinal perforation.
|
Table VI
Demography and clinical presentation of patients with lower gastrointestinal perforation.
| Variables |
Total (n=273) |
Non-survivors (n=31) |
Survivors (n=242) |
P-value |
| Male sex, n (%) |
190 (69.6) |
22 (71.0) |
168 (69.4) |
0.860 |
| Age, years, median IQR (1/4) |
63 (49-73) |
70 (63-78) |
60.5 (47-72) |
<0.001 |
| Smoking history, n (%) |
58 (21.2) |
9 (29.0) |
49 (20.2) |
0.260 |
| Drinking history, n (%) |
31 (11.4) |
6 (19.4) |
25 (10.4) |
0.238 |
| HR, beats/min, mean ± SD |
88.51±18.39 |
96.81±24.33 |
87.72±17.68 |
0.052 |
| HR >90 beats/min, n (%) |
106 (38.8) |
17 (54.8) |
89 (36.8) |
0.052 |
| Fevera, n (%) |
45 (16.5) |
3 (9.7) |
42 (17.4) |
0.278 |
| Body temperatureb, ˚C, mean ± SD |
36.81±0.56 |
36.64±0.51 |
36.84±0.58 |
0.077 |
| Pre-hospitalization symptom duration, h, mean ± SD |
68.27±64.95 |
61.20±61.50 |
37.81±37.45 |
0.074 |
| Shock, n (%) |
42 (15.4) |
11 (35.5) |
31 (12.8) |
0.002 |
| Etiology, n (%) |
|
|
|
0.579 |
| Tumor |
87 (31.9) |
12 (38.7) |
75 (31.0) |
|
| Ulceration |
10 (3.7) |
2 (6.5) |
8 (3.3) |
|
| Inflammatory |
138 (50.5) |
15 (48.4) |
123 (50.8) |
|
| Foreign body |
17 (6.2) |
1 (3.2) |
16 (6.6) |
|
| Iatrogenic |
21 (7.7) |
1 (3.2) |
20 (8.3) |
|
| Hyperkalemiac, n (%) |
10 (3.7) |
4 (12.9) |
6 (2.5) |
0.016 |
| Diabetes, n (%) |
20 (7.3) |
3 (9.7) |
17 (7.0) |
0.594 |
| Severe heart disease, n (%) |
29 (10.6) |
4 (12.90) |
25 (10.3) |
0.662 |
| Hypertension, n (%) |
63 (23.1) |
9 (29.0) |
54 (22.3) |
0.403 |
| WBC count <3.5x109 or >20x109 cells/l, n (%) |
67 (24.5) |
15 (48.4) |
52 (21.5) |
0.001 |
| CRP, mg/l, mean ± SD |
78.59±62.26 |
92.94±69.37 |
71.09±65.80 |
0.118 |
| sCr, µmol/l, mean ± SD |
81.19±62.74 |
126.68±131.91 |
75.65±45.62 |
0.052 |
Discussion
GI perforation often causes serious abdominal infection, which frequently leads to sepsis with shock. Ultimately, it causes multiple organ dysfunction with a poor prognosis (13). It has been reported that the annual incidence of GI ulcer perforation in the general population ranges from 0.004 to 0.014%, with a 30-day mortality rate of 20-50% (14,15). In the present study, the overall mortality rate of patients with GI perforation who underwent surgery was 10.59%, which may be due to the study not including cases that died following ineffective conservative treatment.
Surgical control of infection is key to the treatment GI perforation, as it is the most fundamental means of preventing the further dissemination of pathogenic microorganisms in the abdominal cavity (16). Previous studies have revealed that the type and extent of peritoneal contamination depend on the location, size and duration of the perforation and the physiology of the patient, including time from the last meal and coexisting diseases (17,18). Consistent with this, the present study found that patients who were elderly, in shock on admission, had elevated sCr levels or a WBC count <3.5x109 or >20x109 cells/l tended to have a poor prognosis. Therefore, it is recommended that clinicians should pay more attention to such patients and fully communicate the risks and benefits of surgery to them.
In the present study, older patients had a higher mortality rate than younger patients. Various factors may explain the higher postoperative mortality in elderly patients undergoing emergency laparotomy. First, older patients are likely to have more comorbidities than younger patients, as demonstrated in the present and other studies; in particular, cardiac and renal insufficiency are associated with increased postoperative mortality (19,20). Furthermore, older patients are more likely to have skeletal sarcopenia, and the age-related loss of skeletal muscle mass is a strong predictor of mortality in patients undergoing emergency abdominal procedures (21). Hajibandeh et al (22) noted that patients >80 years old with an American Society of Anesthesiologists score of >3 had an elevated risk of postoperative death following emergency laparotomy. In addition, Møller et al (7) revealed that age >65 years, shock upon admission, preoperative metabolic acidosis and an elevated concentration of creatinine upon admission were significantly associated with death <30 days after surgery.
Shock is an important variable that was found to be significantly associated with poor prognosis in the present study. In patients with shock, the blood flow to the brain and heart is maintained due to the redistribution of blood away from the peripheral organs. This disrupts the integrity barrier of the gut and enhances bacterial translocation, leading to sepsis and multi-organ dysfunction (11). Therefore, when patients with acute abdominal pain present with signs of shock, it is essential to treat them promptly because the timely treatment of this complication is essential for prognosis.
The present study showed that elevated sCr was an independent risk factor for postoperative mortality, which is similar to previous findings (6,23-26). Elevated sCr may occur due to a combination of factors, including chronic renal insufficiency often combined with advanced age, inadequate renal perfusion in patients in shock, or acute renal failure brought on by sepsis. sCr values are similar to disease mortality, in that both are directly or indirectly influenced by multiple pathological factors.
Renal impairment or hepatorenal syndrome has been shown to be an independent predictor of in-hospital mortality in patients with spontaneous bacterial peritonitis (27). A study of mortality after major surgery found that in patients with acute kidney injury (28), even in cases where complete renal recovery occurred after surgery, the odds of dying were increased compared with those of patients without AKI, regardless of the severity of the AKI (29). It is important to note that these risk factors, including septic shock, AKI and advanced age, may interact with each other, and directly or indirectly cause death. However, the complex mechanisms underlying these interactions were not explored in the present study and remain to be elucidated.
The present study also found that patients had a higher mortality rate when their leukocyte count at admission was <3.5x109 or >20x109 cells/l. Leukocytes are among the most important immune cells involved in sepsis (30). Neutrophils are crucial in fighting pathogens during sepsis. It has previously been reported that patients with a WBC count of >15x109 cells/l have increased mortality when undergoing surgery to treat hepatic abscess (31). In addition, in patients with septic shock, a rising WBC count has been shown to be associated with higher mortality (32). Leukocytosis often predicts severe infection or a poor prognosis, which is consistent with the results of the present study. However, the relationship between significantly lower white blood cell counts and mortality in patients undergoing surgery requires investigation in further prospective studies.
A previous study found that in patients with GI perforation combined with septic shock, minimal delay from admission to the start of surgical intervention is key to the maintenance of hemodynamic stability. The target time to achieve a good outcome was suggested to be within 6 h of admission (33). The present study found that patients who did not survive their stay in hospital had a longer duration of pre-hospitalization symptoms than those who did survive; the difference was close to reaching statistical significance (P=0.083). This may be because the First Affiliated Hospital of Fujian Medical University admits numerous patients from remote areas, who are often initially seen at local hospitals before being referred to the institution. This may have led to confounding bias.
It is worthy of note that in the subgroup analysis of upper GI perforation, smoking history was indicated to be associated with prognosis. Smoking is a known risk factor for GI ulcers; previous studies have established a link between smoking and peptic ulcers (34,35). A study performed by Jha et al (36) suggested that smoking accounts for almost half of all male mortality in the lowest social strata of various countries. The poor prognosis in patients with smoking and GI perforation has been suggested be due to smoking impeding the therapeutic efficacy of histamine-2 antagonists, inducing the reflux of duodenal contents into the stomach, and increasing Helicobacter pylori infection risk and associated adverse effects, pepsin secretion, free radical production, vasopressin levels, pituitary secretion, endothelin secretion by the gastric mucosa, and platelet-activating factor production. In addition, smoking adversely affects the protective mechanisms of the mucous membranes; it decreases blood flow to the gastric mucosa and inhibits gastric motility (37).
The gut microbiome (GM) has been shown to play a crucial role in the development of postoperative complications (38). Daniel et al (39) demonstrated that the recent use of antibiotics by outpatients, particularly in the preceding 30 days, is associated with upper GI perforation. Study of the microbiome has expanded in the past few decades, and it is known that the gut microbiota gradually changes as individuals age and is influenced by various factors. Critical illness induces notable alterations in the human GM, resulting in a disruption of gut barrier function, which is implicated in the development of multiple organ dysfunction. Bacterial translocation, in which bacteria and their byproducts cross the intestinal barrier, has been identified as the underlying mechanism of sepsis (40).
Matsuda et al (28) evaluated the role of the endotoxin activity assay (EAA) in the assessment of severity in patients who underwent emergency surgery for colorectal perforation and were subsequently admitted to the intensive care unit. The study indicated that the EAA may be used as a predictor of outcomes in such patients. The relationship of the GM composition or endotoxin levels with the poor prognosis of patients with GI perforation has not yet been studied. However, probiotics administration and fecal microbiota transplantation may have potential application value in patients with GI perforation or abdominal infection.
Aging is associated with low-level chronic inflammation involving elevated levels of circulating inflammatory mediators, and inflammation is a risk factor for morbidity and mortality in older adults. Furthermore, the GM is markedly disrupted with age, and there is evidence to suggest that age-associated dysbiosis of the GM is responsible for systemic inflammation in the elderly (41,42). While inflammation is crucial in protecting against harmful bacteria, viral infections and environmental factors during healing, it must be noted that some forms of inflammation are not beneficial. Prolonged and persistent inflammation can be detrimental and destructive (43). Although the present study did not establish a direct association between inflammatory markers and a poor prognosis, this may be attributed to the lack of specificity exhibited by the markers that were collected. Further investigation into the management of inflammation during the acute phase of GI perforation and the control of chronic inflammation in inflammatory bowel disease may provide valuable insights.
There are certain limitations to the present study. First, this is a study of patients who underwent surgery. The data may underestimate the overall incidence of poor prognosis in patients with GI perforation, considering that patients who did not undergo surgery may have not received surgical treatment due to their extremely critical condition. Secondly, since there is no standardized definition of a significantly abnormal WBC count, this may lead to classification errors. Finally, the study is retrospective, and the effect of unmeasured confounding factors cannot be excluded, which may lead to a misinterpretation of its potential predictive value.
In conclusion, the present study indicated that advanced age, shock on admission, elevated sCr and significantly abnormal WBC count are important mortality predictors for patients undergoing emergency laparotomy for GI perforation.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets generated and analyzed during the current study are not publicly available to maintain the confidentiality of patient data but are available from the corresponding author on reasonable request.
Authors' contributions
WDW, QYW and YXY were responsible for conceptualization, methodology and software implementation. YXY, WDW and YLW performed data curation and wrote the original draft of the manuscript. WDW and YXY performed data visualization and investigation. YLW and WZC contributed to software implementation and validation. QYW and WDW reviewed and edited the manuscript. YXY, WDW, YLW, WZC and QYW confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study was approved by the Ethics Committee of First Affiliated of Fujian Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Thorsen K, Søreide JA, Kvaløy JT, Glomsaker T and Søreide K: Epidemiology of perforated peptic ulcer: Age- and gender-adjusted analysis of incidence and mortality. World J Gastroenterol. 19:347–354. 2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Tan S, Wu G, Zhuang Q, Xi Q, Meng Q, Jiang Y, Han Y, Yu C, Yu Z and Li N: Laparoscopic versus open repair for perforated peptic ulcer: A meta analysis of randomized controlled trials. Int J Surg. 33:124–132. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kim CW, Kim JW, Yoon SN, Oh BY and Kang BM: Laparoscopic repair of perforated peptic ulcer: A multicenter, propensity score matching analysis. BMC Surg. 22(230)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chen P, Gao J, Li J, Yu R, Wang L, Xue F, Zheng X, Gao L and Shang X: Construction and efficacy evaluation of an early warning scoring system for septic shock in patients with digestive tract perforation: A retrospective cohort study. Front Med (Lausanne). 9(976963)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dadfar A and Edna TH: Epidemiology of perforating peptic ulcer: A population-based retrospective study over 40 years. World J Gastroenterol. 26:5302–5313. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Singh R, Kumar N, Bhattacharya A and Vajifdar H: Preoperative predictors of mortality in adult patients with perforation peritonitis. Indian J Crit Care Med. 15:157–163. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Møller MH, Shah K, Bendix J, Jensen AG, Zimmermann-Nielsen E, Adamsen S and Møller AM: Risk factors in patients surgically treated for peptic ulcer perforation. Scand J Gastroenterol. 44:145–152. 2009.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hoshino N, Endo H, Hida K, Kumamaru H, Hasegawa H, Ishigame T, Kitagawa Y, Kakeji Y, Miyata H and Sakai Y: Laparoscopic surgery for acute diffuse peritonitis due to gastrointestinal perforation: A nationwide epidemiologic study using the national clinical database. Ann Gastroenterol Surg. 6:430–444. 2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Paterson-Brown S: Emergency laparoscopic surgery. Br J Surg. 80:279–283. 1993.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Whelton PK, Carey RM, Mancia G, Kreutz R, Bundy JD and Williams B: Harmonization of the American college of cardiology/American heart association and European society of cardiology/European Society of hypertension blood pressure/hypertension guidelines. Eur Heart J. 43:3302–3311. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, et al: The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 315:801–810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Haemmerli A, Janata J and Brown HM: Ion-selective electrode for intracellular potassium measurements. Anal Chem. 52:1179–1182. 1980.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Sartelli M, Viale P, Catena F, Ansaloni L, Moore E, Malangoni M, Moore FA, Velmahos G, Coimbra R, Ivatury R, et al: 2013 WSES guidelines for management of intra-abdominal infections. World J Emerg Surg. 8(3)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Søreide K, Thorsen K, Harrison EM, Bingener J, Møller MH, Ohene-Yeboah M and Søreide JA: Perforated peptic ulcer. Lancet. 386:1288–1298. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Langell JT and Mulvihill SJ: Gastrointestinal perforation and the acute abdomen. Med Clin North Am. 92:599–625, viii-ix. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Skrupky LP, Tellor BR and Mazuski JE: Current strategies for the treatment of complicated intraabdominal infections. Expert Opin Pharmacother. 14:1933–1947. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Putcha RV and Burdick JS: Management of iatrogenic perforation. Gastroenterol Clin North Am. 32:1289–1309. 2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kocer B, Surmeli S, Solak C, Unal B, Bozkurt B, Yildirim O, Dolapci M and Cengiz O: Factors affecting mortality and morbidity in patients with peptic ulcer perforation. J Gastroenterol Hepatol. 22:565–570. 2007.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Steffens NM, Tucholka JL, Nabozny MJ, Schmick AE, Brasel KJ and Schwarze ML: Engaging patients, health care professionals, and community members to improve preoperative decision making for older adults facing high-risk surgery. JAMA Surg. 151:938–945. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shahrokni A, Tin AL, Sarraf S, Alexander K, Sun S, Kim SJ, McMillan S, Yulico H, Amirnia F, Downey RJ, et al: Association of geriatric comanagement and 90-day postoperative mortality among patients aged 75 years and older with cancer. JAMA Netw Open. 3(e209265)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hajibandeh S, Hajibandeh S, Jarvis R, Bhogal T and Dalmia S: Meta-analysis of the effect of sarcopenia in predicting postoperative mortality in emergency and elective abdominal surgery. Surgeon. 17:370–380. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hajibandeh S, Hajibandeh S, Shah J, Martin J, Abdelkarim M, Murali S, Maw A, Mansour M and Satyadas T: The risk and predictors of mortality in octogenarians undergoing emergency laparotomy: A multicentre retrospective cohort study. Langenbecks Arch Surg. 406:2037–2044. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kao SS, Kim SW, Horwood CM, Hakendorf P, Li JY and Thompson CH: Variability in inpatient serum creatinine: its impact upon short- and long-term mortality. QJM. 108:781–787. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Horiuchi A, Watanabe Y, Doi T, Sato K, Yukumi S, Yoshida M, Yamamoto Y, Sugishita H and Kawachi K: Evaluation of prognostic factors and scoring system in colonic perforation. World J Gastroenterol. 13:3228–3231. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ahmed M, Mansoor T, Rab AZ and Rizvi SAA: Risk factors influencing postoperative outcome in patients with perforated peptic ulcer: A prospective cohort study. Eur J Trauma Emerg Surg. 48:81–86. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Patel S, Kalra D, Kacheriwala S, Shah M and Duttaroy D: Validation of prognostic scoring systems for predicting 30-day mortality in perforated peptic ulcer disease. Turk J Surg. 35:252–258. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Terg R, Gadano A, Cartier M, Casciato P, Lucero R, Muñoz A, Romero G, Levi D, Terg G, Miguez C and Abecasis R: Serum creatinine and bilirubin predict renal failure and mortality in patients with spontaneous bacterial peritonitis: A retrospective study. Liver Int. 29:415–419. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Matsuda K, Aoki T, Watanabe M, Tomioka K, Tashiro Y, Tomokazu K, Koizumi T, Satoru G, Yamazaki K, Otsuka K and Murakami M: Comparison of endotoxin activity assay and various biomarkers for severity assessment in colorectal perforation patients. Am Surg. 89:2854–2856. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Korenkevych D, Ozrazgat-Baslanti T, Thottakkara P, Hobson CE, Pardalos P, Momcilovic P and Bihorac A: The pattern of longitudinal change in serum creatinine and 90-day mortality after major surgery. Ann Surg. 263:1219–1227. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Urrechaga E, Bóveda O and Aguirre U: Improvement in detecting sepsis using leukocyte cell population data (CPD). Clin Chem Lab Med. 57:918–926. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Christein JD, Kendrick ML and Que FG: What affects mortality after the operative management of hepatic abscess? HPB (Oxford). 8:175–178. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Rimmer E, Garland A, Kumar A, Doucette S, Houston BL, Menard CE, Leeies M, Turgeon AF, Mahmud S, Houston DS and Zarychanski R: White blood cell count trajectory and mortality in septic shock: A historical cohort study. Can J Anaesth. 69:1230–1239. 2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Azuhata T, Kinoshita K, Kawano D, Komatsu T, Sakurai A, Chiba Y and Tanjho K: Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Crit Care. 18(R87)2014.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ainley CC, Forgacs IC, Keeling PW and Thompson RP: Outpatient endoscopic survey of smoking and peptic ulcer. Gut. 27:648–651. 1986.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Collatuzzo G, Alicandro G, Bertuccio P, Pelucchi C, Bonzi R, Palli D, Ferraroni M, Ye W, Plymoth A, Zaridze D, et al: Peptic ulcer as mediator of the association between risk of gastric cancer and socioeconomic status, tobacco smoking, alcohol drinking and salt intake. J Epidemiol Community Health. (jech-2022-219074)2022.PubMed/NCBI View Article : Google Scholar : (Epub ahead of print).
|
|
36
|
Jha P, Peto R, Zatonski W, Boreham J, Jarvis MJ and Lopez AD: Social inequalities in male mortality, and in male mortality from smoking: Indirect estimation from national death rates in England and Wales, Poland, and North America. Lancet. 368:367–370. 2006.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Eastwood GL: Is smoking still important in the pathogenesis of peptic ulcer disease? J Clin Gastroenterol. 25 (Suppl 1):S1–S7. 1997.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Schmitt FCF, Brenner T, Uhle F, Loesch S, Hackert T, Ulrich A, Hofer S, Dalpke AH, Weigand MA and Boutin S: Gut microbiome patterns correlate with higher postoperative complication rates after pancreatic surgery. BMC Microbiol. 19(42)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Daniel VT, Francalancia S, Amir NS, Ayturk MD, Sanders SB, Wisler JR, Collins CE, Ward DV, Kiefe CI, McCormick BA and Santry HP: Upper gastrointestinal perforations: A possible danger of antibiotic overuse. J Gastrointest Surg. 24:2730–2736. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Mustansir Dawoodbhoy F, Patel BK, Patel K, Bhatia M, Lee CN and Moochhala SM: Gut microbiota dysbiosis as a target for improved post-surgical outcomes and improved patient care: A review of current literature. Shock. 55:441–454. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Teissier T, Boulanger E and Cox LS: Interconnections between inflammageing and immunosenescence during ageing. Cells. 11(359)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Shintouo CM, Mets T, Beckwee D, Bautmans I, Ghogomu SM, Souopgui J, Leemans L, Meriki HD and Njemini R: Is inflammageing influenced by the microbiota in the aged gut? A systematic review. Exp Gerontol. 141(111079)2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rea IM, Gibson DS, McGilligan V, McNerlan SE, Alexander HD and Ross OA: Age and age-related diseases: Role of inflammation triggers and cytokines. Front Immunol. 9(586)2018.PubMed/NCBI View Article : Google Scholar
|

















