Introduction
Endothelial cells produce a variety of inflammatory
agents that intensify endothelial dysfunction in inflammatory
conditions (1).
Inflammation-induced endothelial dysfunction has a crucial impact
on the development of multiple vascular diseases, such as
hypertension, atherosclerosis and vascular complications associated
with diabetes (2,3). Previous research provides substantial
evidence to suggest that endothelial cells may have a crucial role
in the pathophysiology of sepsis, which is a serious inflammatory
response throughout the body to infection triggered by an improper
stimulation of the host's immune system by pathogenic elements
(4). The pathogenic impact of
endothelial activation and dysfunction in sepsis was clearly
demonstrated by the unusual increase in cell adhesion molecules,
which are involved in various processes related to the adhesion and
coagulation of endothelial cells (5,6).
This led to a surge in leukocyte migration, coagulation, vascular
permeability and inflammation, all of which are typical
characteristics of sepsis (7).
Studies suggest that cell adhesion molecules and pro-inflammatory
agents are enhanced through the activation of the NF-κB signaling
pathway (8,9). Thus, mitigating abnormal vascular
endothelial activation and the expression of pro-inflammatory
mediators could offer substantial therapeutic value in treating
inflammation-induced endothelial dysfunction.
Nuclear factor erythroid-derived 2-related factor 2
(Nrf2) is a crucial transcription factor necessary for the cellular
response against a wide range of oxidative stressors. A study
suggested that Nrf2 demonstrates cytoprotective effects in a sepsis
model in mice (10). Caffeic acid
(CA) phenethyl ester (CAPE) has been noticed for its role in
modulating peripheral immune functions, which includes the
expression of heme oxygenase (HO)-1 via the control of Nrf2, a
primary transcription factor of HO-1(11). HO-1, induced by various oxidative
stresses, performs a significant cytoprotective function against
oxidative cell damage. The ability of lipopolysaccharide
(LPS)-challenged macrophages to defend themselves from the aberrant
overproduction of inflammatory mediators such as cyclooxygenase 2
(COX-2) by amplifying HO-1 expression has been reported (12). Furthermore, it has been observed
that HO-1 attenuates the overproduction of cytokines such as TNF-α
in LPS-challenged RAW264.7 cells (13). In addition, induction of HO-1 has
been associated with increased survival rates in fatal endotoxemia
(10). An animal study indicated
that enhanced expression of HO-1 correlates with significant
activation of the Nrf2 pathway (14).
Ester derivatives of CA are found abundantly in
numerous natural plants. These include derivatives such as CA
methyl ester (CAME), CAPE, CA isopropenyl ester and CA benzyl ester
(15-17).
Research indicates that they display an array of biological
effects, including anti-oxidant, anti-inflammatory, anti-microbial,
anti-tumor and anti-acetylcholinesterase activities (17-20).
CAPE, for instance, is known to restrain cytokine-induced NF-κB
signaling in macrophage cells (21), and plays a critical part in
regulating the host's immune response (22). CAPE can also counteract allergic
reactions by interfering with MAPK and NF-κB signaling in HMC-1
human mast cells that had been activated (23). CAME has been found to exhibit a
variety of pharmacological benefits, such as anti-inflammatory and
neuroprotective activities (24).
Since CAME demonstrates extensive anti-inflammatory effects and
CAPE exhibits anti-allergic characteristics, it is plausible to
hypothesize that CAME could also display anti-inflammatory activity
in endothelial cells. Therefore, the present study aims to explore
the potential anti-inflammatory capabilities of CAME and the
underlying mechanism in LPS-stimulated HUVECs, with the goal of
identifying a possible therapeutic agent capable of alleviating
diverse inflammatory vascular conditions.
Materials and methods
Reagents and cell culture
Escherichia coli serotype 055:B5's bacterial
LPS was obtained from Sigma-Aldrich (Merck KGaA). CAME and CA were
isolated and identified from the bark of Lonicera maackii as
described previously (25). To
determine whether Lonicera maackii is classified as an
endangered species, a thorough search was conducted on Convention
of International Trade in Endangered Species of Wild Fauna and
Flora (CITES; https://cites.org/eng). The search
confirmed that Lonicera maackii is not categorized as an
endangered species. Furthermore, Lonicera maackii is
commonly found in various Asian countries including Korea, Japan
and China. To briefly explain the separation and identification
process of CAME and CA, the dried bark of Lonicera maackii,
which were collected in August 2014 from Samaksan (Korea) and
identified by Dr Yong-Soo Kwon (College of Pharmacy, Kangwon
National University, Chuncheon, Korea). The confirmed sample (no.
KNUPH-S-14-01) is currently stored in the medicinal plant
laboratory of the College of Pharmacy, Kangwon National University
(Chuncheon, Korea). The plant material was dried (2.4 kg), cut into
small pieces for use and extracted with 15 l methanol at room
temperature. The methanolic extract (230 g) was then partitioned
sequentially using hexane, chloroform and butanol. The obtained
fractions were subjected to structural determination using NMR and
mass spectrometry techniques. In addition, their purity was
confirmed to be >95% through high-performance liquid
chromatography analysis, which was performed using the Waters e2695
system, and the detector used was the Waters 2489 UV-vis detector
(Waters Corp.), measuring at 260 nm. The isolated compounds were
identified as CAME and CA by comparing their characteristics with
those reported in the compound literature library. Lonicera
maackii is a shrub of the Caprifoliaceae family, found in
Korea, China, Japan and other regions. Its flower buds, leaves and
bark are used in traditional medicine for colds and flu. It
contains flavonoids, iridoids, caffeoyl quinic acids and
phytosterols (25). CAME and CA
were dissolved in DMSO and added to the culture media at the
required concentrations, with 1 ppm of DMSO in the culture media.
Human umbilical vein endothelial cells (HUVECs) were purchased from
the American Type Culture Collection (cat. no. CRL-1730) and were
cultured on a 2% gelatin-coated plate in M199 medium (Hyclone;
Cytiva) (26) supplemented with
20% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc.), 3
ng/ml basic fibroblast growth factor (Gibco; Thermo Fisher
Scientific, Inc.), 100 U/ml penicillin-streptomycin (Gibco; Thermo
Fisher Scientific, Inc.) and 5 U/ml heparin (Sigma-Aldrich; Merck
KGaA) (culture medium) in an incubator maintained at ~95% humidity,
at a temperature of 37˚C with 5% CO2. Cells between
passages two and six were used in the experiments. The cells were
grown on 6-well plates (Corning; Merck KGaA) coated with gelatin
and incubated in M199 medium containing 1% fetal bovine serum and
100 U/ml penicillin-streptomycin for 6 h, which is classified as
the starvation medium (26). The
cells were then incubated with designated concentrations of CAME
and CA before being treated with LPS (1 µg/ml). In this study, the
micromolar concentration range of CAME was used, as no significant
cell toxicity was observed in previous studies (27).
Cytokine and prostaglandin
(PG)E2 assays
HUVECs were incubated with CAME (1-100 µM) for 24 h
and then stimulated in the absence or presence of LPS (1 µg/ml) for
24 h. ELISA kits were used to detect TNF-α (cat. no. MTA00B;
R&D systems), IL-1β (cat. no. MLB00C; R&D systems) and
PGE2 (cat. no. ADI-900-001; Enzo Life Sciences, Inc.)
secreted into the culture media of HUVECs according to the
manufacturer's instructions.
Western blot analysis
HUVECs were pretreated with CAME for 3 h before LPS
stimulation. HUVECs were rinsed with ice-cold PBS and lysed in
PRO-PREP lysis buffer (iNtRON Biotechnology, Inc.). Protein lysates
(10 µg per lane) were separated by 10% SDS-PAGE. These proteins
were then transferred to Hypond PVDF membranes (Amersham; Cytiva)
and blocked for 1 h at room temperature in Tris-buffered saline
containing Tween-20 (TBST) with 5% skimmed milk. Specific
antibodies against inducible nitric oxide synthase (iNOS; 1:1,000
dilution; cat. no. 610329; BD Pharmingen; BD Biosciences), COX-2
(1:1,000 dilution; cat. no. 12282; Cell Signaling Technology,
Inc.), HO-1 (1:1,000 dilution; cat. no. ab13243; abchem), Nrf2
(1:1,000 dilution; cat. no. bs-2013R; Bioss Inc.), phosphorylated
(p)-Nrf2 (1:1,000 dilution; cat. no. ab76026; abchem), p65 (1:1,000
dilution; cat. no. 8242; Cell Signaling Technology, Inc.), p-p65
(1:1,000 dilution; cat. no. 3033; Cell Signaling Technology, Inc.),
inhibitor of kB (IkB; 1:1,000 dilution; cat. no. 9242; Cell
Signaling Technology, Inc.), p-IkB (1:1,000 dilution; cat. no.
2589; Cell Signaling Technology, Inc.) and β-actin (1:2,500
dilution; cat. no. A5441; Sigma-Aldrich; Merck KGaA) were diluted
in TBST containing 5% milk. After being washed intensely with TBST,
the following HRP-conjugated secondary antibodies were added and
incubated overnight at room temperature: Anti-mouse IgG (1:1,000
dilution; cat. no. 7076; Cell Signaling Technology, Inc.) or
Peroxidase AffiniPure goat anti-rabbit IgG (1:1,000 dilution; cat.
no. 111-035-144; Jackson ImmunoResearch, Inc.). The blots were then
developed and detected with the use of an enhanced
chemiluminescence agent (cat. no. RPN3004; Amersham; Cytiva).
Statistical analysis
Values are expressed as the mean ± SD from three
independent experiments. Results were statistically analyzed using
SPSS 20.0 (IBM Corp.). To assess the differences between multiple
groups, one-way analysis of variance was used, and Dunnett's
multiple-comparison test was implemented. P<0.05 was considered
to indicate a statistically significant difference.
Results
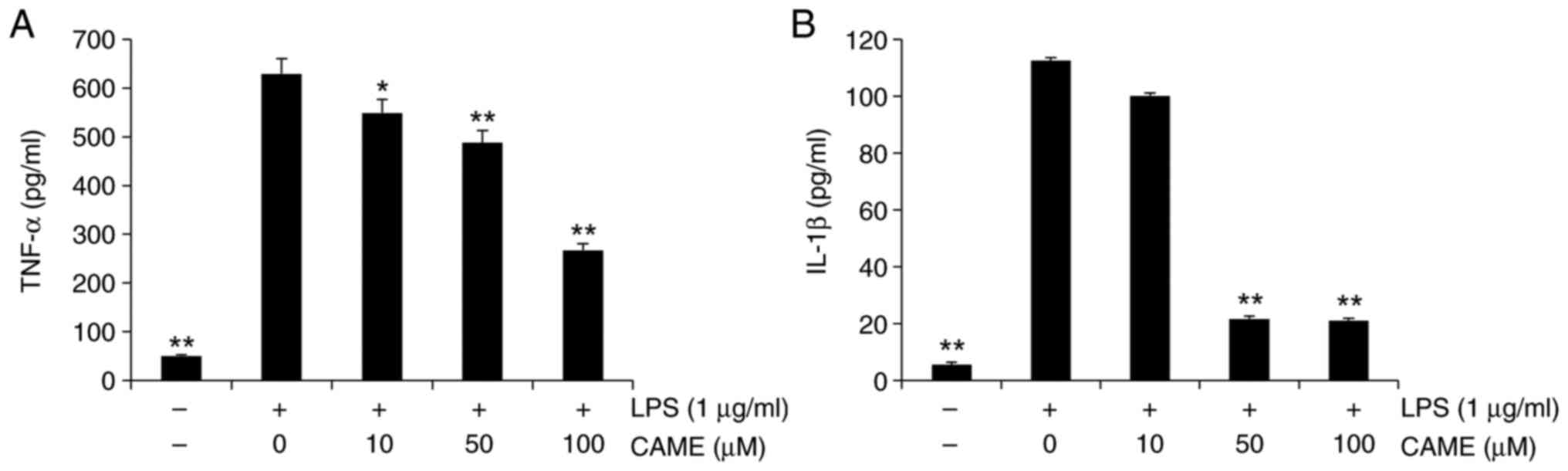
CAME inhibits TNF-α and IL-1β
secretion in LPS-challenged HUVECs
Research studies have reported that inflammatory
cytokines such as TNF-α and IL-1β have a vital influence on the
progression of inflammation (28,29).
Hence, the impact of CAME on the extracellular secretion of TNF-α
and IL-1β was examined in LPS-challenged HUVECs. The cells were
treated with CAME for 24 h prior to LPS treatment (1 µg/ml). LPS
increased the secretion of TNF-α and IL-1β in HUVECs and CAME
significantly suppressed the extracellular release of TNF-α and
IL-1β in LPS-stimulated HUVECs in a concentration-dependent manner
(Fig. 1). Of note, there was no
evident cytotoxicity of CAME within the concentration ranges used
(data not shown).
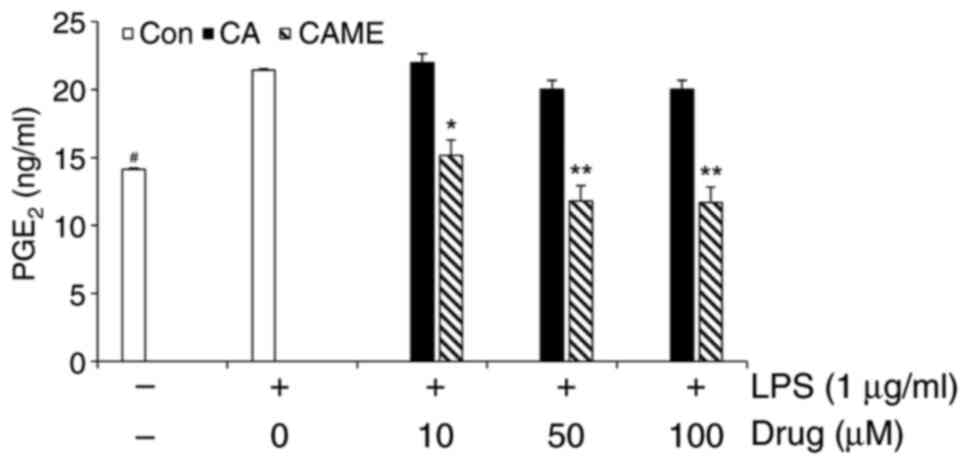
CAME inhibits LPS-induced
PGE2 release and expression of COX-2 and iNOS
Given the previous report that increased levels of
COX-2 are associated with inflammatory response leading to the
production of pro-inflammatory mediator PGE2 (22), PGE2 secretion and COX-2
expression were examined in LPS-challenged HUVECs. LPS treatment
resulted in increased secretion of PGE2 (Fig. 2). To compare the potency of CAME,
CA was utilized as a reference compound. Of note, at the
concentrations used in the present study, CA exerted no noticeable
attenuation on the LPS-induced PGE2 secretion, whereas
CAME showed a significant inhibition of LPS-induced PGE2
production (Fig. 2), suggesting
that CAME may be more potent than CA. Furthermore, the expression
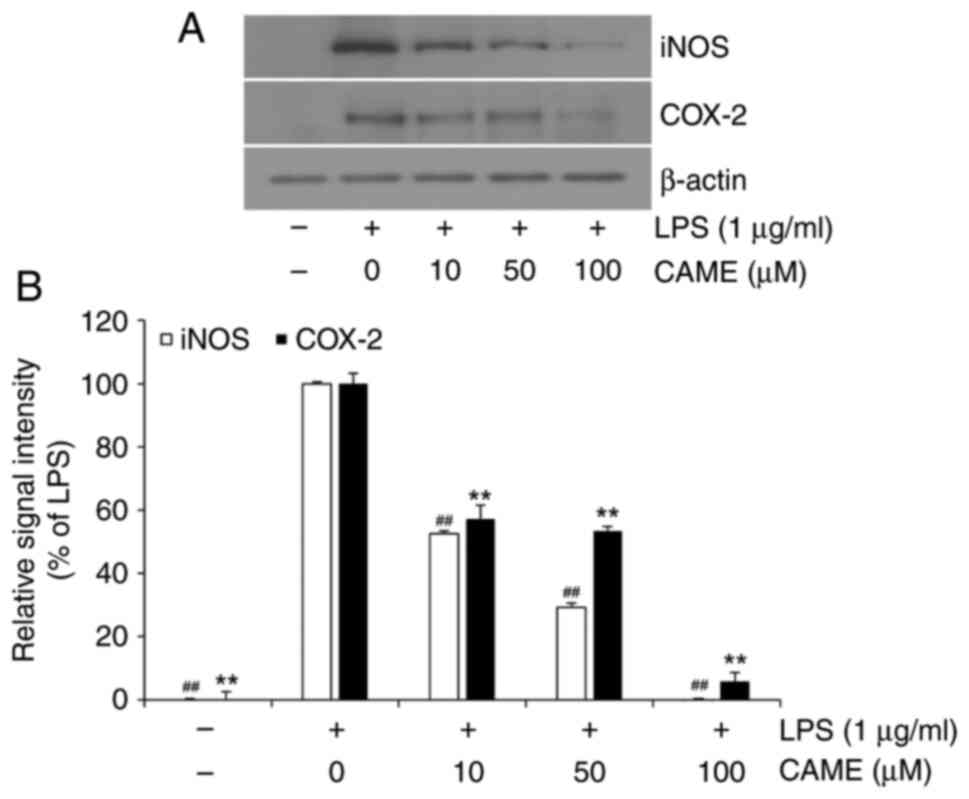
level of COX-2 was examined. In accordance with PGE2
secretion, CAME significantly suppressed LPS-induced COX-2
expression in HUVECs (Fig. 3A).
Quantitative analysis of COX-2 expression revealed considerable
inhibition of COX-2 expression in a concentration-dependent manner
(Fig. 3B). In addition, effects on
iNOS, a pro-inflammatory protein related to endothelial
inflammation, were examined. CAME significantly inhibited
LPS-induced iNOS expression depending on the concentration of the
compound in LPS-challenged HUVECs (Fig. 3), suggesting that CAME suppresses
endothelial inflammation through inhibition of the expression of
pro-inflammatory proteins such as COX-2 and iNOS in HUVECs.
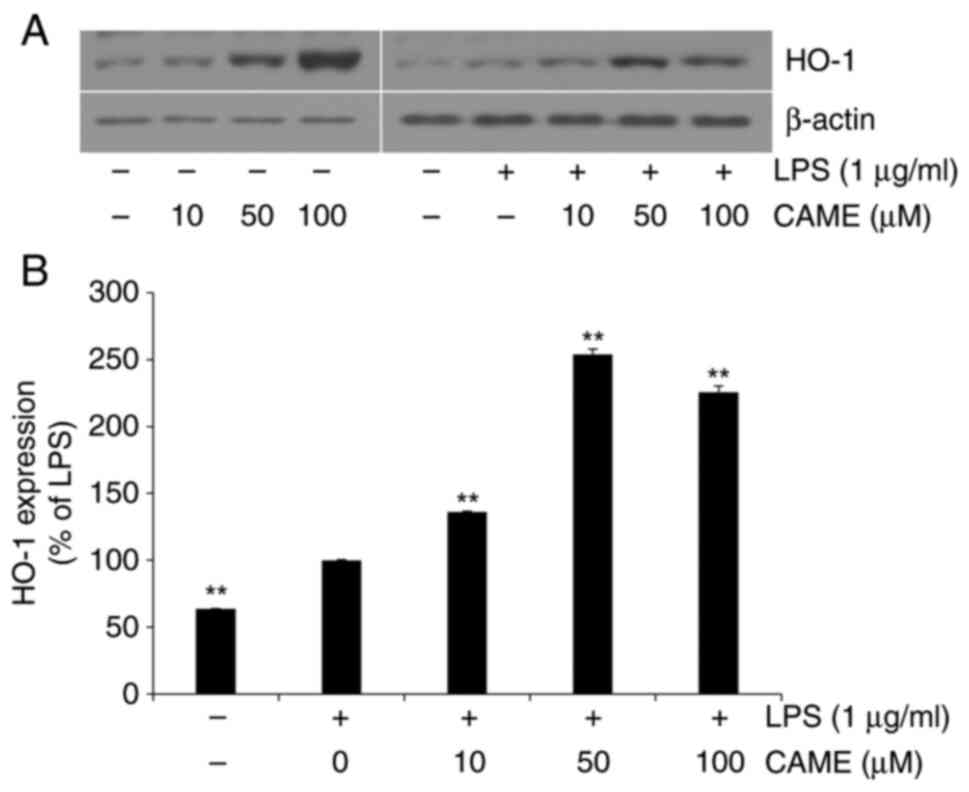
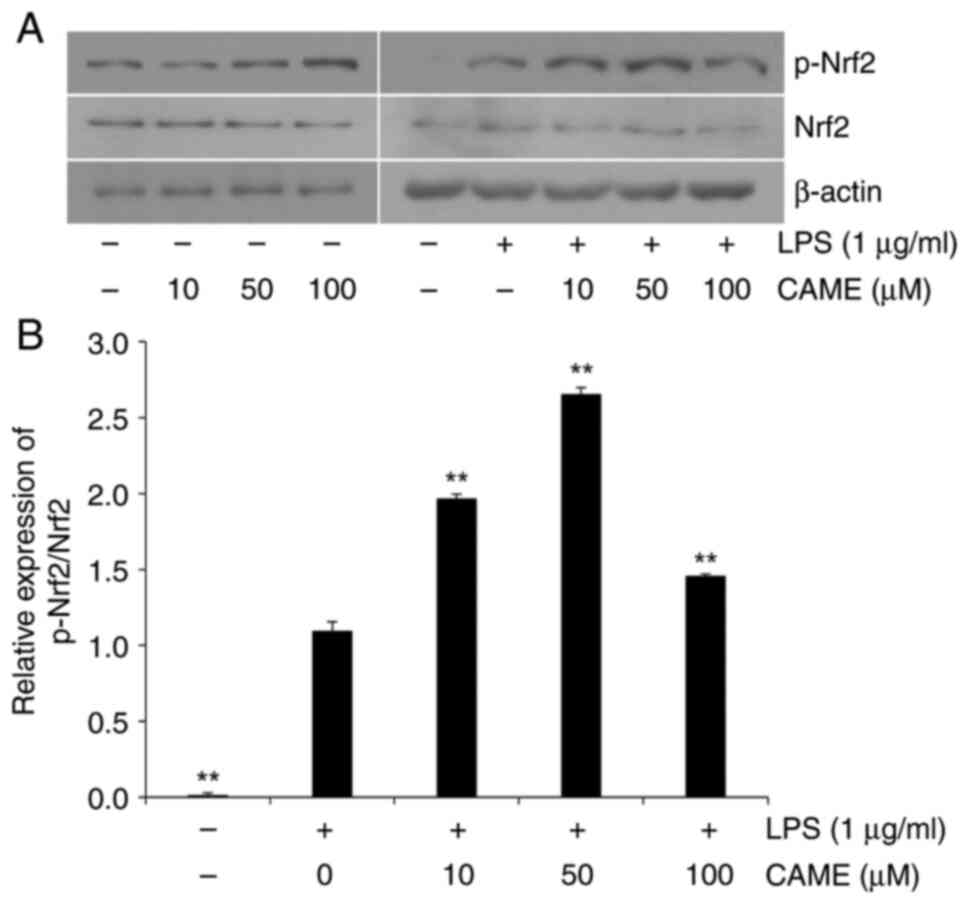
CAME activates the HO-1/Nrf2 pathway
in LPS-challenged HUVECs
HO-1, which is expressed through the transcription
of Nrf2, has been reported to exert cytoprotective effects against
a wide range of cellular stresses (30,31).
Thus, in the present study, the effect of CAME on the expression of
HO-1 was determined. In the absence of LPS challenge, CAME produced
an increase in the expression of HO-1 depending on the
concentration of the compound in HUVECs (Fig. 4). CAME resulted in increased
expression of HO-1 in the presence of LPS challenge, suggesting
that CAME exerts cytoprotective effects irrespective of the absence
or presence of cellular stresses. Furthermore, the phosphorylation
level of Nrf2, the transcription factor of HO-1, was also examined.
In accordance with the level of HO-1, Nrf2 phosphorylation was
increased with CAME in the absence or presence of LPS treatment
(Fig. 5). These results strongly
indicate that CAME may exert its cytoprotective activity through
activation of the HO-1/Nrf2 pathway.
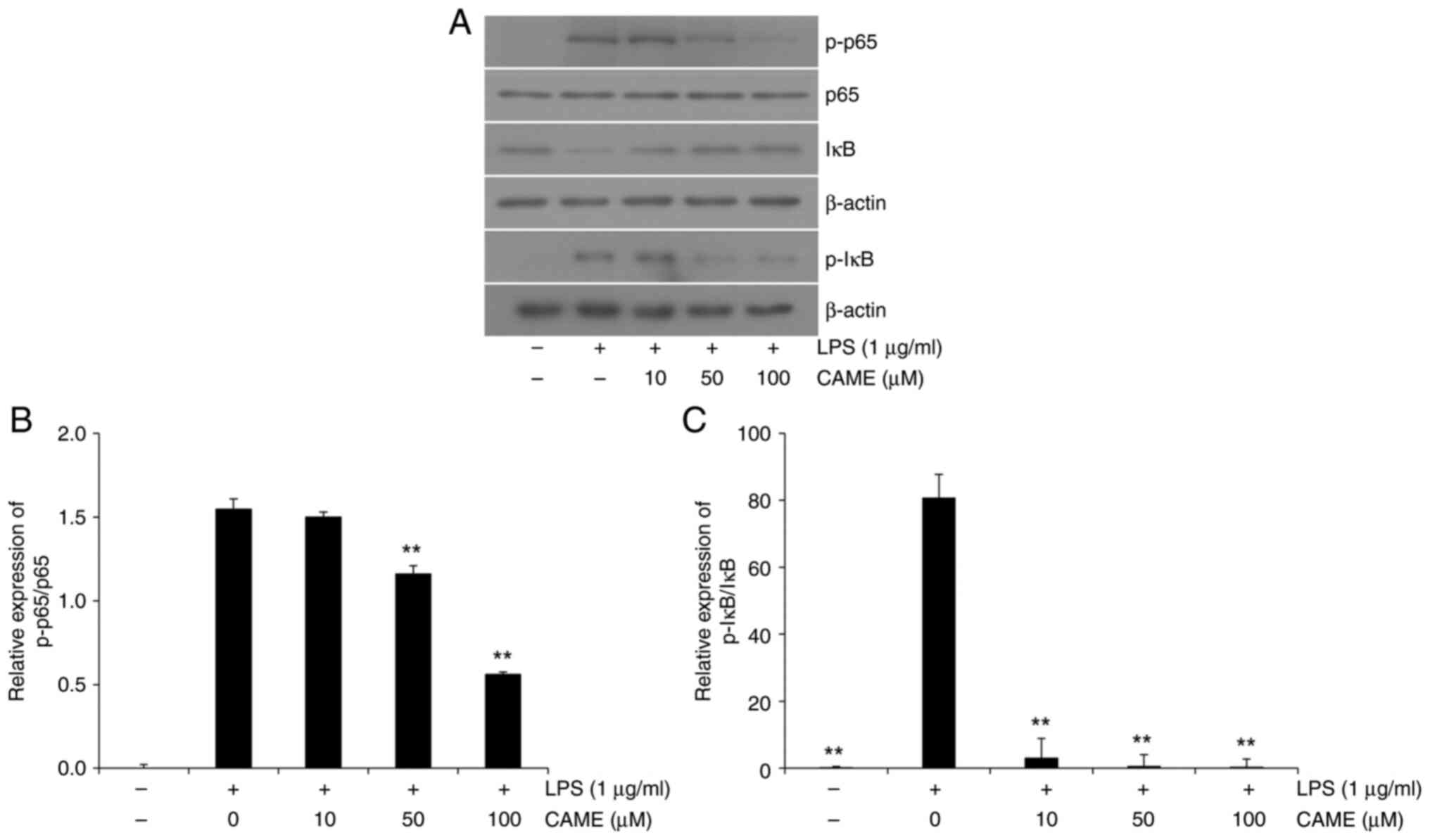
CAME inhibits NF-κB phosphorylation,
and IκB phosphorylation and degradation
NF-κB is a pivotal transcription factor of
pro-inflammatory genes in various inflammatory conditions (29). In the present study, the impact of
CAME on LPS-induced activation of NF-κB was analyzed in
LPS-challenged HUVECs. LPS treatment produced significantly
increased phosphorylation of NF-κB a concentration-dependent manner
in HUVECs (Fig. 6). Representative
immunoblots showed increased phosphorylation of the p65 subunit and
a gradual decrease with CAME (Fig.
6A), and quantitative analysis showed a significant decrease of
p65 phosphorylation in a concentration-dependent manner (Fig. 6B). LPS-induced phosphorylation of
IκB was significantly attenuated by CAME in a
concentration-dependent manner (Fig.
6C). As IκB prevents the translocation of the phosphorylated
p65 subunit of NF-κB into the nucleus (29), the total level of IκB was also
examined. IκB levels were significantly attenuated with the LPS
treatment in HUVECs and CAME suppressed IκB degradation in a
dose-dependent manner (Fig. 6A and
C). Presumably, sustained levels
of IκB by CAME sequester NF-κB in the cytosol, preventing the
NF-κB-mediated transcription of pro-inflammatory genes. Thus, CAME
inhibited LPS-induced NF-κB activation through suppression of IκB
degradation.
Discussion
The findings of the present study demonstrated that
CAME is effective in reducing inflammatory responses triggered by
LPS in vascular endothelial cells. CAME significantly reduced the
levels of cytokines secreted, as well as the expression of COX-2
and iNOS in HUVECs challenged with LPS. CAME significantly
activated the HO-1/Nrf2 pathway and suppressed the aberrantly
activated NF-κB pathway in HUVECs.
CA and their derivatives are known to possess a
diverse range of biological properties, such as antitumor,
anti-inflammatory, antimicrobial, immunosuppressive and
neuroprotective activities (32-34).
In a previous study by our group, trihydroxycinnamic acid (THC), a
derivative of CA, was shown to exert anti-inflammatory effects in
LPS-challenged BV2 microglial cells (35). Our group also reported that THC
leads to a reduction of LPS-induced inflammatory reactions in
RAW264.7 macrophage cells and enhanced survival rates in mice in an
LPS-induced endotoxemia model (9).
Pathologic endothelial activation has been observed
in sepsis and numerous cardiovascular conditions, including
atherosclerosis, hypertension and hemolysis (36,37).
During the inflammatory response, vascular endothelial cells
excessively produce a range of cytokines and mediators, thereby
exacerbating the inflammatory injury (38). In the present study, the
LPS-induced overproduction of cytokines, such as TNF-α and IL-1β,
was significantly reduced with CAME treatment. The vascular
endothelium acts as a barrier that selectively regulates the
transfer of plasma and cells between the bloodstream and adjacent
tissues (39). In a previous study
by our group, it was observed that LPS-induced vascular leakage in
multiple organs, encompassing the spleen, kidney and liver, was
substantially curtailed by THC, a CA derivative, in a mouse model
of sepsis (9). Furthermore, THC
significantly reduced the infiltration of macrophages in the
kidneys in an LPS-induced mouse model of sepsis (9). These results suggest that ester
derivatives including CAME may also possess protective properties
against vascular damage.
Nrf2 has been shown to have an essential role in
maintaining cellular homeostasis and suppressing inflammatory
conditions through the expression of cytoprotective enzymes and
stress-responsive proteins (40,41).
In experiments involving mice treated with sulforaphane, activation
of Nrf2 and an enhancement of HO-1 were observed (42), while the absence of Nrf2 signaling
led to increased vulnerability to inflammation (43). In addition, the upregulation of
HO-1 contributed to a decrease in oxidative stress by eliminating
free heme and simultaneously increasing the level of
anti-inflammatory substances (44). Studies have indicated that the
activation of the HO-1/Nrf2 signaling pathway may be facilitated
via a variety of signaling pathways (9,10,35).
CAPE has been reported to activate the Nrf2 pathway in colonic
inflammation (45) and
osteoarthritis progression (46).
In the present study, treatment with CAME resulted in increased
levels of phosphorylated Nrf2, which in turn induced the increased
expression of HO-1 in HUVECs in the absence or presence of LPS
stimulation. These results imply that the HO-1/Nrf2 signaling
pathway may be induced with activators such as CAME, irrespective
of the presence of cellular stressors. Furthermore, the HO-1/Nrf2
pathway may also be activated as a defense mechanism against
cellular stressors. This suggests that activating the HO-1/Nrf2
pathway may prepare cells for the potential stressor under normal
conditions and also provide a defense against damage under stress
conditions. Given the cytoprotective role of HO-1, the
pharmacological induction of HO-1 expression with CAME may serve as
a promising therapeutic approach in combating sepsis and
inflammatory vascular conditions. However, further studies CA
derivatives activate Nrf2 phosphorylation.
NF-κB is the key transcription factor responsible
for inflammatory responses (35).
It is widely recognized that NF-κB is implicated in the generation
of pro-inflammatory cytokines such as IL-1β and TNF-α, as well as
proteins such as COX-2 and iNOS (47,48).
LPS has been known to activate the IκB kinase (IKK) complex,
composed of two catalytic subunits (IKKα and IKKβ) and a regulatory
subunit (NEMO/IKKγ). Once activated, IKK phosphorylates the IκB
proteins, targeting ubiquitination and subsequent proteasomal
degradation. This allows the NF-κB dimers (p50/p65) to translocate
to the nucleus and induce the transcription of target genes. Given
the fact that inhibition of IKK can prevent the activation and
nuclear translocation of NF-κB, IKK inhibitors are potential drugs
to suppress the production of cytokines and other inflammatory
mediates, ultimately reducing inflammation in tissue damage in
various disorders, such as rheumatoid arthritis, inflammatory bowel
disease and psoriasis (49,50).
Previously, it was reported that CA regulates the NF-κB signaling
pathway by acting on the IKK pathway (51). In the present study, CAME
significantly suppressed LPS-induced IκB degradation, suggesting a
possibility that CAME may directly interfere with the metabolizing
action of IKK. Therefore, further studies assessing the involvement
of IKK by determining its phosphorylation level and investigating
nuclear translocation of NF-κB through a nuclear fractionation
assay may offer a more comprehensive understanding of the complete
role of the NF-κB pathway.
Of note, the present study had a limitation. CAME,
used in the present study, was derived from Lonicera maackii
and had a purity of >95%. However, it contained certain
impurities. There is a chance that these impurities may have
influenced the study's anti-inflammatory results. To address this
limitation, future studies should compare the effects with CAME
commercially purified to 100% to validate the findings of the
present study. In addition to CAME, various other compounds, such
as 5-caffeoylquinic acid n-butyl ester, methyl 3,4-dicaffeoyl
quinate, 3,5-dicaffeoyl quinic acid n-butyl ester, loganin and CA
have been isolated from Lonicera maackii. While the current
study investigated the anti-inflammatory properties of CAME, it
does not offer a comprehensive understanding of the biological
effects of Lonicera maackii. Consequently, in-depth
investigations into each individual compound, as well as the whole
extract, are crucial for an extensive understanding of the
biological properties of Lonicera maackii.
In conclusion, the findings of the present study
provide clear evidence that CAME effectively suppresses
LPS-triggered inflammatory responses by activating the
cytoprotective HO-1/Nrf2 pathway and inhibiting pro-inflammatory
NF-κB signaling in LPS-challenged HUVECs. CAME can activate
HO-1/Nrf2 pathway under normal conditions to preemptively prepare
cells for potential stressors and also provide protection against
stressors in inflammatory conditions. The results clearly suggest
that CAME is a promising therapeutic agent having dual beneficial
properties against inflammatory vascular disorders.
Acknowledgements
Not applicable.
Funding
Funding: This work was supported by the National Research
Foundation of Korea grant funded by the Korea government (grant no.
2021-R1A4A1031574) and the Korea Basic Science Institute (National
Research Facilities and Equipment Center) grant funded by the
Ministry of Education (grant no. 2022R1A6C101A739).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JYP and MY conducted the experiments and analyzed
the data. ETH, WSP and JHH performed data analysis for the study.
YSK isolated and identified CAME and CA from Lonicera
maackii. HJL performed the statistical analysis of the results.
WC conceptualized the study and wrote, reviewed and edited the
manuscript. YSK and WC confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yisireyili M, Hayashi M, Wu H, Uchida Y,
Yamamoto K, Kikuchi R, Shoaib Hamrah M, Nakayama T, Wu Cheng X,
Matsushita T, et al: Xanthine oxidase inhibition by febuxostat
attenuates stress-induced hyperuricemia, glucose dysmetabolism, and
prothrombotic state in mice. Sci Rep. 7(1266)2017.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gray SP and Jandeleit-Dahm KAM: The role
of NADPH oxidase in vascular disease-hypertension, atherosclerosis
& stroke. Curr Pharm Des. 21:5933–5944. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Han JM, Li H, Cho MH, Baek SH, Lee CH,
Park HY and Jeong TS: Soy-leaf extract exerts atheroprotective
effects via modulation of Krüppel-like factor 2 and adhesion
molecules. Int J Mol Sci. 18(373)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kang JS, Jeon YJ, Park SK, Yang KH and Kim
HM: Protection against lipopolysaccharide-induced sepsis and
inhibition of interleukin-1beta and prostaglandin E2 synthesis by
silymarin. Biochem Pharmacol. 67:175–181. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Reinhart K, Bayer O, Brunkhorst F and
Meisner M: Markers of endothelial damage in organ dysfunction and
sepsis. Crit Care Med. 30 (5 Suppl):S302–S312. 2002.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yano K, Liaw PC, Mullington JM, Shih SC,
Okada H, Bodyak N, Kang PM, Toltl L, Belikoff B, Buras J, et al:
Vascular endothelial growth factor is an important determinant of
sepsis morbidity and mortality. J Exp Med. 203:1447–1458.
2006.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Shapiro NI, Khankin EV, Van Meurs M, Shih
SC, Lu S, Yano M, Castro PR, Maratos-Flier E, Parikh SM, Karumanchi
SA and Yano K: Leptin exacerbates sepsis-mediated morbidity and
mortality. J Immunol. 185:517–524. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Lee HJ, Lim HJ, Lee DY, Jung H, Kim MR,
Moon DC, Kim KI, Lee MS and Ryu JH: Carabrol suppresses LPS-induced
nitric oxide synthase expression by inactivation of p38 and JNK via
inhibition of I-kappaBalpha degradation in RAW 264.7 cells. Biochem
Biophys Res Commun. 391:1400–1404. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lee JW, Kwon JH, Lim MS, Lee HJ, Kim SS,
Lim SY and Chun W: 3,4,5-Trihydroxycinnamic acid increases
heme-oxygenase-1 (HO-1) and decreases macrophage infiltration in
LPS-induced septic kidney. Mol Cell Biochem. 397:109–116.
2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ha YM, Ham SA, Kim YM, Lee YS, Kim HJ, Seo
HG, Lee JH, Park MK and Chang KC: β1-adrenergic receptor-mediated
HO-1 induction, via PI3K and p38 MAPK, by isoproterenol in RAW
264.7 cells leads to inhibition of HMGB1 release in LPS-activated
RAW 264.7 cells and increases in survival rate of CLP-induced
septic mice. Biochem Pharmacol. 82:769–777. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Fidan H, Sahin O, Yavuz Y, Kilbas A,
Cetinkaya Z, Ela Y, Ozen OA and Altuntas I: Caffeic acid phenethyl
ester reduces mortality and sepsis-induced lung injury in rats.
Crit Care Med. 35:2822–2829. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Park SY, Seetharaman R, Ko MJ, Kim DY, Kim
TH, Yoon MK, Kwak JH, Lee SJ, Bae YS and Choi YW: Ethyl linoleate
from garlic attenuates lipopolysaccharide-induced pro-inflammatory
cytokine production by inducing heme oxygenase-1 in RAW264.7 cells.
Int Immunopharmacol. 19:253–261. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tsoyi K, Lee TY, Lee YS, Kim HJ, Seo HG,
Lee JH and Chang KC: Heme-oxygenase-1 induction and carbon
monoxide-releasing molecule inhibit lipopolysaccharide
(LPS)-induced high-mobility group box 1 release in vitro and
improve survival of mice in LPS- and cecal ligation and
puncture-induced sepsis model in vivo. Mol Pharmacol. 76:173–182.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Park EJ, Lim JH, Nam SI, Park JW and Kwon
TK: Rottlerin induces heme oxygenase-1 (HO-1) up-regulation through
reactive oxygen species (ROS) dependent and PKC delta-independent
pathway in human colon cancer HT29 cells. Biochim. 92:110–115.
2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Clifford MN: Chlorogenic acids and other
cinnamates-nature, occurrence and dietary burden†. J Sci Food
Agric. 79:362–372. 1999.
|
|
16
|
Macheix JJ, Fleuriet A and Billot J: Fruit
phenolics. CRC Press, FL,. 113:pp41–43. 1990.
|
|
17
|
Murtaza G, Karim S, Akram MR, Khan SA,
Azhar S, Mumtaz A and Bin Asad MH: Caffeic acid phenethyl ester and
therapeutic potentials. Biomed Res Int. 2014(145342)2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bose JS, Gangan V, Jain SK and Manna SK:
Novel caffeic acid ester derivative induces apoptosis by expressing
FasL and downregulating NF-KappaB: Potentiation of cell death
mediated by chemotherapeutic agents. J Cell Physiol. 218:653–662.
2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Collins W, Lowen N and Blake DJ: Caffeic
acid esters are effective bactericidal compounds against
paenibacilluslarvae by altering intracellular oxidant and
antioxidant levels. Biomolecules. 9(312)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gießel JM, Loesche A, Csuk R and Serbian
I: Caffeic acid phenethyl ester (CAPE)-derivatives act as selective
inhibitors of acetylcholinesterase. Eur J Med Chem. 177:259–268.
2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schoonbroodt S, Legrand-Poels S,
Best-Belpomme M and Piette J: Activation of the NF-kappaB
transcription factor in a T-lymphocytic cell line by hypochlorous
acid. Biochem J. 321:777–785. 1997.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou Y, Yang Q, Xu H, Zhang J, Deng H, Gao
H, Yang J, Zhao D and Liu F: miRNA-221-3p enhances the secretion of
interleukin-4 in mast cells through the phosphatase and tensin
homolog/p38/nuclear factor-kappaB pathway. PLoS One.
11(e0148821)2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Cho MS, Park WS, Jung WK, Qian ZJ, Lee DS,
Choi JS, Lee DY, Park SG, Seo SK, Kim HJ, et al: Caffeic acid
phenethyl ester promotes anti-inflammatory effects by inhibiting
MAPK and NF-κB signaling in activated HMC-1 human mast cells. Pharm
Biol. 52:926–932. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Shin KM, Kim IT, Park YM, Ha J, Choi JW,
Park HJ, Lee YS and Lee KT: Anti-inflammatory effect of caffeic
acid methyl ester and its mode of action through the inhibition of
prostaglandin E2, nitric oxide and tumor necrosis factor-alpha
production. Biochem Pharmacol. 68:2327–2336. 2004.PubMed/NCBI View Article : Google Scholar
|
|
25
|
An GY, Chung SW, Cho HC, Park JR, Kim MJ,
Kim HP, Yang HJ, Chun W and Kwon Y: Phytochemical constituents of
Lonicera maackii stems. Korean J Pharmacogn. 49:103–107.
2018.
|
|
26
|
Kim DY, Park JA, Kim Y, Noh M, Park S, Lie
E, Kim E, Kim YM and Kwon YG: SALM4 regulates angiogenic functions
in endothelial cells through VEGFR2 phosphorylation at Tyr1175.
FASEB J. 33:9842–9857. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Park JY, Lee HJ, Han ET, Han JH, Park WS,
Kwon YS and Chun W: Caffeic acid methyl ester inhibits mast cell
activation through the suppresion of MAPKs and NF-κB signaling in
RBL-2H3 cells. Heliyon. 9(e16529)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hu Frisk JM, Kjellén L, Melo FR, Öhrvik H
and Pejler G: Mitogen-activated protein kinase signaling regulates
proteoglycan composition of mast cell secretory granules. Front
Immunol. 9(1670)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rehman MU, Yoshihisa Y, Miyamoto Y and
Shimizu T: The anti-inflammatory effects of platinum nanoparticles
on the lipopolysaccharide-induced inflammatory response in RAW
264.7 macrophages. Inflamm Res. 61:1177–1185. 2012.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bhatt NP, Park JY, Lee HJ, Kim SS, Kwon YS
and Chun W: Apocynin protects mesangial cells from
lipopolysaccharide-induced inflammation by exerting heme oxygenase
1-mediated monocyte chemoattractant protein-1 suppression. Int J
Mol Med. 40:1294–1301. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Subedi L, Lee JH, Yumnam S, Ji E and Kim
SY: Anti-inflammatory effect of sulforaphane on LPS-activated
microglia potentially through JNK/AP-1/NF-κB inhibition and
Nrf2/HO-1 activation. Cells. 8(194)2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Nagasaka R, Chotimarkorn C, Shafiqul IM,
Hori M, Ozaki H and Ushio H: Anti-inflammatory effects of
hydroxycinnamic acid derivatives. Biochem Biophys Res Commun.
358:615–619. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kim YC: Neuroprotective phenolics in
medicinal plants. Arch Pharm Res. 33:1611–1632. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee JW, Cheong IY, Kim HS, Lee JJ, Lee YS,
Kwon YS, Kim MJ, Lee HJ, Kim SS and Chun W: Anti-inflammatory
activity of 1-docosanoyl cafferate isolated from rhus verniciflua
in LPS-stimulated BV2 microglial cells. Korean J Physiol Pharmacol.
15:9–15. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee JW, Bae CJ, Choi YJ, Kim SI, Kim NH,
Lee HJ, Kim SS, Kwon YS and Chun W: 3,4,5-Trihydroxycinnamic acid
inhibits LPS-induced iNOS expression by suppressing NF-κB
activation in BV2 microglial cells. Korean J Physiol Pharmacol.
16:107–112. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ross R: Atherosclerosis-an inflammatory
disease. N Engl J Med. 340:115–126. 1999.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bains SK, Foresti R, Howard J, Atwal S,
Green CJ and Motterlini R: Human sickle cell blood modulates
endothelial heme oxygenase activity: Effects on vascular adhesion
and reactivity. Arterioscler Thromb Vasc Biol. 30:305–312.
2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu XH, Pan LL, Yang HB, Gong QH and Zhu
YZ: Leonurine attenuates lipopolysaccharide-induced inflammatory
responses in human endothelial cells: Involvement of reactive
oxygen species and NF-κB pathways. Eur J Pharmacol. 680:108–114.
2012.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fisher M: Injuries to the vascular
endothelium: Vascular wall and endothelial dysfunction. Rev Neurol
Dis. 5 (Suppl 1):S4–S11. 2008.PubMed/NCBI
|
|
40
|
Lee IS, Lim J, Gal J, Kang JC, Kim HJ,
Kang BY and Choi HJ: Anti-inflammatory activity of xanthohumol
involves heme oxygenase-1 induction via NRF2-ARE signaling in
microglial BV2 cells. Neurochem Int. 58:153–160. 2011.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Surh YJ, Kundu JK and Na HK: Nrf2 as a
master redox switch in turning on the cellular signaling involved
in the induction of cytoprotective genes by some chemopreventive
phytochemicals. Planta Med. 74:1526–1539. 2008.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Lin W, Wu RT, Wu T, Khor TO, Wang H and
Kong AN: Sulforaphane suppressed LPS-induced inflammation in mouse
peritoneal macrophages through Nrf2 dependent pathway. Biochem
Pharmacol. 76:967–973. 2008.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yates MS, Tran QT, Dolan PM, Osburn WO,
Shin S, McCulloch CC, Silkworth JB, Taguchi K, Yamamoto M, Williams
CR, et al: Genetic versus chemoprotective activation of Nrf2
signaling: Overlapping yet distinct gene expression profiles
between Keap1 knockout and triterpenoid-treated mice.
Carcinogenesis. 30:1024–1031. 2009.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kawakami T, Takahashi T, Shimizu H,
Nakahira K, Takeuchi M, Katayama H, Yokoyama M, Morita K, Akagi R
and Sassa S: Highly liver-specific heme oxygenase-1 induction by
interleukin-11 prevents carbon tetrachloride-induced
hepatotoxicity. Int J Mol Med. 18:537–546. 2006.PubMed/NCBI
|
|
45
|
Kim H, Kim W, Yum S, Hong S, Oh JE, Lee
JW, Kwak MK, Park EJ, Na DH and Jung Y: Caffeic acid phenethyl
ester activation of Nrf2 pathway is enhanced under oxidative state:
Structural analysis and potential as a pathologically targeted
therapeutic agent in treatment of colonic inflammation. Free Radic
Biol Med. 65:552–562. 2013.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sun W, Xie W, Huang D, Cui Y, Yue J, He Q,
Jiang L, Xiong J, Sun W and Yi Q: Caffeic acid phenethyl ester
attenuates osteoarthritis progression by activating NRF2/HO-1 and
inhibiting the NF-κB signaling pathway. Int J Mol Med.
50(134)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Wan M, Liu J and Ouyang X:
Nucleotide-binding oligomerization domain 1 regulates Porphyromonas
gingivalis-induced vascular cell adhesion molecule 1 and
intercellular adhesion molecule 1 expression in endothelial cells
through NF-κB pathway. J Periodontal Res. 50:189–196.
2015.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang L, Xu Y, Yu Q, Sun Q, Xu Y, Gu Q and
Xu X: H-RN, a novel antiangiogenic peptide derived from hepatocyte
growth factor inhibits inflammation in vitro and in vivo through
PI3K/AKT/IKK/NF-κB signal pathway. Biochem Pharmacol. 89:255–265.
2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Antonia RJ, Hagan RS and Baldwin AS:
Expanding the view of IKK: New substrates and new biology. Trends
Cell Biol. 31:166–178. 2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Mulero MC, Huxford T and Ghosh G: NF-κB,
IκB, and IKK: Integral components of immune system signaling. Adv
Exp Med Biol. 1172:207–226. 2019.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Kim SR, Jung YR, Kim DH, An HJ, Kim MK,
Kim ND and Chung HY: Caffeic acid regulates LPS-induced NF-κB
activation through NIK/IKK and c-Src/ERK signaling pathways in
endothelial cells. Arch Pharm Res. 37:539–547. 2014.PubMed/NCBI View Article : Google Scholar
|