Introduction
Acute pancreatitis (AP) is an inflammatory process
affecting the pancreas and is characterized by severe abdominal
pain and increased levels of amylase and lipase (1). AP in pregnancy (APIP) is a rare
complication (incidence between 1 in 12,000 and 1 in 1,000) that
threatens the health of the patient and their offspring (2,3).
APIP is associated with increased risk of prematurity,
preeclampsia, post-partum hemorrhage, maternal death and fetal
demise. In pregnant women with AP, delivery is associated with
increased risk of requiring transfusions, developing venous
thromboembolisms, acute respiratory failure and disseminated
intravascular coagulation (4,5).
Hyperlipidemia (HL) AP in pregnancy has been reported to be
associated with a maternal mortality rate of ~20% (6). HLAP can cause serious complications,
such as fetal distress, intrauterine death, maternal multiple organ
failure, abdominal compartment syndrome and pancreatic
encephalopathy, when not treated in a timely manner (7).
HL has emerged as an important cause of APIP in the
Chinese female population (8).
Among Chinese women, HL is the second leading cause of APIP after
cholelithiasis (6). HLAP is
increased in patients with plasma triglyceride (TG) levels
>1,000 mg/dl (11.3 mmol/l) compared with patients with plasma TG
levels ≤1,000 mg/dl. Excess TGs are hydrolyzed by lipase enzymes
excreted by pancreatic acinar cells to produce free fatty acids
(FFAs). FFAs have a direct cytotoxic effect on acinar and vascular
endothelial cells and elevated levels of FFAs trigger an
inflammatory response (9). Local
inflammation, triggered by the activation of pancreatic proteolytic
enzymes within acinar tissues, may progress to systemic
inflammation (10).
HLAP in pregnancy has been reported to be associated
with a higher risk of severe AP (SAP) and poor maternal and fetal
outcomes compared with AP due to other causes (11). TG levels have been demonstrated to
be positively associated with AP severity, and a prospective
multicenter study of 716 patients indicated a dose-dependent
relationship between TG levels and AP severity, and high TG levels
were associated with organ failure in patients with AP (12). An observational study of 54
pregnant patients with AP showed that HL is associated with more
intrauterine fetal distress and worse fetal outcomes compared with
AP from other etiologies (13).
Severe pancreatitis is often complicated by varying degrees of
multi-organ failure such as acute respiratory failure, acute renal
failure, heart failure and arrhythmias, gastrointestinal bleeding,
and pancreatic encephalopathy (3).
SAP is a serious complication during pregnancy and the main cause
of maternal and fetal death, with its fetal mortality rate at
10-30% (14). The 2012 revised
Atlanta Classification classifies the severity of AP as follows:
Mild (M) AP, patients without organ failure and local
complications; moderately severe AP, patients with organ failure
for <48 h or local complications; SAP, patients with organ
failure for >48 h (15).
HL can originate from a primary (genetic)
abnormality of lipid metabolism or it may be caused by other
diseases that cause an overproduction of TG, such as diabetes,
visceral obesity and pregnancy (16). HL is defined as >11.3 mmol/l or
5.6-11.3 mmol/l TG with chyle blood (17). Severe HL also increases the risk of
AP. A previous study reported that, compared with a control group
of individuals with <150 mg/dl TG, patients with 150-300 mg/dl
TG have an AP hazard ratio of 1.5, whilst individuals with >500
mg/dl TG had a hazard ratio of 3.2. Moreover, the same authors
reported a 4% increase in incidence of AP for every 1,000 mg/dl
increase in serum TG (18).
HLAP in pregnancy usually occurs in pregnant
individuals with preexisting abnormalities of lipid metabolism such
as obesity, fatty liver disease and diabetes before pregnancy
(16). The diagnosis of HLAP meets
the AP diagnostic criteria: i) Persistent pain in the upper
abdomen; ii) serum amylase and/or lipase concentrations are at
least 3 times higher than the upper limit of normal; and iii)
imaging findings of AP. AP is diagnosed if two of the three
criteria are met. The following criteria should also be met for the
diagnosis of HLAP: 1+3 or 2+3, where 1 indicates serum TG levels
≥11.3 mmol/l, 2 indicates serum TG levels between 5.65 and 11.3
mmol/l, and 3 indicates blood sample appeared chylous to exclude
other causes of acute pancreatitis (19). However, early diagnosis of HLAP in
pregnant patients can be delayed as its early clinical symptoms,
such as abdominal pain, can be confused with abdominal pain caused
by contractions in late pregnancy and abdominal pain caused by AP
(20).
HLAP is mainly caused by elevated serum
triglycerides and its treatment principles are similar to those of
management of non-pregnant patients; however, multidisciplinary
cooperation is required to ensure the safety of mother and baby.
Specific treatment measures include nutrient solution support,
fasting, gastrointestinal decompression, inhibition of pancreatic
enzyme activity, suppression of pancreatic secretion, maintenance
of insulin, heparin use and antibiotic use in infected patients
(21).
As HLAP can lead to adverse maternal and infant
outcomes, there is a need for early diagnosis and assessment of
disease severity to improve the prognosis of HLAP in pregnancy. The
present study reported on the early diagnosis and management of a
case of HLAP in a pregnant patient with diabetes and obesity and
emphasized the diagnostic and treatment challenges associated with
this condition.
Case Report
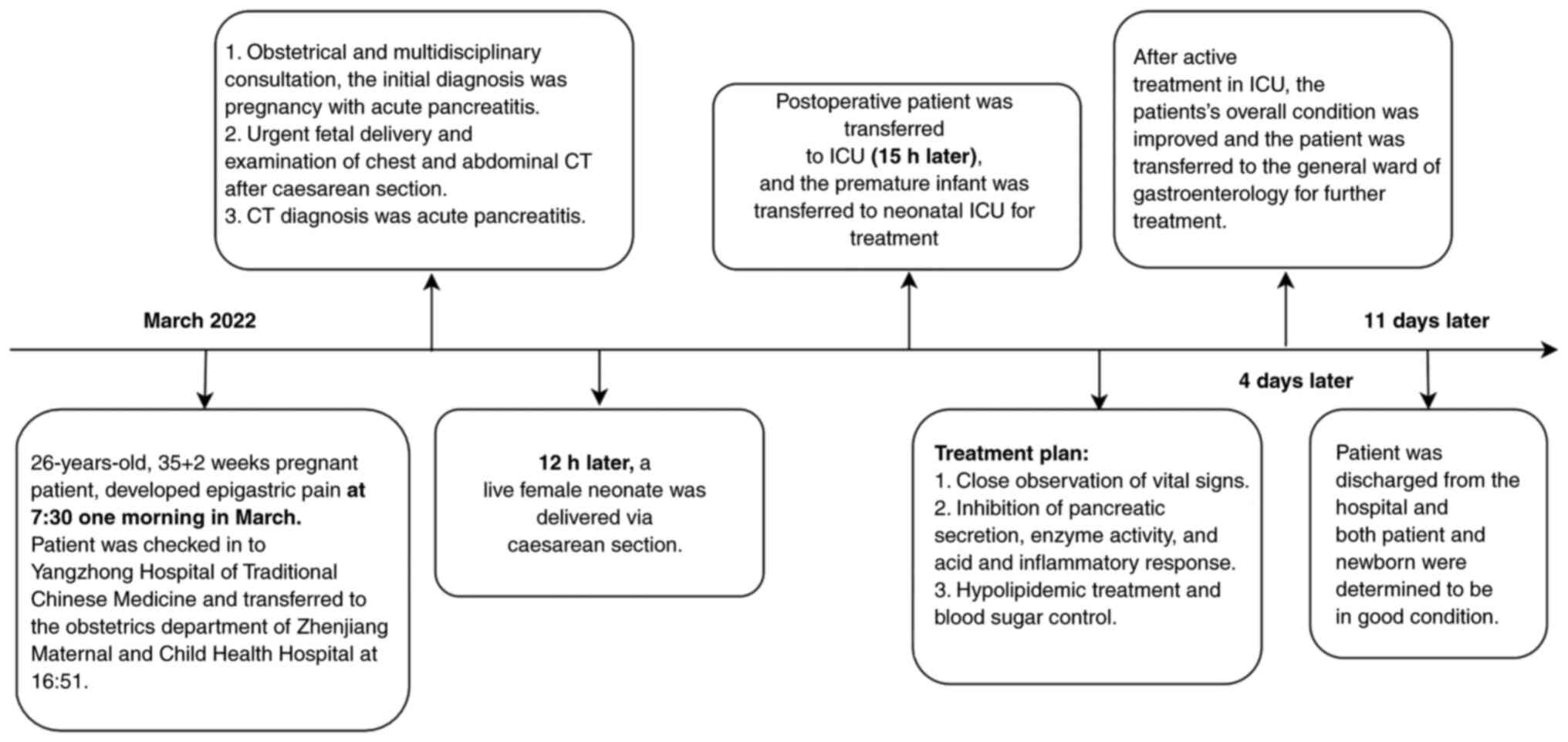
A 26-year-old patient at 35 weeks and 2 days of
gestation of their first pregnancy was transferred from Zhenjiang
Yangzhong County Hospital of Traditional Chinese Medicine
(Zhenjiang, China) to Maternity and Child Health Hospital of
Zhenjiang (Zhenjiang, China). They were hospitalized in March 2022
due to epigastric pain, which was dull with paroxysmal
intensification. The patient presented with a body temperature of
36.8˚C, blood pressure at 138/88 mmHg, respiratory rate of 17
breaths/min and pulse at 78 beats/min. Physical examination of the
abdominal pregnancy indicated a soft abdomen with tenderness under
the xiphoid process and no rebound pain reported in the left upper
abdomen. Bowel sounds were reduced to 1-2 times/min. Medical and
family history reported diagnoses of diabetes and obesity. The
patient's weight before pregnancy was 103 kg, with a BMI of 36.40
kg/m2.
Laboratory test results at admission, including
coagulation parameters, are presented in Table I. Levels of total cholesterol (TC),
TG and low-density lipoprotein (LDL) cholesterol were increased at
admission compared with their corresponding normal ranges and
high-density lipoprotein cholesterol levels reached 9.89 mmol/l. In
addition, blood samples appeared chylous. Other parameters were
within normal ranges.
 | Table ILaboratory tests of hyperlipidemia
acute pancreatitis during pregnancy. |
Table I
Laboratory tests of hyperlipidemia
acute pancreatitis during pregnancy.
| Main
parameters | Admission day | Admission
day-ICU | 4 days- ICU | Discharge day | Normal range |
|---|
| White blood count,
x109/l | 13.27 | 12.99 | 11.56 | 6.35 | 4.00-10.00 |
| Neutrophils,
x109/l | 11.23 | 8.94 | 9.77 | 6.61 | 1.80-6.30 |
| Neutrophil ratio,
% | 84.6% | 81.80 | 79.20 | 55.90 | 40.0-75.0 |
| C-reactive protein,
mg/l | 105.90 | 112.30 | 148.20 | 4.30 | 0.00-8.00 |
| Lipase, U/l | 425.00 | 355.00 | 305.00 | Untested | 23.00-300.00 |
| Amylase, IU/l | 147.60 | 168.00 | 49.90 | 47.40 | 37.00-53.00 |
| Uroamylase,
IU/l | 157.50 | 894.90 | 340.00 | 33.00 | 0.00-600.00 |
| Total protein,
g/l | 128.00 | 81.80 | 45.90 | 56.30 | 60.00-83.00 |
| ALB, g/l | 34.80 | 26.00 | 23.00 | 34.70 | 37.00-53.00 |
| GLOB, g/l | 93.20 | 19.10 | 20.50 | 21.60 | 20.00-32.00 |
| ALB/GLOB ratio | 0.37 | 0.49 | 1.12 | 1.61 | 1.20-2.40 |
| Lactic
dehydrogenase, IU/l | 212.00 | 345.00 | 194.00 | 193.00 | 103.00-227.00 |
| Glucose,
mmol/l | 9.62 | 9.74 | 8.37 | 5.69 | 3.89-6.11 |
| Creatinine,
µmol/l | 138.90 | 42.50 | 44.40 | 39.80 | 45.00-84.00 |
| Magnesium,
mmol/l | 0.59 | 0.71 | 0.71 | 1.02 | 0.70-1.08 |
| Total cholesterol,
mmol/l | 22.00 | 19.40 | 8.88 | 4.05 | 3.10-5.20 |
| Triglycerides,
mmol/l | 26.30 | 27.50 | 6.86 | 5.66 | 0.40-1.70 |
| Low density
lipoprotein cholesterol, mmol/l | 10.17 | 4.90 | 3.99 | 2.66 | 0.00-3.37 |
| High density
lipoprotein cholesterol, mmol/l | 9.89 | 0.70 | 0.72 | 1.46 | 1.03-1.55 |
| FG, g/l | 5.76 | 8.39 | 9.60 | 3.88 | 2.38-4.98 |
| FG degradation
product, µg/ml | 5.80 | 7.20 | 5.80 | 4.75 | 0.00-5.00 |
| Antithrombin III,
% | 20.40 | 16.00 | 57.00 | 76.00 | 83.00-128.00 |
| D-dimer, ng/ml | 2447.00 | 2054.00 | 1385.00 | 1345.00 | 0.00-255.00 |
The patient presented with the following pregnancy
measures: Uterine height, 41 cm; abdominal circumference, 120 cm;
fetal orientation, left occiput anterior; fetal heart rate, 150
beats/min; head exposure. Abdominal ultrasonography using the
emergency bedside B-ultrasound (GEE LOGIQ S8 ultrasound;
parameters: MI 1.0 TIS 1.6 C1-5 abdominal mode) showed a hypoechoic
pancreatic head (images not available). The Acute Physiologic
Assessment and Chronic Health Evaluation II (APACHEII) scoring
system was used, which includes an acute physiology score, chronic
health score and age score. APACHEII scores range between 1 and 71
points in total, and the higher the score, the more severe the
condition (22). The patient in
the present study was rated with six points. For an AP diagnosis,
at least two of the following three criteria need to be met: i)
Abdominal pain in the upper abdomen at the onset of AP; ii)
biochemical evidence of pancreatitis (serum amylase and/or lipase
>3 times the upper limit of the normal range); and iii) typical
abdominal imaging findings (19).
A diagnosis of HLAP can then be made based on the following
criteria established by the Chinese guidelines for HLAP: AP
diagnosis and TG ≥11.3 mmol/l (>1,000 mg/dl) at the time of
onset or TG between 5.65-11.3 mmol/l (500-1,000 mg/dl) without
other underlying conditions, such as gallstone and alcoholism, or
medications such as diuretics, hormonal drugs and antineoplastic
drugs (19). Diagnosis of AP in
the patient in the present study was based on symptoms, clinical
findings (the patient was in epigastric pain, which was dull with
paroxysmal intensification.), elevated TG, amylase and lipase and
ultrasound examination.
Caesarean section was performed at 12 h later. The
newborn was female, with a birth weight of 3,500 g and an
Appearance, Pulse, Grimace, Activity and Respiration score at 1 and
5 min after birth of 9 and 10, respectively (23). The newborn was sent to the neonatal
intensive care unit (ICU) due to the premature birth. Treatment for
the newborn included: Warmth, monitoring of vital signs, prevention
of nosocomial infections and developmental support. The newborn was
healthy despite prematurity.
A 200-mLlfatty effusion was found in the abdominal
cavity at the time of the caesarean section, a drainage tube was
inserted for drainage and AP was confirmed by CT after the
caesarean section. CT (64 rows of 128-slice Light Speed spiral CT;
GE Healthcare; 64 rows of 128 layers; parameters: tube voltage, 120
kV; tube current, 350 mA; pitch, 0.9; matrix, 512X512; layer
thickness, 5 mm; layer pitch, 5 mm; supine position) scanning after
caesarean section demonstrated peripancreatic fat stranding and
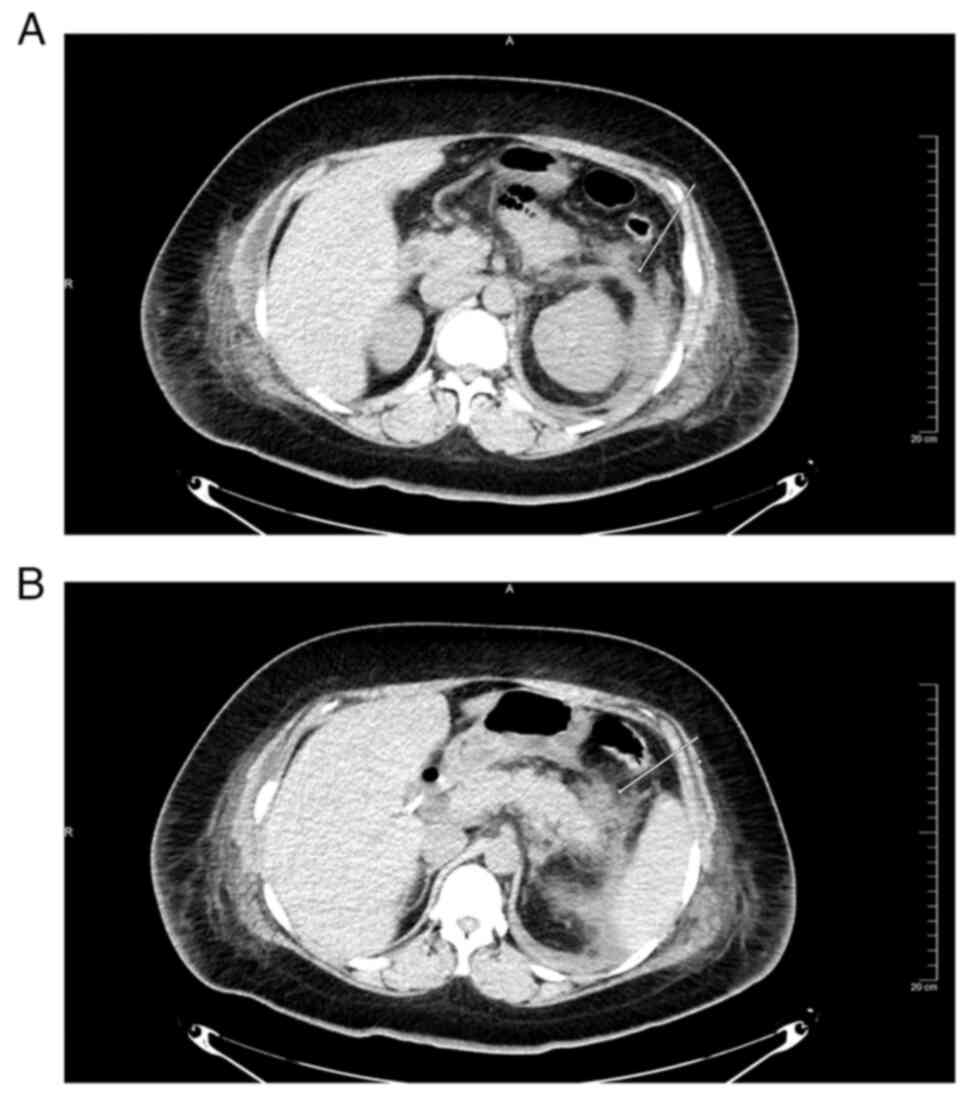
left anterior renal vein thickening (Fig. 1A and B) but no evidence of pancreatic necrosis.
CT images of pancreatic cancer demonstrate pancreatic morphological
variation, localized enlargement, loss of fat around the pancreas,
narrowing of the pancreatic duct, dilation and compression of large
blood vessels (24). Therefore,
pancreatic cancer was excluded in this patient.
Following diagnosis of HLAP, the Pancreatic Surgery
Group, Surgical Branch of Chinese Medical Association guidelines
(19) state that treatment should
continue to be performed with treatment routines such as aggressive
hydration, analgesia and close observation. TG levels should be
measured as early as possible and HL should be reduced within 48 h
from the onset of AP (25). After
obstetric and ICU consultation, the patient in the present study
was referred to ICU (15 h later) for further treatment. Following
ICU admission, supportive care with fasting, fluid resuscitation,
pain and blood sugar control, blood lipid-lowering drugs,
anti-infective agents (once every 12 h: Ceftazidime, 2 g,
cefoperazone sodium, 3 g and sulbactam sodium, 3 g) and inhibition
of pancreatic enzyme secretion and activity (intravenous drip once
every 8 h: Octreotide acetate, 0.3 mg and intravenous drip every 6
h: ulinastatin, 100,000 units). The Pancreatic Surgery Group,
Surgical Branch of Chinese Medical Association guidelines state
that patients with severe HLAP should continue treatment with
lipid-lowering agents, including anti-HL drugs, insulin infusion,
heparin and plasmapheresis treatments. The patient in the present
study was administered fibric acid derivative bezafibrate (0.1 g,
orally, three times/day), human insulin mixed injection (6 units,
twice a day, subcutaneously) and low molecular weight calcium
heparin (0.6 ml, every 12 h, subcutaneous injection).
Plasmapheresis was utilized as a lipid- and inflammatory
factors-reducing treatment, and three sessions of hemoperfusion
(HP; 2 h; blood flow, 200-220 ml/min) and continuous renal
replacement therapy (CRRT) were performed. A right internal jugular
venous cannula was used for indwelling with a single-needle
double-lumen catheter. Extracorporeal circulation CRRT treatment
was performed using a blood filter connected in series with a HP
device. Replacement fluid volume was 2,000 ml/h, blood flow was
controlled at 200-220 ml/min and liquid clearance was 200 ml/min.
Sodium bicarbonate was instilled at a uniform rate, the filter was
replaced once at 24 h and treatment was continued for 3 days. The
patient exhibited hypoproteinemia and ALB (10 g per day for 5 days)
was administered. After 4 days in ICU, abdominal pain improved,
amylase levels returned to normal, blood lipid levels (TC, 8.88
mmol/l; TG, 6.86 mmol/l; LDL-cholesterol, 3.99 mmol/l) markedly
decreased and respiration and circulation were stable (Table I). However, WBC, neutrophils, NR
and CRP levels indicated that there was still inflammation
(Table I). Treatment continued
after transfer to gastroenterology from ICU after 4 days with full
improvement of clinical and laboratory parameters. The patient
recovered after 11 days with laboratory tests demonstrating reduced
serum TG levels and normalization of amylase, TC, LDL, glucose,
WBC, neutrophils, NR and CRP levels (Table I). The patient was discharged from
hospital after 11 days with AP symptoms improved and both the
patient and infant were determined to be in good condition. The
clinical course of the patient from hospitalization to discharge is
demonstrated in Fig. 2.
The mother and baby were followed up at 42 days and
6 months postpartum and the mother and baby were healthy.
Discussion
APIP is an inflammatory syndrome that can occur
following pancreatic injury, with a variable acute inflammatory
response and numerous local (such as pancreatic abscess and
pancreatic pseudocyst) and systemic (such as acute respiratory
failure, acute renal failure, heart failure, gastrointestinal
bleeding and pancreatic encephalopathy) complications (26). Obesity (defined as BMI >30
kg/m2) can be characterized by a grade inflammatory
state and has been reported to be harmful to the human body
(27). It also increases the risk
of insulin resistance and diabetes (28). The present case study demonstrated
that the presence of obesity and diabetes pre-pregnancy are
important risk factors in the development of APIP. Previous studies
reported that a family history of HL and type 2 diabetes increased
the risk of hyperlipidemic pancreatitis in pregnancy (29,30).
Zeng et al (31) reported
that mean platelet volume (MPV) was elevated in patients with HLAP,
which has a certain predictive value for AP and disease severity
during pregnancy. MPV is a blood parameter used for measuring
platelet size and is an indicator of thrombocytic activity.
Elevated MPV facilitates platelet adhesiveness and aggregation,
which may lead to a high prothrombotic potential and impairment of
pancreatic microcirculation in HL-induced SAP during pregnancy
(32). In the present case, no MPV
abnormalities were found.
Diabetes and obesity are also associated with
elevated TG levels and increased concentrations of very-LDL and
chylomicrons, which can lead to HLAP. It has been reported that TG
levels >11.3 mmol/l (1,000 mg/dl) can trigger AP and associated
complications (10). Pregnancy is
associated with physiological increases in blood TC and TG, which
are not associated with AP alone; however, a preexisting abnormal
lipid metabolism may aggravate the increase in TG during pregnancy,
leading to HLAP (10). Therefore,
diabetes, overweight and obesity should be considered as potential
cofactors in the etiology of HLAP. Uncontrolled pre-pregnancy
obesity and diabetes are important risk factors for gestational
HLAP (33), as demonstrated in the
present case report. The patient had diabetes and obesity
pre-pregnancy and became pregnant without controlling these
conditions.
Lipid-lowering therapy is particularly important in
the treatment of HLAP, which includes the administration of
lipid-lowering drugs and blood purification. In the present case,
the patient was administered oral lipid-lowering drugs such as
fibrate, non-oral lipid-lowering drugs, heparin and insulin, and
underwent blood purification. This is consistent with other reports
(34,35). Fibrates, a class of drugs belonging
to category C medications, work to raise HDL and lower TG through
the upregulation of lipoprotein lipase metabolism. In addition,
non-oral drugs, such as heparin and insulin, can stimulate
lipoprotein esterase activity and promote chylomicron degradation,
thereby reducing blood lipids. The combination of heparin and
insulin can be used as first-line therapy for severe HLAP (36). Several studies have reported that
sequential hemofiltration therapy with HP can quickly decrease
serum amylase and TG levels in patients with HLAP, effectively
preventing pancreatic inflammation and necrosis, delaying disease
progression and reducing case fatality (37,38).
In the present study, amylase, lipase and TG were notably lower
after HP-CRRT treatment, consistent with the aforementioned
studies. Other clinically available lipid-modifying drugs include
statins, niacin, resins and cholesterol absorption inhibitors. Drug
treatment of diabetes includes oral drugs such as sulfonylureas,
biguanide hypoglycemic drugs, α glucosidase inhibitors, insulin
sensitizers and insulin therapy. Insulin preparations include
animal insulin, human insulin and insulin analogues (39).
HLAP in pregnancy is associated with a much higher
risk of SAP and is strongly associated with poor maternal-fetal
outcomes compared with AP due to other causes (40). Apart from maternal complications
such as acute renal failure, sepsis and acute respiratory distress
syndrome, there are also potential fetus complications with HLAP.
Risks to the fetus of HLAP in pregnancy include preterm,
prematurity and in-utero fetus death. APIP usually presents with a
sudden onset of severe epigastric pain that radiates to the back.
Bowel sounds are reduced, and a positive Murphy's sign may be
present. Early monitoring of the level of inflammatory indicators
is important for the assessment of the severity and classification
of AP. In the present case, the inflammation-related indicators,
CRP, WBC, neutrophils and neutrophil ratio, continuously increased
for several days. CRP is an acute-phase protein produced by the
liver in response to inflammation. The main biological function of
CRP is reported to be host defense against bacterial pathogens and
clearance of apoptotic and necrotic cells (41). CRP is sensitive to the degree of
inflammation and can markedly change with the degree of
inflammation (42). Previous
studies have reported that a systemic inflammatory state with
abnormal indexes such as serum WBC, neutrophils, NR and CRP, is
closely related to the severity of AP (43,44).
In the early stage of AP, neutrophils are activated to produce
numerous inflammatory factors such as IL-1β, IL-6 and IL-17, and
oxygen-free radicals, leading to vascular endothelial cell damage
and pancreatic microcirculation disturbance (45). At the same time, immune cells are
recruited and activated to participate in the systemic inflammatory
response, aggravating the damage to the pancreas and other organs
such as the lung (46). AP itself
causes elevated WBC, neutrophils and CRP, however this can change
as the disease progresses. In MAP, WBC is only slightly elevated or
unchanged. Activation of white blood cells is an important reason
for the evolution of MAP to SAP. A previous study has reported that
a systemic inflammatory state with abnormal indexes, such as serum
WBC, neutrophils, NR and CRP, is closely associated with the
severity of AP (47). The present
case demonstrated that the levels of leukocytes, CRP and
neutrophils change with the progression of HLAP disease. Therefore,
the combined detection of CRP, leukocytes, neutrophils and
neutrophil ratios is recommended to assess the severity of
HLAP.
Fibrinogen (FG), also known as coagulation factor I,
is a coagulation factor produced by the liver which serves an
important physiological role in the coagulation process. Increased
FG content is an independent risk factor for vascular thrombosis.
D-dimer is the product of fibrinolytic hydrolysis after activation
and crosslinking of fibrin monomers. D-dimer is a marker used to
assess hypercoagulability and thrombosis in vivo (48). FG degradation product (FDP) is a
byproduct produced after FG and fibrin are decomposed by plasma
elements, which reflects the intensity of fibrinolytic activity
in vivo (49). In the
present case, the levels of FG, D-dimer and FDP were increased,
whereas antithrombin Ⅲ levels were decreased. This suggested that
the patient had abnormal coagulation which could cause disseminated
intravascular coagulation and thrombosis. Additionally, a transient
increase in TP and globulin and a persistent decrease in ALB were
demonstrated. ALB is a plasma protein produced by the liver and the
level of ALB can reflect the leakage of capillaries (50). SAP is often complicated with
hypoalbuminemia and the mechanism may involve: i) Increased ALB
extravasation; ii) digestive and absorption disorders; iii)
decomposition increases. Several studies have reported that
hypoalbuminemia serves a role as a booster in the pathological
process of SAP (51,52). Elevated amylase and/or lipase are
the diagnostic hallmarks of AP; however, amylase levels in HLAP may
be reported as normal or low in >50% of patients (53). As reported in other studies
(54,55), the patient in the present case had
increased serum amylase and lipase levels which did not reach
levels three times the upper limit of the normal range, as in
patients with pancreatitis who had HL.
Chinese guidelines for the diagnosis and treatment
of AP (19) do not recommend
routine use of prophylactic antibiotics in patients with AP,
consistent with the 2013 International Association of
Pancreatology/American Pancreatic Association Acute Pancreatitis
Guidelines (56) and the 2018
American Gastroenterological Association Institute Clinical
Guidelines Committee (57). AP is
a nonbacterial inflammation and prophylactic use of antibiotics is
controversial; however, the appropriate use of antibiotics in the
case of suspected infection is not controversial. In the present
case, the patient's inflammatory indicators, leukocytes, CRP, WBCs
and neutrophils, were very high and a bacterial infection was
suspected. A puncture of the bacterial culture specimen was
required for bacterial detection, but the patient and family did
not agree to the procedure. Therefore, antibiotics were
administered.
The clinical symptoms and findings from ultrasound
and CT imaging in the present case are summarized as follows: i)
The clinical symptoms of the present case are atypical as they
manifested only as epigastric pain, without obvious nausea,
vomiting and radiation pain; ii) Increased serum amylase and lipase
levels were measured, but they did not reach levels three times the
upper limit of the normal range; iii) Serum lipids were markedly
elevated, especially TG, which was >11.3 mmol/l, and there were
serum chyloform changes; iv) Inflammatory markers, such as
leukocytes, neutrophils, and CRP, were markedly elevated; v) The
patient had pre-pregnancy HL and diabetes mellitus; vi) Abdominal
ultrasonography demonstrated a hypoechoic pancreatic head; vii) CT
scan images demonstrated peripancreatic fat stranding and left
anterior renal vein thickening.
To conclude, the present case study demonstrated
that early and accurate diagnosis of the patient in the obstetrics
department, timely fetal delivery, transfer to ICU and prompt
treatment served key roles in the good maternal health and infant
condition. Additionally, the treatment of HLAP in pregnancy
requires multidisciplinary collaboration to develop personalized
management plans. The treatment plan in the present case was
developed after multidisciplinary discussions that included the
obstetrics, ICU and gastroenterology departments.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the open project of
Zhenjiang Clinical Medical Research Center for Obstetrics and
Gynecology (grant no. SS2022003-KFB03).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
QW and BX obtained the clinical data. CH and XN
performed the investigation. MG and LM conceived the methodology.
JC and YX supervised the study. WC wrote the draft of the
manuscript and XW wrote and reviewed the manuscript. JC and YX
analyzed and interpreted the data. WC made substantial
contributions to conception and design, acquisition, analysis and
interpretation of data, and writing/editing/revising the
manuscript. XW contributed to the conception and design of the
study. WC and XN confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the ethics
committee of the Maternity and Child Health Hospital of Zhenjiang
(approval no. F201931; Zhenjiang, China). Informed consent was
obtained from the patient.
Patient consent for publication
The patient who participated in the study provided
written informed consent for the publication of any associated
data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cain MA, Ellis J, Vengrove MA, Wilcox B,
Yankowitz J and Smulian JC: Gallstone and severe
hypertriglyceride-induced pancreatitis in pregnancy. Obstet Gynecol
Surv. 70:577–583. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mali P: Pancreatitis in pregnancy:
Etiology, diagnosis, treatment, and outcomes. Hepatobiliary
Pancreat Dis Int. 15:434–438. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jain P: Acute pancreatitis in pregnancy:
An unresolved issue. World J Gastroenterol. 16:2065–2066.
2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hacker FM, Whalen PS, Lee VR and Caughey
AB: Maternal and fetal outcomes of pancreatitis in pregnancy. Am J
Obstet Gynecol. 213:568.e1–e5. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cai E, Czuzoj-Shulman N and Abenhaim HA:
Perinatal outcomes in pregnancies complicated by acute
pancreatitis. J Perinat Med. 50:68–73. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Yang AL and McNabb-Baltar J:
Hypertriglyceridemia and acute pancreatitis. Pancreatology.
20:795–800. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Athar S, Ramawat J and Aziz MA:
Hypertriglyceridemia induced acute pancreatitis in pregnancy:
Learning experiences and challenges of a case report. Clin J Obstet
Gynecol. 2:6–12. 2019.
|
|
8
|
Pancreas Study Group, Chinese Society of
Gastroenterology, Chinese Medical Association, Editorial Board of
Chinese Journal of Pancreatology, Editorial Board of Chinese
Journal of Digestion. Chinese guidelines for the management of
acute pancreatitis (Shenyang, 2019). J Clin Hepatol. 35:2706–2711.
2019.
|
|
9
|
Pascual I, Sanahuja A, García N, Vázquez
P, Moreno O, Tosca J, Peña A, Garayoa A, Lluch P and Mora F:
Association of elevated serum triglyceride levels with a more
severe course of acute pancreatitis: Cohort analysis of 1457
patients. Pancreatology. 19:623–629. 2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vipperla K, Somerville C, Furlan A,
Koutroumpakis E, Saul M, Chennat J, Rabinovitz M, Whitcomb DC,
Slivka A, Papachristou GI and Yadav D: Clinical profile and natural
course in a large cohort of patients with hypertriglyceridemia and
pancreatitis. J Clin Gastroenterol. 51:77–85. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Schepers NJ, Bakker OJ, Besselink MG,
Ahmed Ali U, Bollen TL, Gooszen HG, van Santvoort HC and Bruno MJ:
Dutch Pancreatitis Study Group. Impact of characteristics of organ
failure and infected necrosis on mortality in necrotising
pancreatitis. Gut. 68:1044–1051. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Mosztbacher D, Hanák L, Farkas N, Szentesi
A, Mikó A, Bajor J, Sarlós P, Czimmer J, Vincze Á, Hegyi PJ, et al:
Hypertriglyceridemia induced acute pancreatitis: A prospective,
multicenter, international cohort analysis of 716 acute
pancreatitis cases. Pancreatology. 20:608–616. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Wang Q, Wang G, Qiu Z, He X and Liu C:
Elevated serum triglycerides in the prognostic assessment of acute
pancreatitis: A systematic review and meta-analysis of
observational studies. J Clin Gastroenterol. 51:586–593.
2017.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Van Dijk SM, Hallensleben NDL, van
Santvoort HC, Fockens P, van Goor H, Bruno MJ and Besselink MG:
Dutch Pancreatitis Study Group. Acute pancreatitis: Recent advances
through randomised trials. Gut. 66:2024–2032. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS: Acute Pancreatitis
Classification Working Group. Classification of acute
pancreatitis-2012: Revision of the atlanta classification and
definitions by international consensus. Gut. 62:102–111.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Khatua B, El-Kurdi B and Singh VP: Obesity
and pancreatitis. Curr Opin Gastroenterol. 33:374–382.
2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Simmons SC, Dorn DP, Walton CM, Williams
LA III and Pham HP: Hypertriglyceridemia in pregnancy. Transfusion.
57:2824–2825. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rawla P, Sunkara T, Thandra KC and
Gaduputi V: Hypertriglyceridemia-induced pancreatitis: Updated
review of current treatment and preventive strategies. Clin J
Gastroenterol. 11:441–448. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Pancreatic Surgery Group, Surgical Branch
of Chinese Medical Association. Guidelines for the diagnosis and
treatment of acute pancreatitis in China (2021). Chinese Journal of
Practical Surgery. 41:739–746. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Benson M, Arena Goncharov D and Jain S:
Diagnosis and management of acute pancreatitis in pregnancy. Clin
Obstet Gyneco1. 66:237–249. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Garg R and Rustagi T: Management of
hypertriglyceridemia induced acute pancreatitis. Biomed Res Int.
2018(4721357)2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Knaus WA, Draper EA and Wagner DPl: APACHE
Ⅱ: A severity of disease classification system. Crit Care Med.
13:818–829. 1985.PubMed/NCBI
|
|
23
|
Witcher TJ, Jurdi S, Kumar V, Gupta A,
Moores RR Jr, Khoury J and Rozycki HJ: Neonatal Resuscitation and
Adaptation Score vs Apgar: Newborn assessment and predictive
ability. J Perinatol. 38:1476–1482. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Jin J: Application of multi-slice spiral
CT in the diagnosis of pancreatic cancer. Modern Diagnosis and
Treatment. 32:1094–1095. 2021.(In Chinese).
|
|
25
|
Amin T, Poon LC, Teoh TG, Moorthy K,
Robinson S, Neary N and Valabhji J: Management of
hypertriglyceridemia-induced acute pancreatitis in pregnancy. J
Matern Fetal Neonatal Med. 28:954–958. 2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Papachristou GI and Whitcomb DC:
Inflammatory markers of disease severity in acute pancreatitis.
Clin Lab Med. 25:17–37. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Shah AP, Mourad MM and Bramhall SR: Acute
pancreatitis: Current perspectives on diagnosis and management. J
Inflamm Res. 11:77–85. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Laufs U, Parhofer KG, Ginsberg HN and
Hegele RA: Clinical review on triglycerides. Eur Heart J.
41:99–109c. 2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Pedersen SB, Langsted A and Nordestgaard
BG: Non-fasting mild-to-moderate hypertriglyceridemia and risk of
acute pancreatitis. JAMA Intern Med. 176:1834–1842. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cruciat G, Nemeti G, Goidescu I, Anitan S
and Florian A: Hypertriglyceridemia triggered acute pancreatitis in
pregnancy-giagnonostic approach, management and follow-up care.
Lipids Health Dis. 19(2)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zeng L, Cai X, Chen J, Jin G and Zheng Y:
Role of mean platelet volume in hypertriglyceridemia-induced acute
pancreatitis during pregnancy. BMC Pregnancy Childbirth.
20(592)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Lei JJ, Zhou L, Liu Q, Xiong C and Xu CF:
Can mean platelet volume play a role in evaluating the severity of
acute pancreatitis. World J Gastroenterol. 23:2404–2413.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Valaiyapathi B, Sunil B and Ashraf AP:
Approach to hypertriglyceridemia in the pediatric population.
Pediatr Rev. 8:424–434. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Gupta M, Liti B, Barrett C, Thompson PD
and Fernandez AB: Prevention and management of
hypertriglyceridemia-induced acute pancreatitis during pregnancy: A
systematic review. Am J Med. 135:709–714. 2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Padmanabhan A, Connelly-Smith L, Aqui N,
Balogun RA, Klingel R, Meyer E, Pham HP, Schneiderman J, Witt V, Wu
Y, et al: Guidelines on the use of therapeutic apheresis in
clinical practice-evidence-based approach from the writing
committee of the American society for apheresis: The eighth special
issue. J Clin Apher. 34:171–354. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kuchay MS, Farooqui KJ, Bano T, Khandelwal
M, Gill H and Mithal A: Heparin and insulin in the management of
hypertriglyceridemia-associated pancreatitis: Case series and
literature review. Arch Endocrinol Metab. 61:198–201.
2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
James TW and Crpckett SD: Management of
acute pancreatitis in the first 72 hours. Curr Opin Gastroenterol.
34:330–335. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu S, Zheng B and He Y: Effect of early
plasma exchange combined with continuous blood filtration in
treatment of severe acute pancreatitis. Chin J Modern Med.
19:3323–3325. 2009.(In Chinese).
|
|
39
|
Noel P, Patel K, Durgampudi C, Trivedi RN,
de Oliveira C, Crowell MD, Pannala R, Lee K, Brand R, Chennat J, et
al: Per-pancreatic fat necrosis worsens acute pancreatitis
independent of pancreatic necrosis via unsaturated fatty acids
increased in human pancreatic necrosis collections. Gut.
65:100–111. 2016.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kilinc F, Senates E, Demircan F, Pekkolay
Z, Gozel N, Guven M, Bahcecioglu IH and Tuzcu AK: Are There
Differences in the Management of Acute Pancreatitis Cases Due to
Severe Hypertriglyceridemia in Pregnant Women? Med Sci Monit.
24:5619–5623. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Landry A, Docherty P, Ouellette S and
Cartier LJ: Causes and outcomes of markedly elevated C-reactive
protein levels. Can Fam Physician. 63:e316–e323. 2017.PubMed/NCBI
|
|
42
|
Komolafe O, Pereira SP, Davidson BR and
Gurusamy KS: Serum C-reactive protein, procalcitonin, and lactate
dehydrogenase for the diagnosis of pancreatic necrosis. Cochrane
Database Syst Rev. 4(CD012645)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Demirkol ME, Aktas G, Bilgin S, Kahveci G,
Kurtkulagi O, Atak BM and Duman TT: C-reactive protein to
lymphocyte count ratio is a promising novel marker in hepatitis C
infection: The clear hep-c study. Rev Assoc Med Bras (1992).
68:838–841. 2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Sendler M, Van den Brandt C, Glaubitz J,
Wilden A, Golchert J, Weiss FU, Homuth G, De Freitas Chama LL,
Mishra N, Mahajan UM, et al: NLRP3 inflammasome regulates
development of systemic inflammatory response and compensatory
anti-inflammatory response syndromes in mice with acute
pancreatitis. Gastroenterology. 158:253–269. e14. 2020.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cao W, Ni X, Wang Q, Li J, Li Y, Chen T
and Wang X: Early diagnosis and precision treatment of right
ovarian veinand inferior vena cava thrombosis following caesarean
section: A case report. Exp Ther Med. 19:2923–2926. 2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Watanabe T, Kudo M and Strober W:
Immunopathogenesis of pancreatitis. Mucosal Immunol. 10:283–298.
2017.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Xi L and Yingchun Z: Value of combined
detection of serum amylase, C-reactive protein and leukocytes in
the differential of acute pancreatitis. Laboratory Medicine and
Clinic (Chinese). 16:161–163. 2019.
|
|
48
|
Salomone T, Tosi P, Palareti G, Tomassetti
P, Migliori M, Guariento A, Saieva C, Raiti C, Romboli M and Gullo
L: Coagulative disorders in human acute pancreatitis: Role for the
D-dimer. Pancreas. 26:111–116. 2003.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Badhal SS, Sharma S, Saraya A and
Mukhopadhyay AK: Prognostic significance of D-dimer, natural
anti-coagulants and routine coagulation parameters in acute
pancreatitis. Trop Gastroenterol. 33:193–199. 2012.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Hong W, Lin S, Zippi M, Geng W, Stock S,
Basharat Z, Cheng B, Pan J and Zhou M: Serum albumin is
independently associate d with persistent organ failure in acute
pancreatitis. Can J Gastroenterol Hepatol.
2017(5297143)2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Horwich TB, Kalantar-Zadeh K, MacLellan RW
and Fonarow GC: Albumin levels predict survival in patients with
systolic heart failure. Am Heart J. 155:883–889. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Li Y, Lin S, Shu J, Hong W and Pan J:
Bedside severity index plus serum albumin test for acute
pancreatitis in early assessment of acute pancreatitis severity.
Chinese Journal of Digestion. 39:868–871. 2019.(In Chinese).
|
|
53
|
Ismail OZ and Bhayana V: Lipase or amylase
for the diagnosis of acute pancreatitis? Clin Biochem.
50:1275–1280. 2017.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Alam A, Rahman MFU and Rahman M: A case of
acute pancreatitis with normal serum lipase. Bangladesh Med J.
43:162–164. 2016.
|
|
55
|
Hameed AM, Lam VW and Pleass HC:
Significant elevations of serum lipase not caused by pancreatitis:
A systematic review. HPB (Oxford). 17:99–112. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
IAP/APA Evidence-Based Guidelines for the
Management of Acute Pancreatitis. Working Group IAP/APA Acute
Pancreatitis Guidelines. Pancreatology. 13 (Suppl. 2):e1–e15.
2013.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Crockett SD, Wani S, Gardner TB,
Falck-Ytter Y and Barkun AN: American Gastroenterological
Association Institute Clinical Guidelines Committee. American
Gastroenterological Association Institute Guideline on Initial
Management of Acute Pancreatitis. Gastroenterology. 154:1096–1101.
2018.PubMed/NCBI View Article : Google Scholar
|