Introduction
Acute pancreatitis (AP) is one of the most common
acute abdominalgia, manifesting globally in 13 to 45 cases per
100,000 individuals each year. Characterized by its acute onset,
rapid progression and unpredictable outcomes, AP can culminate in
severe complications such as sepsis, multi-organ failure and
systemic inflammatory response syndrome, potentially leading to
fatality (1). The inflammatory
damage in AP is initiated by premature activation of trypsinogen in
pancreatic acinar cells, resulting in a cell-damaging proteolytic
cascade (2). The damaged acinar
cells and necrotic tissue activate immune cells to release
inflammatory mediators, such as tumor necrosis factor-α,
interleukin (IL)-1, IL-6 and IL-8(3), which cause a cascade reaction,
resulting in system inflammatory response syndrome and multiple
organ dysfunction syndrome (1).
To the best of our knowledge, the mechanism
underlying abnormal trypsin activation remains unclear. However,
pathological calcium signaling and the colocalization of zymogen
granules with lysosomes may be key early steps in the pathogenesis
of pancreatitis (4). Cathepsin B,
a lysosomal enzyme, converts trypsinogen to trypsin in these cells
upon colocalization. Notably, the colocalization of lysosomal and
zymogen fractions has been reported to be an autophagic process
(5).
IL-22, a member of the IL-10 family, is produced by
T-helper (Th)-17 cells, Th22 cells, γδ T cells, natural killer T
cells and innate lymphoid cells (6). Unlike most cytokines, IL-22 acts
primarily on non-hematopoietic epithelial cells and fibroblasts, as
determined by its idiographic receptor. IL-22 receptor 1 (IL-22R1)
is expressed only in the stromal and epithelial cells of specific
tissues, with the highest expression observed in pancreatic acinar
cells, followed by the intestinal tract and skin (7). The binding of IL-22 to IL-22R1 forms
a unique receptor complex (IL-22/IL-22R1 subunit) and enables
secondary combination with the IL-10 receptor 2 subunit. This
initiates the activation of receptor-associated Janus kinase 1 and
leads to the phosphorylation of STAT proteins (mainly STAT3)
(8). In addition to STAT
signaling, IL-22 binding activates the MAPK and PI3K-Akt signaling
pathways (9).
Previous studies have reported that IL-22 mediates
the protection and regeneration of epithelial tissues (10,11).
Furthermore, it serves an important role in triggering
antimicrobial immunity and maintaining the integrity of the mucosal
barriers (12). A previous study
has reported that serum IL-22 levels are significantly elevated in
patients with AP (13). It has
also been reported that IL-22 can stimulate the production of
proteins such as regenerating islet-derived (Reg)3β, Reg3γ and
osteopontin (14), and induce the
transcription of anti-apoptotic genes, Bcl-2 and Bcl-Xl (15), thereby serving an important role in
the immune response to AP.
Previous studies have reported that the mechanism of
IL-22-mediated treatment of AP is mainly related to its
anti-inflammatory effect and apoptosis inhibition (16,17).
However, the relationship between trypsinogen activation in
pancreatic acinar cells and IL-22 treatment requires further study,
given it is a key step in the initiation of AP. Proteomics is one
of the most effective methods for identifying molecular markers and
drug targets. To the best of our knowledge, there has been no
proteomic analysis of IL-22-treated pancreatic acinar cells, which
indicated that certain possible mechanisms might not have been
elucidated. Therefore, the present study assessed the possible
molecular mechanisms underlying IL-22-mediated treatment of AP by
inducing pancreatic acinar cell injury in vitro and
performing quantitative proteomics to identify important targets
and the clinical potential of IL-22 in the treatment of this
condition.
Materials and methods
Cell culture and establishment of AP
model
Rat pancreatic cancer AR42J cells (China Center for
Type Culture Collection) were cultured at 37˚C in Ham's F-12K basal
medium (HyClone; Cytiva) containing 20% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) in a humidified, 5% CO2
incubator (Thermo Fisher Scientific, Inc.). L-arginine (2.5 or 5
mg/ml; Sigma-Aldrich; Merck KGaA) was added to establish the AP
model. Hu et al (18)
reported that an arginine dose of 2.5 or 5 mg/ml, as used in the
present study, induced characteristic changes in pancreatic acinar
cells, including an upsurge in apoptosis and autophagy, alongside
abnormal activation of tryptic enzymes. After further incubation at
37˚C for 24 h, the cells and the medium supernatant were collected
for subsequent experiments.
Assessment of cell viability using
Cell Counting Kit-8 (CCK-8) assay
In a 96-well plate (Eppendorf), 5x104
cells per well were seeded. The experimental group was treated with
2.5 or 5 mg/ml arginine, while the control group remained
untreated. Subsequently, cells were treated with 10 µl CCK-8
reagent (MedChemExpress) at specified time intervals (0, 6, 24 and
48 h), and incubated at 37˚C for 1.5 h. Optical density was
measured at 450 nm using a microplate reader (Bio-Rad Laboratories,
Inc.) and a proliferation curve was drawn.
Assessment of apoptosis using flow
cytometry
In a 6-well plate (Eppendorf), 2x106
cells per well were seeded. The experimental group cells were
treated with either 2.5 mg/ml arginine or a combination of 2.5
mg/ml arginine and 10 ng/ml recombinant (r)IL-22, incubated at 37˚C
for 24 h. Untreated cells served as a negative control group. The
choice of IL-22 concentration was based on a previous study
(19), and a preliminary
experiment was performed to ascertain the optimal concentration for
use in the present study (Fig.
S1). Briefly, 5x104 cells per well were seeded in a
96-well plate. After 24 h of co-administration with varying
concentrations (0, 0.1, 1, 5, 10, 15 and 20 ng/ml) of rIL-22 and
2.5 mg/ml arginine, cell survival was assessed using a CCK-8 assay
(MedChemExpress) and incubated for 1.5 h at 37˚C. Post-treatment,
cells from each group were digested with EDTA-free trypsin (Gibco;
Thermo Fisher Scientific, Inc.) and centrifuged at 300 x g for 5
min at 4˚C for collection. Binding buffer (1X) (BD Biosciences) was
added to resuspend the cells and adjust the cell density to
1x106-5x106 cells/ml. Subsequently, 100 µl of
cell suspension was mixed with 5 µl annexin V-FITC and the mixture
was incubated at room temperature in the dark for 5 min. PI
staining solution (10 µl) and 400 µl binding buffer (1X) were added
to the samples, and flow cytometry was performed using the Attune
NxT Flow Cytometer (Thermo Fisher Scientific, Inc.). The results
were analyzed and processed using FlowJo software (version 10.8.1;
FlowJo LLC).
Assessment of cell death using
terminal deoxynucleotidyl-transferase-mediated dUTP nick end
labeling (TUNEL) assay
Briefly, a total of 2.5x105 cells were
seeded in a 24-well plate (Eppendorf) and incubated for 24 h with
arginine and IL-22 as aforementioned. Post-incubation, cells were
fixed in 4% formaldehyde for 30 min at room temperature and
permeabilized using 0.3% Triton X-100 for 5 min at room
temperature. Subsequently, the cells were incubated with terminal
deoxynucleotidyl transferase enzyme and fluorescein-dUTP for 2 h at
37˚C, allowing the enzyme to incorporate fluorescein-labeled dUTPs
to the 3'-OH ends of fragmented DNA. The reaction was terminated by
washing the cells with PBS (Wuhan Servicebio Technology Co., Ltd.),
which were then counterstained with DAPI (5 µg/ml) (Sigma-Aldrich;
Merck KGaA) for 15 min at room temperature. The stained cells were
then visualized under an EVOS M7000 microscope (Invitrogen; Thermo
Fisher Scientific, Inc.) in fluorescence mode.
Observation of cell ultrastructure
using transmission electron microscopy (TEM)
A total of 2.5x105 cells were seeded on
glass coverslips (Corning Inc.) in a 24-well plate and then
incubated for 24 h with arginine and IL-22, as aforementioned.
Post-incubation, the culture was discarded and cells were fixed
using a 2.5% glutaraldehyde electron microscope fixative solution
(Wuhan Servicebio Technology Co., Ltd.) at 4˚C for 2-4 h. Samples
were rinsed in isotonic cacodylate buffer (Shanghai Xinyu
Biotechnology Co., Ltd.) and post-fixed with 1% OsO4 in
isotonic cacodylate buffer at 4˚C for 30 min. After dehydrating the
cells in 50, 75, 95 and 100% ethanol (2x15 min for each
concentration), the cells were infiltrated and embedded at 37˚C
overnight using a gradient of acetone and 812 embedding agent
(Structure Probe, Inc.). Thin sections of 70 nm were cut using an
ultramicrotome (Leica Microsystems GmbH) and stained with uranyl
acetate at room temperature for 20 min and then with lead citrate
at room temperature for 5 min. The specimens were visualized using
TEM (Hitachi, Ltd.) and images were captured for analysis.
Assessment of rat α-amylase using
ELISA
The activity of α-amylase in the cell culture
supernatant was evaluated employing a commercial ELISA kit (cat.
no. SP12818; Wuhan Saipei Biotechnology Co., Ltd.), following the
manufacturer's protocol. Optical density was measured at 450 nm
using a microplate reader (Bio-Rad Laboratories, Inc.).
Analysis of autophagy-related proteins
using western blotting
RIPA lysis solution (Beijing Solarbio Science &
Technology Co., Ltd.) with protease inhibitor was added to
arginine- and IL-22-treated cells to extract the proteins. Protein
concentration was determined by the BCA assay (Beyotime Institute
of Technology). Protein samples (20 µg/lane) were separated on a
10% gel (w/v) by SDS-PAGE and transferred onto a polyvinylidene
difluoride membrane. The nonspecific sites were blocked with a
solution containing 5% skimmed milk powder for 1 h at room
temperature and the membrane was then incubated with primary
anti-ATG5 (1:1,000; cat. no. A0203; ABclonal Biotech Co., Ltd.),
anti-ATG7 (1:30,000; cat. no. ab133528; Abcam), anti-beclin-1
(1:1,000; cat. no. ab210498; Abcam), anti-LC3B (1:2,000; cat. no.
ab192890; Abcam) and anti-β-actin (1:2,000; cat. no. gb15003;
Beijing Solarbio Science & Technology Co., Ltd.) antibodies,
diluted in 0.05% TBST (1X) (Wuhan Servicebio Technology Co., Ltd.)
containing 5% bovine serum albumin overnight at 4˚C with gentle
shaking. After the membrane was washed with TBST (1X), it was
incubated with HRP Goat Anti-Rabbit IgG (H+L) (1:10,000; cat. no.
AS014; ABclonal Biotech Co., Ltd.) at room temperature for 1 h.
Protein bands were visualized using ECL (Wuhan Servicebio
Technology Co., Ltd.). β-actin was used as the internal reference.
Quantity One® 1-D software (version 4.6.6; Bio-Rad
Laboratories, Inc.) was used to analyze the gray-scale values and
the target protein/internal reference was used to assess the
relative expression level of each protein.
Proteomic analysis. Protein
extraction
Two groups, each containing 1x107 cells,
were prepared. The negative control group was treated with 2.5
mg/ml arginine at 37˚C for 24 h. Simultaneously, the experimental
group was treated with 2.5 mg/ml arginine and 10 ng/ml IL-22 under
identical conditions for 24 h. The cell samples were then sonicated
three times on ice using a lysis buffer composed of 8 M urea and 1%
protease inhibitor cocktail (cat. no. HY-K0010; MedChemExpress),
with a high-intensity ultrasonic processor (Ningbo Scientz
Biotechnology Co., Ltd.). The remaining debris was removed by
centrifugation at 12,000 x g at 4˚C for 10 min. Finally, the
supernatant was collected and the protein concentration was
determined using the BCA kit (Beyotime Institute of Technology),
performed according to the manufacturer's instructions.
Trypsin digestion. The protein solution was
reduced with 5 mM dithiothreitol (Sigma-Aldrich; Merck KGaA) for 30
min at 56˚C and then alkylated with 11 mM iodoacetamide
(Sigma-Aldrich; Merck KGaA) for 15 min at room temperature in
darkness. The protein sample was then diluted, by adding 100 mM
tetraethylammonium borohydride (Sigma-Aldrich; Merck KGaA) until a
urea concentration of <2 M was reached. Finally, trypsin
(Promega Corporation) was added at a ratio of 1:50
(trypsin-to-protein mass) for the first digestion overnight at 37˚C
and a ratio of 1:100 (trypsin-to-protein mass) for the second 4-h
digestion at the same temperature.
High-performance liquid chromatography (HPLC)
fractionation. The sample (20 µl) was fractionated using high
pH reverse-phase HPLC on an Agilent 1260 Infinity II (Agilent
Technologies, Inc.), utilizing an Agilent Zorbax 300Extend-C18
column (Agilent Technologies, Inc.; particles, 5 µm; internal
diameter, 4.6 mm; length, 250 mm). Wavelength was set to 214 nm,
column oven temperature was set to 35˚C, and 95% buffer A (2%
acetonitrile, pH 9.0 adjusted by ammonia) and 5% buffer B (98%
acetonitrile, pH 9.0 adjusted by ammonia) were used to equilibrate
the column for ≥30 min. A stepwise gradient method was used after
the baseline was flattened. Subsequently, 1 ml buffer A was added
to the peptide sample and vortexed to dissolve it. The mixture was
centrifuged at 12,000 x g for 5 min at 4˚C and transferred to a new
tube. The mixture was centrifuged again under the same conditions
to remove the supernatant and the sample (20 µl) was loaded for
HPLC analysis. The sample was separated and simultaneously
collected in the automatic collector. The sample was collected at 1
min/tube from tubes 11-46, a total of 36 tubes. The HPLC was
conducted at a flow rate of 1 ml/min. Finally, tubes 11-20, 21-30,
31-40 and 41-46 were combined to form four separate fractions, and
dried using vacuum centrifuging at 12,000 x g for 30 min at
4˚C.
Liquid chromatography (LC)-tandem mass
spectrometry (MS/MS) analysis. Tryptic peptides were dissolved
in 0.1% formic acid (solvent A) and directly loaded onto a
home-made reverse-phase analytical column (length, 15 cm; internal
diameter, 75 µm). The gradient comprised a solvent increasing from
6 to 23% (solvent B; 0.1% formic acid in 98% acetonitrile) in 26
min, 23 to 35% in 8 min and increasing to 80% in 3 min, then
holding at 80% for the last 3 min. This was performed at a constant
flow rate of 400 nl/min on an EASY-nLC 1000 ultra-performance
(UP)LC (Thermo Fisher Scientific, Inc.) system. The LC-MS/MS was
operated in positive ion mode, with a nitrogen gas temperature at
320˚C and nebulizer pressure optimized at 55 psi for the instrument
used. The peptides were then analyzed using the Q Exactive™ Plus
Hybrid Quadrupole-Orbitrap™ MS (Thermo Scientific™; Thermo Fisher
Scientific, Inc.), coupled online to the UPLC system. The
electrospray voltage applied was 2.0 kV. The m/z scan range was
350-1,800 for a full scan and intact peptides were assessed in the
Orbitrap at a resolution of 70,000. Peptides were then selected for
MS/MS using a normalized collision energy setting of 28 and the
fragments were assessed using the Orbitrap at a resolution of
17,500. A data-dependent procedure alternated between one MS scan
followed by 20 MS/MS scans with a 15.0 s dynamic exclusion. The
automatic gain control was set to 5E4. The first fixed mass was set
to 100 m/z.
Database search. Resulting MS/MS data were
processed using the MaxQuant search engine (v.1.5.2.8; http://www.maxquant.org/). Tandem mass spectra were
searched against the rat UniProt database (UniProt Consortium,
2019; https://www.uniprot.org/) and
concatenated with a reverse decoy database. Promega trypsin was
specified as the cleavage enzyme, allowing for ≤4 missing
cleavages. The mass tolerance for the precursor ions was set to 20
ppm in the first search and 5 ppm in the main search, and the mass
tolerance for the fragment ions was set to 0.02 Da. Carbamidomethyl
on Cys was specified as a fixed modification, whilst acetylation
modification and oxidation on Met were specified as variable
modifications. False discovery rate was adjusted to <1% and the
minimum score for the modified peptides was set at >40.
Functional enrichment analysis. Gene Ontology
(GO) enrichment and Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway analyses were performed using the GO database (http://geneontology.org) and KEGG database (http://www.genome.jp/kegg/), respectively. Statistical
significance was assessed using Fisher's exact test. Genes with an
enrichment factor >1.5 and P<0.05 were deemed statistically
significant, defining enriched GO and KEGG terms accordingly.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 18.0; SPSS, Inc.). Data are expressed as mean ±
standard deviation. Unpaired Student's t-test was used to compare
two groups and one-way analysis of variance (ANOVA) was used to
compare multiple groups, followed by the Tukey-Kramer post hoc
test. Each experiment was performed independently in triplicate.
All reported P-values were two-tailed and P<0.05 was considered
to indicate a statistically significant difference.
Results
IL-22 treatment ameliorates
arginine-induced acinar cell injury in vitro
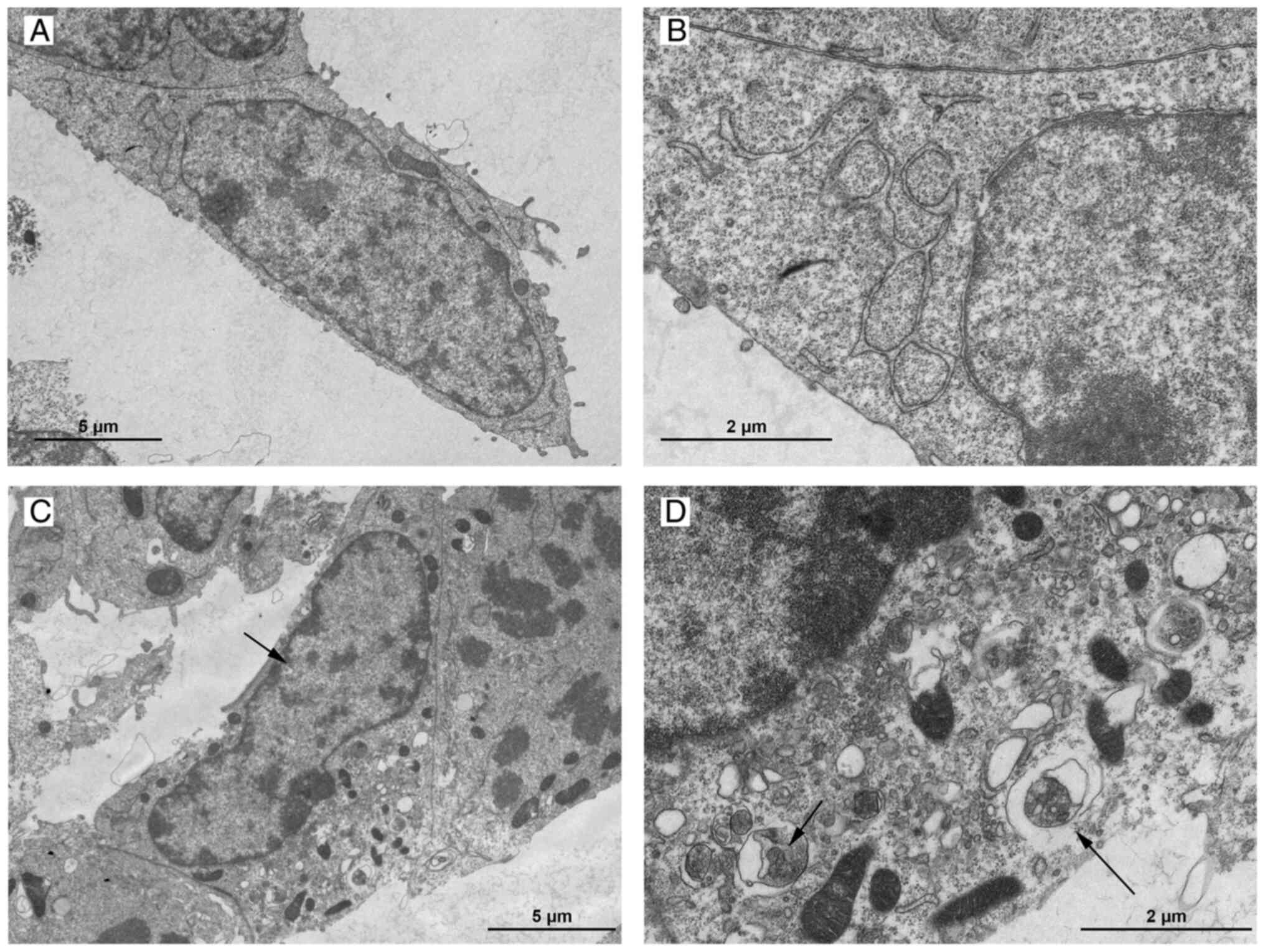
Characteristic changes of the cell ultrastructure in
the arginine-induced group, as observed by TEM, included a notable
reduction of zymogen granules, cytoplasmic vacuolization, nuclear
chromatin margination and marked appearance of autophagic bilayer
membranes containing organelles (Fig.
1). These changes are commonly observed in cells undergoing
autophagy or apoptosis (20).
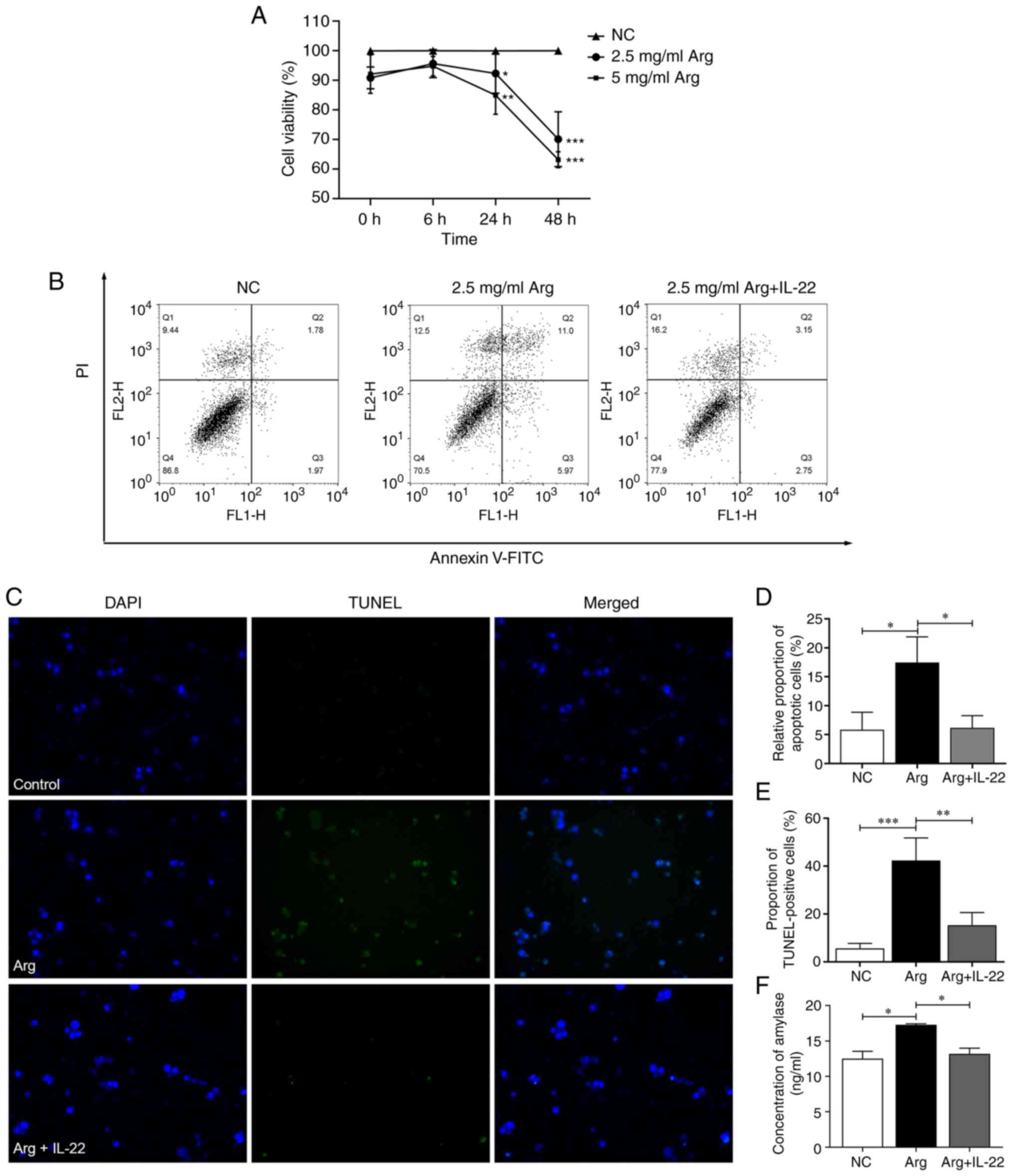
The CCK-8 assay demonstrated that exposure of AR42J
cells to arginine was associated with a notable reduction in cell
viability in a concentration-dependent manner (Fig. 2A), with a significant difference
between the control and arginine-induced groups. A concentration of
2.5 mg/ml arginine was chosen for subsequent studies. This
concentration effectively elicited the observed effects while
minimizing potential toxicity, aligning with the study's objectives
and ensuring the reliability and relevance of subsequent
investigations.
To determine the effects of IL-22 treatment on
arginine-induced acinar cell injury, cell viability, apoptosis and
acinar cell inflammation were assessed. A notable increase in
necrotic (Q1) and apoptotic (Q2 and Q3) cells was demonstrated in
the arginine-induced group compared with the control group.
However, following IL-22 treatment, there was no marked difference
in the number of apoptotic cells compared with the control group,
presenting a decrease compared with that in the arginine-induced
group (Fig. 2B). Notably, a slight
increase in the number of necrotic (Q1) cells was observed. Despite
this, the enhancement in the overall percentage of live cells was
marked. This is further demonstrated in Fig. 2D, where the relative number of
apoptotic cells is illustrated for clarity. The arginine-induced
group had a significant surge in apoptotic cells (17.46±4.42%)
compared with the control group (5.82±3.03%). By contrast, in the
IL-22-treated group, the relative number of apoptotic cells
(6.16±2.11%) was comparatively lower, aligning closely with the
control group. The protective effect of IL-22 and the impact on
arginine-induced cell death were further evaluated by comparing the
proportion of TUNEL-positive cells between the treatment groups
(Fig. 2C and E). The arginine-induced group displayed
high levels of apoptosis (42.4±1.52%), whereas the IL-22-treated
group exhibited markedly diminished apoptosis levels (15.3±5.3%).
This significant reduction in cell death in the IL-22-treated group
compared with the arginine-induced group underscores the effective
therapeutic potential of IL-22.
Amylase is an important serological marker for the
clinical diagnosis of AP (21),
therefore, acinar cell inflammatory damage was evaluated by
measuring amylase levels in the culture medium supernatant. The
amylase concentration in the arginine-induced group (17.28±0.15
ng/ml) was significantly increased compared with the control group
(12.52±1.02 ng/ml), however in the IL-22-treated group, the amylase
concentration (13.20±0.79 ng/ml) was lower and not markedly
different from the control group (Fig.
2F).
The aforementioned changes in cell survival and
functional status demonstrated that an in vitro model of
pancreatic acinar cell injury was successfully established by
adding L-arginine to the culture medium, and that IL-22 was able to
ameliorate arginine-induced apoptosis and inflammation of the
acinar cells.
Proteomic analysis demonstrates the
possible mechanism of IL-22-mediated alleviation of
arginine-induced pancreatic acinar cell injury. Differentially
expressed proteins in IL-22-treated acinar cells compared with
arginine-induced cells
LC-MS/MS analysis identified a total of 48,298
specific peptides. Among these, quantitative information was
garnered for 4,108 distinct proteins. With fold-changes of
≥1.5-fold or ≤0.67-fold included, the IL-22-treated group
demonstrated marked changes in 321 proteins compared with the
arginine-induced group, of which 174 proteins were upregulated and
147 proteins were downregulated.
Proteomics analysis demonstrated that proteins
involved in DNA repair, such as histone H2A.V and 26S proteasome
non-ATPase regulatory subunit 7, were notably upregulated. By
contrast, acyl-CoA-binding domain-containing protein 5, which acts
as the peroxisome receptor for pexophagy, and the regulator complex
protein late endosomal/lysosomal adaptor, MAPK and MTOR activator
5, which is involved in lysosomal enzyme activity and transport,
were downregulated after IL-22 treatment compared to the
arginine-induced group.
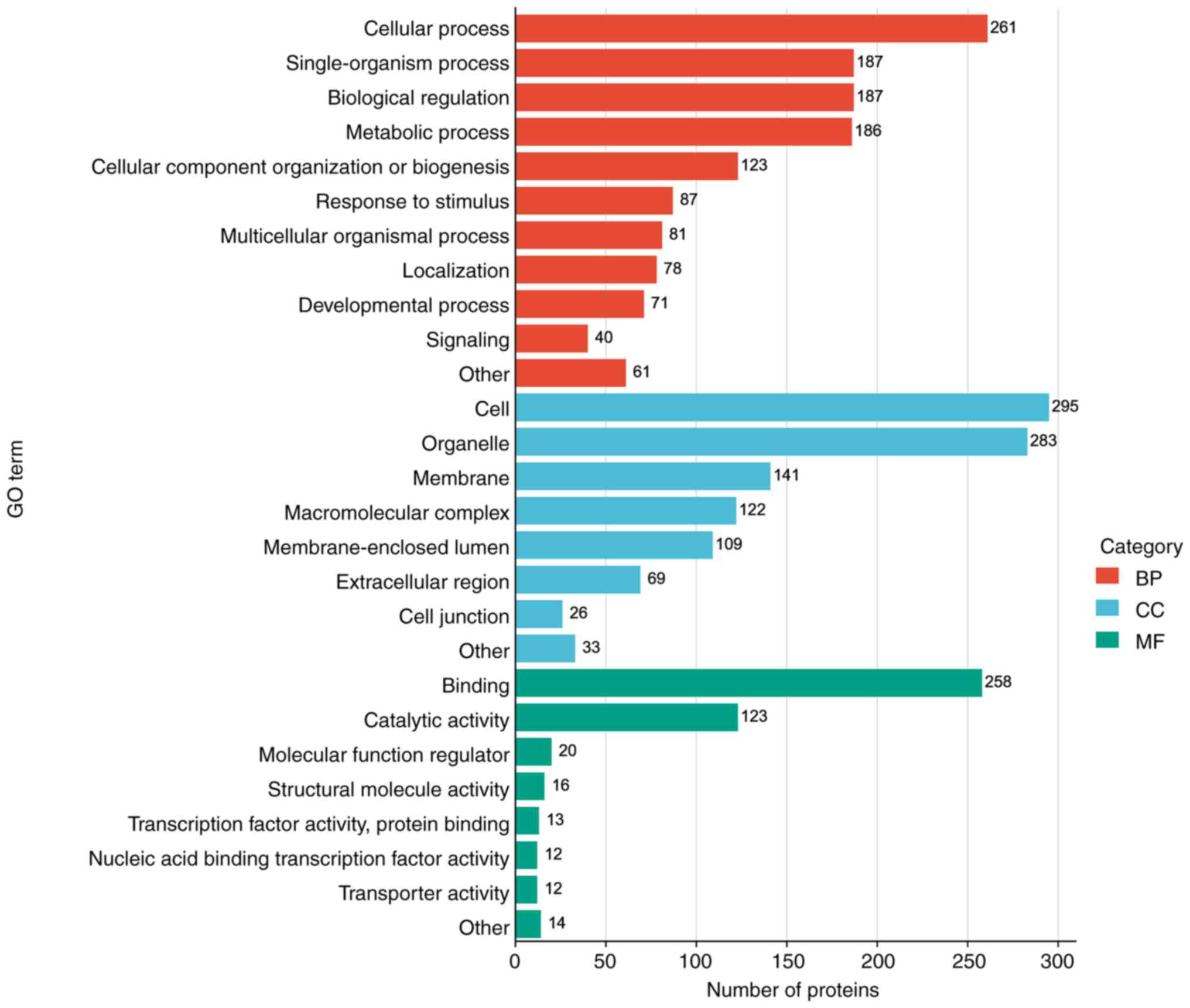
GO analysis of biological processes, cellular
compartments and molecular functions was performed using notably
altered proteins, and independent functional annotation
classifications were performed for upregulated and downregulated
proteins (Fig. 3). The results
demonstrated that proteins with marked changes were mainly in the
cellular biological process (46.8%), such as ‘cellular process’,
‘single-organism process’, ‘biological regulation’, ‘metabolic
process’ and ‘cellular component organization or biogenesis’. Most
proteins classified under component category (37.1%) were involved
in the ‘cell’, ‘organelle’, ‘membrane’, ‘macromolecular complex’
and ‘membrane-enclosed lumen’. In addition, the proteins assessed
were also associated in eight molecular functional GO terms, mainly
‘binding’ and ‘catalytic activity’.
These results collectively highlighted the
substantial impact of IL-22 on various protein expression levels,
underscoring its significant role in modulating a multitude of
cellular functions and activities.
Regulated proteins involved in cellular
composition and biological process are related to the intracellular
vesicle transport system. Analyses of GO terms and KEGG
pathways in which the differentially expressed proteins were
enriched were performed to determine whether these proteins were
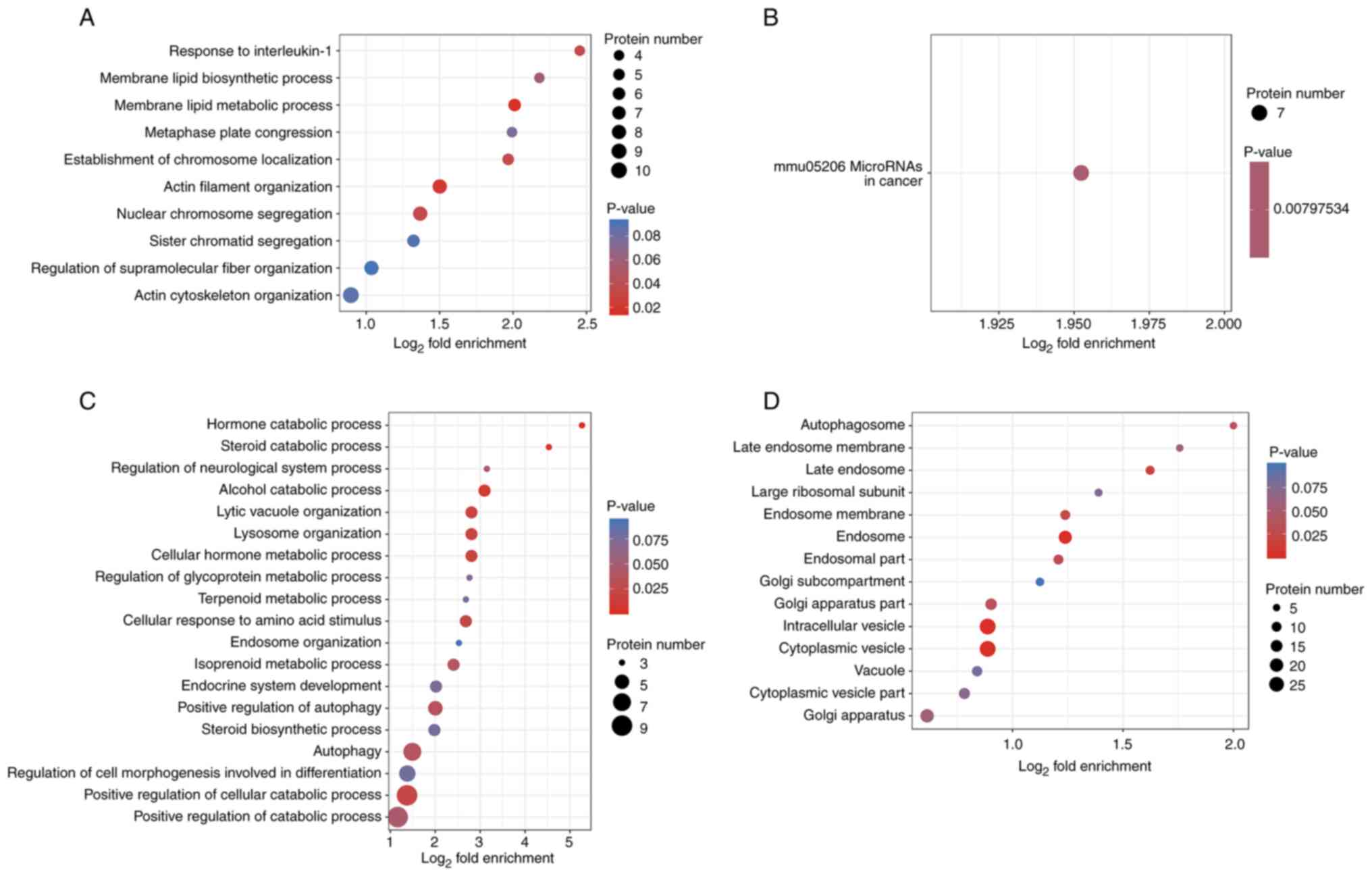
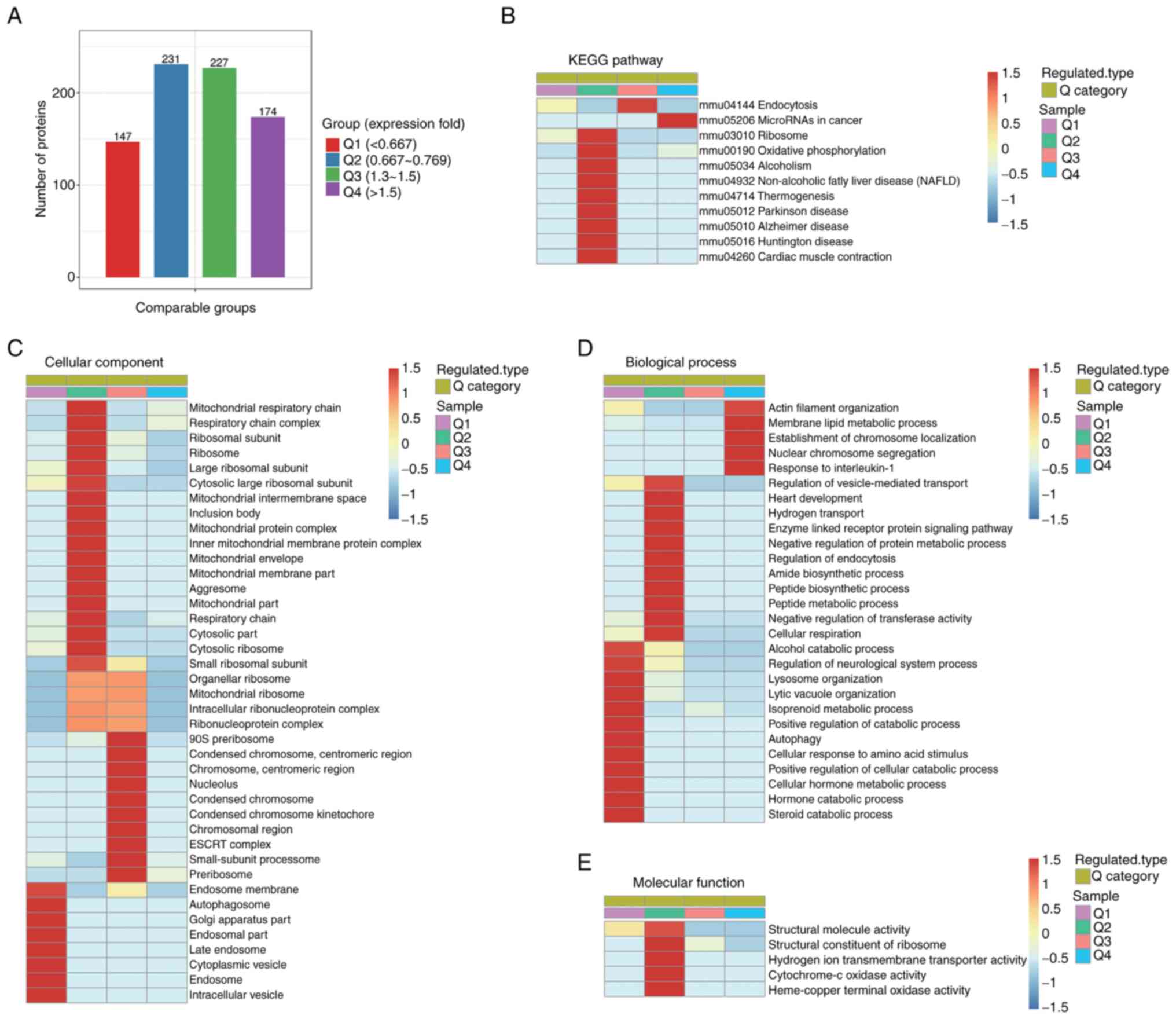
significantly enriched in various functional types (Fig. 4). In total, GO enrichment analysis
identified five significantly enriched biological processes based
on 174 upregulated proteins, four of which were related to actin
organization and function. These included ‘membrane lipid metabolic
process’, ‘actin filament organization’, ‘nuclear chromosome
segregation’, ‘response to IL-1’ and ‘establishment of chromosome
localization’ (Fig. 4A). Important
proteins involved in these biological processes included Abl
interactor 2 (Abi2), vasodilator-stimulated phosphoprotein (Vasp1),
epidermal growth factor receptor kinase substrate 8, monooxygenase
MICAL1 (Mical-1) and sororin. KEGG pathway enrichment analysis
demonstrated only one statistically significant enriched pathway,
‘mmu05206 microRNAs in cancer’ (Fig.
4B). Out of the 147 downregulated proteins, GO enrichment
analysis identified 10 significantly enriched biological processes
(Fig. 4C), including ‘hormone
catabolic process’, ‘alcohol catabolic process’, ‘lytic vacuole
organization’, ‘lysosome organization’ and ‘autophagy’. These
downregulated proteins, mainly vacuolar protein sorting (Vps)18,
Vps11 and beclin-1, are associated with autophagy, lysosome
organization and lytic vacuole organization, in addition to their
enrichment in the catabolic processes of multiple substances (such
as hormone, steroid and alcohol). Furthermore, eight cellular
components were significantly enriched, namely ‘endosome’,
‘cytoplasmic vesicle’, ‘intracellular vesicle’, ‘late endosome’,
‘endosome membrane’, ‘endosomal part’, ‘Golgi apparatus part’ and
‘autophagosome’ (Fig. 4D). The
related proteins included beclin-1, Eps15 homology
domain-containing protein 4, neuropilin-1 and multiple vacuolar
protein sorting-associated proteins (Vps18 and Vps11). These
cellular components were associated with intracellular vesicle to
lysosomes transport system (endocytosis and autophagy), which serve
a key role in the premature activation of trypsinogen in pancreatic
acinar cells (22).
This comprehensive analysis elucidated the
significant roles of regulated proteins in cellular composition and
related biological processes, particularly within the intracellular
vesicle transport system. These findings underscore the potential
inhibitory role of IL-22 in the premature activation of trypsinogen
in pancreatic acinar cells.
Autophagy is significantly inhibited after IL-22
treatment, whilst endocytosis is activated. The differentially
expressed proteins were divided into four groups (Q1-4) based on
differential expression levels to assess the association between
protein function and differential fold expression (Fig. 5A). Compared with the
arginine-induced group, the Q1 group included proteins with fold
expression <0.667 and the Q4 group included proteins with fold
expression >1.5. For each group, GO terms and KEGG enrichment
analysis were performed separately, and cluster analyses were
performed (Fig. 5B-E). The results
demonstrated that proteins in Q1, including Vps18, Vps11 and
beclin-1, were associated with cellular components such as
‘autophagosome’, ‘endosome’, ‘late endosome’ and ‘intracellular
vesicle’ (Fig. 5C). Specifically,
these proteins were crucially involved in biological processes such
as ‘autophagy’, ‘lysosome organization’ and ‘lytic vacuole
organization’ (Fig. 5D). In
addition, the regulation of biological process such as
‘vesicle-mediated transport’, ‘negative regulation of protein
metabolic process’, ‘regulation of endocytosis’ and ‘peptide
biosynthetic process’ were enriched in proteins in Q2 (Fig. 5D), which demonstrated that these
processes were subtly inhibited after IL-22 treatment. Proteins
such as 60S ribosomal proteins, 39S ribosomal proteins, tuberin and
ubiquilin-2 were involved in these processes. However,
‘endocytosis’, a KEGG signaling pathway, was notably enriched in
proteins in Q3 (Fig. 5B). The
proteins involved included Ras-related protein Rab-5A, Vps36,
Vps37b, Rab35, Sorting nexin-4 and Vps37b. Fig. 5E reveals a majority of proteins
impacting molecular function within the Q2 group, suggesting that
IL-22 plays a role in regulating molecular function to some
extent.
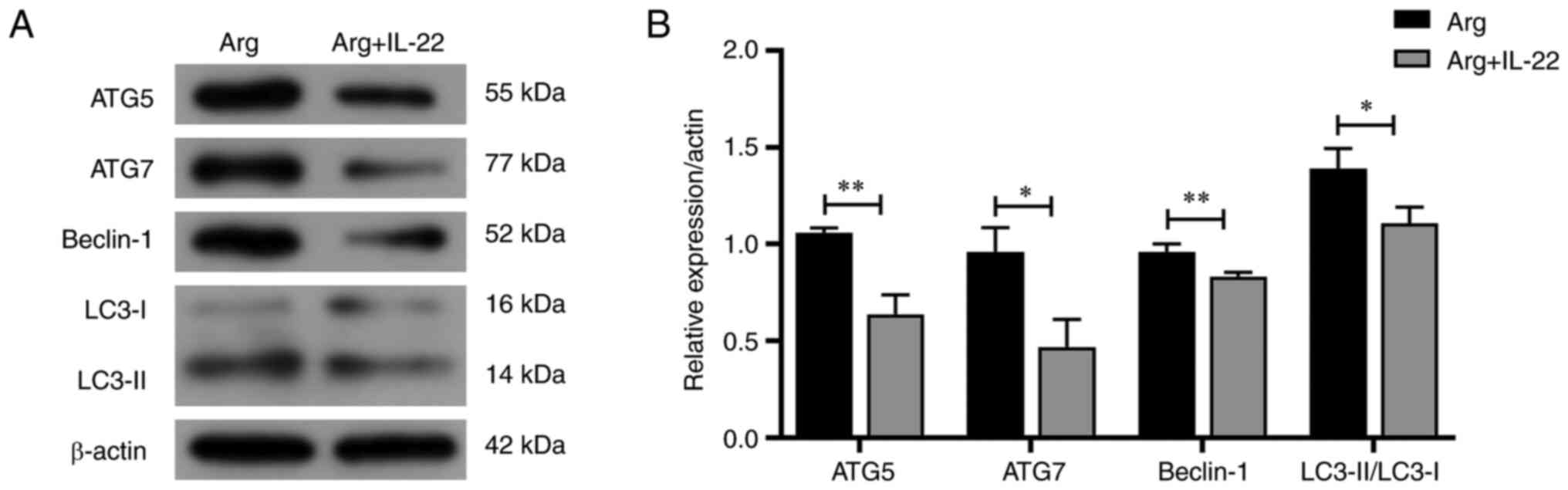
The effect of IL-22 on the expression of
autophagy-related proteins, including beclin-1, autophagy-related
gene (ATG)5, ATG7 and microtubule-associated protein 1 light chain
3 (LC3)-I/II, were assessed to determine whether IL-22 treatment
regulated autophagy. Western blotting results revealed a
significant reduction in the expression of beclin-1, ATG5, ATG7 and
LC3-I/II following IL-22 treatment (Fig. 6).
These findings enhance the understanding of the
impact of IL-22 on acute pancreatitis treatment. Specifically,
IL-22 inhibits the intracellular vesicular transport systems,
particularly autophagy, while sustaining endocytosis and other
molecular functions. Through this mechanism, IL-22 effectively
prevents the activation of trypsinogen, helping to preserve
cellular functionality during the initial stages of
arginine-induced stimulation in pancreatic acinar cells.
Discussion
AP is an inflammatory condition of the pancreas that
can be caused by bile stones and excessive alcohol consumption. It
is characterized by acute pancreatic inflammation and histological
acinar cell destruction (23). The
present study demonstrated that exposure of AR42J cells to arginine
led to changes commonly observed in cells undergoing apoptosis or
autophagy and which resemble the morphological changes seen in AP
(24). Arginine also decreased the
viability of AR42J cells in a concentration-dependent manner, as
demonstrated by results from the CCK-8 assay, and flow cytometry
and TUNEL assays demonstrated that the change was mainly manifested
as a significant increase in apoptosis and necrosis. In the
arginine-induced group, the concentration of amylase in the cell
culture supernatant was significantly increased, compared with that
in the negative control, suggesting that pancreatin in the acinar
cells was abnormally activated and released from the cytoplasm
following arginine exposure. The results indicated that arginine
stimulation triggered apoptosis of the acinar cells, cytoplasmic
vacuole formation and zymogen granules fusion with the lysosomes.
Combined with previous studies, we hypothesized that under this
mechanism, trypsinogen would subsequently be activated in
pancreatic acinar cells, thereby initiating pancreatitis (25,26).
A clinical study by Vasseur et al (13) reported high plasma IL-22 levels in
patients with AP, regardless of the disease severity. In our
previous studies, the therapeutic potential of exogenous IL-22 in
attenuating the severity of arginine-induced severe AP and
associated lung and kidney injury was identified (16,27).
To further evaluate the role of IL-22 in acinar cell damage
associated with AP, the present study established an in
vitro pancreatic acinar cell injury model using an arginine
concentration of 2.5 mg/ml, due to the relatively stable mortality
and survival rate exhibited by cells at this concentration. After
treatment with rIL-22, the amylase concentration in the supernatant
and the number of apoptotic cells were both significantly reduced
compared with those in the untreated group. Proteomic analysis then
demonstrated that the expression of protective proteins, such as
those involved in DNA damage repair and apoptosis regulation, were
notably upregulated after IL-22 treatment. These changes indicated
that IL-22 can effectively ameliorate arginine-induced pancreatic
acinar cell injury.
Moreover, IL-22 treatment might serve a role in
maintaining the cytoskeleton, thereby contributing to the
maintenance of cell morphology, material transportation and
organelle movement. Actin is a component of the cytoskeleton
(28), and its organization and
function were significantly increased by upregulated proteins
(Vasp1, Mical-1, Eps and Abi2) after IL-22 treatment in the present
study. In addition to participating in redox regulation of the
actin cytoskeleton, Mical-1 acts as a negative regulator of
apoptosis (29), which may explain
the reduced apoptosis demonstrated in the present study after IL-22
treatment.
The expression levels of proteins related to
biological processes, such as ‘autophagy’, ‘lysosome organization’
and ‘lytic vacuole organization’, were notably reduced after IL-22
treatment, according to GO analysis. Among the downregulated
proteins, Vps18, Vps11 and beclin-1 garnered particular attention
due to their involvement in the composition of the endosome, as
well as their association with autophagosomes and lysosomes.
Cluster analysis of the notably regulated proteins identified was
used to assess the change in expression levels and associations of
these proteins. Proteins with a fold change of <0.667 were
significantly enriched in the biological processes of ‘autophagy’,
‘lysosome organization’ and ‘lytic vacuole organization’.
Furthermore, western blotting demonstrated that the protein
expression of beclin-1, ATG5, ATG7 and LC3-II/LC3-I was
significantly decreased in the IL-22-treated group, compared with
the arginine-induced only group.
In the arginine-induced pancreatic acinar cell
injury model in the present study, autophagy and lysosomal
organization were significantly inhibited following IL-22
treatment. This inhibitory effect may have contributed to the
alleviation of premature activation of pancreatic enzymes that
originated from the colocalization of organelles. Autophagy, a type
of programmed cell death other than apoptosis that is used to
degrade organelles, proteins and other components (30), is essential for maintaining
cellular homeostasis, and its impairment is related to the
pathogenesis of a number of diseases, such as cancer,
neurodegenerative diseases, and infectious diseases (31). The system responsible for
transporting intracellular vesicles to lysosomes, primarily
involving autophagy, plays a role in the early activation of
trypsinogen during the initial stages of AP (30). Abnormal autophagic responses have
been observed in experimental models of AP and studies have
reported that impaired autophagy results in mitochondrial
dysfunction that leads to ER stress, dysregulated lipid metabolism
and increased severity of pancreatitis (4,32).
However, inhibition of autophagy can suppress trypsinogen
activation and reduced pancreatic damage (33).
Autophagosome maturation is a complex process
involving several vesicle trafficking components. The key step in
autophagic degradation of components is vesicle fusion, for which
vesicle tethering is a prerequisite. The tethering events in the
yeast endosome/vacuole (equivalent to mammalian lysosomes) have
been studied and reported to require the class C Vps complex
(34). Vps11 and Vps18 regulate
the fusion between endosomes, lysosomes and autophagosomes by
forming the core class C Vps complex. Beclin-1, an important marker
to monitor autophagy levels, is involved in autophagic vesicle
enucleation and autophagolysosome maturation (35). LC3 is a ubiquitin-like modifier
involved in autophagosomes formation, and the ratio of LC3-II and
LC3-I is commonly used to determine autophagy flux (36). ATG5 and ATG7 are proteins involved
in autophagy-vesicle formation (37). The decreased protein expression
levels of beclin-1, ATG5, ATG7 and LC3-II/LC3-I in the present
study indicated that IL-22 inhibited the accumulation of
autophagosomes at the initial step, whereas the reduced expression
of LC3-II demonstrated that IL-22 treatment alleviated the blockage
of autophagy flux. Beclin-1 is also involved in other pathways
besides autophagy, including endocytic trafficking and
LC3-associated phagocytosis (38).
ATG5 also serves an important role in augmenting susceptibility to
apoptosis. It likely interacts with mitochondrial components and
participates in the activation of key apoptotic molecules, such as
Bcl-xL (39). The present study
demonstrated that IL-22 alleviated pancreatic acinar cell injury in
the early stages of AP by inhibiting the intracellular lysosomal
degradative system, based on the reduced expression of these
proteins in the IL-22-treated cells.
Similar to autophagy, endocytosis is the
internalization of macromolecules and surface proteins by cells.
Endocytosis serves a pivotal role in the uptake of signaling
molecules, which in turn activates cascades that can result in
pathophysiological conditions (40). Studies have reported that
endocytosis affects the degradation of trypsin in pancreatic acinar
cells (41,42), and colocalization of organelles is
hypothesized to result from the endocytosis of damaged acinar cells
or their components (4).
Endocytosis shares many effector proteins with autophagy, including
Ras-like GTPases (Rabs) and Vps-associated proteins (43). These common effectors indicate an
association between budding and fusion of membrane-bound vesicles.
The crosstalk between autophagy and endocytosis implicates a novel
endocytic regulatory pathway of autophagy. In the present study,
hierarchical clustering of the KEGG pathway demonstrated that
‘endocytosis’ was enriched in the Q3 proteins. Some of these
proteins were involved in both endocytosis and autophagy. For
example, Rab5, a component of the Vps34-beclin-1 complex, is an
essential component for early endosome biogenesis and endosomal
fusion (44). Moreover, it serves
a role in membrane elongation during macroautophagy (45). The adjustment of these co-effector
proteins suggests that the crosstalk between autophagy and
endocytosis may serve a role in IL-22-mediated treatment of
arginine-induced pancreatic acinar cell injury.
Various methods are currently available to induce
experimental pancreatitis, including the use of agents such as
arginine, cerulein and taurolithocholic acid 3-sulfate. Despite the
diversity in drugs and induction techniques, several other
proteomic studies offer comprehensive insights into the development
of this disease (46-48).
Findings from the present study align with these findings,
particularly with regard to modifying elements like Vps, Rabs, and
ubiquitinated proteins. Collectively, these studies underscore the
pivotal role of intricate processes such as apoptosis, autophagy,
endocytosis, mitochondrial function and cytoskeletal dynamics in
the activation of pancreatic enzymes, induction of acinar cell
damage and promotion of cellular repair mechanisms. The present
study demonstrated that following IL-22 treatment, endocytosis and
autophagy showed opposite regulation. This differential regulation
was further evaluated by analyzing the roles of the two
aforementioned processes in acinar cell injury in AP. In the early
stages of AP, impaired autophagy serves an important role in the
premature activation of trypsinogen, which is manifested by
impaired fusion of autophagosomes and lysosomes, leading to
excessive accumulation of autophagolysosomes in the cytoplasm and
incomplete substrate degradation (30). In addition, impaired autophagy
causes mitochondrial dysfunction, resulting in endoplasmic
reticulum stress, dyslipidemia and increased severity of
pancreatitis (32). Therefore,
after IL-22 treatment in the present study, autophagy-related
proteins may have been downregulated to reduce cell and tissue
damage caused by premature activation of trypsin. However, despite
the co-effector proteins shared by endocytosis and autophagy,
maintaining normal endocytosis is necessary to alleviate the
disease because of its role in the uptake of signaling molecules
and transport of necessary substances (49). Therefore, certain proteins (Rab-5A,
Vps36, Vps37b, Rab35, Sorting nexin-4 and Vps37b) involved in
endocytosis were demonstrated to have been upregulated to maintain
cellular physiological functions.
The present study has several limitations. First,
proteomic analysis was not performed on untreated control cells.
This omission restricts the comprehensive understanding of baseline
protein expression and potential changes unrelated to specific
treatments. This may have hindered the ability of the study to
definitively determine the impact of IL-22 treatment on protein
expression. Additionally, comparative experiments involving similar
cell lines were not performed to ascertain the reproducibility of
the outcomes across various cellular models. Whilst the AR42J cell
line is commonly used to model AP (50-52),
excluding other cell lines with similar traits restricts its
broader applicability. Moreover, limitations arise from the complex
interplay between endocytosis and autophagy in pancreatitis.
Although the present study suggests a connection between these
processes, complete understanding of how they interact during AP
progression remains a prospect for future investigation.
In conclusion, the present study demonstrated that
the transport system from intracellular vesicles to lysosomes, with
a particular emphasis on autophagy, serves an important role in the
pathogenesis and treatment of pancreatitis. IL-22 treatment
downregulated the expression of autophagy-related proteins (Vps18,
Vps11, beclin-1, ATG5, ATG7 and LC3-Ⅱ/LC3-Ⅰ), further inhibiting
autophagosome maturation and fusion with lysosomes. Inhibition of
autophagy helps to reduce the premature activation of trypsin and
protects acinar cells from arginine-induced damage (53). Relative activation of the endocytic
pathway occurs during this process, and has been reported to be
related to the maintenance of cell signal transduction and
physiological needs (54). Further
research into the transport system from intracellular vesicles to
lysosomes and the crosstalk between endocytosis and autophagy will
help to elucidate the regulatory mechanism of IL-22. In summary,
the present study demonstrates the potential of IL-22 as a new
therapeutic molecule for treating AP by repairing impaired
autophagy.
Supplementary Material
Impact of varied IL-22 concentrations
on cell viability. IL-22, interleukin-22; rIL-22, recombinant
IL-22.
Acknowledgements
The authors would like to thank Dr Jianfeng Li for
language polishing. Proteomic analysis was supported by the PTM
Bioinformatics Team (Zhejiang, China). An earlier version of the
paper was orally presented at the 20th Congress of Gastroenterology
China, 29-31 October 2020 in Zhuhai, China.
Funding
Funding: The present study was financially supported by the
National Natural Science Foundation of China (grant. no. 82170650)
and the Natural Science Foundation of Shandong Province (grant. no.
ZR2020MH057).
Availability of data and materials
The proteomic datasets generated and analyzed during
the current study are available in the ‘figshare’ repository
(https://doi.org/10.6084/m9.figshare.21077194.v1) and
the other datasets used and analyzed during the current study are
available from the corresponding author on reasonable request.
Authors' contributions
QX conceived the study and wrote the manuscript. QX,
XF and ZX performed the experiments and analyzed the data. HY and
XM collected and processed the samples. ML constructed figures and
interpreted the data. CX, BL and SZ contributed to the design of
the study, interpretation of the data and the writing of the
manuscript. HX designed and supervised the study, and critically
revised the manuscript. QX and XF confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lankisch PG, Apte M and Banks PA: Acute
pancreatitis. Lancet. 386:85–96. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhan X, Wan J, Zhang G, Song L, Gui F,
Zhang Y, Li Y, Guo J, Dawra RK, Saluja AK, et al: Elevated
intracellular trypsin exacerbates acute pancreatitis and chronic
pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol.
316:G816–G825. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Dambrauskas Z, Giese N, Gulbinas A, Giese
T, Berberat PO, Pundzius J, Barauskas G and Friess H: Different
profiles of cytokine expression during mild and severe acute
pancreatitis. World J Gastroenterol. 16:1845–1853. 2010.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Saluja A, Dudeja V, Dawra R and Sah RP:
Early intra-acinar events in pathogenesis of pancreatitis.
Gastroenterology. 156:1979–1993. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gukovsky I, Pandol SJ, Mareninova OA,
Shalbueva N, Jia W and Gukovskaya AS: Impaired autophagy and
organellar dysfunction in pancreatitis. J Gastroenterol Hepatol. 27
(Suppl 2):S27–S32. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Keir M, Yi Y, Lu T and Ghilardi N: The
role of IL-22 in intestinal health and disease. J Exp Med.
217(e20192195)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ouyang W and O'Garra A: IL-10 family
cytokines IL-10 and IL-22: From basic science to clinical
translation. Immunity. 50:871–891. 2019.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yao Y, Yang G, Lu G, Ye J, Cui L, Zeng Z,
Chen J and Zhou J: Th22 Cells/IL-22 serves as a protumor regulator
to drive poor prognosis through the JAK-STAT3/MAPK/AKT signaling
pathway in non-small-cell lung cancer. J Immunol Res.
2022(8071234)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Costa MM, Saraceni PR, Forn-Cuní G, Dios
S, Romero A, Figueras A and Novoa B: IL-22 is a key player in the
regulation of inflammation in fish and involves innate immune cells
and PI3K signaling. Dev Comp Immunol. 41:746–755. 2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Lindemans CA, Calafiore M, Mertelsmann AM,
O'Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM,
Lawrence G, et al: Interleukin-22 promotes
intestinal-stem-cell-mediated epithelial regeneration. Nature.
528:560–564. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Geng H, Bu HF, Liu F, Wu L, Pfeifer K,
Chou PM, Wang X, Sun J, Lu L, Pandey A, et al: In inflamed
intestinal tissues and epithelial cells, interleukin 22 signaling
increases expression of H19 long noncoding RNA, which promotes
mucosal regeneration. Gastroenterology. 155:144–155.
2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zindl CL, Lai JF, Lee YK, Maynard CL,
Harbour SN, Ouyang W, Chaplin DD and Weaver CT: IL-22-producing
neutrophils contribute to antimicrobial defense and restitution of
colonic epithelial integrity during colitis. Proc Natl Acad Sci
USA. 110:12768–12773. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Vasseur P, Devaure I, Sellier J, Delwail
A, Chagneau-Derrode C, Charier F, Tougeron D, Tasu JP, Rabeony H,
Lecron JC and Silvain C: High plasma levels of the pro-inflammatory
cytokine IL-22 and the anti-inflammatory cytokines IL-10 and IL-1ra
in acute pancreatitis. Pancreatology. 14:465–469. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Huan C, Kim D, Ou P, Alfonso A and Stanek
A: Mechanisms of interleukin-22's beneficial effects in acute
pancreatitis. World J Gastrointest Pathophysiol. 7:108–116.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bai J, Bai J and Yang M: Interleukin-22
attenuates acute pancreatitis-associated intestinal mucosa injury
in mice via STAT3 activation. Gut Liver. 15:771–781.
2021.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Qiao YY, Liu XQ, Xu CQ, Zhang Z and Xu HW:
Interleukin-22 ameliorates acute severe pancreatitis-associated
lung injury in mice. World J Gastroenterol. 22:5023–5032.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jin M, Zhang H, Wu M, Wang Z, Chen X, Guo
M, Zhou R, Yang H and Qian J: Colonic interleukin-22 protects
intestinal mucosal barrier and microbiota abundance in severe acute
pancreatitis. FASEB J. 36(e22174)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hu G, Shen J, Cheng L, Guo C, Xu X, Wang
F, Huang L, Yang L, He M, Xiang D, et al: Reg4 protects against
acinar cell necrosis in experimental pancreatitis. Gut. 60:820–828.
2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Berner A, Bachmann M, Bender C,
Pfeilschifter J, Christen U and Mühl H: Though active on RINm5F
insulinoma cells and cultured pancreatic islets, recombinant IL-22
fails to modulate cytotoxicity and disease in a protocol of
streptozotocin-induced experimental diabetes. Front Pharmacol.
6(317)2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rost-Roszkowska MM, Vilimová J, Tajovský
K, Chachulska-Żymełka A, Sosinka A, Kszuk-Jendrysik M, Ostróżka A
and Kaszuba F: Autophagy and apoptosis in the midgut epithelium of
millipedes. Microsc Microanal. 25:1004–1016. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Furey C, Buxbaum J and Chambliss AB: A
review of biomarker utilization in the diagnosis and management of
acute pancreatitis reveals amylase ordering is favored in patients
requiring laparoscopic cholecystectomy. Clin Biochem. 77:54–56.
2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
De Faveri F, Chvanov M, Voronina S, Moore
D, Pollock L, Haynes L, Awais M, Beckett AJ, Mayer U, Sutton R, et
al: LAP-like non-canonical autophagy and evolution of endocytic
vacuoles in pancreatic acinar cells. Autophagy. 16:1314–1331.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Garber A, Frakes C, Arora Z and Chahal P:
Mechanisms and management of acute pancreatitis. Gastroenterol Res
Pract. 2018(6218798)2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Eşrefoğlu M, Gül M, Ateş B and Selimoğlu
MA: Ultrastructural clues for the protective effect of melatonin
against oxidative damage in cerulein-induced pancreatitis. J Pineal
Res. 40:92–97. 2006.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sherwood MW, Prior IA, Voronina SG, Barrow
SL, Woodsmith JD, Gerasimenko OV, Petersen OH and Tepikin AV:
Activation of trypsinogen in large endocytic vacuoles of pancreatic
acinar cells. Proc Natl Acad Sci USA. 104:5674–5679.
2007.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sendler M, Maertin S, John D, Persike M,
Weiss FU, Krüger B, Wartmann T, Wagh P, Halangk W, Schaschke N, et
al: Cathepsin B activity initiates apoptosis via digestive protease
activation in pancreatic acinar cells and experimental
pancreatitis. J Biol Chem. 291:14717–14731. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu X, Qiao Y, Xu C, Zhu S and Xu H:
Protective effects of interleukin-22 on severe acute
pancreatitis-associated kidney injury in mice. Austin Intern Med.
2(1016)2017.
|
|
28
|
Tashiro M, Schäfer C, Yao H, Ernst SA and
Williams JA: Arginine induced acute pancreatitis alters the actin
cytoskeleton and increases heat shock protein expression in rat
pancreatic acinar cells. Gut. 49:241–250. 2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Zhou Y, Adolfs Y, Pijnappel WW, Fuller SJ,
Van der Schors RC, Li KW, Sugden PH, Smit AB, Hergovich A and
Pasterkamp RJ: MICAL-1 is a negative regulator of MST-NDR kinase
signaling and apoptosis. Mol Cell Biol. 31:3603–3615.
2011.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gukovskaya AS, Gukovsky I, Algül H and
Habtezion A: Autophagy, inflammation, and immune dysfunction in the
pathogenesis of pancreatitis. Gastroenterology. 153:1212–1226.
2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang Y and Klionsky DJ: Autophagy and
disease: Unanswered questions. Cell Death Differ. 27:858–871.
2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Biczo G, Vegh ET, Shalbueva N, Mareninova
OA, Elperin J, Lotshaw E, Gretler S, Lugea A, Malla SR, Dawson D,
et al: Mitochondrial dysfunction, through impaired autophagy, leads
to endoplasmic reticulum stress, deregulated lipid metabolism, and
pancreatitis in animal models. Gastroenterology. 154:689–703.
2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Iwahashi K, Hikita H, Makino Y, Shigekawa
M, Ikezawa K, Yoshioka T, Kodama T, Sakamori R, Tatsumi T and
Takehara T: Autophagy impairment in pancreatic acinar cells causes
zymogen granule accumulation and pancreatitis. Biochem Biophys Res
Commun. 503:2576–2582. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liang C, Lee JS, Inn KS, Gack MU, Li Q,
Roberts EA, Vergne I, Deretic V, Feng P, Akazawa C and Jung JU:
Beclin1-binding UVRAG targets the class C Vps complex to coordinate
autophagosome maturation and endocytic trafficking. Nat Cell Biol.
10:776–787. 2008.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Kang R, Zeh HJ, Lotze MT and Tang D: The
Beclin 1 network regulates autophagy and apoptosis. Cell Death
Differ. 18:571–580. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Schaaf MB, Keulers TG, Vooijs MA and
Rouschop KM: LC3/GABARAP family proteins: Autophagy-(un)related
functions. FASEB J. 30:3961–3978. 2016.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Nishida Y, Arakawa S, Fujitani K,
Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y
and Shimizu S: Discovery of Atg5/Atg7-independent alternative
macroautophagy. Nature. 461:654–658. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Green DR and Levine B: To be or not to be?
How selective autophagy and cell death govern cell fate. Cell.
157:65–75. 2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Yousefi S, Perozzo R, Schmid I, Ziemiecki
A, Schaffner T, Scapozza L, Brunner T and Simon HU:
Calpain-mediated cleavage of Atg5 switches autophagy to apoptosis.
Nat Cell Biol. 8:1124–1132. 2006.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Panda PK, Mukhopadhyay S, Das DN, Sinha N,
Naik PP and Bhutia SK: Mechanism of autophagic regulation in
carcinogenesis and cancer therapeutics. Semin Cell Dev Biol.
39:43–55. 2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Messenger SW, Thomas DD, Cooley MM, Jones
EK, Falkowski MA, August BK, Fernandez LA, Gorelick FS and
Groblewski GE: Early to late endosome trafficking controls
secretion and zymogen activation in rodent and human pancreatic
acinar cells. Cell Mol Gastroenterol Hepatol. 1:695–709.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Messenger SW, Jones EK, Holthaus CL,
Thomas DDH, Cooley MM, Byrne JA, Mareninova OA, Gukovskaya AS and
Groblewski GE: Acute acinar pancreatitis blocks vesicle-associated
membrane protein 8 (VAMP8)-dependent secretion, resulting in
intracellular trypsin accumulation. J Biol Chem. 292:7828–7839.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mahapatra KK, Panigrahi DP, Praharaj PP,
Bhol CS, Patra S, Mishra SR, Behera BP and Bhutia SK: Molecular
interplay of autophagy and endocytosis in human health and
diseases. Biol Rev Camb Philos Soc. 94:1576–1590. 2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Trofimenko E, Homma Y, Fukuda M and
Widmann C: The endocytic pathway taken by cationic substances
requires Rab14 but not Rab5 and Rab7. Cell Rep.
37(109945)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Ao X, Zou L and Wu Y: Regulation of
autophagy by the Rab GTPase network. Cell Death Differ. 21:348–358.
2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Chu J, Ji H, Lu M, Li Z, Qiao X, Sun B,
Zhang W and Xue D: Proteomic analysis of apoptotic and oncotic
pancreatic acinar AR42J cells treated with caerulein. Mol Cell
Biochem. 382:1–17. 2013.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li Z, Lu M, Chu J, Qiao X, Meng X, Sun B,
Zhang W and Xue D: Early proteome analysis of rat pancreatic acinar
AR42J cells treated with taurolithocholic acid 3-sulfate.
Pancreatology. 12:248–256. 2012.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Yu JH, Yun SY, Lim JW, Kim H and Kim KH:
Proteome analysis of rat pancreatic acinar cells: Implication for
cerulein-induced acute pancreatitis. Proteomics. 3:2446–2453.
2003.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wu LG, Hamid E, Shin W and Chiang HC:
Exocytosis and endocytosis: Modes, functions, and coupling
mechanisms. Annu Rev Physiol. 76:301–331. 2014.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Sun S, Han Y, Zhang C, Liu H, Wang B, Cao
S, Yuan Q, Wei S and Chen Y: adenosine kinase inhibition prevents
severe acute pancreatitis via suppressing inflammation and acinar
cell necroptosis. Front Cell Dev Biol. 10(827714)2022.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Huang H, Wang M, Guo Z, Wu D, Wang H, Jia
Y, Liu H, Ding J and Peng J: Rutaecarpine alleviates acute
pancreatitis in mice and AR42J cells by suppressing the MAPK and
NF-κB signaling pathways via calcitonin gene-related peptide.
Phytother Res. 35:6472–6485. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Zhang Z, Liu Q, Zang H, Shao Q and Sun T:
Oxymatrine protects against l-arginine-induced acute pancreatitis
and intestine injury involving Th1/Th17 cytokines and MAPK/NF-κB
signalling. Pharm Biol. 57:595–603. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Xiao J, Feng X, Huang XY, Huang Z, Huang
Y, Li C, Li G, Nong S, Wu R, Huang Y and Long XD: Spautin-1
ameliorates acute pancreatitis via inhibiting impaired autophagy
and alleviating calcium overload. Mol Med. 22:643–652.
2016.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Mukherjee S, Ghosh RN and Maxfield FR:
Endocytosis. Physiol Rev. 77:759–803. 1997.PubMed/NCBI View Article : Google Scholar
|