Introduction
End-stage renal disease (ESRD) is a global health
concern (1), and hemodialysis
access is of utmost importance to patients with ESRD who require
renal replacement therapy. According to the Kidney Disease Outcomes
Quality Initiative (2), autogenous
arteriovenous (AV) fistula (AVF) is the recommended vascular access
point for hemodialysis. However, for some patients with exhausted
upper limb autogenous access options, neither renal transplantation
nor peritoneal dialysis is a suitable option. As a result, the
creation of a complex vascular access using an expanded
polytetrafluoroethylene (PTFE) graft or access via the lower limbs
may be required for such patients (3). Therefore, these patients often
require more complex access procedures.
Several complex access procedures have been reported
in patients with challenging circumstances. In 2008, Morsy et
al (4) reported their
experience with the utilization of axillary-axillary arteriovenous
bypass grafts as a final alternative for patients who have
experienced failure of autogenous access sites, and for whom
peritoneal dialysis or transplantation are not feasible options.
The procedure involves the creation of a necklace graft using
internally reinforced prostheses, which are anastomosed end-to-side
to the axillary artery and contralateral vein, and then tunneled
directly through the subcutaneous space before reaching the
sternum. The findings of this study demonstrate that these bypass
grafts offer a viable solution for complex cases, with reasonable
patency rates and minimal complications (4). Other options include internal jugular
vein bypasses, axillary loops, femorofemoral AV crossover bypasses,
superficial femoral vein transpositions, axillary artery to
popliteal vein bypasses and femoral artery to right atrium bypasses
(5,6). When an arteriovenous fistula cannot
be established in the upper or lower limb, arterioarterial
prosthetic loops have been utilized as an alternative for
hemodialysis access in situations where no other option is feasible
(7,8).
Although a number of complex access procedures have
been described, the use of the external jugular vein (EJV) for
outflow in patients with particularly challenging venous disease
has rarely been reported. The external jugular vein was identified
as a viable outflow conduit for arteriovenous fistulas of the upper
extremity in cases where the axillary veins are occluded,
exhibiting acceptable patency rates (9). In another study, the use of an
ipsilateral internal jugular vein (IJV) as an AV outflow vein was
examined in patients with subclavian or axillary vein stenosis or
occlusion (10). The results
indicated that a brachial-jugular AV graft may achieve satisfactory
results in terms of patency and complication rate, although the
primary patency rate decreases significantly over time. The use of
the IJV as an outflow vein should be the last option for using a
particular arm due to the risk of complications such as steal
syndrome, seroma, hematoma, swollen arm, infections,
pseudoaneurysm, bleeding from puncture site, stenosis and
thrombosis (10). In the present
case, a prosthetic AV loop between the brachial artery (BA) and the
EJV was used for a patient whose vascular conditions did not allow
for the creation of another type of upper limb access. The current
study presents the experience of utilizing a PTFE graft loop
anastomosis with the BA and EJV as an AV bypass graft for
hemodialysis access.
Case report
A 70-year-old male patient had received maintenance
hemodialysis for >10 years. In June 2015, the patient presented
to Lishui Municipal Central Hospital (Lishui, China) with a
complaint of lower extremity dialysis access thrombosis for 25 h.
The medical history included diabetes mellitus, diabetic
nephropathy, hypertension and peripheral vascular disease. Nine
years previously, a left upper extremity arteriovenous fistula was
created, but it lost functionality three years postoperatively. Six
years previously, a right upper extremity arteriovenous fistula was
established. Three years previously, a left lower extremity
artificial vascular access was created using a bypass graft from
the left femoral artery to the deep femoral artery. However, after
16 months, the artificial vascular access was removed due to
bleeding. Two years previously, a lower extremity artificial
vascular access was performed using the great saphenous vein.
A percutaneous mechanical thrombectomy was
unsuccessful. A pre-operative assessment was initially performed,
which included upper extremity and central venograms. Venograms of
the upper arm and central veins had excluded central stenosis for
the patient. The BAs were evaluated using color Doppler ultrasound
(data not included). A temporary right femoral vein catheter was
used for hemodialysis. Finally, the EJV was selected as the outflow
vein, and inflow was performed from the left BA. It was decided
that a BA-EJV AV bypass graft procedure would be performed as a
permanent vascular access option.
The procedures were performed under general
anesthesia, which was considered appropriate by the surgical team.
The surgical procedures included the exposure of the BA and EJV,
the subcutaneous placement of an expanded PTFE prosthesis with a
6-mm diameter as the loop, and a PTFE graft loop extending down
into the mid-forearm, for which a forearm incision was required.
Following the separation of the BA and EJV, a PTFE graft was
positioned following the configuration of a subcutaneously tunneled
loop on the upper limb. A 6-0 polypropylene suture was used in the
creation of side-to-end or end-to-end anastomoses between the BA or
EJV and graft. The length of the implanted graft was 60 cm. The
duration of the surgery was 90 min, and the amount of blood lost
was 100 ml.
Post-operative complications included temporary arm
swelling for 2 weeks. Subcutaneous administration of low molecular
weight heparin (4,000 units) once daily was initiated for a
duration of 3 days and subsequently discontinued. Upon discharge,
the patient was prescribed aspirin (100 mg/day) as an antiplatelet
therapy. The patient was assessed at a 2-week post-operative
follow-up by the surgeon. Subsequently, the access nurse evaluated
the graft during dialysis sessions to ensure the patency. In
addition, nephrology physicians advise against repeated punctures
at the same site and recommend puncturing at different points with
intervals of >1 week. Blood flow of the graft was excellent for
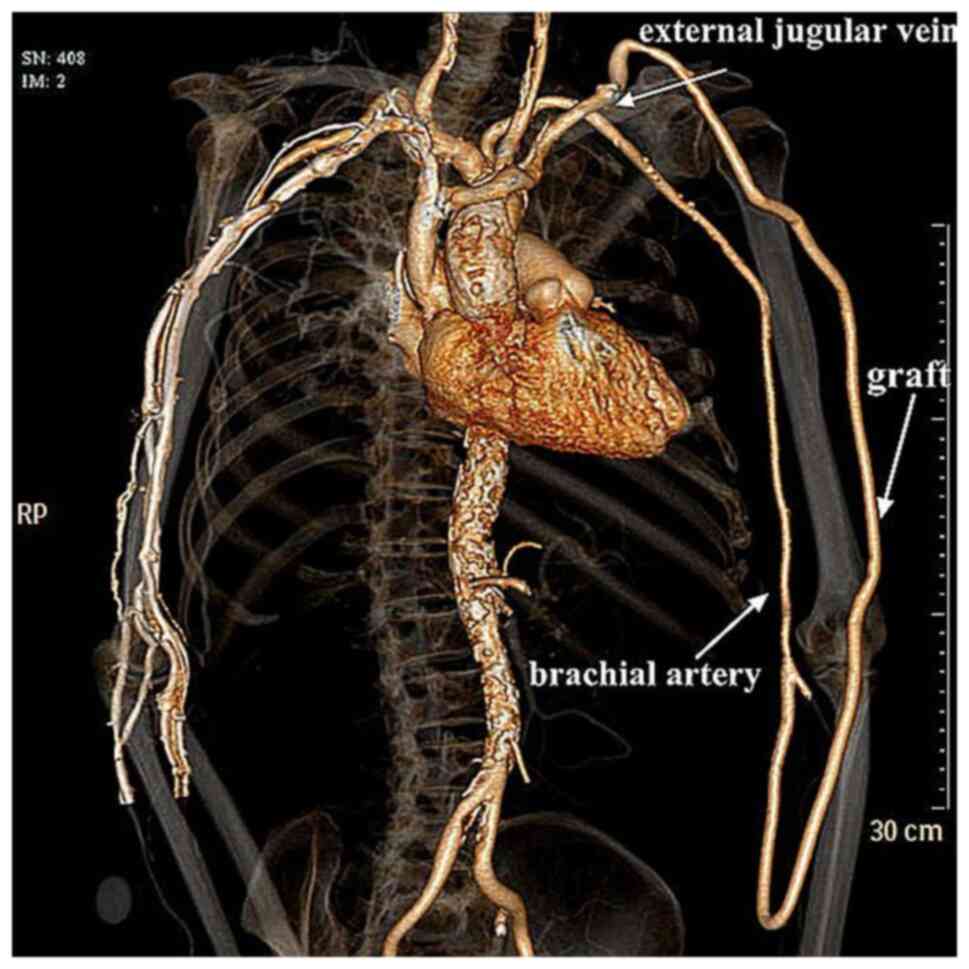
hemodialysis. Post-operative computed tomography angiography of the
access revealed a patent anastomosis (Fig. 1). The patency of the graft
continued for 20 months. The graft became thrombosed 20 months
after the placement. However, the patient refused to undergo a
thrombectomy surgery. A cuffed catheter was placed in the right
femoral vein for long-term hemodialysis. The patient succumbed to
hypertensive intracerebral hemorrhage unrelated to hemodialysis
access, 25 months post-surgery.
Discussion
Optimal vascular access for hemodialysis is
characterized by a low rate of associated complications, the
ability to deliver adequate flow rates and long-lasting patency. It
is generally known that AVF has fewer associated complications, a
longer patency, fewer interventions required and lower costs than
AVGs or catheters. Therefore, AVF is the primary choice for
hemodialysis access (11).
However, the number of patients who have multiple AVF failures and
require complex vascular access is increasing. In the case
described in the present study, the patient had exhausted all other
options for upper extremity access. For patients without options
via the upper extremities, AV or arterial-arterial grafts on the
lower extremities have been previously reported (7,8,12,13).
However, a femoral vein transposition or arterioarterial prosthetic
loops are associated with a high risk of ischemic complications,
and are not suitable for patients with peripheral artery disease.
Therefore, lower extremity vascular access should be carefully
tailored to each individual patient (12).
For patients in whom upper limb access is not
obtainable, thigh AV access has been reported as a viable option
(14). It has been shown that
prosthetic AV access in the leg is associated with a low risk of
Steal syndrome, including symptoms/complaints of pain, numbness,
coldness and weakness in the affected limb; however, it is
accompanied by low primary and secondary patency rates, a
particularly high infection rate and more frequent surgical
revisions (15). Axillorenal AVG
and right atrial bypass grafting have also been previously reported
(16,17). However, the loop requires an
anastomosis to the renal vein or to the right atrial appendage
through a median sternotomy, which is considered to be a complex
access configuration. In addition, chest wall or arm AVGs based on
the axillary artery and ipsilateral axillary vein are well
described in the absence of central vein occlusion (18-20).
In the present case, AVF construction had failed and the creation
of complex grafts based on the axillary vein was not feasible for
the patient. Thus, a novel AVG procedure for hemodialysis based on
the EJV and BA was created as the upper body access option.
In the present patient, previous upper or lower limb
vascular access and central venous catheter use had failed multiple
times, and the incidence of central venous stenosis was high. The
presence of central venous stenosis or obstruction is a
contraindication to this BA-EJV access. Thus, it is suggested that
pre-operative venography is required when the clinical suspicion or
history of central venous stenosis exists. If central venous
stenosis is identified, percutaneous transluminal angioplasty or a
stent is required. If central venous occlusion is identified,
re-cannulation of the central vein is necessary. According to
previous studies, lower limb access is required in patients whose
occlusion cannot be treated (13,18).
In the case in the present study, an increase in the risk of
developing thrombosis after the BA-EJV procedure was considered;
therefore, the patient was treated with a post-operative oral
antithrombotic drug. However, the risk of post-operative
hemorrhagic complications needs to be carefully evaluated.
In the present patient, the BA-EJV graft maintained
patency for >20 months. The patient also did not experience any
severe complications, such as hemorrhaging, infection and
aneurysmal dilatation. These results were considered acceptable
with respect to the lack of vascular access in the patient. Based
on the present study, it is considered that the BA-EJV may
represent a novel hemodialysis access option.
Although BA-EJV grafting is an acceptable procedure
for establishing upper limb hemodialysis access, this process may
be accompanied by complications that require early treatment and
recognition. For example, graft infection requires the use of
systemic antibiotics, or a total or subtotal graft excision
(21). In addition, venous
anastomotic stenosis leads to prosthetic graft thrombosis. Previous
studies have demonstrated that venous anastomotic stenosis
typically occurs in the first year after the procedure (22,23).
Furthermore, heart failure is the most frequent cardiovascular
disease associated with grafting or fistula formation, due to the
marked hemodynamic changes related to the large increase in blood
flow (24-26).
It may be suggested that patients with heart failure classified as
New York Heart Association Class IV are not suitable candidates for
BA-EJV access (27). Finally,
BA-EJV access may result in hematomas, arterial steal syndrome,
ischemic monomelic neuropathy or the formation of an aneurysm
(27). Previous studies have
suggested that pseudoaneurysms occur in ~10% of PTFE grafts
(23,28). The use of BA-EJV GRAFT may also
lead to the development of pseudoaneurysms resulting from repeated
puncture or graft material deterioration. In the present case,
pseudoaneurysm formation was not observed, as a careful puncture
technique was applied.
It should be noted that although acceptable results
were obtained for the patient described in the present study, the
lack of a control group was one limitation to the report. There
were also specific neck mobility issues in the patient.
Furthermore, it was noted that the graft crossed the elbow crease
in two areas, which could lead to the kinking of the graft upon
flexion at the elbow. The indications for BA-EJV GRAFT need to be
clearly defined due to the aforementioned potential complications.
AVF remains the preferred access option for hemodialysis, and
BA-EJV GRAFT may be utilized as a second line procedure only for
patients in whom other upper extremity possibilities for AV access
creation are exhausted.
In conclusion, the present study describes the case
of a patient in which the BA-EJV GRAFT was used as an alternative
approach for hemodialysis access as all other conventional vascular
access options had been exhausted. However, further carefully
designed studies are required in the future to perform more robust
comparisons of BA-EJV GRAFT with other access methods.
Acknowledgements
Not applicable.
Funding
Funding: The present study was financially supported by the
Medical and Health Science Technology Plan Project from Zhejiang,
China (grant no. 2020ZH082).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WL contributed to the conception and design of the
study, treated and followed up patients, and collected the data. JX
advised on patient treatment and surgery. HL was responsible for
obtaining medical images and analyzing patient data. WL and JX
confirm the authenticity of all the raw data. All authors have read
and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of their data and any related
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Anderson S, Halter JB, Hazzard WR,
Himmelfarb J, Horne FM, Kaysen GA, JW Nayfield SG, Schmader K, Tian
Y, et al: Prediction, progression, and outcomes of chronic kidney
disease in older adults. J Am Soc Nephrol. 20:1199–1209.
2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Vascular Access 2006 Work Group. Clinical
practice guidelines for vascular access. Am J Kidney Dis. 48 (Suppl
1):S176–S247. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hedin U: Long-term results of PTFE grafts.
J Vasc Access. 16 (Suppl 9):S87–S92. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Morsy MA, Khan A and Chemla ES: Prosthetic
axillary-axillary arteriovenous straight access (necklace graft)
for difficult hemodialysis patients: A prospective single-center
experience. J Vasc Surg. 48:1251–1254.e1. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Hazinedaroğlu S, Karakayali F, Tüzüner A,
Ayli D, Demirer S, Duman N and Yerdel MA: Exotic arteriovenous
fistulas for hemodialysis. Transplant Proc. 36:59–64.
2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Rajan DK: New approaches to arteriovenous
fistula creation. Semin Intervent Radiol. 33:6–9. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lei W, Ji J, Wang J, Jin L and Zou H:
Arterioarterial prosthetic loop as an alternative approach for
hemodialysis access. Medicine (Baltimore). 94(e1645)2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zanow J, Kruger U, Petzold M, Petzold K,
Miller H and Scholz H: Arterioarterial prosthetic loop: A new
approach for hemodialysis access. J Vasc Surg. 41:1007–1012.
2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Moini M, Rasouli MR and Cheraghi A: The
external jugular vein: An alternative outflow for insertion of
upper extremity arteriovenous grafts in patients with obstructed
axillary vein. Ann Vasc Surg. 24(573)2010.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kim MJ, Yun S, Song D, Cho SW, Goo DE, Kim
YJ and Choi D: Alternative venous outflow by brachial to jugular
vein vascular access for hemodialysis in the exhausted upper
extremities. J Vasc Access. 16:269–274. 2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ashby D, Borman N, Burton J, Corbett R,
Davenport A, Farrington K, Flowers K, Fotheringham J, Andrea Fox
RN, Franklin G, et al: Renal association clinical practice
guideline on haemodialysis. BMC Nephrol. 20(379)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wilmink T: Lower limb access. J Vasc
Access. 15 (Suppl 7):S130–S135. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Antoniou GA, Lazarides MK, Georgiadis GS,
Sfyroeras GS, Nikolopoulos ES and Giannoukas AD: Lower-extremity
arteriovenous access for haemodialysis: A systematic review. Eur J
Vasc Endovasc Surg. 38:365–372. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gibbons CP: Vascular access in the lower
limb. Endovasc Surg. 38:373–374. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Oh E, Kim YJ, Goo DE, Yang S and Hong S:
Percutaneous transluminal angioplasty for dysfunctional femoral
hemodialysis graft. Diagn Interv Radiol. 21:154–159.
2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Karp SJ, Hawxby A and Burdick JF:
Axillorenal arteriovenous graft: A new approach for dialysis
access. J Vasc Surg. 40:379–380. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
El-Sabrout RA and Duncan JM: Right atrial
bypass grafting for central venous obstruction associated with
dialysis access: another treatment option. J Vasc Surg. 29:472–478.
1999.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liechty JM, Fisher T, Davis W, Oglesby WC,
Bennett M, Grimsley B and Shutze W: Experience with chest wall
arteriovenous grafts in hemodialysis patients. Ann Vasc Surg.
29:690–697. 2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hunter JP and Nicholson ML: Midterm
experience of ipsilateral axillary-axillary arteriovenous loop
graft as tertiary access for haemodialysis. J Transplant.
2014(908738)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Mohamed IH, Bagul A, Doughman T and
Nicholson ML: Axillary-axillary loop graft for hemodialysis access.
J Vasc Access. 12:262–263. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Nguyen DB, Arduino MJ and Patel PR:
Hemodialysis-associated infections. Chronic Kidney Disease,
Dialysis and Transplantation 389-410.e8, 2019.
|
|
22
|
Martin C and III Pillai R: Dialysis access
anatomy and interventions: A primer. Semin Intervent Radiol.
33:52–55. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Rose DA, Sonaike E and Hughes K:
Hemodialysis access. Surg Clin North Am. 93:997–1012, x.
2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Roca-Tey R: Permanent arteriovenous
fistula or catheter dialysis for heart failure patients. J Vasc
Access. 17 (Suppl 1):S23–S29. 2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wasse H and Singapuri MS: High-output
heart failure: How to define it, when to treat it, and how to treat
it. Semin Nephrol. 32:551–557. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Singh S, Elramah M, Allana SS, Babcock M,
Keevil JG, Johnson MR, Yevzlin AS and Chan MR: A case series of
real-time hemodynamic assessment of high output heart failure as a
complication of arteriovenous access in dialysis patients. Semin
Dial. 27:633–638. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Malik J, Lomonte C, Rotmans J, Chytilova
E, Roca-Tey R, Kusztal M, Grus T and Gallieni M: Hemodialysis
vascular access affects heart function and outcomes: Tips for
choosing the right access for the individual patient. J Vasc
Access. 22 (Suppl 1):32–41. 2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kakkos SK, Topalidis D, Haddad R, Haddad
GK and Shepard AD: Long-term complication and patency rates of
Vectra and IMPRA Carboflo vascular access grafts with aggressive
monitoring, surveillance and endovascular management. Vascular.
19:21–28. 2011.PubMed/NCBI View Article : Google Scholar
|