Introduction
Acute respiratory distress syndrome (ARDS),
characterized by refractory hypoxemia and noncardiogenic pulmonary
edema, is an acute inflammatory process of the lungs induced by
insults to the alveolar-capillary membrane (1-3).
ARDS develops most often in the setting of sepsis, pneumonia,
severe trauma or aspiration of gastric contents and exists in ~10%
of all patients admitted to the intensive care units (ICU)
worldwide (4). Despite progress in
the improvement of treatments of underlying conditions and organ
support, ARDS is still a major cause of ICU morbidity and mortality
(5,6). Therefore, accurate prediction of ARDS
at an early stage would be useful for decreasing its morbidity and
mortality.
The lung injury prediction score (LIPS), proposed by
Trillo-Alvarez et al (7),
can be used to assess the predisposing factors and risks of ARDS.
However, the positive predictive value (PPV) of this score is low
and limits its application in clinic (8). Biomarkers can improve the prediction
of ARDS but they cannot diagnose ARDS definitely (9). Previous studies have identified
several promising candidate biomarkers, including receptor for
advanced glycation end-products (RAGE), angiopoietin-2,
plasminogen-activator-1, interleukin-8, microRNA (miR)-181a,
miR-92a, miR-424, procollagen peptide I and III, surfactant protein
D, Fas and Fas ligand, acetaldehyde, 3-methylheptane, and octane
(3,10,11).
These biomarkers can be integrated into the clinical prediction
models for ARDS risk. For example, integration of angiopoietin-2
levels into LIPS significantly elevates the predictive value for
ARDS with favorable sensitivity and specificity (12).
As a biomarker of lung epithelium injury, RAGE is
associated with the increased risk for occurrence of ARDS (13). In the present study, the
independent associations between LIPS, RAGE and occurrence of ARDS
in critically ill patients with ARDS risk factors were verified,
and the values of LIPS, RAGE and their combination for predicting
occurrence of ARDS were evaluated. The aim was to provide an
accurate tool for the prediction of ARDS occurrence.
Materials and methods
Patients
In this prospective observational study, a
consecutive cohort of 819 patients with risk factors of ARDS were
enrolled from the ICU of Chongqing University Jiangjin Hospital
(Chongqing, China) between May 2020 and April 2021. These 819
patients included 613 male patients (74.8%) and 206 female patients
(25.2%) with a mean age of 60.12±19.15 years (range, 18-91 years).
The inclusion criteria included presence of one or more risk
factors and informed consent (11). The exclusion criteria included: i)
Developing ARDS before initial blood collection and assessment; ii)
<7 days of hospital stay, resulting in unfeasibility of
determining the clinical outcome; iii) rehospitalization; iv)
failure in collecting blood within 24 h of admission into the ICU;
v) mortality of the patient within 6 h of admission; vi) a history
of chronic interstitial lung disease; vii) diagnosed as congestive
heart failure; and viii) failure to conduct chest computed
radiography or computed tomography within 7 days of admission. The
present study was approved by the Ethical Committee of Chongqing
University Jiangjin Hospital (approval no. JJ2020017031) and
carried out strictly following the guidelines of the Declaration of
Helsinki. Written informed consent was obtained from the study
participants prior to study commencement.
Data collection
Demographic data, baseline clinical information,
ARDS risk factors, ARDS risk modifiers and laboratory parameters
were collected. LIPS was computed within 6 h of admission into the
ICU as previously described (7,14).
At the same time, the Acute Physiology and Chronic Health
Evaluation (APACHE) II score was computed within 24 h of admission
to evaluate the severity index. Blood collection was performed
within 24 h of admission.
RAGE detection
Blood samples were collected using EDTA as an
anti-coagulant within 24 h of admission into the ICU, and
centrifugation of 1,006.2 x g for 10 min at room temperature was
performed to obtain plasma. The plasma concentration of RAGE was
detected using a human receptor for advanced glycation endproducts
ELISA kit (cat. no. ZN2383; Beijing Baiolaibo Technology Co., Ltd.)
following the manufacturer's instructions strictly. This kit has a
detection range of 78-5,000 pg/ml and sensitivity of <2
pg/ml.
Primary outcome
The primary endpoint was ARDS occurrence within 7
days. ARDS was diagnosed by two experienced clinicians (Department
of Critical Care Medicine, Chongqing University Jiangjin Hospital,
Chongqing, China) independent from the present study according to
the Berlin definition for ARDS (1). The two clinicians were blinded to the
concentration of plasma RAGE and LIPS. The diagnosis of sepsis,
severe sepsis and septic shock were determined according to the
previously reported criteria (15).
Statistical analysis
SPSS version 20.0 (IBM Corp.) was used to carry out
statistical analysis. The Kolmogorov-Smirnov test was employed to
assess the normality of continuous variables. For normally
distributed variables, Student's t-test was employed to perform
univariate analysis (intergroup comparison between ARDS group and
non-ARDS group). The χ2 test was employed to perform
univariate analysis of categorical variables. The variables with
P<0.10 in the univariate analysis were then included in binary
logistic regression model to perform multivariate analysis, aiming
for identifying independent associations between LIPS, RAGE levels
and ARDS occurrence. The values of LIPS, RAGE levels and their
combination in predicting ARDS occurrence were assessed using the
receiver operating characteristic (ROC) curve. For the prediction
tool of LIPS combined with RAGE levels, the probability obtained
from binary logistic regression analysis was used as a new
indicator for the prediction of ARDS occurrence. Z test was
employed to perform the comparison of the area under curve (AUC)
between different prediction methods. P<0.05 was considered to
indicate a statistically significant difference.
Results
General information
A total of 819 patients with risk factors of ARDS
were enrolled during the study period, and 551 patients were
included in the final analysis. A total of 45 patients were
excluded due to developing ARDS before initial blood collection and
assessment, 34 patients were excluded due to a hospital stay that
was <7 days, 11 patients were excluded due to rehospitalization,
86 patients were excluded due to failure in collecting blood within
24 h after admission, 2 patients were excluded due to death within
6 h after admission, 17 patients were excluded due to a history of
chronic interstitial lung disease, 14 patients were excluded due to
diagnosed as congestive heart failure and 59 patients were excluded
due to failure in conducting chest computed radiography or computed
tomography within 7 days after admission.
These 551 patients included 414 males (75.1%) and
137 females (24.9%) with an average age of 59.96±19.21 years. The
reasons for admission included respiratory disease (57.0%), trauma
(22.0%), operation (5.8%), acute abdominal disease (5.6%),
cardiopulmonary resuscitation (2.9%) and others (6.7%). Within 7
days after admission into the ICU, ARDS occurred in 176 patients
(31.9%) (Table I).
 | Table IUnivariate analysis results between
the ARDS and non-ARDS groups. |
Table I
Univariate analysis results between
the ARDS and non-ARDS groups.
| Parameter | All patients
(n=551) | ARDS group
(n=176) | Non-ARDS group
(n=375) |
χ2/t-test | P-value |
|---|
| Age, years | 59.96±19.21 | 60.23±18.71 | 59.84±19.45 | 0.225 | 0.830 |
| Male | 441 (75.1%) | 129 (73.3%) | 285 (76.0%) | 0.469 | 0.490 |
| BMI,
kg/m2 | 23.97±3.46 | 24.07±3.41 | 23.92±3.48 | 0.478 | 0.650 |
| Reasons for
admission | | | | | |
|
Operation | 32 (5.8%) | 11 (6.3%) | 21 (5.6%) | 0.093 | 0.760 |
|
Cardiopulmonary
resuscitation | 16 (2.9%) | 5 (2.8%) | 11 (2.9%) | 0.004 | 0.950 |
|
Trauma | 121 (22.0%) | 30(17.0%) | 91 (24.3%) | 3.645 | 0.056 |
|
Respiratory
disease | 314 (57.0%) | 111 (63.1%) | 203 (54.1%) | 3.901 | 0.048 |
|
Acute
abdominal disease | 31 (5.6%) | 7 (4.0%) | 24 (6.4%) | 0.858 | 0.350 |
|
Others | 37 (6.7%) | 12 (6.8%) | 25 (6.7%) | 0.094 | 0.760 |
| LIPS | 5.40±2.43 | 6.17±2.54 | 5.04±2.38 | 4.967 | <0.001 |
| APACHE II
score | 16.81±7.47 | 19.23±7.79 | 15.67±7.32 | 5.098 | <0.001 |
| Length of ICU stay,
days | 7.07±3.25 | 7.38±3.46 | 6.92±3.15 | 1.497 | 0.140 |
| Use of
vasopressors | 145 (26.3%) | 58 (33.0%) | 87 (23.2%) | 5.878 | 0.015 |
| Methods of
respiratory support | | | | | |
|
Invasive
mechanical ventilation | 241 (43.7%) | 80 (45.5%) | 161 (42.9%) | 0.309 | 0.580 |
|
Non-invasive
ventilation | 122 (22.1%) | 41 (23.3%) | 81 (21.6%) | 0.200 | 0.660 |
|
Non-invasive
and invasive mechanical ventilation | 70 (12.7%) | 33 (18.8%) | 37 (9.9%) | 8.523 | 0.004 |
|
Oxygen
inhalation through the nasal tube | 162 (29.4%) | 54 (30.7%) | 108 (28.8%) | 0.204 | 0.650 |
| TC, mmol/l | 4.20±1.40 | 4.24±1.47 | 4.18±1.36 | 0.457 | 0.660 |
| TG, mmol/l | 1.30±0.64 | 1.29±0.62 | 1.31±0.65 | -0.348 | 0.740 |
| HDL-C, mmol/l | 1.29±0.60 | 1.26±0.57 | 1.30±0.61 | -0.751 | 0.460 |
| LDL-C, mmol/l | 2.63±1.12 | 2.68±1.17 | 2.61±1.09 | 0.669 | 0.500 |
| RAGE levels,
µg/l | 1.08±0.38 | 1.85±0.64 | 0.72±0.26 | 22.566 | <0.001 |
Univariate analysis
Univariate analysis (Table I) was conducted between the ARDS
and non-ARDS groups, which demonstrated that LIPS, RAGE levels,
APACHE II score, non-invasive and invasive mechanical ventilation,
use of vasopressors and admission due to respiratory disease were
significantly different (P<0.05), and the remaining variables
were not (P>0.05). However, the P-value of admission due to
trauma was <0.10.
Multivariate analysis
Multivariate analysis was conducted with inclusion
of LIPS, RAGE levels, APACHE II score, non-invasive and invasive
mechanical ventilation, use of vasopressors and admission due to
respiratory disease and trauma. The results demonstrated that LIPS,
RAGE levels and APACHE II score were independently associated with
ARDS occurrence with adjustment for non-invasive and invasive
mechanical ventilation, use of vasopressors, admission due to
respiratory disease and trauma (Table
II).
 | Table IIMultivariate analysis results between
the ARDS and non-ARDS groups. |
Table II
Multivariate analysis results between
the ARDS and non-ARDS groups.
| Parameter | β | SE | Wald
χ2 | OR | 95% CI | P-value |
|---|
| RAGE levels | 0.947 | 0.252 | 6.068 | 2.359 | 1.351-4.813 | <0.001 |
| APACHE II
score | 0.728 | 0.235 | 3.094 | 1.167 | 1.074-1.485 | 0.002 |
| LIPS | 0.531 | 0.196 | 2.397 | 1.282 | 1.108-1.604 | 0.018 |
| Non-invasive and
invasive mechanical ventilation | 0.422 | 0.168 | 1.635 | 1.529 | 0.703-3.072 | 0.117 |
| Use of
vasopressors | 0.294 | 0.103 | 0.538 | 1.396 | 0.592-2.903 | 0.374 |
| Admission due to
respiratory disease | 0.433 | 0.125 | 1.704 | 1.609 | 0.711-4.106 | 0.103 |
| Admission due to
trauma | 0.391 | 0.112 | 1.517 | 1.498 | 0.679-3.104 | 0.122 |
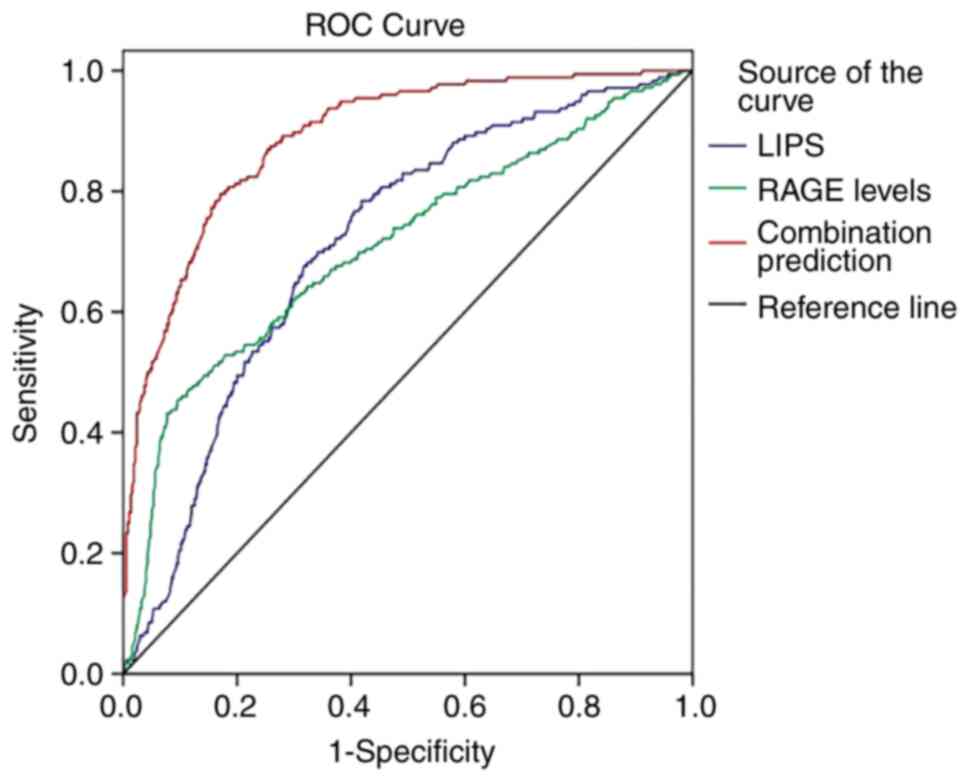
ROC analysis
ROC curves (Fig. 1)
were employed to evaluate the values of LIPS, RAGE levels and their
combination in predicting ARDS occurrence. The results demonstrated
that the AUCs of LIPS, RAGE levels and their combination were 0.714
[standard error (SE), 0.023; 95% confidence interval (CI),
0.670-0.759], 0.709 (SE, 0.025; 95% CI, 0.660-0.758) and 0.889 (SE,
0.014; 95% CI, 0.861-0.917), respectively. The AUC of LIPS combined
with RAGE levels was significantly higher compared with those of
LIPS and RAGE levels alone (0.889 vs. 0.714, Z=6.499, P<0.001;
0.889 vs. 0.709, Z=6.282, P<0.001). The clinical utility indexes
were calculated (Table III),
which demonstrated that the sensitivity, specificity and accuracy
of combination prediction were 87.5, 89.1 and 88.6%,
respectively.
 | Table IIIClinical utility indexes of LIPS,
RAGE levels and their combination in predicting ARDS
occurrence. |
Table III
Clinical utility indexes of LIPS,
RAGE levels and their combination in predicting ARDS
occurrence.
| Parameter | Best cut-off | Sensitivity, % | Specificity, % | Accuracy, % | FPR, % | FNR, % | PPV, % | NPV, % |
|---|
| LIPS combined with
RAGE levels | - | 87.50 | 89.10 | 88.60 | 21.00 | 6.20 | 79.00 | 93.80 |
| LIPS | 5.42 points | 63.60 | 67.70 | 66.40 | 51.90 | 20.10 | 48.10 | 79.90 |
| RAGE levels | 1.13 µg/l | 55.10 | 71.20 | 66.10 | 52.70 | 22.80 | 47.30 | 77.20 |
Discussion
The incidence of ARDS has decreased following
progress in the management of critically ill patients (16). However, mortality among patients
with ARDS still remains high at up to 46.1% for severe ARDS
(6). In order to further decrease
the disease burden of ARDS, it is not adequate to focus on the
treatment following the occurrence of ARDS (17). Firstly, the strategies for
treatment of ARDS are quite limited and there is no effective
strategy other than low-tidal volume ventilation (18). Secondly, preclinical studies have
confirmed the effectiveness of initiating treatment prior to
occurrence of clinical injury (19,20).
Thus, it is important to develop an accurate prediction tool for
the early identification of at-risk patients. The general aim is to
decrease the incidence of ARDS by administering the therapies for
ARDS prevention for at-risk patients.
LIPS can be used to stratify patients at risk for
ARDS by predisposing conditions for ARDS and scoring the risk
factors. It was derived from a multicenter study including
>5,000 patients with risk factors for ARDS and included 22 items
associated with risk modifiers, physiologic data and predisposing
conditions (7). Its predictive
value is relatively high with an AUC of 0.80-0.84. A LIPS exceeding
4 points yields a sensitivity of 69%, specificity of 78% and
negative predictive value (NPV) of 97%, but PPV was only 18%. Kim
et al (21) investigated
the predictive value of LIPS for the occurrence of ARDS in adult
patients admitted to ICUs in the Korean population. Their results
showed that LIPS is significantly correlated with the occurrence of
ARDS, and LIPS >6 points yields a sensitivity of 84.8% and
specificity of 67.2% with an AUC of 0.82 for predicting the
occurrence of ARDS. Moreover, a modified LIPS with adjustment for
severity at ICU admission and age can be applied in predicting ICU
mortality in patients with ARDS (21). Xu et al (12) demonstrated that LIPS is also
associated with ARDS occurrence in critically ill patients with
ARDS risk factors in the Chinese population with an AUC of 0.704
for the prediction of ARDS occurrence. The AUC increased to 0.803
after combining angiopoietin-2 levels with LIPS, and the PPV
increased to 58.19% correspondingly (12).
The biomarkers of ARDS are hypothesized to reflect
its pathophysiological process characterized with high permeability
alveolar oedema, alveolar-capillary membrane injury and migration
of inflammatory cells (22). A
previous study demonstrated that biomarkers associated with
alveolar tissue injury can predict ARDS occurrence, whereas those
associated more with inflammation can predict ARDS mortality
(23). As a biomarker of lung
epithelium injury, RAGE is constitutively expressed on all cells at
low levels, but its expression is significantly upregulated in the
lung epithelium, especially in alveolar type-I cells (24). RAGE is involved in a number of
cellular processes, including vascular smooth muscle proliferation
and migration, microtubule stabilization, apoptosis,
neuroinflammation, excitotoxicity, neurodegeneration, oxidative
stress, corneal healing, and mitochondrial function (25-27).
Its activation can regulate propagation of the inflammatory
response, which is considered to be particularly relevant to ARDS
(28). Calfee et al
(29) reported elevated plasma
levels of RAGE among patients with severe ARDS and its association
with mortality among patients with ARDS ventilated with high tidal
volume. Later studies demonstrated the association of soluble RAGE
(sRAGE) with outcome and severity of patients with ARDS (30,31).
Jabaudon et al (32) showed
that the sRAGE level is higher in patients with ARDS with or
without sepsis compared with that in patients who only have sepsis
but not ARDS. Additionally, the authors indicated that the sRAGE
level is associated with severity of lung injury but not with
outcome. Subsequently, two studies focusing on panels of biomarkers
have indicated the role of RAGE as a valuable candidate for
diagnosing ARDS (33,34). A recent meta-analysis showed that
the plasma RAGE level is positively correlated with increased risk
of ARDS occurrence, but is not correlated with mortality in
patients with ARDS (13).
In the present study, both LIPS and RAGE levels were
independently associated with ARDS occurrence, and could be applied
in predicting ARDS occurrence with medium values (AUC, 0.714 and
0.709). LIPS combined with RAGE levels elevated the predictive
value significantly with an AUC of 0.889, and the clinical utility
indexes also improved significantly, especially PPV up to 79.0%.
Additionally, APACHE II score was also independently associated
with ARDS occurrence in the present study. However, on the one
hand, it has been studied extensively; on the other hand, it needs
a long time to obtain the required parameters and costs more
compared with LIPS. Therefore, the focus was primarily on analyzing
LIPS and RAGE levels as biomarkers for ARDS.
The main limitation of the present study was no
inclusion of all relevant biomarkers, and the prediction tool only
integrated RAGE. In the next step, future studies will develop a
more accurate prediction tool by integrating multiple biomarkers of
different properties.
In conclusion, both LIPS and RAGE levels were
independently associated with ARDS occurrence in critically ill
patients with ARDS risk factors, and could be applied in predicting
ARDS occurrence with medium values. LIPS combined with RAGE levels
elevated the predictive value for ARDS occurrence
significantly.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JY was responsible for acquisition of data and
drafting the article. AW was responsible for acquisition of data
and revising the article. BW was responsible for acquisition of
data and revising the article. JD was responsible for the
conception and design, analysis and interpretation of data, and
critically reviewing the article. JY, AW and BW confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethical Committee of
Chongqing University Jiangjin Hospital (approval no. JJ2020017031)
and carried out strictly following the guidelines of the
Declaration of Helsinki. Written informed consent was obtained from
the study participants prior to study commencement.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
ARDS Definition Task Force. Ranieri VM,
Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E,
Camporota L and Slutsky AS: Acute respiratory distress syndrome:
The Berlin Definition. JAMA. 307:2526–33. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Villar J, Pérez-Méndez L, Blanco J, Añón
JM, Blanch L, Belda J, Santos-Bouza A, Fernández RL and Kacmarek
RM: Spanish initiative for epidemiology, stratification and
therapies for ARDS (SIESTA) network. A universal definition of
ARDS: the PaO2/FiO2 ratio under a standard ventilatory setting-a
prospective, multicenter, validation study. Intensive Care Med.
39:583–92. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
García-Laorden MI, Lorente JA, Flores C,
Slutsky AS and Villar J: Biomarkers for the acute respiratory
distress syndrome: how to make the diagnosis more precise. Ann
Transl Med. 5(283)2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Matthay MA, Zemans RL, Zimmerman GA, Arabi
YM, Beitler JR, Mercat A, Herridge M, Randolph AG and Calfee CS:
Acute respiratory distress syndrome. Nat Rev Dis Primers.
5(18)2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Villar J, Blanco J and Kacmarek RM:
Current incidence and outcome of the acute respiratory distress
syndrome. Curr Opin Crit Care. 22:1–6. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bellani G, Laffey JG, Pham T, Fan E,
Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley
DF, et al: Epidemiology, patterns of care, and mortality for
patients with acute respiratory distress syndrome in intensive care
units in 50 countries. JAMA. 315:788–800. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Trillo-Alvarez C, Cartin-Ceba R, Kor DJ,
Kojicic M, Kashyap R, Thakur S, Thakur L, Herasevich V, Malinchoc M
and Gajic O: Acute lung injury prediction score: Derivation and
validation in a population-based sample. Eur Respir J. 37:604–609.
2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Soto GJ, Kor DJ, Park PK, Hou PC, Kaufman
DA, Kim M, Yadav H, Teman N, Hsu MC, Shvilkina T, et al: Lung
injury prediction score in hospitalized patients at risk of acute
respiratory distress syndrome. Crit Care Med. 44:2182–2191.
2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zheng F, Pan Y, Yang Y, Zeng C, Fang X,
Shu Q and Chen Q: Novel biomarkers for acute respiratory distress
syndrome: genetics, epigenetics and transcriptomics. Biomark Med.
16:217–231. 2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yadav H, Bartley A, Keating S, Meade LA,
Norris PJ, Carter RE, Gajic O and Kor DJ: Evolution of validated
biomarkers and intraoperative parameters in the development of
postoperative ARDS. Respir Care. 63:1331–1340. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhu Z, Liang L, Zhang R, Wei Y, Su L,
Tejera P, Guo Y, Wang Z, Lu Q, Baccarelli AA, et al: Whole blood
microRNA markers are associated with acute respiratory distress
syndrome. Intensive Care Med Exp. 5(38)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Xu Z, Wu GM, Li Q, Ji FY, Shi Z, Guo H,
Yin JB, Zhou J, Gong L, Mei CX and Wang GS: Predictive value of
combined LIPS and ANG-2 level in critically ill patients with ARDS
risk factors. Mediators Inflamm. 2018(1739615)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
van der Zee P, Rietdijk W, Somhorst P,
Endeman H and Gommers D: A systematic review of biomarkers
multivariately associated with acute respiratory distress syndrome
development and mortality. Crit Care. 24(243)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Gajic O, Dabbagh O, Park PK, Adesanya A,
Chang SY, Hou P, Anderson H III, Hoth JJ, Mikkelsen ME, Gentile NT,
et al: Early identification of patients at risk of acute lung
injury: Evaluation of lung injury prediction score in a multicenter
cohort study. Am J Respir Crit Care Med. 183:462–70.
2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bone RC, Balk RA, Cerra FB, Dellinger RP,
Fein AM, Knaus WA, Schein RM and Sibbald WJ: Definitions for sepsis
and organ failure and guidelines for the use of innovative
therapies in sepsis. The ACCP/SCCM consensus conference committee.
American college of chest physicians/society of critical care
medicine. Chest. 101:1644–1655. 1992.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Li G, Malinchoc M, Cartin-Ceba R, Venkata
CV, Kor DJ, Peters SG, Hubmayr RD and Gajic O: Eight-year trend of
acute respiratory distress syndrome: a population-based study in
Olmsted County, Minnesota. Am J Respir Crit Care Med. 183:59–66.
2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yadav H, Thompson BT and Gajic O: Fifty
years of research in ARDS. Is acute respiratory distress syndrome a
preventable disease? Am J Respir Crit Care Med. 195:725–736.
2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kaku S, Nguyen CD, Htet NN, Tutera D, Barr
J, Paintal HS and Kuschner WG: Acute respiratory distress syndrome:
Etiology, pathogenesis, and summary on management. J Intensive Care
Med. 35:723–737. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zarbock A, Singbartl K and Ley K: Complete
reversal of acid-induced acute lung injury by blocking of
platelet-neutrophil aggregation. J Clin Invest. 116:3211–3219.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Jacobson JR, Barnard JW, Grigoryev DN, Ma
SF, Tuder RM and Garcia JG: Simvastatin attenuates vascular leak
and inflammation in murine inflammatory lung injury. Am J Physiol
Lung Cell Mol Physiol. 288:L1026–L1032. 2005.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kim BK, Kim S, Kim CY, Kim YJ, Lee SH, Cha
JH and Kim JH: Predictive role of lung injury prediction score in
the development of acute respiratory distress syndrome in Korea.
Yonsei Med J. 62:417–423. 2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Thompson BT, Chambers RC and Liu KD: Acute
respiratory distress syndrome. N Engl J Med. 377:562–572.
2017.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Terpstra ML, Aman J, van Nieuw Amerongen
GP and Groeneveld AB: Plasma biomarkers for acute respiratory
distress syndrome: A systematic review and meta-analysis*. Crit
Care Med. 42:691–700. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Xie J, Méndez JD, Méndez-Valenzuela V and
Aguilar-Hernández MM: Cellular signalling of the receptor for
advanced glycation end products (RAGE). Cell Signal. 25:2185–2197.
2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Tóbon-Velasco JC, Cuevas E and
Torres-Ramos MA: Receptor for AGEs (RAGE) as mediator of NF-kB
pathway activation in neuroinflammation and oxidative stress. CNS
Neurol Disord Drug Targets. 13:1615–1626. 2014.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nass N, Trau S, Paulsen F, Kaiser D,
Kalinski T and Sel S: The receptor for advanced glycation end
products RAGE is involved in corneal healing. Ann Anat. 211:13–20.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kwon OS, Decker ST, Zhao J, Hoidal JR,
Heuckstadt T, Sanders KA, Richardson RS and Layec G: The receptor
for advanced glycation end products (RAGE) is involved in
mitochondrial function and cigarette smoke-induced oxidative
stress. Free Radic Biol Med. 195:261–269. 2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Walter JM, Wilson J and Ware LB:
Biomarkers in acute respiratory distress syndrome: From
pathobiology to improving patient care. Expert Rev Respir Med.
8:573–86. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Calfee CS, Ware LB, Eisner MD, Parsons PE,
Thompson BT, Wickersham N and Matthay MA: NHLBI ARDS Network.
Plasma receptor for advanced glycation end products and clinical
outcomes in acute lung injury. Thorax. 63:1083–1089.
2008.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mauri T, Masson S, Pradella A, Bellani G,
Coppadoro A, Bombino M, Valentino S, Patroniti N, Mantovani A,
Pesenti A and Latini R: Elevated plasma and alveolar levels of
soluble receptor for advanced glycation end-products are associated
with severity of lung dysfunction in ARDS patients. Tohoku J Exp
Med. 222:105–112. 2010.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nakamura T, Sato E, Fujiwara N, Kawagoe Y,
Maeda S and Yamagishi S: Increased levels of soluble receptor for
advanced glycation end products (sRAGE) and high mobility group box
1 (HMGB1) are associated with death in patients with acute
respiratory distress syndrome. Clin Biochem. 44:601–604.
2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jabaudon M, Futier E, Roszyk L, Chalus E,
Guerin R, Petit A, Mrozek S, Perbet S, Cayot-Constantin S, Chartier
C, et al: Soluble form of the receptor for advanced glycation end
products is a marker of acute lung injury but not of severe sepsis
in critically ill patients. Crit Care Med. 39:480–488.
2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fremont RD, Koyama T, Calfee CS, Wu W,
Dossett LA, Bossert FR, Mitchell D, Wickersham N, Bernard GR,
Matthay MA, et al: Acute lung injury in patients with traumatic
injuries: utility of a panel of biomarkers for diagnosis and
pathogenesis. J Trauma. 68:1121–1127. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ware LB, Koyama T, Zhao Z, Janz DR,
Wickersham N, Bernard GR, May AK, Calfee CS and Matthay MA:
Biomarkers of lung epithelial injury and inflammation distinguish
severe sepsis patients with acute respiratory distress syndrome.
Crit Care. 17(R253)2013.PubMed/NCBI View
Article : Google Scholar
|