Introduction
Ankylosing spondylitis (AS) is a chronic
inflammatory disease that clinically manifests as chronic back pain
and stiffness. AS inflammation tends to accumulate in the
sacroiliac joints at the initial stage and then primarily affects
the spine (1). AS has ~0.25%
prevalence in China and its incidence in males is higher than that
in females (~3:1) (2,3). Long-term spinal involvement may
affect the biomechanical properties of the spine, accompanied by
chronic inflammatory changes. As a key structure connecting the
bone and joint capsule, the synovium often exhibits inflammatory
erosion and hyperplasia in inflammatory disease (4). Under the stimulation of inflammatory
cytokines [such as tumor necrosis factor (TNF-α)] (5), AS fibroblast-like synoviocytes
(AS-FLSs) produce multiple inflammatory signaling molecules,
including IL-6 and IL-4 (6,7),
triggers cytokine cascade effects and recruits inflammatory cells,
thereby aggravating inflammatory responses; these further induce
ongoing joint inflammation and bone destruction (8). Therefore, it is key to control
inflammation as early as possible, improve joint function and
reduce deformities (9). To
pathogenesis of AS is not fully understood. Due to lack of
effective treatment methods, AS pain affects the health and daily
life of patients. Hence, it is crucial to develop novel and
effective drugs for relieving the symptoms of AS.
Key epigenetic regulators, including long non-coding
RNAs (lncRNAs), have multiple biological functions (10,11).
Certain aberrant lncRNA expression profiles contribute to AS
pathogenesis (12,13). lncRNAs may directly regulate
expression of protein-coding genes. For example, Han et al
(14) found that upregulated
lncRNA FOXA2 is associated with AS recurrence and poor outcome
(14). Li et al (15) reported that lncRNA Maternally
expressed gene 3 is downregulated in AS and associated with disease
activity and hospital stay and disease duration. Our previous study
revealed that NONHSAT227927.1 is a key lncRNA involved in AS
inflammation via high-throughput sequencing and bioinformatics
analysis (16).
The Janus kinase/signal transducer and activator of
transcription (JAK/STAT) axis is involved in regulation of cancer,
inflammation and immunity. Numerous cytokines affect JAK/STAT
signaling. When cells are subjected to pro-proliferative stimuli,
JAK2 activates STAT3 to regulate cell survival and proliferation
via its downstream targets (17,18).
The role of JAK/STAT kinase signaling has also been studied in
rheumatoid arthritis. For example, preliminary observations have
suggested that the JAK/STAT kinase signaling cascade regulates the
activation and proliferation of IL17+ effector memory T
cells, showing a potential role in the pathogenesis of AS (19,20).
lncRNA NONHSAT227927.1 is overexpressed in AS and regulates
inflammatory factors by activating the JAK2/STAT3 signaling pathway
and promotes development of AS (21).
Triptolide (TPL), a main compound extracted from the
traditional Chinese medicine Tripterygium wilfordii, has
strong biological activity for treating numerous types of tumor and
autoimmune disease, especially rheumatoid arthritis, systemic lupus
erythematosus and AS (22-24).
Ji et al (25) found that
TPL could inhibit osteoclastogenesis of the spine to alleviate
arthritis in DBA/1 mice. Wang et al (26) reported the anti-ossification
effects of TPL. To the best of our knowledge, however, little is
known about whether TPL affects synovial cells to alleviate AS
inflammation.
Our previous research has confirmed the role of the
NONHSAT227927.1/JAK2/STAT3 combination in regulating inflammation
in AS (21). The diagnostic
potential of NONHSAT227927.1 on AS has also been confirmed through
receiver operating characteristic curve analysis.
The present study aimed to investigate whether TPL
regulates inflammation by targeting the NONHSAT227927.1/JAK2/STAT3
axis and whether it has an anti-inflammatory effect on AS.
Materials and methods
Characteristics of subjects
From March to May 2021, a total of 50 AS patients
were recruited from the First Affiliated Hospital of Anhui
University of Traditional Chinese Medicine in Hefei, Anhui
Province, China. Among these patients, there were 32 males and 18
females, with ages ranging from 19 to 65 years. Additionally, 30
healthy controls were also recruited, who were carefully matched
with the AS patients in terms of both gender and age. The inclusion
criteria were as follows: i) Patients who met the diagnostic
criteria of the 1984 American Society of Rheumatology (27), ii) aged 18-80 years and iii)
patients with complete clinical data. The exclusion criteria were
as follows: i) Severe mental illness or severe liver and kidney
dysfunction, ii) pregnancy and iii) history of immunosuppressive
drugs. All participants provided written informed consent. The
present study was approved by the Medical Ethics Committee of Anhui
University of Traditional Chinese Medicine (approval no.
2015-AH20).
Indicators collection
The general data of 50 cases in the AS group and 30
cases in the NC group were retrieved from the case system of the
First Affiliated Hospital of Anhui University of Traditional
Chinese Medicine: age, gender, disease duration, height, weight,
perception scale scores of patients indicators: Self Rating Anxiety
Scale (SAS), Self-Rating Depression Scale (SDS), The Visual
Analogue Scale (VAS), Bath Ankylosing Spondylitis Disease Activity
Index (DASDAI), Bath Ankylosing Spondylitis Functional Index
(BASFI) and Bath Ankylosing Spondylitis Metrology Index (BASMI),
clinical indicators: Immunoglobulin A (IgA), Immunoglobulin M
(IgM), Immunoglobulin G (IgG), Complement C3 (C3), Complement C4
(C4), erythrocyte sedimentation Rate (ESR), C-reactive Protein
(CRP).
FLS culture
Human primary FLSs isolated from sacroiliac joints
(cat. no. RAB-iCell-s004) and AS-FLSs (cat. no. JDBG200752) were
purchased from Subikang iCell Bioscience, Inc. These cells were
cultured in RPMI-1640 medium [Saibaikang (Shanghai) Biotechnology
Co., Ltd.] containing 1% penicillin-streptomycin and 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc.) in an
incubator (37˚C, 5% CO2) with 100% humidity (11). The medium was refreshed every 2-3
days. At 80-90% confluence, cells were washed twice with
phosphate-buffered saline, detached with 0.25% trypsin and observed
under a light microscope (magnification, x200). When the cells
adhered to the wall and became loose, trypsin was discarded.
Complete medium was added and the cell layer was blown.
Culture of AS-Peripheral blood
mononuclear cells (PBMCs)
Venous blood (4 ml) from patients with AS was
collected using an anticoagulant tube and mixed well with 4 ml PBS.
Then, 4 ml Ficoll solution (cat. no. 17-1440-02; GE Healthcare) was
added into a 15-ml centrifuge tube. The diluted blood was added
slowly to the upper layer of the Ficoll solution, avoiding mixing
the two solutions. Following tube centrifugation (1,150 x g, 37˚C,
20 min) in a horizontal centrifuge, AS-PBMCs were located in the
second white layer from the top. Next, cells were moved to a new
centrifuge tube with PBS (10-15 ml) and then centrifuged (640 x g,
37˚C, 10 min). After removing supernatant, PBS (5-10 ml) before
repeating centrifugation. The cells were resuspended by adding 1 ml
PBS, transferred to a 1.5-ml EP tube and set aside.
AS-PBMC induction and AS-FLS
transfection
AS-PBMCs and AS-FLSs were seeded and cultured in a
Transwell chamber at a ratio of 3:1. PBMCs were added to the apical
chamber and FLSs were placed in the basolateral chamber. Cells were
incubated for 24 h in 37˚C. After growing to 70-90% confluence,
cells in each Transwell well were removed for subsequent
experiments. AS-FLSs were transfected with small interfering RNA
(siRNA)-negative control (NC; cat. no. A06001) and
siRNA-NONHSAT227927.1 (cat. no. A01001; both Shanghai GenePharma
Co, Ltd.) using Lipofectamine® 2000 (cat. no. 11668-019;
Thermo Fisher Scientific, Inc.; 37˚C, 24 h). The transfection
concentration of siRNA was 50 pmol/ml. A total of >5 µg nucleic
acid was used and cells were collected following incubation (37˚C,
48 h). Cell transfection efficiency was detected using reverse
transcription quantitative polymerase chain reaction (RT-qPCR). The
oligonucleotide sequences were as follows: siRNA-NC forward,
5'-UUCUCCGAACGUGUCACGUTT-3' and reverse,
5'-ACGUGACACGUUCGGAGAATT-3' and siRNA-NONHSAT227927.1 forward,
5'-CGACUGACUCGAUCUUUGAAG-3' and reverse,
5'-UCAAAGAUCGAGUCAGUCGGG-3'.
RT-qPCR
The total RNA was extracted from AS-FLSs with
TRIzol® (cat. no. 15596026; Thermo Fisher Scientific,
Inc.). cDNA was synthesized cDNA using the PrimeScript™
RT Reagent kit (cat. no. RR047A, Takara Biotechnology Co., Ltd.)
according to the manufacturer's instructions. Novostart SYBR qPCR
SuperMix Plus (cat. no. E096-01B; Novoprotein Scientific, Inc.) was
used for qPCR following the manufacturer's instructions.
Thermocycling conditions were as follows: Initial denaturation at
95˚C for 1 min, followed by 40 cycles of denaturation at 95˚C for
20 sec and annealing at 60˚C for 1 min. Relative quantitative
analysis was performed using the 2-ΔΔCq method (24) with β-actin as an internal
reference. The sequences of primers were as follows:
NONHSAT227927.1 forward, 5'-TGGGAACTCCTGAGCATACC-3' and reverse,
5'-ATGCTCCAGCAAGTCAGGAT-3' and β-actin forward,
5'-CCCTGGAGAAGAGCTACGAG-3' and reverse,
5'-GGAAGGAAGGCTGGAAGAGT-3'.
ELISA
The levels of IL-4 (cat. no. JYM0142Hu), IL-10 (cat.
no. JYM0155Hu) and TNF-α (cat. no. JYM0110Hu) in the serum of
patients or the supernatant of AS-FLSs were evaluated using ELISA
kits according to the manufacturer's instructions (Wuhan Genomics
Technology Co., Ltd.). Each sample was examined three times
independently.
Cell Counting Kit 8 (CCK-8) assay
The cell viability was measured using a CCK-8 assay
kit (BIOSS) following the manufacturer's protocols. A total of
3x104 AS-FLSs was seeded into 96-well plates and
cultured to 70-90% confluence. The cells were cultured for 0, 12,
24 and 48 h at 37˚C. Then, 10 µl CCK-8 solution was added to each
well for 1-4 h at 37˚C. The cell viability was assessed by
measuring the optical density at 450 nm.
Molecular docking of TPL with
JAK2/STAT3 proteins
Protein Data Bank (PDB) format files of JAK2 and
STAT3 proteins were retrieved in the PDB protein structure database
(rcsb.org/); the mol2 format files of the TPL structure
were downloaded from the Traditional Chinese Medicine Systems
Pharmacology Database and Analysis Platform (TCMSP) database
(tcmsp-e.com). Before molecular docking, the software
pymol 2.3.0 (DeLano Scientific LLC) was used to dehydrate the
target protein receptor molecule and remove the ligand small
molecule. The target protein was hydrogenated by Auto Dock 4.2.6
software (Molecular Graphics Lab at The Scripps Research Institute.
La Jolla, USA). Finally, the receptor protein was molecularly
docked with the ligand small molecule by Auto Dock Vina 1.1.2
software (Molecular Graphics Lab at The Scripps Research
Institute). and visualized by PYMOL 2.3.0 (DeLano Scientific LLC).
The binding energy of receptor protein and ligand small molecule
energy <-5 kcal/mol indicated strong binding force.
Western blot analysis
A total of 600 µl RIPA lysis buffer (cat. no.
P0013B; Beyotime Institute of Biotechnology) was used to extract
total protein in the cells. SDS-PAGE preparation kit (cat. no.
S8010; Beijing Solarbio Science & Technology Co., Ltd.) was
used to prepare the gel (5% stacking gel, 10% separating gel
concentration) and 30 µg protein/lane was added for electrophoresis
on PVDF membranes. The membranes were blocked with 5% skimmed milk
(0.1% Tween 20) for 2 h at room temperature and incubated
(overnight, 4˚C) with primary antibodies as follows:
Anti-phosphorylated p-STAT3 (1:500; cat. no. ab76315; Abcam),
p-JAK2 (1:1,000; cat. no. ab32101; Abcam), JAK2 (1:500; cat. no.
ab39636; Abcam) and STAT3 (1:1,000; cat. no. ab68153; Abcam).
Following washing, horseradish peroxidase-labeled secondary goat
anti-mouse (cat. no. ZB-2305) and anti-rabbit (cat. no. ZB-2301;
both ZSGB-bio) were added at a dilution of 1:1x104 and
membranes were re-probed at room temperature for 2 h. Following
washing, the proteins were visualized using ECL kit (cat. no.
34094; Thermo Fisher Scientific, Inc.). Protein concentration was
determined using the BCA protein concentration assay kit (catalog
number P0012S; Beyotime). The expression of the target proteins was
calculated relative to GAPDH (1:2,000, cat. no. TA-08; Zsbio).
Image J 180 (National Institutes of Health) was used for band
density analysis.
Statistical analysis
Statistical analysis was performed using GraphPad
Prism 8 (GraphPad Software, Inc.; Dotmatics). Data are presented as
mean ± SD or median and interquartile range (IQR) and samples were
compared using paired t test or Wilcoxon paired test based on
normality. One-way ANOVA analysis of variance was used to analyze
multiple groups. χ2 test was used for analysis of
categorical variables. Tukey's post hoc test or Dunn's post hoc
test was used for multiple comparisons. Spearman correlation test
was performed to evaluate the correlation between NONHSAT227927.1
and clinical data. Logistic regression analysis was used to analyze
potential risk factors associated with NONHSAT227927.1. P<0.05
was considered to indicate a statistically significant
difference.
Results
No difference in demographic
characteristics between AS patients and healthy controls
A total of 30 healthy controls [median age, 34 years
(IQR, 24-46)] and 50 patients with AS [median age, 35 years (IQR,
30-45)] were included. There was no significant difference in basic
information (age, sex and BMI) between the two groups (Table I). ESR, CRP, IGA, IgG, IgM and SDS
of the AS group were significantly higher than those of healthy
controls, suggesting that patients with AS exhibited a stronger
inflammatory response and higher risk of depression.
 | Table IClinical immune-inflammatory markers
and perception score of patients with AS and HCs. |
Table I
Clinical immune-inflammatory markers
and perception score of patients with AS and HCs.
| Parameter | AS (n=50) | HC (n=30) |
t/F/χ2-value | P-value |
|---|
| Median age (IQR),
years | 35 (30-45) | 34 (24-46) | 0.84 | 0.40 |
| Sex (%) | | | | 1.00 |
|
Male | 32(64) | 19 (63.33) | | |
|
Female | 18(36) | 11 (36.67) | | |
| Median BMI
(IQR) | 21.19
(18.39-23.80) | 20.34
(18.29-22.25) | 0.58 | 0.36 |
| Median disease
duration (IQR), years | 12.00
(9.75-15.25) | NA | | NA |
| Median ESR (IQR),
mm/h | 39.5
(21.50-58.00) | 5.00
(1.00-7.00) | 0.03 |
<0.01b |
| CRP, mg/l | 34.20±28.30 | 6.35±4.57 | 51.50 |
<0.01b |
| IgA, mmol/l | 4.58±5.67 | 1.01±0.81 | 17.88 |
<0.01b |
| IgG, mmol/l | 11.06±2.98 | 8.41±1.07 | 12.08 |
<0.01b |
| IgM, mmol/l | 1.49±0.81 | 1.00±0.45 | 6.27 | 0.01 |
| C3, g/l | 1.27
(1.13,1.40) | 1.21
(0.98,1.42) | 1.90 | 0.25 |
| C4, g/l | 0.35±0.17 | 0.38±0.20 | 0.64 | 0.43 |
| Median BASDAI score
(IQR) | 4.4
(4.00-5.00) | NA | | NA |
| BASFI score | 4.70±0.53 | NA | | NA |
| BASMI score | 6.82±1.37 | NA | | NA |
| SAS score | 49.85±11.24 | 5.19±1.57 | 0.32 | 0.57 |
| Median SDS score
(IQR) | 51.19
(41.71-73.32) | 7
(17.19-12.11) | 0.09 |
<0.01a |
| Median VAS score
(IQR) | 6.80
(5.82-7.28) | NA | | |
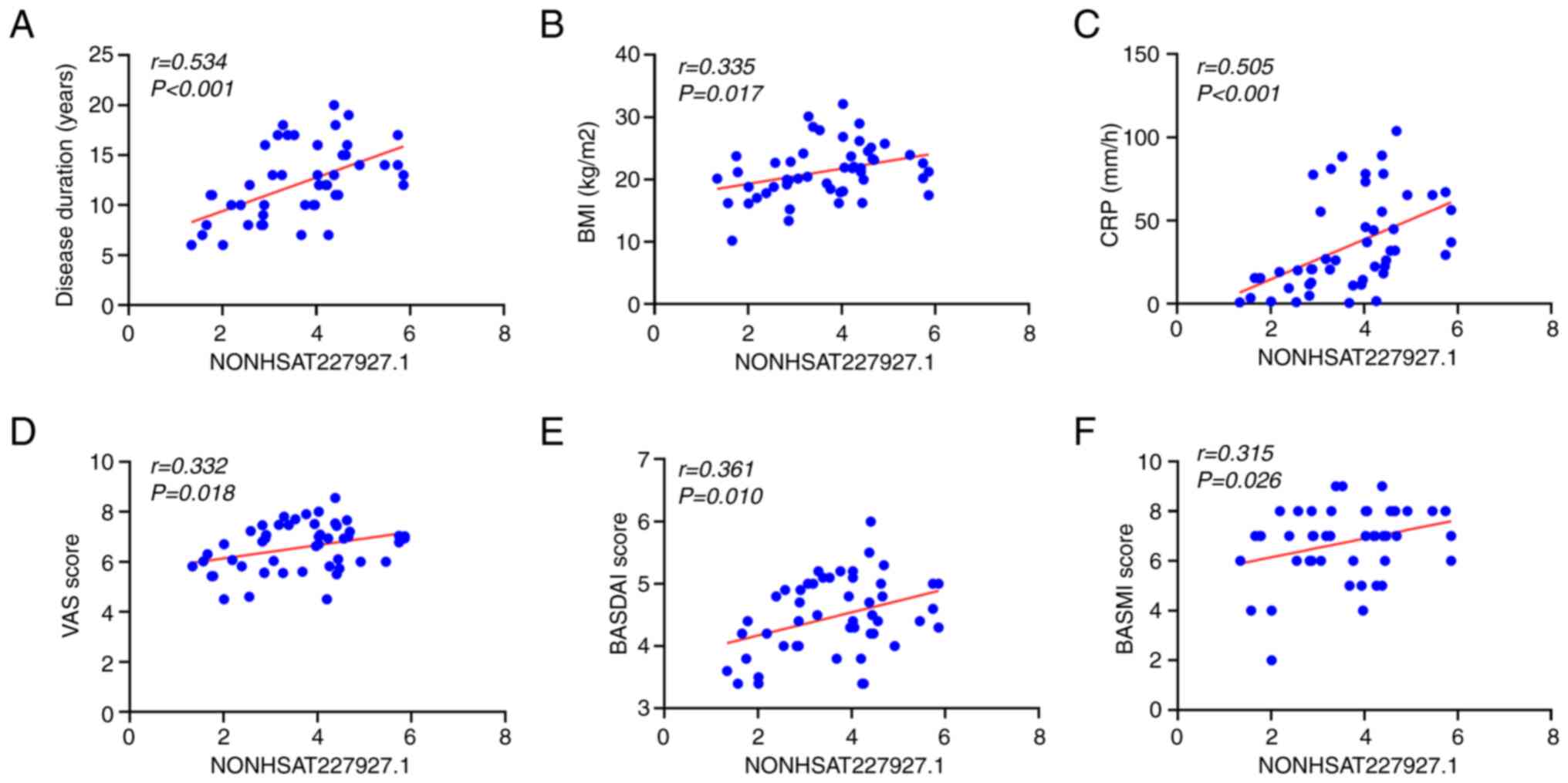
Correlation of NONHSAT227927.1 with
demographic characteristics and clinical indicators in patients
with AS
Our previous study demonstrated that NONHSAT227927.1
had significant diagnostic value in AS (21). To determine whether NONHSAT227927.1
serves as a biomarker in the process of AS, Spearman correlation
analysis was performed to evaluate the correlation of
NONHSAT227927.1 with clinical indicators and basic conditions of
patients with AS. NONHSAT227927.1 was positively correlated with
disease duration (Fig. 1A), BMI
(Fig. 1B), CRP (Fig. 1C), VAS (Fig. 1D), BASDAI (Fig. 1D) and BASMI (Fig. 1E), which suggested that
NONHSAT227927.1 was associated with the progression of AS.
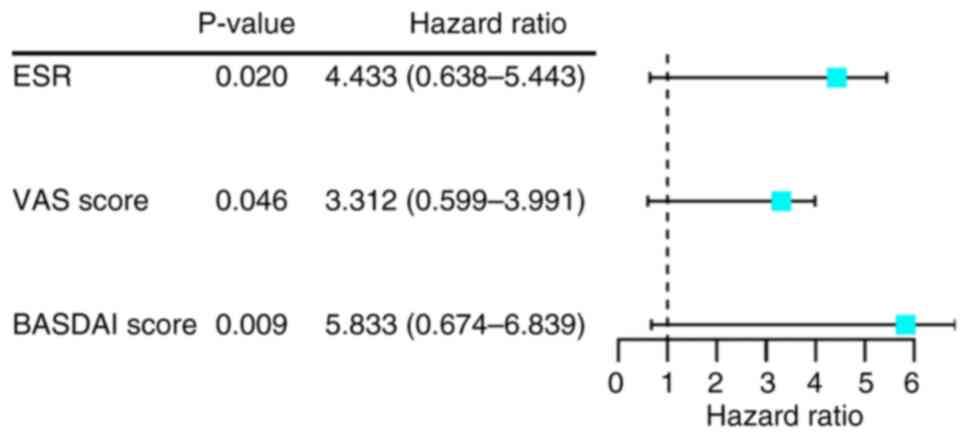
Logistic-regression analysis of
NONHSAT227927.1 with clinical characteristics of patients with
AS
To identify risk factors associated with
NONHSAT227927.1 in patients with AS, logistic regression analysis
was performed. Significant differences in NONHSAT227927.1
expression were associated with ESR (P=0.020), VAS (P=0.046) and
BASDAI (P=0.009; Fig. 2). These
findings indicated that ESR, VAS, and BASDAI were risk factors
associated with NONHSAT227927.1.
TPL inhibits viability of
AS-PBMC-stimulated AS-FLSs
The chemical structure of TPL is shown in Fig. 3A. To determine the optimal
treatment concentration and duration, AS-FLSs were stimulated with
AS-PBMCs to induce inflammatory response before measuring cell
viability following TPL intervention (0, 25, 50 and 100 µg/ml). The
inhibitory ability of AS-FLSs was strongest when TPL concentration
was 100 µg/ml and the intervention time was 48 h (Fig. 3B). Therefore, this TPL
concentration and treatment duration were used for subsequent
experiments.
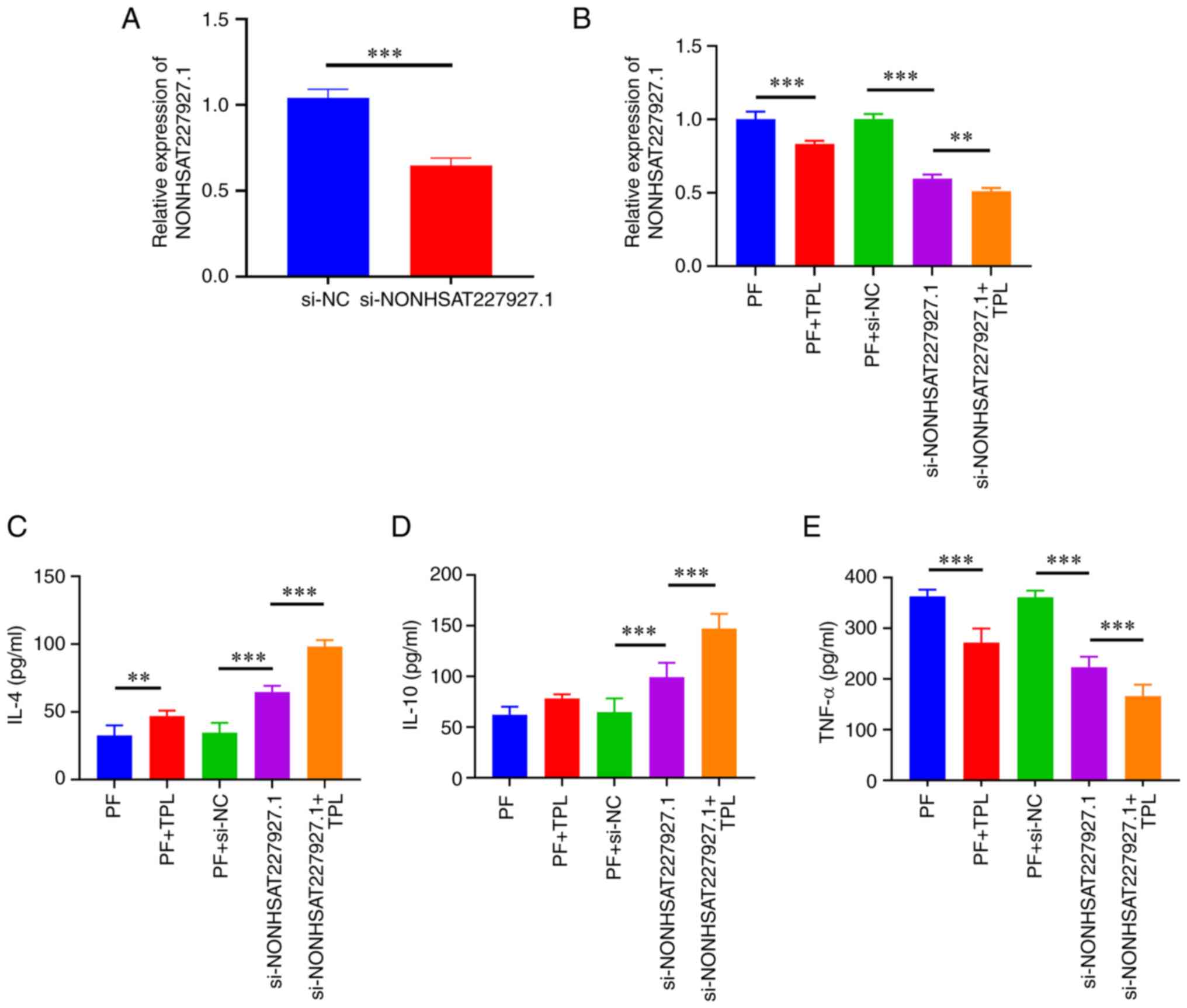
Effect of TPL on inflammation in
AS-PBMC-stimulated AS-FLSs
To verify that TPL exerted therapeutic effects on
AS-FLSs via NONHSAT227927.1, NONHSAT227927.1 was knocked down
(Fig. 4A) and anti-inflammatory
effect of TPL was assessed. RT-qPCR results revealed that TPL
intervention led to a significant decrease in NONHSAT227927.1
expression in FLSs. Compared with the si-NONHSAT227927.1 group, TPL
decreased levels of NONHSAT227927.1 (Fig. 4B). ELISA results indicated that the
addition of TPL notably increased the contents of IL-4 but
decreased the content of TNF-α compared with the PF +si-NC group.
In response to si-NONHSAT227927.1-mediated knockdown of
NONHSAT227927.1, the levels of IL-4 and IL-10 were significantly
increased and levels of TNF-α were decreased; these changes were
significantly greater following addition of TPL (Fig. 4C and D). Taken together, these data indicated
that TPL may play an anti-inflammatory role in AS by regulating
NONHSAT227927.1.
Molecular docking of TPL and
JAK2/STAT3
Our previous study confirmed that activating the
JAK2/STAT3 pathway promotes inflammation in AS (21). Therefore, molecular docking of TPL
with JAK2 and STAT3 protein was performed to investigate the
potential mechanism by which TPL may inhibit the inflammatory
response in AS via the JAK2/STAT3 pathway. The molecular docking
(Fig. 5) showed the binding energy
of JAK2 was -6.5 kcal/mol and the binding energy of STAT3 was -7.9
kcal/mol. The binding effect of the compound and target JAK2 and
STAT3 protein was stronger, indicated by lower binding energy.
PyMOL 2.1 software was used to visualize the compound formed
following docking with the protein and the binding mode between the
compound and protein was obtained. According to the binding mode,
active amino acid residues bound by TPL and JAK2 target proteins
were ARG-335 and ASP-334 and the active amino acid residues bound
by TPL and STAT3 target proteins were ASP-976 and ARG-938. This
compound formed a strong reactive group with the aforementioned
amino acid residues. These interactions improved the stability of
the compound in JAK2 and STAT3 protein pockets, so the compound was
a potentially active small molecule.
TPL regulates the JAK2/STAT3 pathway
via NONHSAT227927.1
It was determined whether TPL participated in
NONHSAT227927.1-regulated cellular inflammation via JAK2/STAT3
signaling in AS-PBMC-stimulated FLSs. Levels of p-JAK and p-STAT3
protein were significantly increased in FLSs, while these increased
levels were inhibited by TPL treatment, implying that TPL inhibited
pro-inflammatory pathways (Fig.
6). Moreover, knockdown of NONHSAT227927.1 reduced the
phosphorylation levels of JAK2 and STAT protein in FLSs. These
results suggested that TPL regulated the JAK2/STAT3 pathway by
regulating NONHSAT227927.1.
Discussion
Considering the inflammatory nature of AS, most
available treatments for AS focus on decreasing the inflammatory
burden (28). Due to the impact of
disease activity on structural damage and function, controlling
inflammation is key in the treatment of AS (29,30).
Despite the improvements in AS treatment, the pathogenesis of AS
has not yet been elucidated. In addition, the treatment effect is
often poor due to the lack of effective therapeutic targets, the
diagnosis and treatment of AS remain a challenge. lncRNAs serve key
roles in various types of autoimmune disease, including AS
(31). lncRNAs can predict AS
recurrence and poor outcomes, representing potential predictive
biomarkers for AS (14,32,33).
Currently, the therapeutic options for AS are limited compared with
those for other rheumatoid diseases (such as rheumatoid or
psoriatic arthritis) and traditional synthetic disease-modifying
antirheumatic drugs or long-term corticosteroids are considered
ineffective in treatment of axial spondyloarthritis (33,34).
Evidence has indicated TPL as an effective oral agent for the
treatment of active AS (35,36).
Our preliminary study demonstrated that lncRNA
NONHSAT227927.1 is highly expressed in patients with AS and might
be a potential AS-specific diagnostic marker (AUC was 0.8463)
(21). The laboratory indicators
(ESR, CRP, IgA, IgG, and IgM) and SDS score of the AS group were
significantly higher than those of healthy controls. Pain is the
main characteristic of inflammation. When there is an inflammatory
reaction, physical pain can lead to anxiety and depression
(37). In addition,
NONHSAT227927.1 was positively correlated with disease duration,
BMI, CRP, VAS, BASDAI and BASMI; these indicated that
NONHSAT227927.1 expression was associated with the disease severity
of AS. Logistic regression found that ESR, VAS and BASDAI were risk
factors for high NONHSAT227927.1 expression.
Studies have shown the key roles of lncRNAs in the
differentiation and function of immune cells (38-40).
When the body is subjected to internal or external stress
responses, non-specific immune cells specifically express
hexamethylene bis-acetamide-inducible protein (HEXIM1) and Nuclear
Enriched Abundant Transcript1 (NEAT1) to regulate immune cell
differentiation and function (41). Li et al (42) found that lncRNA AK001085 is poorly
expressed in the serum of patients with AS and is negatively
correlated with immune-inflammatory markers CRP and ESR. Ding et
al (43) found that 36 lncRNAs
are involved in the Spondyloarthritis/AS competitive regulation of
the immune-validation reactor pathway. Our preliminary
high-throughput sequencing of AS-PBMCs and lncRNAs enriched in
immune-inflammatory responses (fold-change ≥2; P≤0.05) identified
NONHSAT227927.1 for RT-qPCR verification.
T. wilfordii Hook F (TwHF), a traditional
Chinese herb, has been used to treat rheumatoid arthritis and other
autoimmune and inflammatory diseases for a long time (44,45).
TPL (C20H24O6) is a diterpenoid
triepoxide purified from TwHF that possesses potent
immunosuppressive and anti-inflammatory properties (46). According to pharmacological
studies, TPL exerts anti-inflammatory, detoxification,
heat-clearing and dampness-dispelling effects, as well as
inhibitory effects on humoral and cellular immunity (47,48).
A number of randomized controlled trials have shown that TPL is
beneficial in treatment of AS (44,49).
However, the therapeutic potential of TPL is limited due to its
strong toxicity (50,51). Here, TPL at a concentration of 100
µg/ml significantly inhibited cell viability at 48 h. The isolated
AS-FLSs were exposed to TPL (100 µg/ml) to examine the inhibitory
effect of TPL. The present results showed that TPL suppressed the
cytokine metabolism disorder in AS-PBMC-stimulated AS-FLSs. In
addition, the JAK2/STAT3 pathway was significantly inhibited after
NONHSAT227927.1 knockdown.
The JAK2/STAT3 signaling pathway is primarily
responsible for regulating inflammatory responses in AS (52). Together with several STAT proteins,
JAK mediates signaling of extracellular cytokines and affects
various cellular functions. STAT3 is a component of the acute phase
response factor complex activated by IL-6(53). The present study suggested that TPL
inhibited AS progression by mediating JAK2/STAT3 pathway
inactivation and TPL interacted with residues in the JAK2/STAT3
inhibitory interaction pocket.
The efficacy of TPL in treating AS has been
confirmed by previous clinical studies (25,26,36).
To the best of our knowledge, however, research on the specific
mechanism of TPL in treating AS is relatively limited (54). The present study investigated
inflammatory pathway to explore the potential of TPL in treatment
of AS-FLSs, whereas previous studies have used chondrocytes
(25,26). The present study used AS-PBMC and
AS-FLS from patients to co-culture a cell model to verify the
therapeutic effect of TPL. PBMC were taken from the patient's whole
blood, and FLS was taken from the patient's joint. This cell model
has a stronger inflammatory response and can better simulate the
human body environment. The in vitro co-culture cell model
can also provide a usable cell model for subsequent experiments.
TPL prevent the progression of AS by downregulating the expression
of NONHSAT227927.1 and inhibiting the activation of the JAK3/STAT3
pathway, thereby improving the inflammatory response of AS-FLSs
(Fig. 7).
Finally, the present study revealed the binding
modes and sites of TPL and pathway proteins based on molecular
docking. These results provide a scientific basis for T.
wilfordii as a potential therapeutic drug for AS.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by National Nature Fund
Program (grant no. 82104817) and the Key Laboratory of Xin'an
Medicine of the Ministry of Education, Anhui University of Chinese
Medicine (grant no. 2020xayx08).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XD and JL designed the study. YS and XC analyzed
data. XD wrote the manuscript. JL and YS confirm the authenticity
of all the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Medical Ethics
Committee of the First Affiliated Hospital of Anhui University of
Traditional Chinese Medicine (approval no. 2015-AH20). Written
informed consent to participate was obtained from all patients. All
procedures were conducted in accordance with the Medical Ethics
Committee of the First Affiliated Hospital of Anhui University of
Traditional Chinese Medicine protocols.
Patient consent for publication
Not applicable.
Competing interests
The authors declare they have no competing
interests.
References
|
1
|
Braun J and Sieper J: Ankylosing
spondylitis. Lancet. 369:1379–1390. 2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang R and Ward MM: Epidemiology of axial
spondyloarthritis: An update. Curr Opin Rheumatol. 30:137–143.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
van Tubergen A: The changing clinical
picture and epidemiology of spondyloarthritis. Nat Rev Rheumatol.
11:110–118. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Salehi E, Eftekhari R, Oraei M, Gharib A
and Bidad K: MicroRNAs in rheumatoid arthritis. Clin Rheumatol.
34:615–628. 2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
MacFarlane LA, Arant KR, Kostic AM, Mass
H, Jones MH, Collins JE, Losina E and Katz JN: Identifying
inflammation in knee osteoarthritis: Relationship of synovial fluid
white blood cell count to effusion-synovitis on magnetic resonance
imaging. Arthritis Care Res (Hoboken). 75:1783–1787.
2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu L, Chen H, Jiang T and He D:
MicroRNA-106b overexpression suppresses synovial inflammation and
alleviates synovial damage in patients with rheumatoid arthritis.
Mod Rheumatol. 32:1054–1063. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Garrido-Mesa J and Brown MA: T cell
repertoire profiling and the mechanism by which HLA-B27 causes
ankylosing spondylitis. Curr Rheumatol Rep. 24:398–410.
2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shah NG, Keraliya A, Nunez DB, Schoenfeld
A, Harris MB, Bono CM and Khurana B: Injuries to the rigid spine:
What the spine surgeon wants to know. Radiographics. 39:449–466.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gouveia EB, Elmann D and Morales MS:
Ankylosing spondylitis and uveitis: Overview. Rev Bras Reumatol.
52:742–756. 2012.PubMed/NCBI
|
|
10
|
Yu L, Qu H, Yu Y, Li W, Zhao Y and Qiu G:
LncRNA-PCAT1 targeting miR-145-5p promotes TLR4-associated
osteogenic differentiation of adipose-derived stem cells. J Cel Mol
Med. 22:6134–6147. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wang JX, Jing FY, XU YC, Zong HX, Chu YR,
Wang C, Chen KM, Tong WQ, Wang XL and Xu SQ: The potential
regulatory mechanism of lncRNA 122K13.12 and lncRNA 326C3.7 in
ankylosing spondylitis. Front Mol Biosci. 8(745441)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Lalevée S and Feil R: Long noncoding RNAs
in human disease: Emerging mechanisms and therapeutic strategies.
Epigenomics. 7:877–879. 2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Han D, Ouyang G, Pan P and Yuan Y:
Upregulated lncRNA-NEF predicts recurrence and poor treatment
outcomes of ankylosing spondylitis. Immun Inflamm Dis.
10(e627)2022.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Li Y, Zhang S, Zhang C and Wang M: LncRNA
MEG3 inhibits the inflammatory response of ankylosing spondylitis
by targeting miR-146a. Mol Cell Biochem. 466:17–24. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Liu W, Huang L, Zhang C and Liu Z: lncRNA
MEG3 is downregulated in ankylosing spondylitis and associated with
disease activity, hospitalization time and disease duration. Exp
Ther Med. 17:291–297. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Agashe RP, Lippman SM and Kurzrock R: JAK:
Not just another kinase. Mol Cancer Ther. 21:1757–1764.
2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Rosillo MA, Sánchez-Hidalgo M,
Sánchez-Fidalgo S, Aparicio-Soto M, Villegas I and
Alarcón-de-la-Lastra C: Dietary extra-virgin olive oil prevents
inflammatory response and cartilage matrix degradation in murine
collagen-induced arthritis. Eur J Nut. 55:315–325. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li WQ, Dehnade F and Zafarullah M:
Oncostatin M-induced matrix metalloproteinase and tissue inhibitor
of metalloproteinase-3 genes expression in chondrocytes requires
Janus kinase/STAT signaling pathway. J Immunol. 166:3491–3498.
2001.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Raychaudhuri SK and Raychaudhuri SP: Janus
kinase/signal transducer and activator of transcription pathways in
spondyloarthritis. Curr Opin Rheumatol. 29:311–316. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Ding X, Liu J and Sun Y: Expression of
long non-coding RNA NONHSAT227927.1 and its effect on the
JAK2/STAT3 signaling pathway and inflammation in patients with
ankylosing spondylitis. Exp Ther Med. 25(231)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li XJ, Jiang ZZ and Zhang LY: Triptolide:
Progress on research in pharmacodynamics and toxicology. J
Ethnopharmacol. 155:67–79. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yan P and Sun X: Triptolide: A new star
for treating human malignancies. J Cancer Res Ther. 14
(Suppl):S271–S275. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
American Diabetes Association. Diagnosis
and classification of diabetes mellitus. Diabetes Care. 36 (Suppl
1):S67–S74. 2013.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ji W, Lu Y, Ma Z, Gan K, Liu Y, Cheng Y,
Xu J, Liu S, Guo Y, Han S, et al: Triptolide attenuates inhibition
of ankylosing spondylitis-derived mesenchymal stem cells on the
osteoclastogenesis through modulating exosomal transfer of
circ-0110634. J Orthop Translat. 36:132–144. 2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wang G, Cai J, Zhang J and Li C: Mechanism
of triptolide in treating ankylosing spondylitis through the
anti-ossification effect of the BMP/Smad signaling pathway. Mol Med
Rep. 17:2731–2737. 2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
van der Linden S, Valkenburg HA and Cats
A: Evaluation of diagnostic criteria for ankylosing spondylitis. A
proposal for modification of the New York criteria. Arthritis
Rheum. 27:361–368. 1984.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ramiro S, Nikiphorou E, Sepriano A,
Ortolan A, Webers C, Baraliakos X, Landewé RBM, Van den Bosch FE,
Boteva B, Bremander A, et al: ASAS-EULAR recommendations for the
management of axial spondyloarthritis: 2022 Update. Ann Rheum Dis.
82:19–34. 2023.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Landewé R, Dougados M, Mielants H, van der
Tempel H and van der Heijde D: Physical function in ankylosing
spondylitis is independently determined by both disease activity
and radiographic damage of the spine. Ann Rheum Dis. 68:863–867.
2009.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Poddubnyy D, Protopopov M, Haibel H, Braun
J, Rudwaleit M and Sieper J: High disease activity according to the
ankylosing spondylitis disease activity score is associated with
accelerated radiographic spinal progression in patients with early
axial spondyloarthritis: Results from the GErman SPondyloarthritis
inception cohort. Ann Rheum Dis. 75:2114–2118. 2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Qin X, Zhu B, Jiang T, Tan J, Wu Z, Yuan
Z, Zheng L and Zhao J: miR-17-5p regulates heterotopic ossification
by targeting ANKH in ankylosing spondylitis. Mol Ther Nucleic
Acids. 18:696–707. 2019.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhong H and Zhong M: LINC00311 is
overexpressed in ankylosing spondylitis and predict treatment
outcomes and recurrence. BMC Musculoskelet Disord.
20(278)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
van der Heijde D, Ramiro S, Landewé R,
Baraliakos X, Van den Bosch F, Sepriano A, Regel A, Ciurea A,
Dagfinrud H, Dougados M, et al: 2016 update of the ASAS-EULAR
management recommendations for axial spondyloarthritis. Ann Rheum
Dis. 76:978–991. 2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ward MM, Deodhar A, Gensler LS, Dubreuil
M, Yu D, Khan MA, Haroon N, Borenstein D, Wang R, Biehl A, et al:
2019 update of the American college of rheumatology/spondylitis
association of America/spondyloarthritis research and treatment
network recommendations for the treatment of ankylosing spondylitis
and nonradiographic axial spondyloarthritis. Arthritis Rheumatol.
71:1599–1613. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li N, Chen Z, Feng W, Gong Z, Lin C, Chen
J, Chu C and Xu Q: Triptolide improves chondrocyte proliferation
and secretion via down-regulation of miR-221 in synovial cell
exosomes. Phytomedicine. 107(154479)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Ji W, Liu S, Zhao X, Guo Y, Xia S, Lu Y,
Yin M and Xu X: Triptolide inhibits proliferation, differentiation
and induces apoptosis of osteoblastic MC3T3-E1 cells. Mol Med Rep.
16:7391–7397. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Luo X, Gu Y, Tao X, Serhan CN and Ji RR:
Resolvin D5 inhibits neuropathic and inflammatory pain in male but
not female mice: distinct actions of D-series resolvins in
chemotherapy-induced peripheral neuropathy. Front Pharmacol.
10(745)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hur K, Kim SH and Kim JM: Potential
implications of long noncoding RNAs in autoimmune diseases. Immune
Netw. 19(e4)2019.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hamdy SM, Ali MS, Abd El-Hmid RG,
Abdelghaffar NK and Abdelaleem OO: Role of long non coding RNAs,
NEAT1 and Lnc-DC expression in pediatric immune thrombocytopenic
purpura. Rep Biochem Mol Biol. 11:635–643. 2023.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Cai B, Cai J, Yin Z, Jiang X, Yao C, Ma J,
Xue Z, Miao P, Xiao Q, Cheng Y, et al: Long non-coding RNA
expression profiles in neutrophils revealed potential biomarker for
prediction of renal involvement in SLE patients. Rheumatology
(Oxford). 60:1734–1746. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Morchikh M, Cribier A, Raffel R, Amraoui
S, Cau J, Severac D, Dubois E, Schwartz O, Bennasser Y and
Benkirane M: HEXIM1 and NEAT1 long non-coding RNA form a
multi-subunit complex that regulates DNA-mediated innate immune
response. Mol Cell. 67:387–399.e5. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Li X, Chai W, Zhang G, Ni M, Chen J, Dong
J, Zhou Y, Hao L, Bai Y and Wang Y: Down-regulation of
lncRNA-AK001085 and its influences on the diagnosis of ankylosing
spondylitis. Med Sci Monit. 23:11–16. 2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ding M, Guan TJ, Wei CY and Chen BH:
Identification of pathways significantly associated with
spondyloarthropathy/ankylosing spondylitis using the sub-pathway
method. Mol Med Rep. 18:3825–3833. 2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Marcus DM: Comparison of Tripterygium
wilfordii Hook F with methotrexate in the treatment of
rheumatoid arthritis. Ann Rheum Dis. 73(e56)2014.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tao X, Younger J, Fan FZ, Wang B and
Lipsky PE: Benefit of an extract of Tripterygium wilfordii
Hook F in patients with rheumatoid arthritis: A double-blind,
placebo-controlled study. Arthritis Rheum. 46:1735–1743.
2002.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Matta R, Wang X, Ge H, Ray W, Nelin LD and
Liu Y: Triptolide induces anti-inflammatory cellular responses. Am
J Transl Res. 1:267–282. 2009.PubMed/NCBI
|
|
47
|
Chen BJ: Triptolide, a novel
immunosuppressive and anti-inflammatory agent purified from a
Chinese herb Tripterygium wilfordii Hook F. Leuk Lymphoma.
42:253–265. 2001.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Su MX, Zhou WD, Lan J, Di B and Hang TJ:
Rapid and sensitive analysis of multiple bioactive constituents in
tripterygium glycosides tablets using liquid chromatography coupled
with time-of-flight mass spectrometry. J Sep Sci. 38:804–812.
2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Wang QL, Sun M, Wang DT, et al:
Observation on the efficacy of Tripterygium wilfordii
polyglycosides combined with methotrexate in the treatment of
ankylosing spondylitis. World Chin Med. 17:2486–2489. 2002.(In
Chinese).
|
|
50
|
Ji W, Chen Y, Zhao X, Guo Y, Zhong L, Li
H, Wang D and Song Y: Beneficial effects of tripterygium glycosides
tablet on biomarkers in patients with ankylosing spondylitis. Mol
Med Rep. 12:684–690. 2015.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Ye X, Li W, Yan Y, Mao C, Cai R, Xu H and
Yang X: Effects of cytochrome P4503A inducer dexamethasone on the
metabolism and toxicity of triptolide in rat. Toxicol Lett.
192:212–220. 2010.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Lee HI, Kim HJ, Jo S, Shim SC, Kim TH, Won
EJ and Kim TJ: IL-6 activates pathologic Th17 cell via STAT 3
phosphorylation in inflammatory joint of ankylosing spondylitis.
Biochem Biophys Res Commun. 620:69–75. 2022.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Zhong Z, Wen Z and Darnell JE Jr: Stat3: A
STAT family member activated by tyrosine phosphorylation in
response to epidermal growth factor and interleukin-6. Science.
264:95–98. 1994.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Fang YY, Wan L, Dong WZ, Wen JT and Liu J:
Effect of triptolide in improving platelet activation in patients
with ankylosing spondylitis by regulating VEGFA,SDF-1,CXCR4
pathway. Zhongguo Zhong Yao Za Zhi. 44:3520–3525. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|