Introduction
Spinal cord injury (SCI) refers to the damage to the
spinal cord caused by external factors, resulting in sensory, motor
and functional impairments in the corresponding injured and lower
segments (1). The incidence of
traumatic SCI has been reported to be 569.7 per million of the
population, with a significantly higher rate in men compared with
women (753.6 vs. 387.7) (2). SCI
can be categorized into primary and secondary injuries (3). Primary injury results in local
necrosis and apoptosis of neurons and glial cells, while the
subsequent inflammatory response of the nervous system leads to
secondary damage. In the case of secondary injury, spinal cord
ischemia and hypoxia trigger the generation and release of numerous
free radicals and reactive oxygen species, further disrupting the
microenvironment and impairing the functioning of the nervous
system (4,5).
Nuclear factor-erythroid 2-related factor 2 (Nrf2)
is a transcriptional regulatory factor that plays a crucial role in
maintaining homeostasis by activating the expression of antioxidant
and anti-inflammatory genes (6-9).
Nrf2 binds to the antioxidant response element located in the
promoter region of cytoprotective and antioxidant genes, thereby
promoting their transcription (10-12).
Under normal physiological conditions, Nrf2 is associated with
Keap1, which inhibits Nrf2 activity (13,14).
However, during oxidative stress, Nrf2 dissociates from the complex
and translocates into the nucleus, where it stimulates the
expression of anti-inflammatory and antioxidant genes (15-17).
By reducing the levels of reactive oxygen species, Nrf2 can enhance
nerve cell survival at the site of injury and improve the outcome
of SCI.
Melatonin, also known as
N-acetyl-5-methoxytryptamine, is a hormone that plays a crucial
role in regulating biological rhythms due to its fluctuating
concentrations throughout the day and night (18). In the past, the effects of
melatonin were primarily attributed to its ability to promote sleep
initiation and maintenance. However, emerging research indicates
that melatonin possesses broader neuroprotective properties by
inhibiting apoptosis, thereby improving neuronal survival. This has
been observed in various neurological conditions, including
Alzheimer's disease (19) and
Parkinson's disease (20).
Currently, two melatonin receptors have been
identified in mammals: MT1 (Mel1a) and MT2 (Mel1b), along with
nuclear binding sites ROR and RZR (21). Melatonin receptors are widely
distributed in the brain, particularly in the hypothalamus,
hippocampus and pineal gland, as well as in the retina and various
peripheral tissues including the liver and adipose tissue (22). The MT2 receptor has been found to
regulate stem cell activity and attenuate oxidative stress-induced
damage, thereby reducing traumatic injury (14). Importantly, the functionality of
the MT2 receptor partially overlaps with the Nrf2 signaling
pathway.
Nonetheless, the precise mechanism underlying the
effects of melatonin in SCI remains unclear, and the relationship
between melatonin and the Nrf2 signaling pathway in SCI has yet to
be elucidated. It was postulated that the melatonin MT2 receptor
and the Nrf2 signaling pathway collectively contribute to the
secondary injury mechanism in SCI, given that inflammatory
responses and oxidative stress injury are recognized as highly
detrimental factors in this context. In the present study,
4-phenyl-2-propionamidotetralin (4P-PDOT) was used, a specific
antagonist targeting melatonin and MT2 receptors, to treat SCI mice
and investigate the neuroprotective role of melatonin along with
its associated signaling pathways.
Materials and methods
Animal studies
In the present study, male C57BL/6 mice aged 8-12
weeks and weighing between 22-28 g were obtained from the Animal
Research Center of Zhengzhou University (Henan, China). The mice
were housed in a standard animal facility with controlled
environmental conditions of 26˚C temperature, 38.5% humidity, and a
12/12-h light-dark cycle. Mice had ad libitum access to food
and water. A total of 112 mice were included in the present study,
and 8 mice succumbed during the course of the experiment. All
experimental procedures involving animals were conducted in
accordance with the guidelines and regulations approved by the
Ethics Committee of The First Affiliated Hospital of Zhengzhou
University (approval no. ZZUIRB 2022-145) (Zhengzhou, China). A
conceivable effort was made to minimize animal suffering and the
minimum number of animals necessary to achieve our research
objectives, was employed.
Establishment of models
Operation was conducted following previously
described protocols (23). Prior
to the surgical procedure, mice were intraperitoneally injected
with pentobarbital sodium (40 mg/kg) for anesthesia. Following
anesthesia, the back skin was prepared and disinfected using
iodophor solution. The mice were then positioned in the prone
position on the experimental table, with the skin fixed. A 2-cm
incision was made along the midline of the mouse's back after
proper preoperative preparation. The fascia and paraspinal muscles
were carefully separated to expose the T9-T11 vertebrae segment.
SCI was induced using a modified mouse Allen method, wherein a 5-g
hammer was dropped from a height of 3 cm onto the T10 spinal cord
segment. Successful modeling was confirmed by hindlimb twitching.
Following this, the muscle and skin layers were sutured
meticulously and the area was disinfected again with iodophor
solution. For the next three days after the surgery, gentle
abdominal massages were administered to the mice twice daily, and
gentamicin (40 mg/kg) was administrated to prevent urinary tract
infection.
Experimental groups
Mice were randomly assigned to one of the four
groups using a random number table method, as previously described
(24,25) (http://www.randomization.com): i) Sham group, in which
the vertebral plate of the corresponding segments was surgically
removed without any further treatment; ii) Vehicle group (Veh),
receiving intraperitoneal injections of normal saline (10 mg/kg)
immediately after SCI, once daily for 7 days; iii) Melatonin group
(Mel), receiving intraperitoneal injections of melatonin (cat. no.
M813985; Shanghai McLean Biochemical Technology Co., Ltd.) (10
mg/kg) immediately after SCI, once daily for 7 days; iv) Mel + 4PP
group, receiving immediate intraperitoneal injections of melatonin
(10 mg/kg) after SCI, followed by intragastric administration of
4P-PDOT (cat. no. P910644; Shanghai McLean Biochemical Technology
Co., Ltd.) (10 mg/kg), once daily for 7 days. Melatonin was
dissolved in absolute ethanol and then diluted to a concentration
of 1 mg/ml using PBS. 4P-PDOT was dissolved in DMSO and also
diluted to a concentration of 1 mg/ml using PBS. The concentrations
of melatonin and 4P-PDOT were determined based on preliminary
experiments and previous studies (26-30).
Assessment of motor function
The motor function of the hindlimbs in mice was
assessed using the Basso Mouse Scale (BMS) score at 1, 3, 7, 14, 21
and 28 days after SCI, with 6 mice in each group. The BMS score
ranges from 0 to 9, where 0 indicates complete paralysis and 9
indicates normal functioning of the hindlimbs. Prior to the
assessment, the mice were given an opportunity to familiarize
themselves with the open field environment. Two experimenters
independently observed and analyzed the mice's behavior on a
computer, assigning scores that were subsequently averaged
(23). The detailed rules of BMS
scoring are shown in Table I
(31).
 | Table IBasso mouse scale. |
Table I
Basso mouse scale.
| Grading | Scoring
standard |
|---|
| 0 | No ankle joint
movement |
| 1 | Slight ankle joint
movement |
| 2 | Extensive ankle
joint mobility |
| 3 | No weight-bearing,
or occasional, frequent, sustained forefoot standing without
hindfoot standing |
| 4 | Occasional forefoot
standing |
| 5 | Frequent, sustained
forefoot standing with some coordination, but not coordinated; or
frequent, sustained forefoot standing with some coordination, but
toes rotate upon contact and lift-off |
| 6 | Frequent, sustained
forefoot standing with some coordination, toes are steady upon
contact; or frequent, sustained forefoot standing, highly
coordinated, but toes rotate upon contact and lift-off |
| 7 | Frequent, sustained
forefoot standing, highly coordinated, toes are steady upon contact
but rotate during lift-off; or frequent, sustained forefoot
standing, highly coordinated, toes are steady upon contact and
lift-off, but significant trunk |
| 8 | Frequent, sustained
forefoot standing, highly coordinated, toes are steady upon contact
and lift-off, slight trunk instability; or frequent, sustained
forefoot standing, highly coordinated, toes are steady upon contact
and lift-off, trunk stable but tail droops or curls upwards and
then droops |
| 9 | Frequent, sustained
forefoot standing, highly coordinated, toes are steady upon contact
and lift-off, trunk stable, tail raised |
Tissue processing
Gene expression differences were found to be most
significant 7 days after SCI (32). Therefore, on day 7 after the
operation, mice were euthanized, and complete spinal cord tissue
(T9-T11) was collected, with the injury site designated as the
epicenter. Tissues intended for oxidative stress detection, reverse
transcription-quantitative (RT-q) PCR, and western blot analysis
were stored at -80˚C in a refrigerator for subsequent processing.
Additionally, tissues designated for immunohistochemical and
H&E staining were fixed in 4% paraformaldehyde and then
dehydrated using a series of 10, 20 and 30% sugar water solutions
in a refrigerator at 4˚C; each concentration was placed there for
24 h. The spinal cord tissues were sectioned into 15-µm slices
using a cryostat. Once completely dried, the sections were stored
at -80˚C in a refrigerator for follow-up experiments.
Immunohistochemical staining
Immunofluorescence staining was conducted following
previously described protocols (33,34).
The tissue sections were gradually rehydrated and washed with PBS
for 5 min each time, followed by fixation with 4% paraformaldehyde.
Subsequently, the sections were fixed in acetone for 10 min, and
antigen retrieval was performed using a citric acid solution
containing 0.05% Tween. The primary antibodies, including iNOS,
inducible nitric oxide synthase (iNOS; cat. no. AF0199; Affinity
Biosciences, Ltd.), Arginase 1 (Arg1; cat. no. DF6657; Affinity
Biosciences, Ltd.), GFAP (cat. no. 16825-1-AP; Proteintech Group,
Inc.) and NeuN (cat. no. DF6145; Affinity Biosciences, Ltd.), were
diluted in PBST solution containing 0.05% Tween (1:200) and applied
to the sections. The sections were then covered and placed in a
humidified chamber, incubating overnight at 4˚C.
The sections were washed three times with PBS for 15
min each time, ensuring that the entire process was carried out in
the absence of light. The secondary Goat Anti-Rabbit IgG (H+L)
Fluor594-conjugated antibody (1:200; cat. no. S0006; Affinity
Biosciences, Ltd.), was applied to the sections and incubated for 2
h.
After staining, the sections were sealed with 10
µg/ml DAPI (cat. no. C0065; Beijing Solarbio Science &
Technology, Ltd.) staining solution and incubated for 15 min. The
fluorescence microscope was used to locate the visual field in the
anterior horn of the spinal cord, and each image was observed and
analyzed using the same exposure settings. The number of positive
cells in each visual field was quantified using ImageJ
software.
H&E staining
The frozen sections were stained with hematoxylin
solution (cat. no. G1120; Beijing Solarbio Science & Technology
Co., Ltd.) for 2 min, followed by rinsing in running tap water.
Subsequently, the sections were treated with differentiation
solution for 30 sec and immersed in distilled water for 2 min.
Eosin Y stain was applied for 1 min, and then the sections were
washed with distilled water for 1 min. Finally, the sections were
dehydrated sequentially in 30, 50, 75, 95 and 100% alcohol
(35).
Detection of oxidative stress
Oxidative stress levels were assessed 7 days after
SCI using assay kits for measuring superoxide dismutase (SOD) (cat.
no. BC0170), glutathione (GSH) (cat. no. BC1175) and
malondialdehyde (MDA) (cat. no. BC0025; all purchased from Beijing
Solarbio Science & Technology Co., Ltd.). To perform the
measurements, mice were first anesthetized and euthanized. Spinal
cord tissue was collected and subjected to grinding, followed by
centrifugation (8,000 x g for 10 min at 4˚C) to obtain the
supernatant for subsequent experiments (33).
RNA isolation and RT-qPCR
analysis
Total RNA was extracted from spinal cord tissue
using TRIzol reagent (cat. no. CW0580; Jiangsu Cowin Biotech Co.,
Ltd.) following the provided instructions and a previous study
(36). The extracted total RNA (1
µg) was reverse transcribed using the PrimeScript RT reagent kit
(cat. no. RR047A; Takara Bio, Inc.), following the instructions
provided in the manual. qPCR was performed on the transcribed cDNA
using TB Green (cat. no. RR820A; Takara Bio, Inc.) and specific
primers. The thermocycling conditions were as follows: 95˚C for 2
min, followed by denaturation at 95˚C for 15 sec and
annealing/extension at 60˚C for 1 min, for a total of 40 cycles.
Relative gene expression was calculated using the 2-ΔΔCq
method (37). The primer sequences
used were as follows: Bcl-2 forward, 5'-GATGACTTCTCTCGTCGCTAC-3'
and reverse, 5'-GAACTCAAAGAAGGCCACAATC-3'; Bax forward,
5'-TTGCCCTCTTCTACTTTGCTAG-3' and reverse,
5'-CCATGATGGTTCTGATCAGCTC-3'; IL-1β forward,
5'-TTCAGGCAGGCAGTATCACTC-3' and reverse,
5'-GAAGGGTCCACGGGAAAGACAC-3'; IL-4 forward,
5'-GGTCTCAACCCCCAGCTAGT-3' and reverse,
5'-GCCGATGATCTCTCTCAAGTGAT-3'; TNF-α forward,
5'-CACCACCATCAAGGACTCAA-3' and reverse, 5'-GAGACAGAGGCAACCTGACC-3';
β-actin (reference gene) forward, 5'-TTGCTGACAGGATGCAGAAG-3' and
reverse 5'-TTGCTGACAGGATGCAGAAG-3'; Nrf2 forward,
5'-CGGGACTATTGAAGGCTGTGA-3' and reverse, 5'-GGAGTGCTCTGGGGACGCT-3';
and Keap1 forward, 5'-GACTGGGTCAAATACGACTGC-3' and reverse,
5'-GAATATCTGCACCAGGTAGTCC-3'.
Western blot analysis
Protein was prepared from fresh spinal cord tissue
and analyzed according to a previously described protocol (35). Total protein was extracted using
RIPA Lysis Buffer (cat. no. R0010; Beijing Solarbio Science &
Technology Co., Ltd.), and the protein concentration was determined
using the BCA kit (cat. no. PC0020; Beijing Solarbio Science &
Technology Co., Ltd.). Equal amounts of protein (30 µg/lane) were
separated on 8% gels by SDS-polyacrylamide gel electrophoresis and
transferred onto polyvinylidene fluoride (PVDF) membranes. The PVDF
membranes containing proteins were incubated at 4˚C overnight in a
blocking solution (cat. no. SW3012; Beijing Solarbio Science &
Technology Co., Ltd.). The membranes were then probed with specific
primary antibodies and incubated at 4˚C in a refrigerator for 2 h,
including MT2 (1:3,000; cat. no. NLS932; Novus Biologicals, LLC),
Nrf2 (1:3,000; cat. no. A0674; ABclonal Biotech Co., Ltd.), Keap1
(1:3,000; cat. no. A17061; ABclonal Biotech Co., Ltd.), iNOS
(1:3,000; cat. no. AF0199; Affinity Biosciences, Ltd.) and Arg1
(1:3,000; cat. no. DF6657; Affinity Biosciences, Ltd.); followed by
incubation with a secondary Goat Anti-Rabbit IgG (H+L)
HRP-conjugated antibody (1:3,000; cat. no. S0001; Affinity
Biosciences, Ltd.) for 2 h. Protein bands were visualized using
enhanced chemiluminescence (ECL) (cat. no. PE0010; Beijing Solarbio
Science & Technology Co., Ltd.), and the intensity of the bands
was quantified using ImageJ 1.54 software (National Institutes of
Health).
Statistical analysis
Data analysis was performed using SPSS 21.0 software
(IBM Corp.). The results are presented as the mean ± standard
deviation. Prior to analysis, normal distribution and homogeneity
of variance assumptions were checked. For data that followed a
normal distribution and met the assumption of homogeneity of
variance, one-way analysis of variance (ANOVA) was used to compare
multiple groups, and the unpaired Student's t-test was used to
compare two groups. If the data did not meet the assumptions of
normal distribution and homogeneity of variance, the Kruskal-Wallis
nonparametric test was employed, and post-hoc analysis was
conducted using the Bonferroni correction to assess differences
between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Melatonin activates the Nrf2/Keap1
signal pathway through the MT2 receptor
Initially, the protein expression levels of the MT2
receptor and the Nrf2/Keap1 signaling pathway were assessed
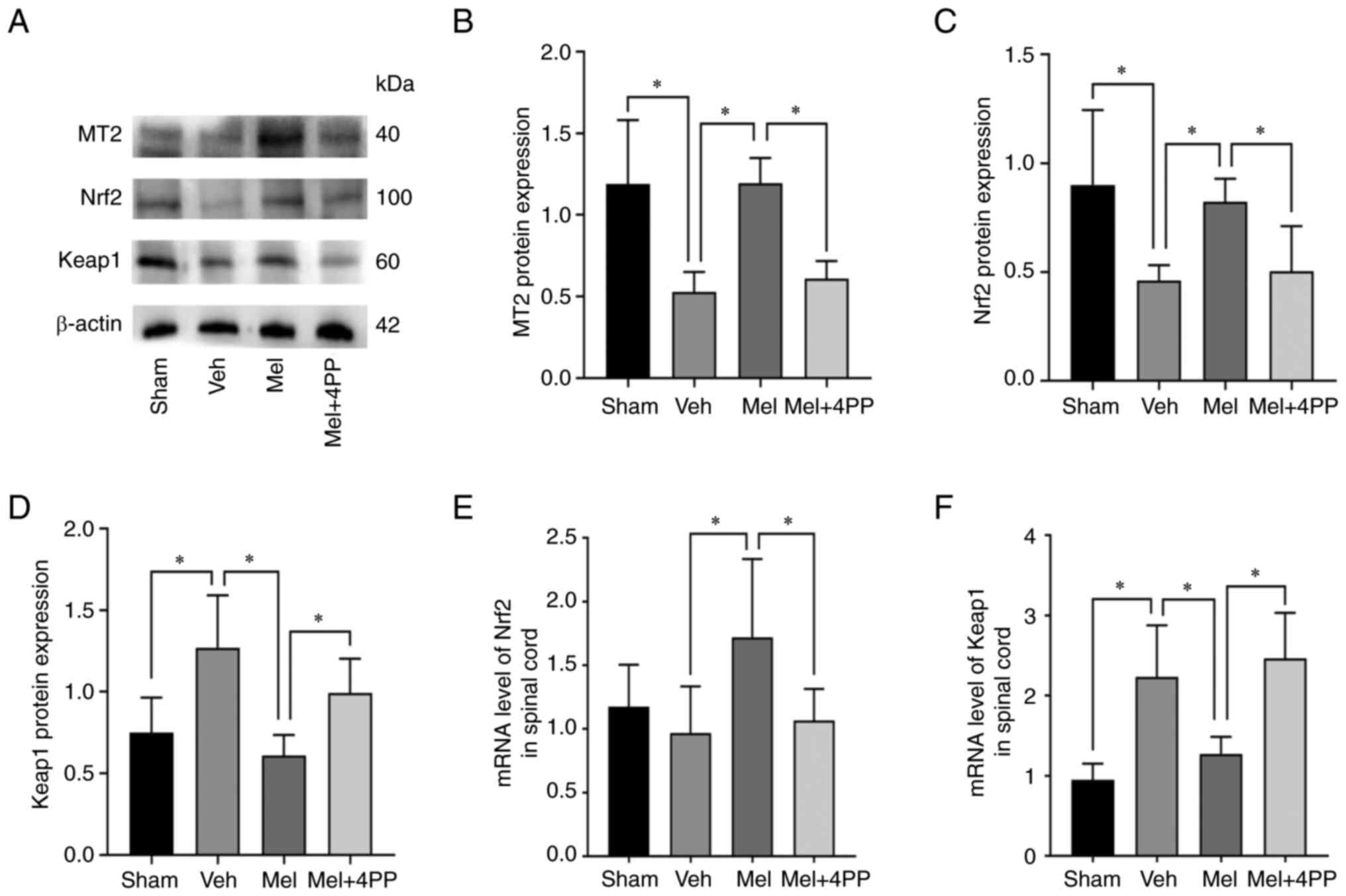
(Fig. 1A). Western blot analysis
revealed that the MT2 receptor protein expression in the Mel group
was significantly higher compared with the Veh group (1.19±0.16 vs.
0.53±0.12, N=5/group, P<0.001). However, the MT2 receptor
blocker, 4P-PDOT, reduced the MT2 receptor protein content in the
Mel + 4PP group (1.19±0.16 vs. 0.61±0.11, N=5/group, P=0.029)
compared with the Mel group (Fig.
1B). The Nrf2 protein expression levels in the Veh group and
the Mel + 4PP group were comparable (0.46±0.07 vs. 0.50±0.21,
N=5/group, P=0.760) (Fig. 1C). By
contrast, the Mel group exhibited significantly increased Nrf2
protein expression compared with both the Veh and Mel + 4PP groups
(0.46±0.07 vs. 0.82±0.21 and 0.50±0.21 vs. 0.82±0.21, N=5/group,
P=0.015 and P=0.029). Additionally, the expression of Keap1 protein
decreased in the Mel group compared with the Veh and Mel + 4PP
groups (1.27±0.32 vs. 0.61±0.13 and 0.99 ± 0.21 vs. 0.61±0.13,
N=5/group, P<0.001 and P=0.018) (Fig. 1D).
In addition to protein expression analysis, the
transcription levels of Nrf2 and Keap1 mRNA were investigated using
RT-qPCR. The results revealed that Nrf2 mRNA expression was
significantly increased in the Mel group compared with the Veh
group (0.97±0.38 vs. 1.72±0.62, N=6/group, P=0.005) (Fig. 1E). Conversely, Keap1 mRNA
expression was decreased in the Mel group compared with the Veh
group (2.23±0.65 vs. 1.27±0.21, N=6/group, P=0.002) (Fig. 1F). However, in the Mel + 4PP group,
these effects were not observed (0.97±0.38 vs. 1.07±0.25,
N=6/group, P=0.683) (2.23±0.65 vs. 2.47±0.57, N=6/group, P=0.381),
indicating that Melatonin could activate the Nrf2/Keap1 signaling
pathway through the MT2 receptor after SCI (Fig. 1B).
Melatonin attenuates inflammation and
oxidative stress through the MT2 receptor
On day 7 after SCI, immunofluorescence staining of
GFAP on frozen sections of the injured spinal cord tissue was
performed (Fig. 2A and B). The immunofluorescence results
demonstrated that melatonin treatment significantly reduced the
number of astrocytes compared with the Veh group (161.33±27.23 vs.
217.00±39.34, N=3/group, P=0.048). However, when the 4P-PDOT MT2
receptor blocker was used, the effect of melatonin on astrocytes
was completely abolished (225.67±28.04 vs. 161.33±27.23, N=3/group,
P=0.029). The number of astrocytes in the Mel + 4PP group was
similar to that in the Veh group but significantly less than that
in the Mel group.
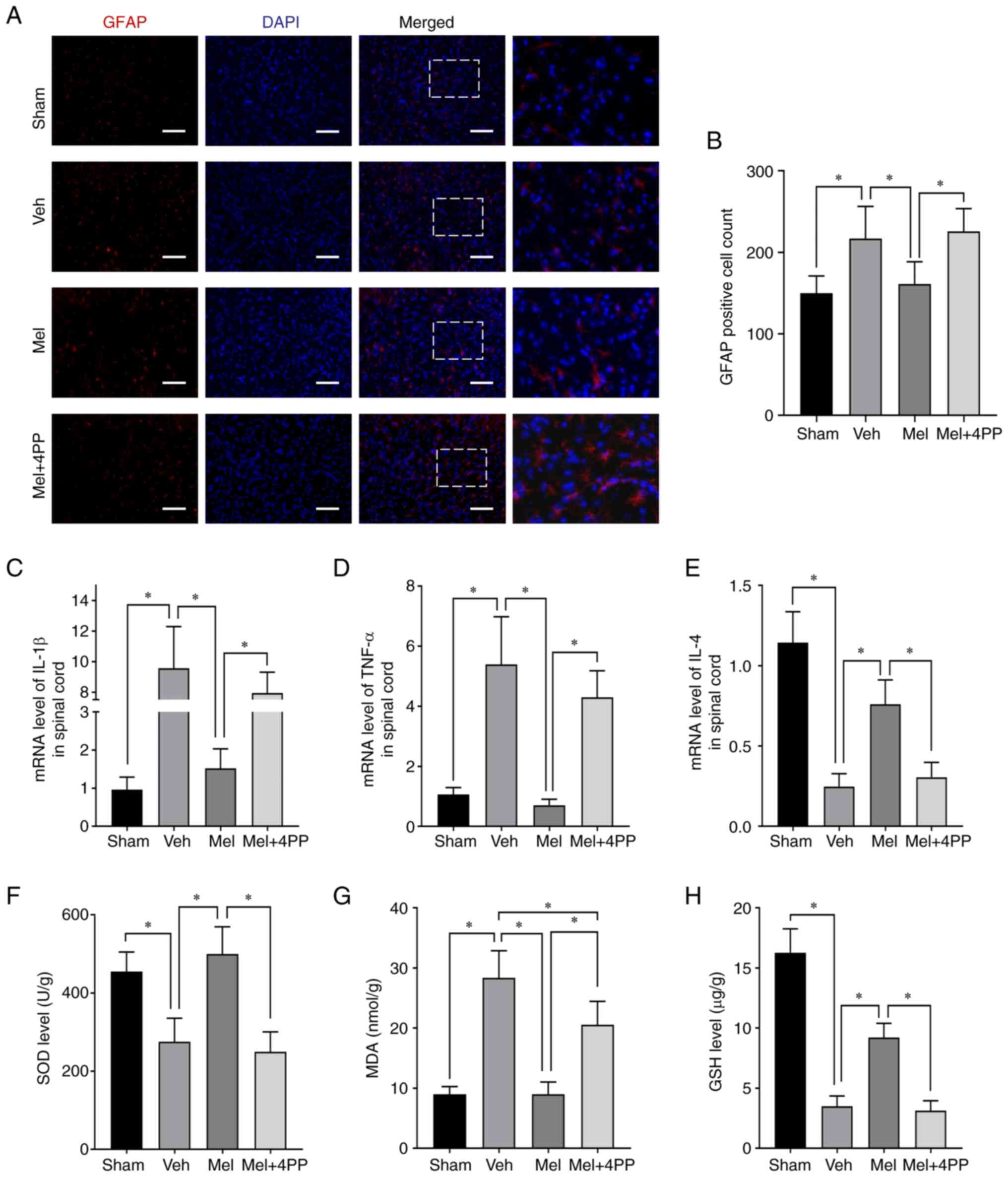 | Figure 2Melatonin exerts its effects by
reducing inflammation and attenuating the oxidative stress
response. (A and B) Representative images of immunofluorescence
staining were obtained to assess the levels of GFAP in the anterior
horn of the spinal cord at the epicenter of the lesion. The scale
bar represents 200 µm. (C-E) mRNA levels of pro-inflammatory
factors IL-1β and TNF-α, as well as the anti-inflammatory factor
TNF-α, were assessed in spinal cord tissue at 7 dpi. (F-H) The
oxidative stress index was evaluated at 7 dpi to assess the levels
of oxidative stress in each group. *P≤0.05. GFAP, glial
fibrillary acidic protein; dpi, days post-injury; SOD, superoxide
dismutase; MDA, malondialdehyde; GSH, reduced glutathione; Mel,
Melatonin; Veh, Vehicle; 4PP, 4-phenyl-2-propionamidotetralin. |
To assess the impact of melatonin on inflammation
following SCI, the levels of cytokines, including IL-1β, IL-4 and
TNF-α, in the SCI site were examined. As expected, the
proinflammatory factors IL-1β and TNF-α exhibited higher expression
after SCI compared with the baseline levels (0.96±0.33 vs.
9.56±2.74, N=6/group, P<0.001) (1.07±0.23 vs. 5.40±1.59,
N=6/group, P<0.001) (Fig. 2C
and D). Similar results were
observed in the Mel + 4PP group. However, in comparison to the Veh
group and the Mel + 4PP group, the Mel group showed a significant
decrease in the expression of these two proinflammatory factors
(1.52±0.52 vs. 9.56±2.74 and 1.52±0.52 vs. 7.93±1.37, N=6/group,
both P<0.001) (0.70±0.20 vs. 5.40±1.59 and 0.70±0.20 vs.
4.30±0.89, N=6/group, both P<0.001). Conversely, the levels of
the anti-inflammatory factor IL-4 showed an opposite trend
(Fig. 2E). In the Mel group, IL-4,
which serves as a marker of favorable prognosis, exhibited an
increase compared with the Veh group (0.76±0.15 vs. 0.25±0.08,
N=6/group, P<0.001) and the Mel + 4PP group (0.76±0.15 vs.
0.30±0.09, N=6/group, P<0.001). Moreover, the IL-4 level in the
Mel + 4PP group did not differ significantly from that of the Veh
group (0.30±0.09 vs. 0.25±0.08, N=6/group, P=0.477), indicating
that 4P-PDOT could block the therapeutic effect of the MT2
receptor.
To assess the antioxidant effect of melatonin, the
levels of three indicators, SOD, MDA and GSH were examined. The
levels of SOD (275.31±60.08 vs. 249.65±50.94, N=6/group, P=0.455)
(Fig. 2F) and GSH (3.49±0.86 vs.
3.13±0.82, N=6/group, P<0.001) (Fig. 2H) in the Veh group and Mel + 4PP
group were similar but lower than those in the Mel group
(499.61±70.09 vs. 275.31±60.08 and 499.61±70.09 vs. 249.65±50.94,
N=6/group, both P<0.001) (9.22±1.18 vs. 3.49±0.86 and 9.22±1.18
vs. 3.13±0.82, N=6/group, both P<0.001). Melatonin treatment
significantly reduced the MDA content (8.99±2.06 vs. 28.38±4.49,
N=6/group, P<0.001) (Fig. 2G).
However, when the MT2 receptor blocker 4P-PDOT was used, the MDA
content in the Mel + 4PP group was higher than that in the Mel
group (20.58±3.87 vs. 8.99±2.06, N=6/group, P<0.001), although
it remained lower than that in the Veh group (20.58±3.87 vs.
28.38±4.49, N=6/group, P<0.001). These findings suggested that
the MT2 receptor plays a crucial role in the antioxidant effect
observed.
Melatonin regulates the direction of
polarization of microglia through the MT2 receptor
Microglia polarization into M1 and M2 types is a
crucial phenomenon in the pathological process of SCI. The
activation of microglia by inflammatory factors such as IL-1β and
TNF-α can lead to their polarization into M1 type microglia
(38). M1 type microglia, in turn,
exacerbate the inflammatory response and cause damage to spinal
cord cells. Conversely, during the progression of the inflammatory
response, the upregulation of IL-4 receptors on microglia can
induce the conversion of M1 type microglia to M2 type microglia
(39). The M2 type microglia
possess anti-inflammatory properties and contribute to tissue
repair and regeneration in the spinal cord.
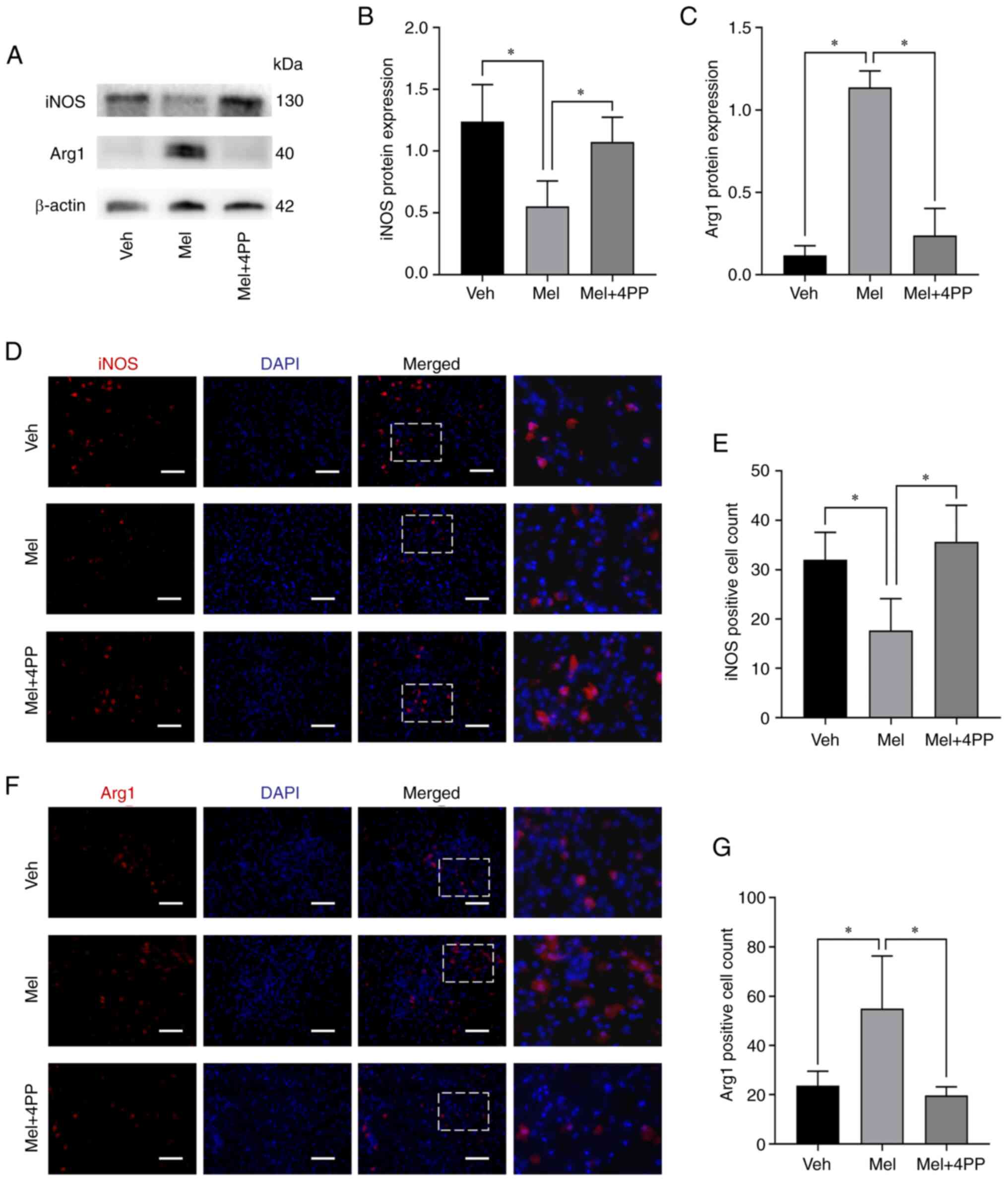
To investigate the effect of melatonin treatment on
microglial polarization, the protein expression of iNOS and Arg1was
examined using western blot analysis (Fig. 3A-C). Treatment with melatonin
significantly increased the expression of Arg1 protein (0.12±0.06
vs. 1.14±0.10, N=5/group, P=0.001) and decreased the expression of
iNOS protein (1.24±0.30 vs. 0.55±0.21, N=5/group, P=0.001).
However, when the MT2 receptor inhibitor 4P-PDOT was used, the
effect of melatonin on microglial polarization was reversed. The
expression of Arg1 protein (0.12±0.06 vs. 0.24±0.16, N=5/group,
P=0.126) and iNOS protein (1.24±0.30 vs. 1.08±0.20, N=5/group,
P=0.293) in the Mel + 4PP group showed no significant difference
compared with the Veh group. These results suggested that melatonin
can induce the polarization of microglia towards the M2 type by
stimulating the MT2 receptor.
Immunofluorescence staining was further employed in
spinal cord sections using markers for M1 type (iNOS) and M2 type
(Arg1) microglia to assess the effect of melatonin treatment on
microglial polarization. Consistent with the previous
immunofluorescence results, the quantitative analysis demonstrated
significant differences among the groups. In comparison to the Veh
group and the Mel + 4PP group, the Mel group exhibited a notable
reduction in the number of iNOS-positive cells (32.00±5.57 vs.
17.67±6.43 and 35.67±7.37 vs. 17.67±6.43, N=3/group, P=0.036 and
P=0.015) (Fig. 3D and E). Conversely, the number of
Arg1-positive cells increased in the Mel group, indicating an
enhanced M2-type polarization (23.67±5.86 vs. 55.00±21.28 and
19.97±3.51 vs. 55.00±21.28, N=3/group, P=0.025 and P=0.015)
(Fig. 3F and G).
Melatonin increases the number of
nerve cells surviving through the MT2 receptor
The NeuN antibody was utilized to label neurons. The
immunofluorescence analysis revealed that treatment with melatonin
resulted in an increased number of viable neurons. In comparison to
the Mel group, the number of neuron-positive cells was
significantly lower in the Veh group (163.67±34.02 vs. 65.67±30.92,
N=3/group, P=0.004) and the Mel + 4PP group (163.67±34.02 vs.
68.00±7.21, N=3/group, P=0.005) following SCI (Fig. 4A and B). These findings collectively indicated
that melatonin exerts a pronounced neuroprotective effect on the
damaged spinal cord through the involvement of the MT2
receptor.
Furthermore, the expression of apoptosis-related
genes, Bcl2 and Bax was assessed. The PCR results demonstrated a
significant decrease in Bax expression (a pro-apoptotic gene)
(4.75±0.32 vs. 1.07±0.21, N=6/group, P<0.001) and an increase in
Bcl2 expression (an anti-apoptotic gene) (0.22±0.09 vs. 2.59±0.92,
N=6/group, P<0.001) in the spinal cord tissues of mice treated
with melatonin compared with the Veh group. The ratio of Bcl2 to
Bax (Bcl2/Bax) was significantly different between the Veh and Mel
groups (0.05±0.02 vs. 2.60±1.30, N=6/group, P<0.001), while
these data were similar between the Veh group and Mel + 4PP group
(0.05±0.02 vs. 0.11±0.03, N=6/group, P=0.869). These findings
indicated that melatonin can effectively inhibit cell apoptosis, as
reflected by the alteration in the expression of Bcl2 and Bax genes
(Fig. 4C-E).
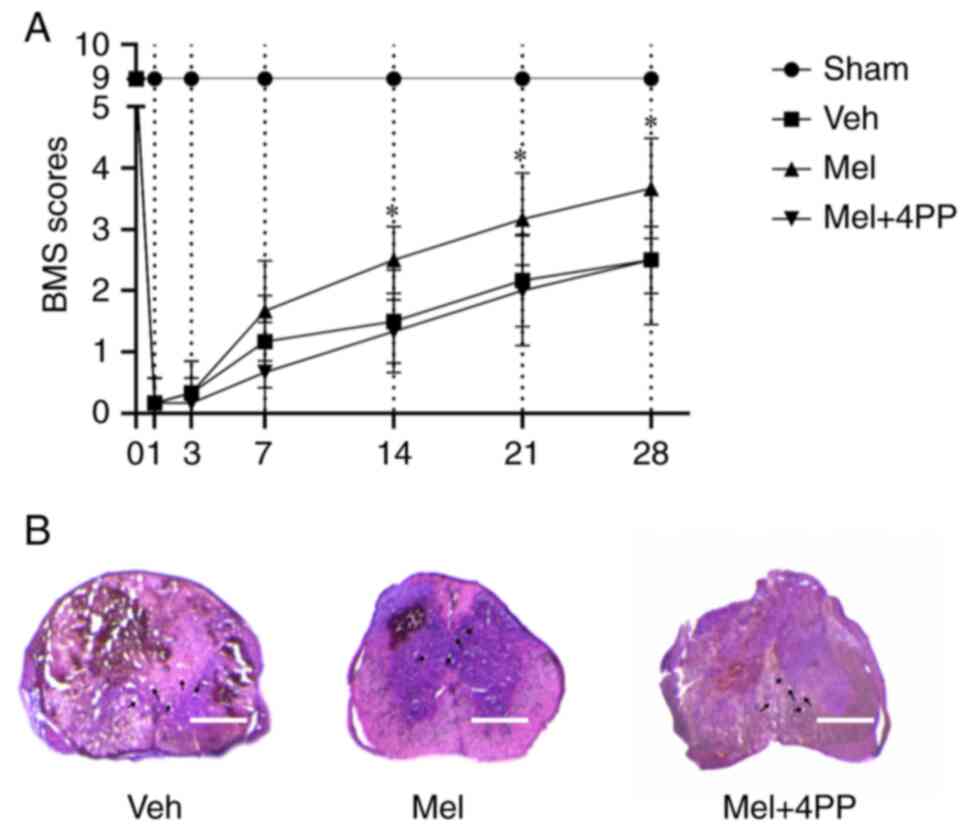
Melatonin promotes functional recovery
after SCI through the MT2 receptor
The BMS scores were used to assess the effect of
melatonin and 4P-PDOT on locomotor function after SCI. The
evaluation was conducted on days 1, 3, 5, 7, 14, 21, and 28
following the injury. During the first week after SCI, there were
no significant differences in the BMS scores among all groups.
However, at 14 days post-injury, mice treated with melatonin
exhibited improved functional ability compared with the Veh group
(2.29±0.76 vs. 1.50±0.84, N=6/group, P=0.039) and the Mel + 4PP
group (2.29±0.76 vs. 1.40±0.55, N=6/group, P=0.028). Meanwhile,
there was no significant difference between the Veh group and the
Mel + 4PP group (1.50±0.84 vs. 1.40±0.55, N=6/group, P=0.798).
Although the BMS score of the Mel + 4PP group was slightly higher
than that of the Veh group on day 21, the difference between the
two groups was not statistically significant (2.17±0.75 vs.
2.20±0.84, N=6/group, P=0.945) (Fig.
5A). These findings indicated that melatonin could enhance
functional recovery following SCI, while the MT2 receptor blocker
4P-PDOT could impede this process.
H&E staining was conducted on the spinal cord
sections from each experimental group (Fig. 5B). The results revealed a
substantial disruption of spinal cord tissue integrity in all three
groups. Vacuoles were observed at the site of injury, and local
bleeding was evident. Furthermore, the boundary between the grey
matter and white matter in the center of the injury appeared
indistinct. However, compared with the Veh group and the Mel + 4PP
group, the Mel group exhibited relatively preserved spinal cord
tissue.
Discussion
SCI is known to cause significant damage to the
nervous, motor and sensory functions of the human body, leading to
a severe decline in patients' quality of life and placing a
substantial burden on society. Long-term statistical analysis of
SCI patients over a decade has revealed a persistently high
mortality rate among the elderly, particularly those over 75 years
of age, which is ~five times higher than that of younger
individuals (40).
In the context of SCI, the initial injury triggers
the rapid death of local neurons and glial cells at the injury site
within min to h. Subsequently, the nervous system undergoes an
inflammatory response that leads to secondary damage (3). Hypoxia, ischemia-reperfusion injury
and microenvironmental imbalances following SCI contribute to the
generation and release of a significant number of free radicals and
reactive oxygen species, further disrupting the local microvascular
system and causing dysfunction in nerve cells (4,5).
Therefore, achieving a swift equilibrium between oxidative stress
and the inflammatory response plays a critical role in safeguarding
the spinal cord and preserving the functionality of spinal cord
nerve cells.
Although the exact mechanism is not fully
understood, Melatonin has gained significant attention in recent
years due to its recognized anti-inflammatory and antioxidant
effects. Melatonin is a neurokinin peptide primarily secreted by
the pineal gland and is widely distributed throughout various parts
of the body, including the bone marrow, lymphocytes,
gastrointestinal tract and skin (41,42).
The discovery of melatonin receptors initially occurred in the
African clawed toad in 1994 and were initially named Mel1a and
Mel1b (43,44). However, they were later renamed MT1
and MT2 according to the official IUPHAR nomenclature. The human
MT1 receptor consists of 350 amino acids, while MT2 receptor
consists of 362 amino acids. These receptors share an overall amino
acid homology of 55% and exhibit a 70% homology within the
structural transmembrane domain (45). Recent studies suggest that MT1
receptors are primarily associated with biological circadian
rhythms. For instance, Giannoni-Guzmán et al (46) found that a prolonged photoperiod
resulted in decreased co-expression levels of Tph2 and Pet-1 in
5-HT neurons, and this modulatory effect of the photoperiod on
TREK-1 was lost in MT1 knockout mice. Li reported that melatonin
reduces the expression of Nox2 and Nox4, thereby decreasing ROS
levels and attenuating the inflammatory response in mice with
ischemic stroke through the activation of MT2 receptors (47).
In the present study, a simultaneous administration
of melatonin and the MT2 receptor blocker 4P-PDOT occurred, to
treat SCI mice. A significant increase in the expression of the MT2
receptor in mice treated with melatonin was observed, while the
administration of 4P-PDOT successfully reduced the expression of
the MT2 receptor. The findings of the present study in SCI mice
demonstrated the following: i) Melatonin increased the expression
of the MT2 receptor and activated the Nrf2/Keap1 signal pathway;
ii) Melatonin reduced the inflammatory response and oxidative
stress after SCI through MT2 receptor activation; iii) Melatonin
regulated the inflammatory microenvironment through MT2 receptor
modulation, thereby improving the polarization direction of
microglia; iv) Melatonin reduced neuronal death and increased
neuronal survival through MT2 receptor activation; and v) Melatonin
promoted the recovery of hindlimb motor function in SCI mice
through MT2 receptor activation.
Tissue destruction and extensive cell necrosis can
result in hypoxia, ischemia-reperfusion injury and microenvironment
imbalance (48). These conditions
can trigger the production and release of a large number of oxygen
free radicals and inflammatory factors, leading to local
microvascular system disorder and dysfunction of nerve cells
(4,5). Therefore, it is crucial to rapidly
restore the balance of oxidative stress and stabilize the
inflammatory microenvironment as a means of treating SCI. The
Nrf2/Keap1 pathway plays a key role in regulating the expression of
antioxidant genes. Keap1, a substrate adaptor protein, is
responsible for degrading Nrf2 through the ubiquitin-26S proteasome
pathway in the cytoplasm (49).
The Nrf2/Keap1 pathway has been shown to play an important role in
hippocampal neurogenesis (50),
but its specific role in how melatonin improves the prognosis of
SCI through the activation of the Nrf2/ARE pathway remains
unclear.
In the present study, the expression of Nrf2 and
Keap1 was simultaneously detected through western blotting and
RT-qPCR analyses. The results revealed an increase in Nrf2
expression, while the expression of Keap1, which inhibits Nrf2,
decreased. However, when the MT2 receptor was blocked using
4P-PDOT, the activation of the Nrf2/Keap1 signaling pathway was
hindered. These findings suggested that melatonin can activate the
Nrf2 signaling pathway through the MT2 receptor after SCI,
indicating its potential role in promoting antioxidant responses
and reducing oxidative stress.
The Nrf2/Keap1 signaling pathway was important in
the regulation of the inflammatory response (51). Based on this knowledge, it was
hypothesized that melatonin could also mitigate the inflammatory
response. Considering that significant changes in gene expression
occur within 28 days after SCI, with the most prominent alterations
observed on day 7(32), it was
chosen to examine the injured tissue on this particular day for the
improved comprehension of the microenvironmental changes within the
tissue. The pathophysiology of SCI is multifaceted, involving
processes such as apoptosis, inflammation, vascular injury,
electrolyte imbalance and mitochondrial dysfunction (52). Injured spinal cord tissue is
characterized by the presence of myelin debris and glial scars, as
well as various factors that restrict neuronal regeneration and
axonal growth (53). The
microenvironment of injured spinal cord tissue poses challenges for
neural tissue regeneration. Following tissue damage, astrocytes
release pro-inflammatory factors (54) and proliferate to form glial scars,
which impede nerve tissue regeneration (55). Moreover, through the
astrocyte-microglial co-regulatory network, astrocytes can
influence the polarization of microglia (56).
Consistent with the findings of the present study on
the Nrf2/Keap1 signaling pathway, it was observed that melatonin
treatment effectively suppressed the release of inflammatory
factors and promoted the release of anti-inflammatory factors.
Additionally, it was also observed an increased expression of SOD
and GSH in the Mel group, while the expression of MDA, a marker of
oxidative stress, decreased. These findings suggested that
melatonin has the potential to attenuate the inflammatory response
and enhance antioxidant defenses, leading to a more favorable
microenvironment for tissue repair and regeneration in the injured
spinal cord.
The inflammatory microenvironment of the injured
spinal cord is closely associated with the polarization of
microglia (57,58). Pro-inflammatory factors such as
IL-1β and TNF-α can induce microglia to polarize into the M1
phenotype (38), which in turn
release large amounts of pro-inflammatory factors, thereby
exacerbating the inflammatory response (59). Interestingly, IL-4 receptors on the
surface of M1 microglia have the ability to convert them into M2
phenotype (39), which release
neurotrophic factors including anti-inflammatory substances,
transforming growth factor-β, and vascular endothelial growth
factor. This M2 phenotype plays a crucial role in restoring in
vivo homeostasis. The concept of M1/M2 macrophages was first
proposed by Mills et al (60). It was also observed that M1
macrophages could inhibit cell proliferation and induce cell death,
while M2 macrophages could promote cell proliferation and tissue
growth (61). The microenvironment
at the site of injury may determine the polarization of microglia.
Following SCI, most microglia tend to polarize toward the M1
phenotype rather than the M2 phenotype. Therefore, modulating the
microenvironment after injury may regulate the polarization of
microglia toward the M2 phenotype.
In the present study, the effects of melatonin on
the inflammatory response after SCI by examining the cytokine
profiles of spinal cord tissue were investigated. Based on the
results of the present study, it was hypothesized that melatonin,
acting through the MT2 receptor, can influence the local
microenvironment, inhibit inflammatory factors, and modulate the
polarization of microglia toward the M2 anti-inflammatory
phenotype. By promoting the shift from M1 to M2 microglia,
melatonin may contribute to the resolution of inflammation and
create a more favorable environment for tissue repair and recovery
after SCI.
The spinal cord coordinates various motor reflexes
in the body, as it contains numerous motor neurons that innervate
skeletal muscles. However, the regenerative capacity of neurons in
the spinal cord is limited, highlighting the importance of
protecting neurons from injury following SCI. The results of the
NeuN staining in the present study demonstrated that melatonin
treatment leads to an increased number of surviving neurons. Based
on the observed differences between the treatment groups, it can be
hypothesized that this neuroprotective effect is mediated through
the MT2 receptor. Furthermore, the present study's analysis of the
apoptotic factors Bcl2 and Bax revealed that melatonin may
partially inhibit neuronal apoptosis. This finding suggested that
melatonin may have a role in preventing cell death and promoting
neuronal survival after SCI.
The assessment of motor function using the BMS score
indicated that melatonin treatment has a significant and sustained
positive impact on the recovery of motor ability in mice over the
medium and long term. Given that motor neurons responsible for
innervating skeletal muscles are located in the anterior horn of
the spinal cord, the observed improvement in motor ability strongly
suggests that melatonin exerts a neuroprotective effect after SCI
by effectively reducing neuronal loss.
Overall, the findings of the present study suggested
that melatonin has the potential to enhance neuronal survival,
inhibit apoptosis, and improve motor function following SCI. These
effects are likely mediated through the MT2 receptor, highlighting
the therapeutic potential of melatonin for neuroprotection in the
context of SCI.
In conclusion, the findings of the present study
provided evidence that melatonin exerts its beneficial effects in
SCI by reducing oxidative stress and suppressing local inflammation
through the activation of the Nrf2/Keap1 signaling pathway via MT2
receptors. This mechanism leads to improvements in the local
microenvironment, reduction in nerve cell apoptosis within the
anterior horn of the spinal cord, increased neuronal survival,
modulation of microglial differentiation, and ultimately, enhanced
long-term prognosis in mice with SCI. These findings suggested that
melatonin holds promise as a potential therapeutic agent for
clinical treatment of SCI.
However, it is important to acknowledge certain
limitations to the present study. Firstly, there was no
investigation into the effects of MT2 receptor agonists, which
could provide additional insights into the mechanisms of action and
further validate the role of the MT2 receptor in mediating the
effects of melatonin. Future studies incorporating MT2 receptor
agonists could provide a more comprehensive understanding of the
therapeutic potential of melatonin in SCI.
Secondly, there was no assessment in the expression
of the MT2 receptor in different tissues of melatonin-treated mice.
Examining the expression of MT2 receptors in various tissues would
provide valuable information regarding the specificity and
distribution of the receptor, and further elucidate its involvement
in the observed neuroprotective effects of melatonin.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by The First Affiliated
Hospital of Zhengzhou University (grant no. YNQN2017042).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
LY made significant contributions to
conceptualization. XH and MZ conducted the investigation and
validated the data. HK interpreted the data and drafted the
manuscript. HL and TC designed and conceptualized the study, and
revised the essential content of the manuscript. All authors read
and approved the final version of the manuscript. LY and XH confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved (approval no. ZZUIRB
2022-145) by the Ethics Committee of The First Affiliated Hospital
of Zhengzhou University (Zhengzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zipser CM, Cragg JJ, Guest JD, Fehlings
MG, Jutzeler CR, Anderson AJ and Curt A: Cell-based and
stem-cell-based treatments for spinal cord injury: Evidence from
clinical trials. Lancet Neurol. 21:659–670. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jiang B, Sun D, Sun H, Ru X, Liu H, Ge S,
Fu J and Wang W: Prevalence, incidence, and external causes of
traumatic spinal cord injury in China: A nationally representative
cross-sectional survey. Front Neurol. 12(784647)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Fleming JC, Norenberg MD, Ramsay DA,
Dekaban GA, Marcillo AE, Saenz AD, Pasquale-Styles M, Dietrich WD
and Weaver LC: The cellular inflammatory response in human spinal
cords after injury. Brain. 129:3249–3269. 2006.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Routhe LJ and Moos T: Handling iron in
restorative neuroscience. Neural Regen Res. 10:1558–1559.
2015.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Fan B, Wei Z, Yao X, Shi G, Cheng X, Zhou
X, Zhou H, Ning G, Kong X and Feng S: Microenvironment imbalance of
spinal cord injury. Cell Transplant. 27:853–866. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Higgins LG, Kelleher MO, Eggleston IM,
Itoh K, Yamamoto M and Hayes JD: Transcription factor Nrf2 mediates
an adaptive response to sulforaphane that protects fibroblasts in
vitro against the cytotoxic effects of electrophiles, peroxides and
redox-cycling agents. Toxicol Appl Pharmacol. 237:267–280.
2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sajadimajd S and Khazaei M: Oxidative
stress and cancer: The role of Nrf2. Curr Cancer Drug Targets.
18:538–557. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Wang J, Fields J, Zhao C, Langer J,
Thimmulappa RK, Kensler TW, Yamamoto M, Biswal S and Doré S: Role
of Nrf2 in protection against intracerebral hemorrhage injury in
mice. Free Radic Biol Med. 43:408–414. 2007.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chang CF, Cho S and Wang J:
(-)-Epicatechin protects hemorrhagic brain via synergistic Nrf2
pathways. Ann Clin Transl Neurol. 1:258–271. 2014.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Dodson M, Castro-Portuguez R and Zhang DD:
NRF2 plays a critical role in mitigating lipid peroxidation and
ferroptosis. Redox Biol. 23(101107)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhang X, Wu Q, Lu Y, Wan J, Dai H, Zhou X,
Lv S, Chen X, Zhang X, Hang C and Wang J: Cerebroprotection by
salvianolic acid B after experimental subarachnoid hemorrhage
occurs via Nrf2- and SIRT1-dependent pathways. Free Radic Biol Med.
124:504–516. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lan X, Han X, Li Q and Wang J:
(-)-Epicatechin, a natural flavonoid compound, protects astrocytes
against hemoglobin toxicity via Nrf2 and AP-1 signaling pathways.
Mol Neurobiol. 54:7898–7907. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ulasov AV, Rosenkranz AA, Georgiev GP and
Sobolev AS: Nrf2/Keap1/ARE signaling: Towards specific regulation.
Life Sci. 291(120111)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang J, Jiang C, Zhang K, Lan X, Chen X,
Zang W, Wang Z, Guan F, Zhu C, Yang X, et al: Melatonin receptor
activation provides cerebral protection after traumatic brain
injury by mitigating oxidative stress and inflammation via the Nrf2
signaling pathway. Free Radic Biol Med. 131:345–355.
2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Suzuki T and Yamamoto M: Stress-sensing
mechanisms and the physiological roles of the Keap1-Nrf2 system
during cellular stress. J Biol Chem. 292:16817–16824.
2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ren H, Han R, Liu X, Wang L, Koehler RC
and Wang J: Nrf2-BDNF-TrkB pathway contributes to cortical
hemorrhage-induced depression, but not sex differences. J Cereb
Blood Flow Metab. 41:3288–3301. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jia P, Wang J, Ren X, He J, Wang S, Xing
Y, Chen D, Zhang X, Zhou S, Liu X, et al: An enriched environment
improves long-term functional outcomes in mice after intracerebral
hemorrhage by mechanisms that involve the Nrf2/BDNF/glutaminase
pathway. J Cereb Blood Flow Metab. 43:694–711. 2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Liu Z, Gan L, Luo D and Sun C: Melatonin
promotes circadian rhythm-induced proliferation through
Clock/histone deacetylase 3/c-Myc interaction in mouse adipose
tissue. J Pineal Res. 62:2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rosales-Corral SA, Acuña-Castroviejo D,
Coto-Montes A, Boga JA, Manchester LC, Fuentes-Broto L, Korkmaz A,
Ma S, Tan DX and Reiter RJ: Alzheimer's disease: Pathological
mechanisms and the beneficial role of melatonin. J Pineal Res.
52:167–202. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Naskar A, Prabhakar V, Singh R, Dutta D
and Mohanakumar KP: Melatonin enhances L-DOPA therapeutic effects,
helps to reduce its dose, and protects dopaminergic neurons in
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinsonism
in mice. J Pineal Res. 58:262–274. 2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hardeland R: Melatonin: Signaling
mechanisms of a pleiotropic agent. Biofactors. 35:183–192.
2009.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Liu J, Clough SJ, Hutchinson AJ,
Adamah-Biassi EB, Popovska-Gorevski M and Dubocovich ML: MT1 and
MT2 melatonin receptors: A therapeutic perspective. Annu Rev
Pharmacol Toxicol. 56:361–383. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang J, Cheng T, Chen Y, Gao F, Guan F
and Yao M: A chitosan-based thermosensitive scaffold loaded with
bone marrow-derived mesenchymal stem cells promotes motor function
recovery in spinal cord injured mice. Biomed Mater.
15(035020)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Han X, Zhao X, Lan X, Li Q, Gao Y, Liu X,
Wan J, Yang Z, Chen X, Zang W, et al: 20-HETE synthesis inhibition
promotes cerebral protection after intracerebral hemorrhage without
inhibiting angiogenesis. J Cereb Blood Flow Metab. 39:1531–1543.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Lan X, Han X, Li Q, Li Q, Gao Y, Cheng T,
Wan J, Zhu W and Wang J: Pinocembrin protects hemorrhagic brain
primarily by inhibiting toll-like receptor 4 and reducing M1
phenotype microglia. Brain Behav Immun. 61:326–339. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Singhal NK, Srivastava G, Patel DK, Jain
SK and Singh MP: Melatonin or silymarin reduces maneb- and
paraquat-induced Parkinson's disease phenotype in the mouse. J
Pineal Res. 50:97–109. 2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Kang JW and Lee SM: Melatonin inhibits
type 1 interferon signaling of toll-like receptor 4 via heme
oxygenase-1 induction in hepatic ischemia/reperfusion. J Pineal
Res. 53:67–76. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ding K, Wang H, Xu J, Li T, Zhang L, Ding
Y, Zhu L, He J and Zhou M: Melatonin stimulates antioxidant enzymes
and reduces oxidative stress in experimental traumatic brain
injury: The Nrf2-ARE signaling pathway as a potential mechanism.
Free Radic Biol Med. 73:1–11. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
El-Sokkary GH, Nafady AA and Shabash EH:
Melatonin administration ameliorates cadmium-induced oxidative
stress and morphological changes in the liver of rat. Ecotoxicol
Environ Saf. 73:456–463. 2010.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Chern CM, Liao JF, Wang YH and Shen YC:
Melatonin ameliorates neural function by promoting endogenous
neurogenesis through the MT2 melatonin receptor in ischemic-stroke
mice. Free Radic Biol Med. 52:1634–1647. 2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Basso DM, Fisher LC, Anderson AJ, Jakeman
LB, McTigue DM and Popovich PG: Basso Mouse Scale for locomotion
detects differences in recovery after spinal cord injury in five
common mouse strains. J Neurotrauma. 23:635–659. 2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Guo L, Lv J, Huang YF, Hao DJ and Liu JJ:
Bioinformatics analyses of differentially expressed genes
associated with spinal cord injury: A microarray-based analysis in
a mouse model. Neural Regen Res. 14:1262–1270. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Cheng T, Wang W, Li Q, Han X, Xing J, Qi
C, Lan X, Wan J, Potts A, Guan F and Wang J: Cerebroprotection of
flavanol (-)-epicatechin after traumatic brain injury via
Nrf2-dependent and -independent pathways. Free Radic Biol Med.
92:15–28. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Han X, Li Q, Lan X, El-Mufti L, Ren H and
Wang J: Microglial depletion with clodronate liposomes increases
proinflammatory cytokine levels, induces astrocyte activation, and
damages blood vessel integrity. Mol Neurobiol. 56:6184–6196.
2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cheng T, Yang B, Li D, Ma S, Tian Y, Qu R,
Zhang W, Zhang Y, Hu K, Guan F and Wang J: Wharton's jelly
transplantation improves neurologic function in a rat model of
traumatic brain injury. Cell Mol Neurobiol. 35:641–649.
2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Li Q, Lan X, Han X and Wang J: Expression
of Tmem119/Sall1 and Ccr2/CD69 in FACS-sorted microglia- and
monocyte/macrophage-enriched cell populations after intracerebral
hemorrhage. Front Cell Neurosci. 12(520)2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kroner A, Greenhalgh AD, Zarruk JG, Passos
Dos Santos R, Gaestel M and David S: TNF and increased
intracellular iron alter macrophage polarization to a detrimental
M1 phenotype in the injured spinal cord. Neuron. 83:1098–1116.
2014.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Fenn AM, Henry CJ, Huang Y, Dugan A and
Godbout JP: Lipopolysaccharide-induced interleukin (IL)-4
receptor-α expression and corresponding sensitivity to the M2
promoting effects of IL-4 are impaired in microglia of aged mice.
Brain Behav Immun. 26:766–777. 2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Barbiellini Amidei C, Salmaso L, Bellio S
and Saia M: Epidemiology of traumatic spinal cord injury: A large
population-based study. Spinal Cord. 60:812–819. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Welp A, Manz B and Peschke E: Development
and validation of a high throughput direct radioimmunoassay for the
quantitative determination of serum and plasma melatonin
(N-acetyl-5-methoxytryptamine) in mice. J Immunol Methods. 358:1–8.
2010.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chen LY, Tiong C, Tsai CH, Liao WC, Yang
SF, Youn SC, Mai FD and Chang HM: Early-life sleep deprivation
persistently depresses melatonin production and bio-energetics of
the pineal gland: Potential implications for the development of
metabolic deficiency. Brain Struct Funct. 220:663–676.
2015.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Ebisawa T, Karne S, Lerner MR and Reppert
SM: Expression cloning of a high-affinity melatonin receptor from
Xenopus dermal melanophores. Proc Natl Acad Sci USA. 91:6133–6137.
1994.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Reppert SM, Godson C, Mahle CD, Weaver DR,
Slaugenhaupt SA and Gusella JF: Molecular characterization of a
second melatonin receptor expressed in human retina and brain: The
Mel1b melatonin receptor. Proc Natl Acad Sci USA. 92:8734–8738.
1995.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Cecon E, Liu L and Jockers R: Melatonin
receptor structures shed new light on melatonin research. J Pineal
Res. 67(e12606)2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Giannoni-Guzmán MA, Kamitakahara A,
Magalong V, Levitt P and McMahon DG: Circadian photoperiod alters
TREK-1 channel function and expression in dorsal raphe serotonergic
neurons via melatonin receptor 1 signaling. J Pineal Res.
70(e12705)2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Li H, Wang Y, Feng D, Liu Y, Xu M, Gao A,
Tian F, Zhang L, Cui Y, Wang Z and Chen G: Alterations in the time
course of expression of the Nox family in the brain in a rat
experimental cerebral ischemia and reperfusion model: Effects of
melatonin. J Pineal Res. 57:110–119. 2014.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Van Broeckhoven J, Sommer D, Dooley D,
Hendrix S and Franssen AJPM: Macrophage phagocytosis after spinal
cord injury: When friends become foes. Brain. 144:2933–2945.
2021.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Das BN, Kim YW and Keum YS: Mechanisms of
Nrf2/Keap1-dependent phase II cytoprotective and detoxifying gene
expression and potential cellular targets of chemopreventive
isothiocyanates. Oxid Med Cell Longev. 2013(839409)2013.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Herrera-Arozamena C, Martí-Marí O, Estrada
M, de la Fuente Revenga M and Rodríguez-Franco MI: Recent advances
in neurogenic small molecules as innovative treatments for
neurodegenerative diseases. Molecules. 21(1165)2016.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zhang Z, Zhang Z, Lu H, Yang Q, Wu H and
Wang J: Microglial polarization and inflammatory mediators after
intracerebral hemorrhage. Mol Neurobiol. 54:1874–1886.
2017.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Jia Y, Lu T, Chen Q, Pu X, Ji L, Yang J
and Luo C: Exosomes secreted from sonic hedgehog-modified bone
mesenchymal stem cells facilitate the repair of rat spinal cord
injuries. Acta Neurochir (Wien). 163:2297–2306. 2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Xi K, Gu Y, Tang J, Chen H, Xu Y, Wu L,
Cai F, Deng L, Yang H, Shi Q, et al: Microenvironment-responsive
immunoregulatory electrospun fibers for promoting nerve function
recovery. Nat Commun. 11(4504)2020.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Yuan J, Chen L, Wang J, Xia S, Huang J,
Zhou L, Feng C, Hu X, Zhou Z and Ran H: Adenosine A2A receptor
suppressed astrocyte-mediated inflammation through the inhibition
of STAT3/YKL-40 axis in mice with chronic cerebral
hypoperfusion-induced white matter lesions. Front Immunol.
13(841290)2022.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Zhang C, Yan Z, Maknojia A, Riquelme MA,
Gu S, Booher G, Wallace DJ, Bartanusz V, Goswami A, Xiong W, et al:
Inhibition of astrocyte hemichannel improves recovery from spinal
cord injury. JCI Insight. 6(e134611)2021.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Linnerbauer M, Wheeler MA and Quintana FJ:
Astrocyte crosstalk in CNS inflammation. Neuron. 108:608–622.
2020.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Lan X, Han X, Li Q, Yang QW and Wang J:
Modulators of microglial activation and polarization after
intracerebral haemorrhage. Nat Rev Neurol. 13:420–433.
2017.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Lan X, Han X, Liu X and Wang J:
Inflammatory responses after intracerebral hemorrhage: From
cellular function to therapeutic targets. J Cereb Blood Flow Metab.
39:184–186. 2019.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhu H, Wang Z, Yu J, Yang X, He F, Liu Z,
Che F, Chen X, Ren H, Hong M and Wang J: Role and mechanisms of
cytokines in the secondary brain injury after intracerebral
hemorrhage. Prog Neurobiol. 178(101610)2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Mills CD, Kincaid K, Alt JM, Heilman MJ
and Hill AM: M-1/M-2 macrophages and the Th1/Th2 paradigm. J
Immunol. 164:6166–6173. 2000.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Mills CD, Shearer J, Evans R and Caldwell
MD: Macrophage arginine metabolism and the inhibition or
stimulation of cancer. J Immunol. 149:2709–2714. 1992.PubMed/NCBI
|