Introduction
Central nervous system (CNS) aspergillosis is relatively uncommon but tends to occur in immunocompromised patients, arising most commonly from hematogenous dissemination of pulmonary aspergillosis or infection spread of the paranasal sinus aspergillosis. Reports (1,2) of CNS aspergillosis attributed to iatrogenic/penetrating trauma, and procedure-related contamination are also available. However, the symptoms of CNS aspergillosis are atypical, often resulting in confusion with cerebral abscesses or space-occupying lesions (3), making diagnosis difficult and leading to a high rate of misdiagnosis and mistreatment (4,5). The current study presents a case of intracranial Aspergillus infection in a patient with acute lymphoid leukemia (ALL), which had the following characteristics: i) The patient had only fever symptoms in the early stage, and no obvious symptoms of sepsis, so the clinical symptoms were mild and easy to miss diagnosis; ii) The source of infection in this case was considered to be lumbar puncture, which is a recognized but rare source of infection and deserves the attention of medical personnel; iii) metagenomic next generation sequencing (NGS) testing method was adopted in this case, which is a relatively sensitive method for intracranial infection detection and worthy of clinical promotion (6).
Case report
Diagnosis and chemotherapy for ALL. A 56-year-old male patient was admitted to Ruijin Hospital Affiliated to Shanghai Jiao Tong University (Shanghai, China) in early February 2022 due to ‘fatigue for a week’. After a bone marrow puncture, a preliminary diagnosis considered ALL. After excluding contraindications, two sessions of chemotherapy were prescribed (specific chemotherapy regimen unknown). Later, the patient was hospitalized at the Hematology Department of Huzhou First People's Hospital (Huzhou, China) on February 22, 2022 and received regular chemotherapy therein. Within eight months, the patient underwent seven sessions of chemotherapy. During this period, the patient also received five lumbar punctures plus intrathecal injections (cytarabine 50 mg + dexamethasone 5 mg) as precautionary measures against intracranial metastasis. During the last hospitalization for chemotherapy between September 26, 2022 and November 10, 2022, a routine blood test of the patient showed a significant decrease in neutrophils, which were 0.04x109/l while white blood cells and lymphocytes were severely suppressed. On October 8, 2022, fever was identified in the patient for the first time, with the body temperature as high as 39˚C. After examination, it was found that the blood culture and 1, 3-β-D-glucan (G) test results were negative and no obvious infection foci were detected in the lung computerized tomography (CT). In response to this, the patient was given the coadministration of cefoperazone sulbactam and fluconazole sodium chloride injection, but the clinical response was not satisfactory. Afterwards, the therapy regimen was changed to imipenem-cilastatin sodium injection in combination with voriconazole tablets. The body temperature became lower as a result. Furthermore, treatment to boost the blood cell count was given. Although the patient had fever, there were no signs of sepsis such as shortness of breath and rapid heartbeat and no symptoms of systemic organ toxicity. However, low-grade fever occurred in the patient on October 16 after ~5 days of normal body temperature, registering a maximum temperature of 38.3˚C. Perianal infection was suspected in this case and vancomycin was administered against positive bacterial infections. The patient's perianal abscess was treated with regular local dressing changes and gradually resolved. During this period, the patient complained of transient blurred vision in the left eye. After a comprehensive cranial magnetic resonance imaging (MRI) examination, an intracranial occupying lesion was considered in the right occipital lobe. However, considering that the patient developed myelosuppression following chemotherapy, including low platelets, no surgical or lumbar puncture examination was performed. The patient was discharged on November 10, 2022 following treatment.
Surgical treatment
The patient was readmitted on November 28, 2022. Preoperative contrast-enhanced MRI (enhanced T1) of the brain revealed a right occipital lobe mass with increased edema compared to the previous imaging result (Fig. 1), suggesting disease progression. The preoperative lung CT scan did not indicate obvious signs of lung infection and the patient had no obvious respiratory symptoms such as fever, coughing, phlegm, or runny nose. In addition, the patient's routine blood test did not exhibit signs of a bacterial infection. Finally, the patient underwent surgical treatment. During the procedure, a few pale-yellow changes were observed on the brain surface and the adhesion of the space-occupying lesion to the surrounding brain tissue was obvious with a thick capsule containing pus. Some of the pus was taken for NGS and culture, and the capsule tissue was sent for routine pathological examination (Fig. 2).
 |
Figure 1
Head magnetic resonance imaging (enhanced T1) of the patient was performed before the operation. The arrow in the picture indicated an intracranial occupying lesion that was considered in the right occipital lobe, but the edema was worse than before, indicating that the disease had progressed.
|
 |
Figure 2
During the operation. (A) A few pale-yellow changes were observed on the brain surface and the adhesion of the space-occupying lesion to the surrounding brain tissue was obvious with a thick capsule containing pus. (B) There was a brain abscess-like lesion with coffee-colored pus inside. Some of the pus was taken for metagenomic next generation sequencing and culture. (C) The capsule was complete and was completely resected.
|
Specimen analysis
The patient's pus specimen was observed under a fluorescence microscope at x400 magnification, where Aspergillus hyphae were detected. The routine pathological examination of the capsule tissue indicated aspergillosis with abscess formation in the right occipital lobe. NGS testing (Genskey Medical Biotechnology Co, Ltd.; report no. MBX127334) of the pus indicated infection with Aspergillus udagawae (Fig. 3). The NGS method was as follows: Samples and negative batch controls were extracted by Genskey Micro DNA Kit (cat. no. 1901; Genskey Medical Biotechnology Co, Ltd.) and measured by Qubit dsDNA HS Assay Kits. The DNA libraries were constructed with an NGS library construction kit (cat. no. 2012B; Genskey Medical Biotechnology Co, Ltd.). The quality of DNA libraries was assessed using the Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.); and the concentration was determined by Qubit and must be >1 ng/µl (standard). Following the thermal denaturation (conventional process: 98˚C for 20 sec, 60˚C for 15 sec and 72˚C for 30 sec, for 10 cycles; and an RNA process: 98˚C for 20 sec, 60˚C for 15 sec and 72˚C for 30 sec, for 16 cycles) and mixing of the library, DNA Nanoball was prepared. All the libraries were sequenced with single-stranded circular DNA and 2-3 quantitative sets were added to obtain DNA nanospheres. The DNA nanospheres were loaded on the sequencing chip and sequenced using the MGISEQ-200 sequencing platform (MGI Tech Co., Ltd.). For sequencing data quality control, quality control-like adapter contamination and low-quality and low-complexity reads, raw reads were filtered by fastp (v0.22.0) (7) and Komplexity (v0.3.6) (https://github.com/eclarke/komplexity) Reads that were mapped to human reference assembly GRCh38 were removed with bowtie2 (v2.3.5.1) (8).
 |
Figure 3
Specimen analysis results. (A) The patient's pus specimen was stained with fluorescent dyes and observed under a microscope (magnification, x400) where Aspergillus hyphae were detected. (B) Metagenomic next generation sequencing testing of the pus indicated infection with Aspergillus udagawae. (C) The routine pathological examination of the capsule tissue indicated aspergillosis with abscess formation in the right occipital lobe (hematoxylin and eosin staining; magnification, x200). The arrow indicates Aspergillus. (D) The routine pathological examination of the capsule tissue indicated aspergillosis with abscess formation in the right occipital lobe (PAS, magnification, x200). The arrow indicates Aspergillus.
|
Patient prognosis
After being treated with voriconazole (0.2 g; Q12 h) for two straight weeks, the patient recovered well, with the body temperature back to normal range. The follow-up CT brain imaging results were also found satisfactory (Fig. 4). Since the patient required further chemotherapy, he was referred to the hematology department of The First People's Hospital of Huzhou for further anti-infective therapy. Voriconazole was used for intensive treatment (0.2 g; Q12 h) in the first 3 months. Following that, the patient's temperature was normal and his condition was generally stable and the patient has now entered maintenance treatment (0.1 g; Q12 h). No additional PCR or NGS tests were performed following surgery, cerebrospinal fluid test and enhanced head MRI were reviewed in June 13, 2023. Fortunately, the patient's head MRI (enhanced T1) showed no obvious enhancement of the disease (Fig. 5). There was no increase in micrototal protein (Pyrogallol red colorimetry) and no decrease in sugar content in cerebrospinal fluid test, suggesting that there was no abnormal index of cerebrospinal fluid infection. Although it cannot be ruled out that the patient has a recessive Aspergillus infection, treatment is still effective at the time of writing. In the later stage, the authors recommend that patients undergo NGS testing of cerebrospinal fluid to further determine whether there is a recurrence of Aspergillus.
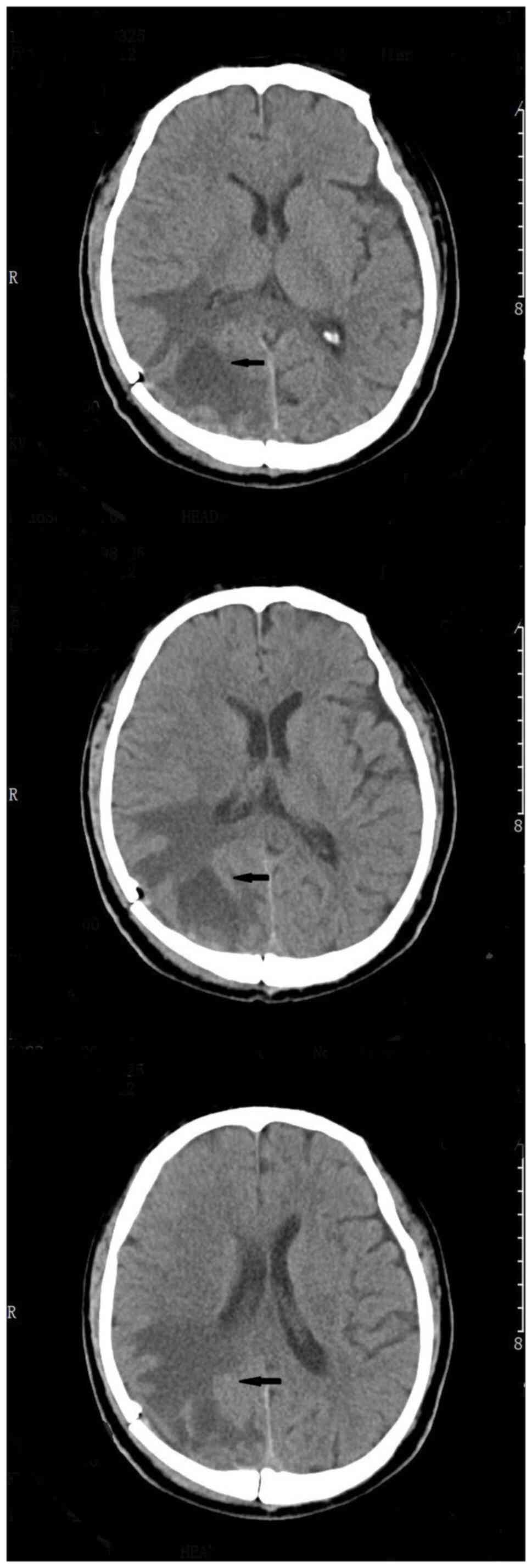 |
Figure 4
Head computed tomography examination after operation showed satisfactory results. The arrows in the images indicate the operating area. Most of the lesions were resected without postoperative bleeding.
|
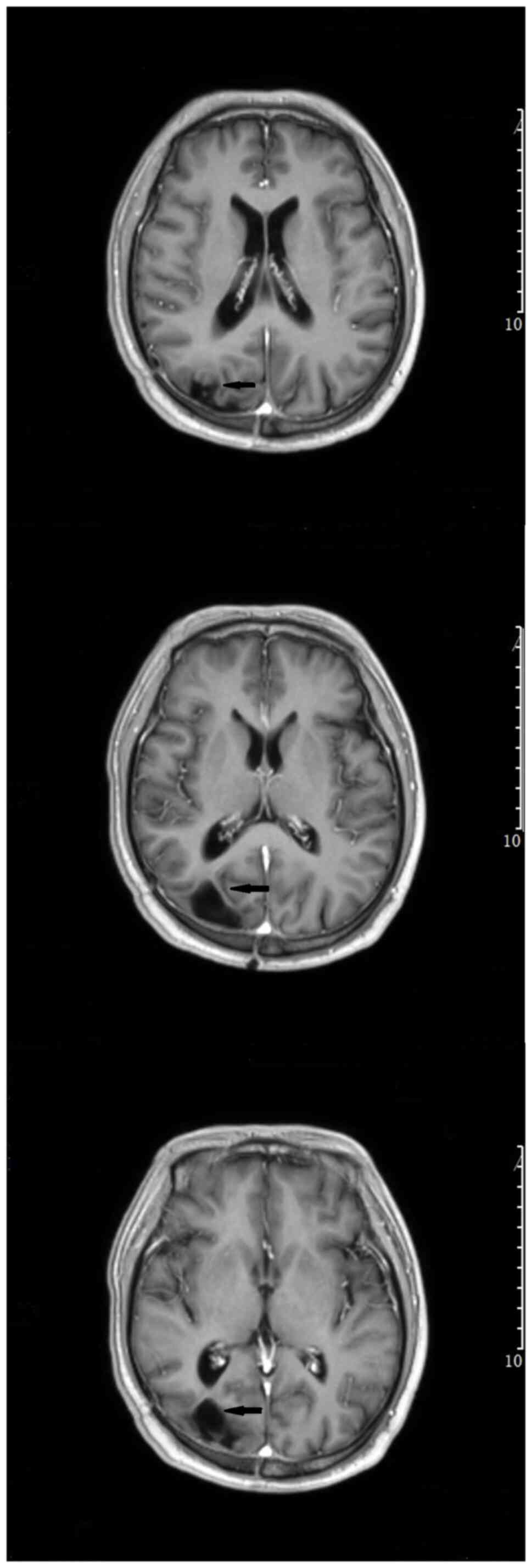 |
Figure 5
Head magnetic resonance imaging (enhanced T1) of the patient was performed at 7 months after surgery. The arrows in the picture indicate no obvious enhancement of the disease.
|
Discussion
Widely present in nature, Aspergillus can adhere to the surface of human skin, but generally does not cause infection. A few aspergilli that enter the human body can be blocked by the body's natural immune system. However, in case the quantity of invasive Aspergillus is relatively large, or the person is under immunosuppression or immunodeficiency especially when neutrophils are reduced, Aspergillus can quickly reproduce, inflicting harm on the body (9). Therefore, the most common accompanying symptom of cerebral aspergillosis reported in the literature is hematological malignancies while neutrophil depletion and the application of corticosteroids are considered the primary risk factors (10). CNS aspergillosis generally results from hematogenous infection of pulmonary aspergillosis and direct invasion of paranasal sinus aspergillosis, as well as some hospital-acquired infections such as surgery or lumbar puncture (11,12). CNS aspergillosis can lead to brain abscess, brain granuloma, cerebrovascular aspergillosis or cerebral mycotic aneurysms, which can cause symptoms such as fever, headache, seizures, hemiplegia and neurological dysfunction in patients. However, most of the clinical manifestations tend to be nonspecific, and most patients do not present with obvious symptoms in the early stage. Furthermore, most imaging results indicate brain abscess or occupying lesions, which makes differential diagnosis difficult. To address those concerns, laboratory tests after surgery are warranted to establish the diagnosis.
For the treatment of CNS aspergillosis, the first step is to treat the underlying disease and eliminate risk factors, such as neutropenia or immunosuppression, as much as possible while discontinuing the dosing of glucocorticoids. Meanwhile, active treatment should be implemented for pulmonary aspergillosis or sinus aspergillosis. In terms of drug selection, itraconazole or amphotericin B are often adopted, because of their broad antibacterial spectrum. Currently, voriconazole (13) is often the choice due to its ability to penetrate the blood-brain barrier with a concentration beyond minimum inhibitory concentration in the cerebrospinal fluid, significantly improving the therapeutic effectiveness of the treatment of CNS aspergillosis (3). In addition, clinicians have learned the drug interaction between voriconazole and cytochrome P450 isoforms, CY3A4, CYP2C9 and CYP2C19 corticosteroids, which may lead to a decline in voriconazole plasma concentration, thus inhibiting its therapeutic effectiveness on Aspergillus (14). Therefore, the voriconazole plasma concentration needs to be analyzed ~5 days following administration, in order to achieve the minimum inhibitory concentration (15). When necessary, CYP2C19 genotyping should be performed to determine the metabolic status predicted with the gene in order to prevent the failure of voriconazole treatment. The selection of the surgical method should be made as following the patient's condition, physical status, intracranial lesions and the surgeon's expertise (16). Considering that CNS aspergillosis mostly occurs in immunocompromised patients with underlying disease and the unintended infections introduced during the surgery, the optimal treatment regimen for those patients is surgical treatments in combination with antifungal therapy.
This is particularly true for patients with hematological malignancies (17,18), with ALL being the most common blood system disease, especially in children. Therefore, for patients with hematological malignancies who are long-term users of immunosuppressants, clinicians should be cautious about the possibility of aspergillosis. Efforts should be made for early detection, diagnosis and treatment, which are vital to achieving a favorable prognosis. For the early diagnosis of intracranial Aspergillus infection, the authors proposed the following methods through literature review combined with the present case: i) Cerebrospinal fluid examination (routine, biochemical and culture) should be performed as soon as possible. The routine and biochemical examination of cerebrospinal fluid in patients with intracranial Aspergillus infection can be normal, or it can also be manifested as increased white blood cell count, increased protein and decreased glucose, with low sensitivity. Some Aspergillus can be cultured, but the positive rate is not high (~10%) (19). Therefore, the cerebrospinal fluid test was repeated a number of times to prevent false negative. ii) 1, 3-β-D-glucan (G) test and galactomannan (GM) test are the most commonly used detection methods for the early diagnosis of invasive fungal disease, with high sensitivity, but not strong specificity for Aspergillus (20), GM test is a common method for the early diagnosis of invasive fungal disease, mainly detecting cerebrospinal fluid and serum, with good sensitivity and specificity and is one of the main early detection methods. Naturally, the two methods should be repeated a number of times. iii) Enhanced head MRI is not highly sensitive to early intracranial Aspergillus infection and when no abscess is formed or there is no evident space occupation, it is often unable to identify the cause. Moreover, when the lesions can be detected by imaging, it is usually in the middle and late stages of the disease and the effect of single drug therapy is no longer useful, but it is still an important imaging examination and can be used as a basis for the progression and prognosis of the disease. In addition, the authors recommended that lung CT and sinus MRI be improved to look for possible sources of infection. iv) Molecular biological examination, PCR + NGS and the application of PCR technology is helpful to further identify Aspergillus species in the sterile tissue of patients and it was found with three mutations. There are certain mutations related to drug resistance of azole drugs, but there is a lack of unified clinical standards at present. NGS is a high-throughput sequencing technology independent of in vitro culture, which can detect the nucleic acid sequence of pathogens in non-targeted clinical specimens and has high application value for the pathogenic diagnosis of infectious diseases. The sensitivity and specificity of intracranial Aspergillus infection diagnosis were 85.7 and 84.6%, respectively (21,22).
The present study collected and summarized the case data of hematological malignancies accompanied by CNS Aspergillus infection through PubMed/Medline (https://pubmed.ncbi.nlm.nih.gov/) in the recent 10 years. In addition to the present study, ~24 cases were reported. Among them, 18 were males, 6 were females and 1 was unknown. The mean age was 43.5±26.9. Fever was the main symptom in 16 cases. There were eight cases of septicemia or sepsis and the rest were related neurological symptoms without specificity. In addition to the unclear source of infection, 15 cases were mostly pulmonary Aspergillus, two cases were related to surgery and three were other causes, while the only cases that considered infection caused by lumbar puncture were reported in the present study. Histopathologic Examination and culture were the most commonly used diagnostic methods, and PCR was used in four cases. NGS was only reported in the present study. The most frequently used drug was voriconazole, accounting for 22 cases and with the improvement of the drug, the disease improvement rate of patients significantly advanced compared with previous use, accounting for 19 cases (Table I) (23-42).
 |
Table I
Clinical profiles of 25 cases of hematologic malignancy with central nervous system aspergillosis in the recent 10 years.
|
Table I
Clinical profiles of 25 cases of hematologic malignancy with central nervous system aspergillosis in the recent 10 years.
| First author, year |
Sex |
Age |
Etiology |
Clinical manifestation |
Source of infection |
Diagnostic method |
Treatment |
Outcome |
(Refs.) |
| Amanati et al, 2020 |
Male |
18 months |
ALL |
Loss of consciousness, seizure, sepsis |
Pulmonary aspergillosis |
HE |
Surgical, MB, voriconazole |
Death |
(1) |
| Le et al, 2020 |
Male |
74 years |
CLL |
Progressive imbalance, unsteady gait, left occipital headache and intermittent confusion |
Uncertain |
HE and CSF culture |
Surgical, voriconazole |
Improved |
(23) |
| McCarter et al, 2019 |
Male |
79 years |
B-ALL |
Confusion, anorexia and failure to thrive |
Pulmonary aspergillosis |
HE, culture |
Voriconazole, caspofungin |
Not reported |
(24) |
| Eichenberge et al r, 2019 |
Male |
62 years |
CLL |
Fevers, aphasia, confusion and profound expressive aphasia |
Pulmonary aspergillosis |
HE, culture |
Voriconazole |
Improved |
(25) |
| Peddada et al, 2018 |
Male |
48 years |
B-ALL |
Left eye progressive vision loss, tearing and redness then declining mental status |
Virulent Aspergillus endophthalmitis |
HE, culture |
Voriconazole, MB |
Improved |
(26) |
| McCaslin et al, 2015 |
Female |
19 years |
ALL |
Extremity weakness and fevers |
Adjacent vertebral osteomyelitis |
Culture, GM assay |
Surgical, voriconazole |
Death |
(27) |
| Lin et al, 2014 |
Female |
32 years |
T-ALL |
Septic shock, febrile neutropenia, acute hypoxic respiratory failure, right facial droop, severe aphasia, with right upper and right lower extremity paresis |
Pulmonary aspergillosis |
GM assay |
Voriconazole |
Improved |
(28) |
| Davoudi et al, 2014 |
Female |
24 years |
AML |
Fever, chills, diarrhea and malaise |
Uncertain |
HE |
Surgical, LMB, voriconazole, caspofungin |
Improved |
(29) |
| Beresford et al, 2019 |
Male |
66 years |
CLL |
Confusion and expressive dysphasia |
Uncertain |
HE, culture and PCR |
Surgical, voriconazole, posaconazole |
Improved |
(30) |
| Peng et al, 2015 |
Male |
53 years |
APL |
Confusion, left lower extremity movement disorder and dyspnea |
Pulmonary aspergillosis |
Culture of sputum |
Itraconazole, caspofungin |
Improved |
(31) |
| Gaye et al, 2018 |
Male |
65 years |
CLL |
Light-headedness, balance disorder, and right hemiparesis |
Pulmonary aspergillosis |
Culture |
Voriconazole, LAMB and reduction of the ibrutinib dose |
Improved |
(32) |
| |
Male |
75 years |
CLL |
Fever, visual impairment and ataxia, generalized seizure |
Pulmonary aspergillosis |
HE, PCR, negative CSF culture |
Voriconazole, prednisone |
Improved |
|
| Rouzaud et al, 2019 |
Female |
39 years |
CLL |
Headache, seizures and fever |
Uncertain |
HE of surgical samples and culture |
LAMB, voriconazole, isavuconazole, ibrutinib |
Not reported |
(33) |
| Pouvaret et al, 2019 |
Female |
52 years |
CLL |
Fever, confusion, behavior disorders and aggression |
Hematogenous dissemination |
Culture of brain biopsy |
LAMB, voriconazole, isavuconazole, ibrutinib |
Improved |
(34) |
| Nyga et al, 2020 |
Male |
69 years |
CLL |
Left miosis and a balance disorder |
Pulmonary aspergillosis |
Culture of bronchoalveolar lavage |
LAMB, voriconazole, isavuconazole |
Improved |
(14) |
| Furtwängler et al, 2017 |
Male |
22 months |
T-ALL |
Fever and lymphadenitis, left sided hemiparesis |
Surgery-related |
HE, negative culture |
LAMB, caspofungin, voriconazole |
Improved |
(2) |
| Turki et al, 2017 |
Male |
52 years |
T-LGL |
Acute strong nausea, vomiting, fever, relapsing focal seizures of right arm, paresthesia and motoric weakness |
Pulmonary aspergillosis |
HE, negative culture and PCR |
Voriconazole, LAMB |
Improved |
(35) |
| Matis et al, 2013 |
Male |
32 years |
AML |
Weight-loss, frequent rhinorrhagia, gum swelling, cephalalgia, fatigue, fever, and somnolence, motor nor sensory deficits |
Uncertain |
HE, culture |
AMB |
Death |
(36) |
| Peri et al, 2018 |
Not reported |
57 years |
CLL |
Fever and dyspnea |
Pulmonary aspergillosis |
HE |
Voriconazole |
Improved |
(37) |
| De Leonardis et al, 2020 |
Female |
3 years |
B-ALL |
Fever and pancytopenia, seizure |
Pulmonary aspergillosis |
MRI, CT and GM assay |
Voriconazole then LAMB and isavuconazole |
Improved |
(38) |
| Matsuo et al, 2020 |
Male |
90 years |
CLL |
Headache, fever with altered mental status |
Uncertain |
GM assay |
Voriconazole |
Improved |
(39) |
| Sakata et al, 2021 |
Male |
15 years |
AML |
Dry cough, high fever, right-sided weakness and impaired consciousness |
Surgery-related |
GM assay |
Voriconazole, LAMB and itraconazole |
Death |
(40) |
| Kural et al, 2018 |
Male |
18 years |
ALL |
Clouding of consciousness and tendency toward sleepiness |
Uncertain |
HE |
surgical,AMB |
Improved |
(41) |
| Sadarangani et al, 2015 |
Male |
3 years |
B-ALL |
Fever, leg pain, spontaneous bruising and a petechial rash, right hemiparesis and aphasia |
Uncertain |
HE, PCR from CSF |
Voriconazole, LAMB |
Improved |
(42) |
| Yang et al, 2023 |
Male |
56 years |
ALL |
Fever, transient blurred vision in the left eye |
Lumbar puncture |
HE, culture, NGS |
Surgical, voriconazole |
Improved |
Present study |
In retrospect, the initial diagnosis made for this patient is ALL with a history of long-term use of chemotherapy and steroids, making the patient susceptible to aspergillosis. The patient had a high-grade fever before surgery, which could have been induced by an aspergillosis abscess. The patient also developed blurred vision, which could be related to the location of the aspergillosis abscess and the resulting edema. However, uncertainty remains regarding the mode of transmission because neither clear signs of pulmonary aspergillosis were observed on chest CT nor signs of sinusitis or maxillary sinusitis on cerebral MRI. Furthermore, hematogenous infections rarely occur on this site. Considering all those factors, hospital-acquired infections were suspected. As the patient had undergone multiple intrathecal injections and Aspergillus is widespread on the body surface, chances are infections will occur if disinfection was not thorough and puncture was performed without following the proper procedures. In addition, The First People's Hospital of Huzhou (Zhejiang, China) is a local grass-roots hospital, lacking drug concentration detection means and combined with the economic cost to the patient, the voriconazole concentration detection and the CYP2C19 genotyping status of the patient could not be performed; this is a limitation of the present study. However, it is recommended that hospitals with the ability to perform such testing should do so to determine the effective concentration of the drug.
In summary, this was a rare case of intracranial Aspergillus infection in a patient with ALL. Although the prognosis of intracranial Aspergillus infection is poor, there are still some patients with good prospects and it should be actively treated. Throughout the present case, the treatment experience aimed to promote communication, improve the attention of neurosurgeons to intracranial Aspergillus infection and understand the early diagnosis and treatment of intracranial Aspergillus infection. Doctors should strengthen the concept of asepsis when performing lumbar puncture or related invasive operations on such patients to reduce the possible iatrogenic infection. Finally, since intracranial Aspergillus infection is mostly sporadic, it is hoped that some researchers can summarize or study a larger number of cases in the future, in order to digest the pathogenesis characteristics of such patients and improve the cure rate of intracranial Aspergillus infection.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. NGS raw data is available from the NCBI database, provided by Genskey Medical Technology Co, Ltd. (https://www.ncbi.nlm.nih.gov/bioproject/PRJNA1027136; accession number. PRJNA1027136).
Authors' contributions
TY was involved in the writing of the original draft, in the writing, reviewing and editing of the manuscript and in the collection of clinical data of the patient. YC was involved in the writing, reviewing and editing of the manuscript, in the surgical treatment of the patient and in the collection of clinical data of the patient. YPZ was involved in the pathological analysis of surgical specimens. All authors have read and approved the final manuscript. TY and YC confirm the authenticity of all the raw data.
Ethics approval and consent to participate
The patient provided signed informed consent for the inclusion of the data in the present case report.
Patient consent for publication
The patient provided signed informed consent for publication.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Amanati A, Lotfi M, Masoudi MS, Jafarian H, Ghasemi F, Bozorgi H and Badiee P: Cerebral and pulmonary aspergillosis, treatment and diagnostic challenges of mixed breakthrough invasive fungal infections: Case report study. BMC Infect Dis. 20(535)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Furtwängler R, Schlotthauer U, Gärtner B, Graf N and Simon A: Nosocomial legionellosis and invasive aspergillosis in a child with T-lymphoblastic leukemia. Int J Hyg Environ Health. 220:900–905. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Xue Q, Lei Y, Zhan C, Liao WQ and Hou LJ: Retrospective analysis of cerebral aspergillosis in mainland China from 2000 to 2019. Chin J Mycol. 14:134–140. 2019.
|
|
4
|
Turgut M, Ozsunar Y, Oncü S, Akyüz O, Ertuğrul MB, Tekin C, Gültekin B and Sakarya S: Invasive fungal granuloma of the brain caused by Aspergillus fumigatus: A case report and review of the literature. Surg Neurol. 69:169–174. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kumar D, Nepal P, Singh S, Ramanathan S, Khanna M, Sheoran R, Bansal SK and Patil S: CNS aspergilloma mimicking tumors: Review of CNS Aspergillus infection imaging characteristics in the immunocompetent population. J Neuroradiol. 45:169–176. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chiu CY and Miller SA: Clinical metagenomics. Nat Rev Gen. 20:341–355. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Chen S, Zhou Y, Chen Y and Gu J: fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 34:i884–i890. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Langmead B and Salzberg S: Fast gapped-read alignment with bowtie 2. Nat Methods. 9:357–359. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cherian T, Giakoustidis A, Yokoyama S, Heneghan M, O'Grady J, Rela M, Wendon J, Heaton DN and Verma A: Treatment of refractory cerebral aspergillosis in a liver transplant recipient with voriconazole: Case report and review of the literature. Exp Clin Transplant. 10:482–486. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhu HM, Jia YP, Chen M, Zhao J and Wen H: Antifungal therapy in severe rhinosino-optical-otic and cerebral invasive aspergillosis caused by Aspergillus flavus. Chin J Mycol. 4:90–92. 2009.
|
|
11
|
Ganesh P, Nagarjuna M, Shetty S, Kumar P, Bhat V and Salins PC: Invasive aspergillosis presenting as swelling of the buccal mucosa in an immunocompetent individual. Oral Surg Oral Med Oral Pathol Oral Radiol. 119:e60–e64. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hersh CM, John S, Subei A, Willis MA, Kosmorsky GS, Prayson RA and Bhimraj A: Optic neuropathy and stroke secondary to invasive Aspergillus in an immunocompetent patient. J Neuroophthalmol. 36:404–407. 2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Schwartz S and Thiel E: Cerebral aspergillosis: Tissue penetration is the key. Med Mycol. 47 (Suppl 1):S387–S393. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nyga R, Delette C, Mabille C, Bennis Y, Chouaki T, Boone M, Maizel J, Marolleau JP and Joseph C: Ibrutinib related cerebral aspergillosis successfully treated with isavuconazole: A case report. Leuk Lymphoma. 61:1760–1762. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li YF and Li HJ: A case of AIDS associated Aspergillus infection in brain. Radiol Pract. 27:1038–1039. 2012.
|
|
16
|
Nadkarni T and Goel A: Aspergilloma of the brain: An overview. J Postgrad Med. 51 (Suppl 1):S37–S41. 2005.PubMed/NCBI
|
|
17
|
Koh S, Ross LA, Gilles FH, Nelson MD Jr and Mitchell WG: Myelopathy resulting from invasive aspergillosis. Pediatr Neurol. 19:135–138. 1998.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Christophe C, Azzi N, Bouché B, Dan B, Levivier M and Ferster A: Magnetic resonance imaging and angiography in cerebral fungal vasculitis. Neuropediatrics. 30:218–220. 1999.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Chong GM, Maertens JA, Lagrou K, Driessen GJ, Cornelissen JJ and Rijnders BJ: Diagnostic performance of galactomannan antigen testing in cerebrospinal fluid. J Clin Microbiol. 54:428–431. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
De Vlieger G, Lagrou K, Maertens J, Verbeken E, Meersseman W and Van Wijngaerden E: Beta-D-glucan detection as a diagnostic test for invasive aspergillosis in immunocompromised critically ill patients with symptoms of respiratory infection: An autopsy-based study. J Clin Microbiol. 49:3783–3787. 2011.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Xing XW, Zhang JT, Ma YB, He MW, Yao GE, Wang W, Qi XK, Chen XY, Wu L, Wang XL, et al: Metagenomic next-generation sequencing for diagnosis of infectious encephalitis and meningitis: A large, prospective case series of 213 patients. Front Cell Infect Microbiol. 10(88)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Xing XW, Yu SF, Zhang JT, Tan RS, Ma YB, Tian X, Wang RF, Yao GE, Cui F, Gui QP and Yu SY: Metagenomic next-generation sequencing of cerebrospinal fluid for the diagnosis of cerebral aspergillosis. Front Microbiol. 12(787863)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Le TH, Kumar V, Gondal K, Barnes M, Siddique H, Buttar B and Kaell A: Isolated central nervous system aspergillosis infection in a chronic lymphocytic leukemia patient on Ibrutinib: A case report. BMC Infect Dis. 20(175)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
McCarter SJ, Vijayvargiya P, Sidana S, Nault AM, Lane CE, Lehman JS, Wilson JW, Parikh SA, Nowakowski GS and Al-Kali A: A case of ibrutinib-associated aspergillosis presenting with central nervous system, myocardial, pulmonary, intramuscular, and subcutaneous abscesses. Leuk Lymphoma. 60:559–561. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Eichenberger EM, Saullo J, Brander D, Wang SH, Perfect JR and Messina JA: A case of CNS aspergillosis in a patient with chronic lymphocytic leukemia on first-line ibrutinib therapy. Med Mycol Case Rep. 27:17–21. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Peddada K, Khan NM, Rubin J, Zakaryan H, Liu Y, Popnikolov N, Sangani R and Li W: Diagnosis of vitreoretinal aspergillosis with transvitreal retinochoroidal biopsy. Case Rep Ophthalmol Med. 2018(8306163)2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
McCaslin AF, Lall RR, Wong AP, Lall RR, Sugrue PA and Koski TR: Thoracic spinal cord intramedullary Aspergillus invasion and abscess. J Clin Neurosci. 22:404–406. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lin C, Barrio GA, Hurwitz LM and Kranz PG: Cerebral air embolism from angioinvasive cavitary aspergillosis. Case Rep Neurol Med. 2014(406106)2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Davoudi S, Anderlini P, Fuller GN and Kontoyiannis DP: A long-term survivor of disseminated Aspergillus and mucorales infection: An instructive case. Mycopathologia. 178:465–470. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Beresford R, Dolot V and Foo H: Cranial aspergillosis in a patient receiving ibrutinib for chronic lymphocytic leukemia. Med Mycol Case Rep. 24:27–29. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Peng HL, Yi YF, Shen XH, Yin YF and Zhang GS: Dramatic response to itraconazole in central nervous system aspergillosis complicating acute promyelocytic leukemia. Infect Dis (Lond). 47:104–106. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Gaye E, Le Bot A, Talarmin JP, Le Calloch R, Belaz S, Dupont M and Tattevin P: Cerebral aspergillosis: An emerging opportunistic infection in patients receiving ibrutinib for chronic lymphocytic leukemia? Med Mal Infect. 48:294–297. 2018.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Rouzaud C, Jullien V, Herbrecht A, Palmier B, Lapusan S, Morgand M, Guéry R, Dureault A, Danion F, Puget S, et al: Isavuconazole diffusion in infected human brain. Antimicrob Agents Chemother. 63:e02474–18. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Pouvaret A, Guery R, Montillet M, Molina TJ, Duréault A, Bougnoux ME, Galliot R, Lanternier F, Delarue R and Lortholary O: Concurrent cerebral aspergillosis and abdominal mucormycosis during ibrutinib therapy for chronic lymphocytic leukaemia. Clin Microbiol Infect. 25:771–773. 2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Turki AT, Rashidi-Alavijeh J, Dürig J, Gerken G, Rath PM and Witzke O: Successful treatment of cerebral aspergillosis: Case report of a patient with T-cell large granular lymphocytic leukemia (T-LGL). BMC Infect Dis. 17(797)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Matis GK, Voultsinou D, Chrysou O, Birbilis T and Geroukis T: Cerebral aspergillosis and acute myeloid leukemia. J Neurosci Rural Pract. 4 (Suppl 1):S134–S135. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Peri AM, Bisi L, Cappelletti A, Colella E, Verga L, Borella C, Foresti S, Migliorino GM, Gori A and Bandera A: Invasive aspergillosis with pulmonary and central nervous system involvement during ibrutinib therapy for relapsed chronic lymphocytic leukaemia: Case report. Clin Microbiol Infect. 24:785–786. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
De Leonardis F, Novielli C, Giannico B, Mariggiò MA, Castagnola E and Santoro N: Isavuconazole treatment of cerebral and pulmonary aspergillosis in a pediatric patient with acute lymphoblastic leukemia: Case report and review of literature. J Pediatr Hematol Oncol. 42:e469–e471. 2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Matsuo T, Mori N, Sakurai A and Furukawa K: Aspergillus meningitis in a patient with chronic lymphocytic leukemia. J Infect Chemother. 26:622–624. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Sakata N, Okano M, Masako R, Tanaka A, Yamashita Y, Karasuno T, Imadome KI, Okada M and Sugimoto K: Donor-derived myelodysplastic syndrome after allogeneic stem cell transplantation in a family with germline GATA2 mutation. Int J Hematol. 113:290–296. 2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Kural C, Ozer MI, Ezgu MC, Mehtiyev R, Yasar S, Kutlay AM, Daneyemez MK, Onguru O, Erdogan E and Izci Y: Intracavitary amphotericin B in the treatment of intracranial aspergillosis. J Clin Neurosci. 51:75–79. 2018.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sadarangani M, Harvey M, McDonald A, Speert DP and Dix D: Brain abscesses due to Aspergillus nidulans Infection during induction chemotherapy for acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 37:e384–e386. 2015.PubMed/NCBI View Article : Google Scholar
|



















