Introduction
A total of 18,000,000 individuals die from
cardiovascular diseases worldwide annually, accounting for 31% of
all mortalities for any reason (1). Coronary artery disease (CAD) is
caused by a narrowing of the coronary artery lumen due to
atherosclerosis of the vessel wall. CAD is the main cause of death
from cardiovascular disease, accounting for 45% of total mortality
cases (2). Risk factors for CAD
include hypertension, diabetes, smoking and hypercholesterolemia
(3).
Studies have suggested that vascular inflammation
plays an important role in the initiation, progression, plaque
instability and eventual rupture of atherosclerosis (4,5).
Therefore, various inflammatory biomarkers have attracted
attention, including the systemic immune inflammation index (SII;
calculated as neutrophils x platelets/lymphocytes),
neutrophil-to-lymphocyte ratio (NLR) and platelet-to-lymphocyte
ratio (PLR). Other studies have suggested that these inflammatory
parameters can indicate CAD occurrence, development and severity
(6-8).
They can also be used as independent predictors of cardiovascular
risk, all-cause mortality and CAD (9,10).
Lymphocyte-to-C-reactive protein (CRP) ratio (LCR) is a novel
immune system inflammatory indicator. Okugawa et al
(11) found that LCR can predict
the clinical prognosis of colorectal cancer. Zhang et al
(12) found that LCR is associated
with disease severity in patients with coronavirus disease 2019.
Liu et al (13) found that
LCR can predict the clinical prognosis of ST-segment elevation
myocardial infarction. However, the association between LCR and CAD
occurrence and progression remains unclear. Thus, the present study
aimed to investigate the relationship between LCR and CAD.
Materials and methods
Participants
Patients who underwent concurrent coronary
angiography at the Department of Cardiology, The 904th Hospital of
Joint Logistics Support Force of People's Liberation Army (Wuxi,
China) between January 2019 and December 2021 were included in the
present study. Inclusion criteria of this study were as follows:
Patients who were hospitalized due to typical symptoms of chest
tightness and chest pain, had suspected CAD and underwent improved
percutaneous coronary angiography during hospitalization. The study
exclusion criteria were as follows: i) Previous myocardial
infarction, coronary intervention therapy or coronary artery bypass
grafting; ii) acute cerebral infarction occurring within 6 months;
iii) other heart diseases, such as congenital heart disease,
valvular heart disease or great vascular disease; iv) presence of a
malignant tumor, hematological disease or autoimmune disease; and
v) complications of acute and chronic infectious diseases. CAD was
diagnosed according to the American College of Cardiology/American
Heart Association clinical guidelines for CAD: <50% stenosis of
any of the following major coronary arteries, including the left
main trunk, left anterior descending branch, left circumflex
branch, right coronary artery or its major branches with a vessel
diameter >1.5 mm (for example, diagonal branch, obtuse margin
branch, posterior left ventricular branch and posterior descending
branch) (14).
After admission, all patients were treated with 300
mg chewable aspirin and a 180-mg loading dose of ticagrelor. The
demographic characteristics of all patients were collected,
including hypertension, diabetes, smoking status, height and
weight. The cohort consisted of 736 males and 371 females, and the
median age was 64 years (range, 26-90 years). Body mass index [BMI;
weight (kg)/height (m2)] was also calculated. The
present study was approved by the Ethics Committee of the 904th
Hospital of Joint Logistic Support Force of People's Liberation
Army, and all patients provided written informed consent (approval
no. 2022-05-24).
Laboratory parameters
After admission, each patient fasted for 10-12 h.
Venous blood was collected the next morning and subjected to
routine blood marker and biochemical tests, including white blood
cell (WBC) count and triglyceride, total cholesterol (TC),
high-density lipoprotein (HDL-C), low-density lipoprotein (LDL-C),
creatinine and CRP levels. LCR was calculated as lymphocyte count
(103/µl)/CRP (mg/l). SII (neutrophils x platelets / lymphocytes),
NLR and PLR were also calculated.
Angiography
Judkins-style coronary angiography was performed of
each patient, and the degree of coronary stenosis was determined by
at least two interventional cardiologists based on the angiography
results (14). The Gensini score
was used to determine coronary stenosis severity (15,16).
The Gensini score evaluation criteria included coronary stenosis
degree and lesion site scores. i) Coronary stenosis degree score:
1-25%, 1 point; 26-50%, 2 points; 51-75%, 4 points; 76-90%, 8
points; 91-99%, 16 points; and 100%, 32 points. ii) Lesion site
score: Left trunk, 5 points; proximal left anterior descending
branch, 2.5 points; middle left anterior descending branch, 1.5
points; aorta and first diagonal branch, 1 point; second diagonal
branch, 0.5 point; distal left anterior descending branch, 1 point;
proximal left circumflex branch, 2.5 points; middle left circumflex
branch, 2.5 points; distal left circumflex branch, 1 point; blunt
edge branch, 0.5 point; proximal segment of the right coronary
artery, 1 point; middle segment of the right coronary artery, 1
point; distal segment of the right coronary artery, 1 point; distal
segment of right coronary artery, 1 point; posterior descending
branch, 1 point; and posterior branch of left ventricle, 0.5 point.
The Gensini score is the sum of the coronary stenosis degree and
lesion site scores as follows: 0 points, normal; 1-30 points, mild
CAD; and ≥30 points, severe CAD.
Statistical analyses
Three ofmore experimental repeats were performed.
The counting data are expressed as number of cases and percentage,
while the non-normally distributed measurement data are expressed
as median (interquartile range). The χ2 test was used to
examine counting data, while the non-parametric test (Mann-Whitney
U test) was used to examine non-normally distributed measurement
data. The Kolmogorov-Smirnov test was used to detect whether the
data were normally distributed.
The propensity score matching (PSM) method was used
to balance the clinical baseline data of the two groups, and the
patients were included in the regression model variables by the
logistic regression method, including sex, age, hypertension,
diabetes, age, BMI and LDL-C and the 1:1 nearest neighbor matching
method (caliper value=0.02) was adopted to facilitate matching
accuracy. If the control group contained multiple observation
objects that met the matching criteria, one participant was
randomly selected. Pearson and Spearman correlation coefficients
were used for the correlation analysis. The data were dichotomized
according to the median and included in the univariate logistic
regression analysis. The relevant influencing factors were screened
according to the standard of P<0.1 and included in the
multivariate logistic regression analysis to determine the
independent risk factors for CAD and severe coronary artery
stenosis. SPSS version 26.0 (IMB Corp.) was used to analyze the
data. All comparisons were two-tailed and values of P<0.05 were
considered to indicate a statistically significant difference.
Results
Baseline characteristics of the
unmatched group
A total of 1,107 patients [736 men, 371 women;
median age, 64 years (55-70 years)] were included in the present
study. According to the results of the coronary angiography, the
patients were divided into normal control and CAD groups. The
variables of sex, smoking history, hypertension, diabetes and age
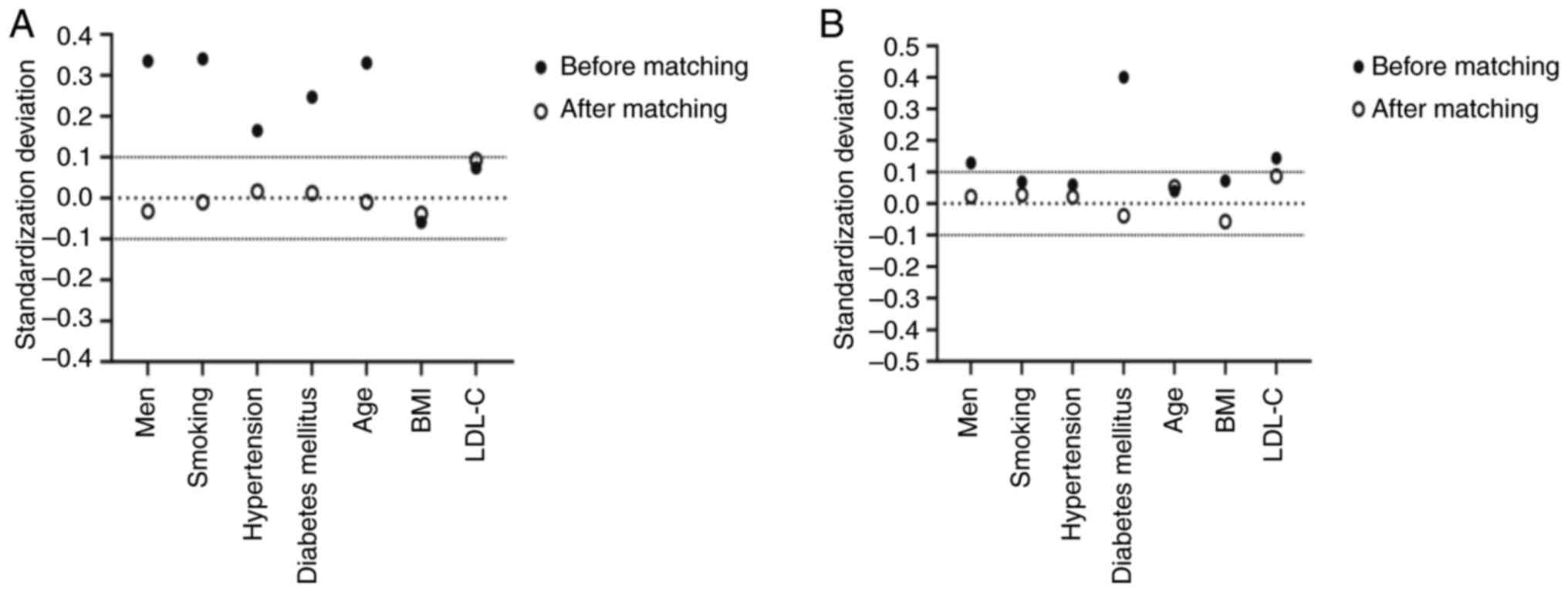
differed significantly between the two groups (P<0.05; Table I). After PSM, there were 384 cases
in both groups with no statistically significant differences in
characteristics such as sex, smoking history, hypertension,
diabetes, or age (P>0.05; Table
I). The absolute value of the standardized deviation after PSM
was <0.1, indicating good matching (Fig. 1A). The CAD group was further
divided into mild and severe CAD groups according to Gensini score
(Table II; Fig. 1B).
 | Table IBaseline characteristics of patients
with and without coronary heart disease before and after
matching. |
Table I
Baseline characteristics of patients
with and without coronary heart disease before and after
matching.
| | Before matching | After matching |
|---|
| Variable | CAD(-) (n=479) | CAD(+) (n=628) | P-value | CAD(-) (n=384) | CAD(+) (n=384) | P-value |
|---|
| Men, n (%) | 278 (58.04) | 458 (72.93) |
<0.001a | 247 (64.32) | 241 (62.76) | 0.708 |
| Smoking, n (%) | 162 (34.11) | 319 (51.12) |
<0.001a | 155 (40.36) | 153 (39.84) | 0.941 |
| Hypertension, n
(%) | 310 (64.72) | 453 (72.13) | 0.010a | 255 (66.41) | 258 (67.19) | 0.878 |
| Diabetes mellitus, n
(%) | 89 (18.86) | 189 (30.19) |
<0.001a | 85 (22.14) | 87 (22.66) | 0.931 |
| Age, years | 61 (54, 68) | 65 (57, 72) |
<0.001a | 63 (56, 70) | 64 (54.75, 70) | 0.979 |
| BMI,
kg/m2 | 24.80 (22.74,
27.00) | 24.80 (22.49,
26.67) | 0.220 | 24.77 (22.60,
26.99) | 24.79 (22.32,
26.83) | 0.472 |
| LDL-C, mmol/l | 2.3 0 (1.78,
2.79) | 2.36 (1.78,
2.89) | 0.301 | 2.30 (1.73,
2.79) | 2.36 (1.78,
2.91) | 0.236 |
| LVEF, % | 60 (60, 62) | 60 (60, 62) | 0.052 | 60 (60, 62) | 60 (60, 62) | 0.407 |
| Triglyceride,
mmol/l | 1.43 (0.98,
2.00) | 1.47 (0.99,
2.17) | 0.425 | 1.45 (0.98,
1.96) | 1.54 (1.00,
2.24) | 0.087 |
| TC, mmol/l | 4.31 (3.65,
4.96) | 4.26 (3.58,
4.93) | 0.498 | 4.22 (3.63,
4.94) | 4.27 (3.65,
4.98) | 0.614 |
| HDL-C, mmol/l | 1.16 (1.01,
1.35) | 1.10 (0.96,
1.26) |
<0.001a | 1.15 (1.00,
1.34) | 1.11 (0.95,
1.28) | 0.009a |
| CRP, mg/l | 1.40 (0.70,
3.00) | 2.00
(0.90,4.40) |
<0.001a | 1.40 (0.77,
2.92) | 1.80 (0.90,
4.00) | 0.001a |
| Creatinine,
µmol/l | 69 (59, 78) | 74 (64, 85) |
<0.001a | 69 (62, 80) | 71 (60, 82) | 0.772 |
| Hemoglobin,
g/dl | 13.70 (12.50,
14.90) | 13.90 (12.70,
15.03) | 0.302 | 13.80 (12.60,
15.00) | 13.80 (12.70,
15.00) | 0.848 |
| Neutrophil,
103/µl | 3.60 (2.80,
4.55) | 4.00 (3.12,
5.11) |
<0.001a | 3.59 (2.80,
4.46) | 3.79 (3.00,
4.89) | 0.012a |
| Lymphocyte,
103/µl | 1.7 0 (1.35,
2.10) | 1.73 (1.38,
2.16) | 0.317 | 1.76 (1.43,
2.16) | 1.77 (1.41,
2.22) | 0.761 |
| White blood cell,
103/µl | 6.08 (5.01,
7.19) | 6.48 (5.46,
7.89) |
<0.001a | 6.16 (5.04,
7.24) | 6.28 (5.26,
7.78) | 0.031a |
| Platelet,
103/µl | 192 (162, 229) | 198 (166, 235) | 0.051 | 184 (154, 217) | 198 (164, 237) | 0.002a |
| LCR | 1.28 (0.50,
2.28) | 0.90 (0.40,
1.81) |
<0.001a | 1.34 (0.54,
2.38) | 0.96 (0.44,
1.98) | 0.003a |
| SII,
103/µl | 389 (287, 575) | 448 (314, 661) |
<0.001a | 360 (272, 504) | 413 (296, 607) |
<0.001a |
| NLR | 2.08 (1.56,
2.81) | 2.30 (1.67,
3.17) |
<0.001a | 1.99 (1.52,
2.59) | 2.16 (1.62,
2.92) | 0.019a |
| PLR | 110 (88, 144) | 112 (92, 142) | 0.300 | 103 (85, 127) | 110 (89, 137) | 0.010a |
| Gensini score | 0.00
(0.00,3.50) | 18.50 (8.50,
36.00) |
<0.001a | 0.00
(0.00,3.00) | 18.00 (8.75,
36.00) |
<0.001a |
 | Table IIBaseline characteristics of two
groups of patients with coronary heart disease severity before and
after matching. |
Table II
Baseline characteristics of two
groups of patients with coronary heart disease severity before and
after matching.
| | Before
matching | After matching |
|---|
| Variable | Mild CAD
(n=399) | Severe CAD
(n=229) | P-value | Mild CAD
(n=212) | Severe CAD
(n=212) | P-value |
|---|
| Men, n (%) | 283 (70.93) | 175 (76.42) | 0.162 | 157 (74.06) | 159(75) | 0.911 |
| Smoking, n (%) | 198 (49.87) | 121 (53.3) | 0.458 | 109 (51.42) | 112 (52.83) | 0.846 |
| Hypertension, n
(%) | 284 (71.18) | 169 (73.8) | 0.540 | 154 (72.64) | 156 (73.58) | 0.913 |
| Diabetes mellitus,
n (%) | 91 (22.92) | 98 (42.79) |
<0.001a | 87 (41.04) | 83 (39.15) | 0.766 |
| Age, years | 65 (57, 71) | 65 (56, 72) | 0.651 | 65 (57, 71) | 65 (56, 72) | 0.525 |
| BMI
(kg/m2) | 24.8 (22.60,
26.45) | 24.80 (22.32,
26.99) | 0.522 | 24.80 (22.79,
26.68) | 24.66 (22.32,
26.83) | 0.563 |
| LDL-C (mmol/l) | 2.33 (1.75,
2.82) | 2.42 (1.81,
2.99) | 0.066 | 2.37 (1.90,
2.79) | 2.42 (1.82,
2.98) | 0.355 |
| LVEF (%) | 60 (60, 62) | 60 (60, 62) | 0.727 | 60 (60, 62) | 60 (60, 62) | 0.356 |
| Triglyceride
(mmol/l) | 1.46 (0.95,
2.16) | 1.49 (1.03,
2.19) | 0.455 | 1.53 (1.02,
2.42) | 1.46 (1.02,
2.19) | 0.527 |
| TC (mmol/l) | 4.26 (3.56,
4.93) | 4.27 (3.63,
4.93) | 0.59 | 4.34 (3.69,
4.94) | 4.29 (3.69,
4.93) | 0.861 |
| HDL-C (mmol/l) | 1.14 (0.98,
1.30) | 1.05 (0.91,
1.20) |
<0.001a | 1.13 (0.97,
1.26) | 1.06 (0.91,
1.20) | 0.003a |
| CRP (mg/l) | 1.70 (0.90,
3.40) | 2.60(1.30,
6.50) |
<0.001a | 1.70(0.90,
3.32) | 2.45 (1.28,
6.43) |
<0.001a |
| Creatinine
(µmol/l) | 73 (63, 84) | 75 (64, 86) | 0.326 | 73.5 (63, 86) | 75 (64, 86) | 0.757 |
| Hemoglobin,
g/dl | 139 (128, 151) | 138 (125, 149) | 0.245 | 139 (129, 151) | 139 (125, 151) | 0.377 |
| Neutrophil,
103/µl | 3.89 (3.00,
4.88) | 4.12 (3.37,
5.49) | 0.003a | 3.84 (3.02,
4.87) | 4.10 (3.39,
5.43) | 0.004a |
| Lymphocyte,
103/µl | 1.75 (1.38,
2.16) | 1.7 0(1.38,
2.18) | 0.600 | 2.00 (1.62,
2.42) | 1.71 (1.39,
2.11) |
<0.001a |
| White blood cell,
103/µl | 6.43 (5.32,
7.73) | 6.59 (5.70,
8.24) | 0.016a | 6.70 (5.51,
7.88) | 6.56 (5.69,
8.23) | 0.373 |
| Platelet,
103/µl | 198 (164, 235) | 197 (167, 232) | 0.992 | 182 (155, 222) | 197 (169, 230) | 0.007a |
| LCR | 1.04 (0.49,
2.13) | 0.69 (0.25,
1.33) |
<0.001a | 1.17 (0.58,
2.39) | 0.7 (0.26,
1.38) |
<0.001a |
| SII,
103/µl | 433(302, 624) | 479 (340, 696) | 0.017a | 342 (256, 483) | 480 (345, 681) |
<0.001a |
| NLR | 2.19 (1.63,
3.05) | 2.52 (1.84,
3.31) | 0.004a | 1.88 (1.44,
2.47) | 2.52 (1.85,
3.28) |
<0.001a |
| PLR | 113 (92, 140) | 112 (93, 146) | 0.770 | 94 (79, 110) | 112 (90, 147) |
<0.001a |
| Gensini score | 12.00 (7.00,
18.00) | 48.00 (36.00,
66.00) |
<0.001a | 11.50 (6.00,
18.00) | 48.00 (36.00,
66.00) |
<0.001a |
Matched groups
After matching, the neutrophil, WBC and platelet
counts as well as the SII, NLR and PLR were higher in the CAD vs.
control group, whereas the HDL-C level and LCR were significantly
lower in the CAD vs. control group (P<0.05; Table I). The neutrophil and platelet
counts as well as the CRP level, SII, NLR and PLR were
significantly higher in the severe CAD vs. mild CAD group after
matching, whereas the lymphocyte count and LCR were significantly
lower in the severe CAD vs. mild CAD group (P<0.05; Table II).
After the PSM analysis, the risk factors for CAD
were explored. As shown in Table
III, the results of the univariate logistic regression analysis
suggested that HDL-C level, CRP level, neutrophil count, platelet
count, LCR, SII, NLR and PLR were possible risk factors for CAD
(P<0.1). However, LCR was strongly correlated with CRP level
(r=-0.831, P<0.001) (data not shown), and single risk factors
were not as stable as combined risk factors; therefore, CRP level
was excluded from further analysis. HDL-C level, neutrophil and
platelet counts, LCR, SII, NLR and PLR were included in the
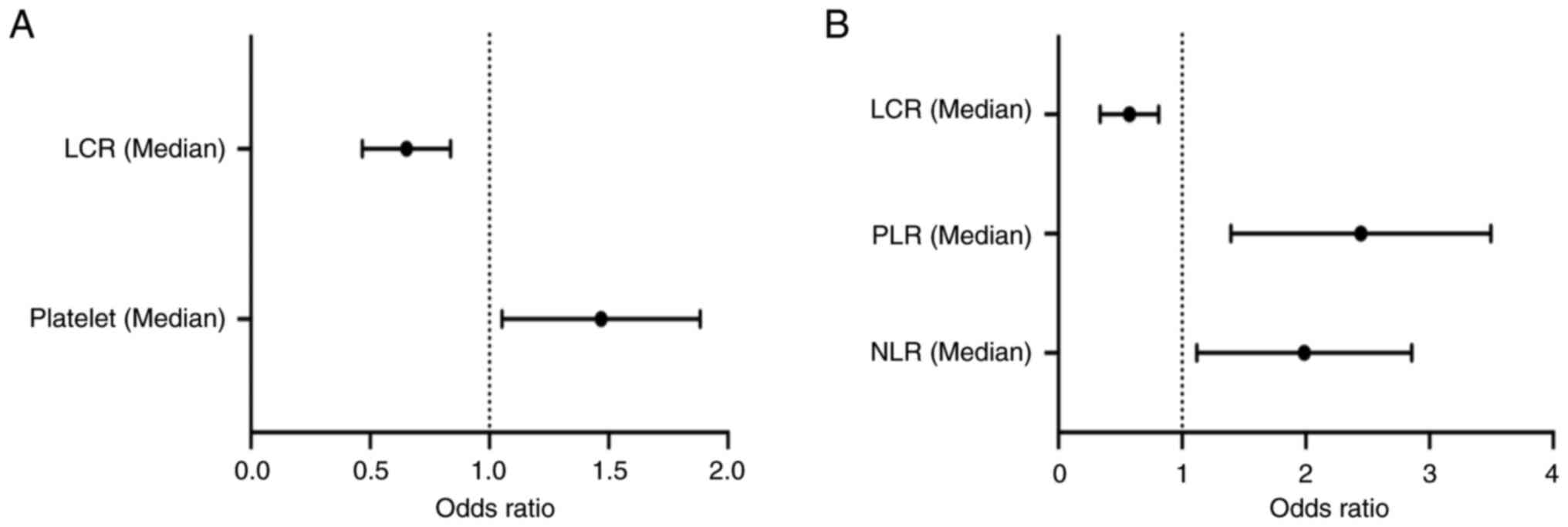
multivariate logistic analysis. Platelet count [odds ratio (OR),
1.429; 95% confidence interval (CI), 1.074-1.902; P=0.014] was an
independent risk factor for CAD, while LCR (OR, 0.635; 95% CI,
0.477-0.845; P=0.002) was an independent protective factor for CAD
(Table III; Fig. 2A). The univariate logistic
regression analysis showed that HDL-C, CRP, lymphocytes, platelets,
LCR, SII, PLR and PLR were correlated with CAD severity. Similarly,
LCR was strongly correlated with CRP level (r=-0.739, P<0.001),
which was then excluded from the further analysis.
 | Table IIIMultivariate logistic regression
analysis of patients with or without coronary heart disease after
propensity score matching. |
Table III
Multivariate logistic regression
analysis of patients with or without coronary heart disease after
propensity score matching.
| | Univariate
analysis | Multivariable
analysis |
|---|
| Variable | P-value | OR | 95% CI | P-value | OR | 95% CI |
|---|
| HDL-C (median) | 0.061 | 0.762 | 0.574 | 1.012 | | | | |
| CRP (median) | <0.001 | 1.858 | 1.396 | 2.474 | | | | |
| Neutrophil
(median) | 0.037 | 1.353 | 1.019 | 1.797 | | | | |
| Platelet
(median) | 0.012 | 1.441 | 1.085 | 1.915 | 0.014 | 1.429 | 1.074 | 1.902 |
| LCR (median) | 0.002 | 0.631 | 0.475 | 0.839 | 0.002 | 0.635 | 0.477 | 0.845 |
| SII (median) | 0.009 | 1.457 | 1.096 | 1.935 | | | | |
| NLR (median) | 0.083 | 1.284 | 0.967 | 1.705 | | | | |
| PLR (median) | 0.017 | 1.411 | 1.063 | 1.875 | | | | |
The remaining factors were included in the
multivariate logistic analysis, and the results suggested that NLR
(OR, 1.862; 95% CI, 1.189-2.914; P=0.007) and PLR (OR, 2.295; 95%
CI, 1.478-3.564; P<0.001) were independent risk factors for CAD
severity. LCR (OR, 0.541; 95% CI, 0.354-0.825; P=0.004) was an
independent protective factor against severe CAD (Table IV; Fig. 2B).
 | Table IVMultivariate logistic regression
analysis of coronary heart disease severity group after propensity
score matching. |
Table IV
Multivariate logistic regression
analysis of coronary heart disease severity group after propensity
score matching.
| | Univariate
analysis | Multivariable
analysis |
|---|
| Variable | P-value | OR | 95%CI | P-value | OR | 95%CI |
|---|
| HDL-C (median) | 0.012 | 0.611 | 0.416 | 0.896 | | | | |
| CRP (median) | 0.001 | 1.910 | 1.299 | 2.810 | | | | |
| Lymphocyte
(median) | <0.001 | 0.422 | 0.286 | 0.623 | | | | |
| Platelet
(median) | 0.020 | 1.576 | 1.074 | 2.312 | | | | |
| LCR (median) | <0.001 | 0.382 | 0.258 | 0.566 | 0.004 | 0.541 | 0.354 | 0.825 |
| SII (median) | <0.001 | 2.616 | 1.768 | 3.870 | | | | |
| NLR (median) | <0.001 | 3.074 | 2.069 | 4.567 | 0.007 | 1.862 | 1.189 | 2.914 |
| PLR (median) | <0.001 | 3.337 | 2.241 | 4.969 | <0.001 | 2.295 | 1.478 | 3.564 |
Discussion
CAD is the leading cause of morbidity and mortality
worldwide (17). CAD is a
multifactorial disease for which dyslipidemia, abnormal blood
glucose levels, smoking and genetic susceptibility are all risk
factors. Atherosclerosis is the leading cause of CAD (5). In recent years, atherosclerosis has
been increasingly recognized as a chronic inflammatory disease of
the arterial wall (18) and an
active inflammatory process instead of a simple passive injury
caused by lipid infiltration (19). Vascular inflammation plays an
important role in plaque formation, progression and even rupture
and can have serious consequences such as local thrombosis and
hypoxia-related myocardial injury (20).
The immune system is divided into innate and
adaptive systems. Neutrophil and platelet counts are important
indicators of innate immunity (21,22),
whereas lymphocytes primarily mediate adaptive immunity (23). NLR, PLR and SII reflect the balance
between innate and adaptive immunity well (24-26).
The NLR, PLR and SII are correlated with CAD severity and prognosis
(6). In patients with cancer, LCR
is a novel systemic inflammation indicator with a stronger
predictive effect compared with other indicators, such as NLR and
PLR (11). After PSM, the present
study balanced the traditional risk factors for cardiovascular
disease, including sex, smoking history, hypertension, diabetes,
age, BMI and LDL-C. Platelet count and a lower LCR were risk
factors for CAD, whereas PLR, NLR and a lower LCR were risk factors
for severe CAD.
LCR is the ratio of lymphocytes to CRP level, and a
decreased lymphocyte count and increased CRP level can downregulate
LCR (13). Atherosclerotic plaques
are characterized by the infiltration of monocytes/macrophages and
lymphocyte cells that migrate from the blood to the lower arterial
endothelium, thereby reducing the number of lymphocytes in the
circulating blood when plaques form (27). This has been confirmed in
lymphocyte-deficient mice, in which the atherosclerotic burden
induced by a high-cholesterol diet can be reduced by 80% (28). In clinical studies, Horne et
al (19) found that WBC count
is an independent predictor of prognosis of patients with CAD.
However, a high neutrophil or low lymphocyte count has a stronger
predictive power, and a low lymphocyte count increases the risk of
cardiovascular disease. Similarly, Adamstein et al (10) reported the protective effect of a
high lymphocyte count on atherosclerosis.
Although mechanistic studies suggest that
lymphocytes can be both atheroprotective and atherogenic, the
lymphopenia-induced effect of atherosclerosis may reflect a more
general process such as frailty (29,30).
CRP, a part of the innate immunity that aggregates or binds to
ligands to activate the classical complement pathway, also binds to
the phospholipids of damaged cells and subsequently activates the
complement system in a limited manner, enhancing the uptake of
these cells by macrophages, which have atherogenic properties
(31). A previous study suggests
that plasma CRP levels can predict the risk of vascular disease
with a predictive ability as high as that of TC or HDL-C (20). In the CANTOS trial, decreased CRP
levels were closely associated with reduced rates of cardiovascular
events and all-cause mortality (31). Platelets are involved in various
vascular inflammation-related diseases, including atherosclerosis,
myocardial infarction and autoimmune diseases (32). NLR and PLR can be used to assess
CAD development and severity before coronary angiography (33). In the present study, LCR was
closely related to CAD occurrence and severity.
The present study had several limitations. First,
only patients initially diagnosed with CAD were included, while
those previously diagnosed with CAD were excluded. Therefore, the
results can be applied to only a narrow population. Second, only a
single blood sample was collected from patients after admission,
and the LCR was not regularly assessed. Therefore, the long-term
predictive effect of LCR remains unclear. Thirdly, the present
study did not further explore the predictive efficacy of LCR
compared with traditional inflammatory biomarkers NLR and PLR in
predicting CAD and severe coronary artery stenosis. Therefore, the
aforementioned issues will be further improved in future research.
Finally, this was a single-center study with a small sample size.
Subsequent multicenter studies will aim to include more patients to
provide high-quality clinical evidence.
In conclusion, LCR, a simple and easy-to-acquire
indicator in clinical studies, is an independent protective factor
against CAD occurrence. It is also related to CAD severity and an
independent protective factor against severe CAD. LCR
determinations may guide the early screening and assessment of CAD
severity.
Acknowledgements
The authors would like to thank Professor Gangjun
Zong and Professor Yehong Liu from Department of Cardiology, The
904th Hospital of Joint Logistic Support Force of People's
Liberation Army for their assistance in manuscript editing and
language polishing.
Funding
Funding: This study was supported by the Natural Science
Foundation of Jiangsu Province (grant no. BK20201139), the Major
Project of Jiangsu Provincial Health Commission (grant no.
ZD2021020) and the Wuxi Science and Technology Development Fund
(grant no. Y20212011).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
KC, YHL and GJZ conceived and designed the study.
KC, BDX, TY, LC and GYW contributed to the data acquisition. KC and
YHL performed the statistical analyses. KC and YHL drafted the
manuscript. All authors have read and approved the final
manuscript. KC, YHL and GJZ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
This study was performed in accordance with the
Declaration of Helsinki and was approved by the Ethics Committee of
the 904th Hospital of the Joint Logistic Support Force of People's
Liberation Army (approval no. 2022-05-24). All participants
provided written informed consent prior to participating.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mensah GA, Roth GA and Fuster V: The
global burden of cardiovascular diseases and risk factors: 2020 and
beyond. J Am Coll Cardiol. 74:2529–2532. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Aengevaeren VL, Mosterd A, Sharma S,
Prakken NHJ, Möhlenkamp S, Thompson PD, Velthuis BK and Eijsvogels
TMH: Exercise and coronary atherosclerosis: Observations,
explanations, relevance, and clinical management. Circulation.
141:1338–1350. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Avis SR, Vernon ST, Hagström E and Figtree
GA: Coronary artery disease in the absence of traditional risk
factors: A call for action. Eur Heart J. 42:3822–3824.
2021.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Koenig W and Khuseyinova N: Biomarkers of
atherosclerotic plaque instability and rupture. Arterioscler Thromb
Vasc Biol. 27:15–26. 2007.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liberale L, Montecucco F, Schwarz L,
Lüscher TF and Camici GG: Inflammation and cardiovascular diseases:
Lessons from seminal clinical trials. Cardiovasc Res. 117:411–422.
2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu Y, Ye T, Chen L, Jin T, Sheng Y, Wu G
and Zong G: Systemic immune-inflammation index predicts the
severity of coronary stenosis in patients with coronary heart
disease. Coron Artery Dis. 32:715–720. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhang S, Diao J, Qi C, Jin J, Li L, Gao X,
Gong L and Wu W: Predictive value of neutrophil to lymphocyte ratio
in patients with acute ST segment elevation myocardial infarction
after percutaneous coronary intervention: A meta-analysis. BMC
Cardiovasc Disord. 18(75)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Balta S and Ozturk C: The
platelet-lymphocyte ratio: A simple, inexpensive and rapid
prognostic marker for cardiovascular events. Platelets. 26:680–681.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yang YL, Wu CH, Hsu PF, Chen SC, Huang SS,
Chan WL, Lin SJ, Chou CY, Chen JW, Pan JP, et al: Systemic
immune-inflammation index (SII) predicted clinical outcome in
patients with coronary artery disease. Eur J Clin Invest.
50(e13230)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Adamstein NH, MacFadyen JG, Rose LM, Glynn
RJ, Dey AK, Libby P, Tabas IA, Mehta NN and Ridker PM: The
neutrophil-lymphocyte ratio and incident atherosclerotic events:
Analyses from five contemporary randomized trials. Eur Heart J.
42:896–903. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Okugawa Y, Toiyama Y, Yamamoto A,
Shigemori T, Ide S, Kitajima T, Fujikawa H, Yasuda H, Hiro J,
Yoshiyama S, et al: Lymphocyte-C-reactive protein ratio as
promising new marker for predicting surgical and oncological
outcomes in colorectal cancer. Ann Surg. 272:342–351.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang JN, Gao Y, Wang XT, Li NN, Du X,
Tang YJ, Lai QQ, Chen PF, Yue CS, Wu JH, et al:
Lymphocyte-C-reactive protein ratio can differentiate disease
severity of COVID-19 patients and serve as an assistant screening
tool for hospital and ICU admission. Front Immunol.
13(957407)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Y, Ye T, Chen L, Xu B, Wu G and Zong
G: Preoperative lymphocyte to C-reactive protein ratio: A new
prognostic indicator of post-primary percutaneous coronary
intervention in patients with ST-segment elevation myocardial
infarction. Int Immunopharmacol. 114(109594)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ryan TJ, Bauman WB, Kennedy JW, Kereiakes
DJ, King SB III, McCallister BD, Smith SC and Ullyot DJ: Guidelines
for percutaneous transluminal coronary angioplasty. A report of the
American heart association/american college of cardiology task
force on assessment of diagnostic and therapeutic cardiovascular
procedures (committee on percutaneous transluminal coronary
angioplasty). Circulation. 88:2987–3007. 1993.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gensini GG: A more meaningful scoring
system for determining the severity of coronary heart disease. Am J
Cardiol. 51(606)1983.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sullivan DR, Marwick TH and Freedman SB: A
new method of scoring coronary angiograms to reflect extent of
coronary atherosclerosis and improve correlation with major risk
factors. Am Heart J. 119:1262–1267. 1990.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Gaba P, Gersh BJ, Ali ZA, Moses JW and
Stone GW: Complete versus incomplete coronary revascularization:
Definitions, assessment and outcomes. Nat Rev Cardiol. 18:155–168.
2021.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Saigusa R, Winkels H and Ley K: T cell
subsets and functions in atherosclerosis. Nat Rev Cardiol.
17:387–401. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Horne BD, Anderson JL, John JM, Weaver A,
Bair TL, Jensen KR, Renlund DG and Muhlestein JB: Intermountain
Heart Collaborative Study Group. Which white blood cell subtypes
predict increased cardiovascular risk? J Am Coll Cardiol.
45:1638–1643. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ridker PM: From C-reactive protein to
interleukin-6 to interleukin-1: Moving upstream to identify novel
targets for atheroprotection. Circ Res. 118:145–156.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Elzey BD, Sprague DL and Ratliff TL: The
emerging role of platelets in adaptive immunity. Cell Immunol.
238:1–9. 2005.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kobayashi SD and DeLeo FR: Role of
neutrophils in innate immunity: A systems biology-level approach.
Wiley Interdiscip Rev Syst Biol Med. 1:309–333. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
23
|
Bonilla FA and Oettgen HC: Adaptive
immunity. J Allergy Clin Immunol. 125 (Suppl 2):S33–S40.
2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fest J, Ruiter R, Ikram MA, Voortman T,
van Eijck CHJ and Stricker BH: Reference values for white
blood-cell-based inflammatory markers in the Rotterdam Study: A
population-based prospective cohort study. Sci Rep.
8(10566)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gong S, Gao X, Xu F, Shang Z, Li S, Chen
W, Yang J and Li J: Association of lymphocyte to monocyte ratio
with severity of coronary artery disease. Medicine (Baltimore).
97(e12813)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Fani L, van Dam-Nolen DHK, Vernooij M,
Kavousi M, van der Lugt A and Bos D: Circulatory markers of
immunity and carotid atherosclerotic plaque. Atherosclerosis.
325:69–74. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Falk E, Shah PK and Fuster V: Coronary
plaque disruption. Circulation. 92:657–671. 1995.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Montarello NJ, Nguyen MT, Wong DTL,
Nicholls SJ and Psaltis PJ: Inflammation in coronary
atherosclerosis and its therapeutic implications. Cardiovasc Drugs
Ther. 36:347–362. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Núñez J, Sastre C, D'Ascoli G, Ruiz V,
Bonanad C, Miñana G, Valero E, Garcia-Blas S, Mollar A, Villaescusa
A, et al: Relation of low lymphocyte count to frailty and its
usefulness as a prognostic biomarker in patients >65 years of
age with acute coronary syndrome. Am J Cardiol. 125:1033–1038.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ekerstad N, Swahn E, Janzon M, Alfredsson
J, Löfmark R, Lindenberger M and Carlsson P: Frailty is
independently associated with short-term outcomes for elderly
patients with non-ST-segment elevation myocardial infarction.
Circulation. 124:2397–2404. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Hoffman M, Blum A, Baruch R, Kaplan E and
Benjamin M: Leukocytes and coronary heart disease. Atherosclerosis.
172:1–6. 2004.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Morrell CN, Pariser DN, Hilt ZT and Vega
Ocasio D: The platelet napoleon complex-small cells, but big immune
regulatory functions. Annu Rev Immunol. 37:125–144. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sari I, Sunbul M, Mammadov C, Durmus E,
Bozbay M, Kivrak T and Gerin F: Relation of
neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio with
coronary artery disease severity in patients undergoing coronary
angiography. Kardiol Pol. 73:1310–1316. 2015.PubMed/NCBI View Article : Google Scholar
|