Introduction
Tophaceous gout is a metabolic disorder mostly
affecting the metatarsal-phalangeal joint, the elbow and the knee
(1). Caused by the high serum uric
acid levels, the majority of cases of tophaceous gouts suffer from
primary hyperuricemia due to the decreased uric acid excretion.
Owing to the high uric acid dietary changes over the past few
decades, the prevalence of tophaceous gout has increased
significantly (2). Spinal gout is
also reported and due to the asymptomatic development and insidious
onset in the majority of patients, the incidence of spinal gout may
be highly underestimated (3).
Spinal gout may present with different clinical
symptoms, varying from back pain to symptoms of neural compression.
More severe cases may present with acute onset paraparesis
(4). Spinal gout may affect the
vertebral bodies, the joint facets, the flavum ligament and the
pedicles (5). Differential
diagnoses of spinal gout include ossification of the ligamentum
flavum, stenosis of the spinal canal, lumbar disc herniation and
other degenerative spinal disorders. Due to the rareness and
atypical symptoms in most of the cases, the definite diagnosis of
spinal gout is usually challenging to orthopedic surgeons (3). In the present study, the clinical,
imaging and pathologic findings of a patient with radiculopathy
caused by this unusual presentation of spinal gout are
reported.
Case report
A 57-year-old man was admitted to the Department of
Orthopedics of Tianjin Hospital (Tianjin, China) in August 2022 due
to lower back pain and bilateral sciatic pain for the past 12
months, with gradual deterioration, resulting in gait impairment in
the last month. The patient had 20 years' history of gout with
palpable tophi in both the left and right hand, the knee and the
foot (Fig. 1). The medical history
showed no diabetes, no renal diseases and no malignancies.
Nonsteroidal anti-inflammatory drugs were not able to significantly
alleviate the pain. On neurological examination, hypoesthesia was
observed in the posterolateral lower leg and anterolateral foot on
the left side. The bilateral Achilles tendon reflex was reduced and
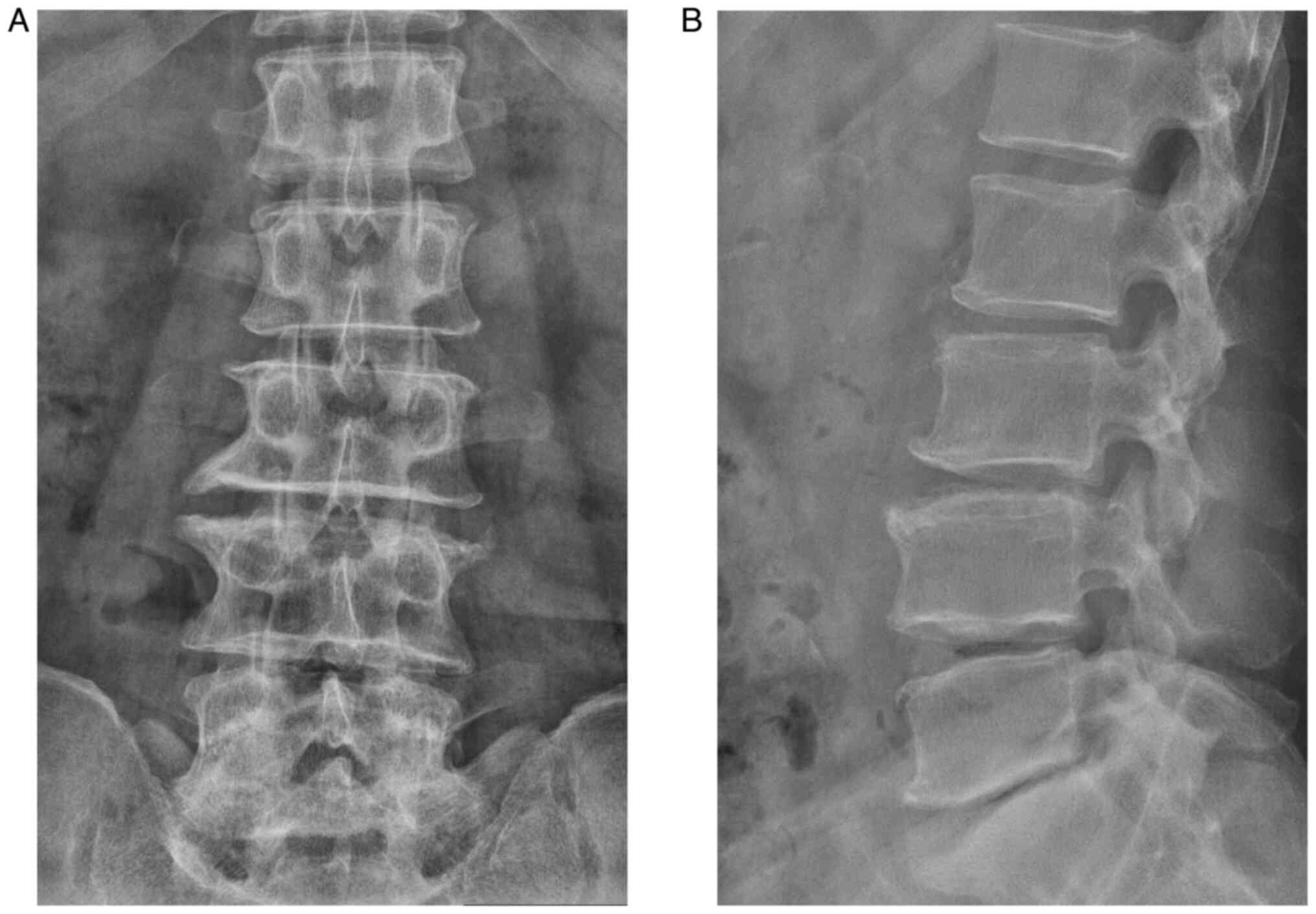
the bilateral Laseque sign was negative. Anterior-posterior and
lateral X-ray radiographs revealed degenerative features of the
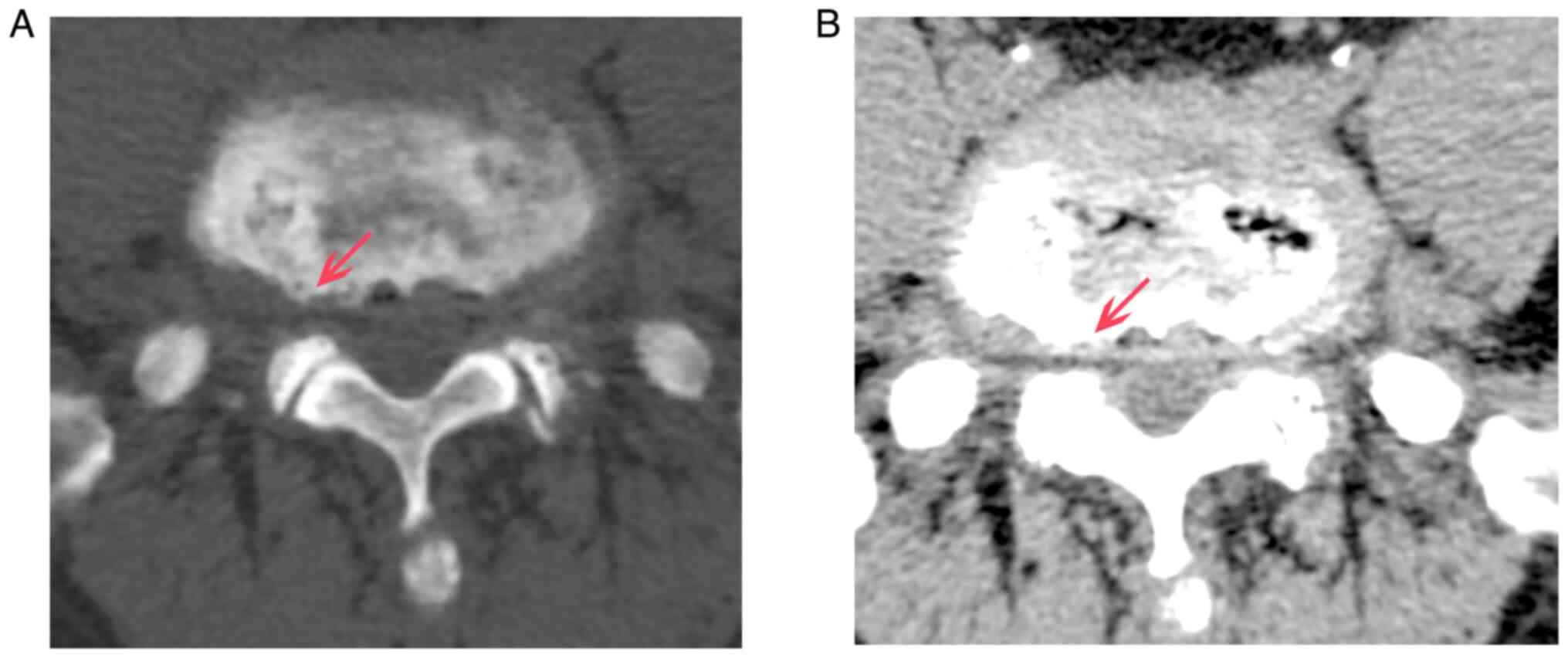
lower spine (Fig. 2). CT and MRI
scans revealed bilateral stenosis in the L5/S1 intervertebral
foramen without any significant stenosis in the vertebral canal and
lateral recess (Figs. 3 and
4).
The bone mineral density detected by quantitative
computed tomography was 124.3 mg/cm3 (normal range:
>120 mg/cm3). The abnormal laboratory results were as
follows: Serum uric acid, 541 µmmol/l (normal range: 208-428
µmol/l); serum C-reactive protein, 49 mg/l (normal: 0-6 mg/l);
hemoglobin, 112 g/l (normal: 130-175 g/l); serum triglyceride, 2.73
mmol/l (normal: 0-1.7 mmol/l); and serum D-dimer, 700 µg/l (normal:
0-300 µg/l).
On admission, the patient was diagnosed with
bilateral L5/S1 foraminal stenosis and far-lateral lumbar disc
herniation. Both the neurological examination and the radiological
examination confirmed the compression of the bilateral L5 nerve
root. However, spinal gout had not been initially considered. In
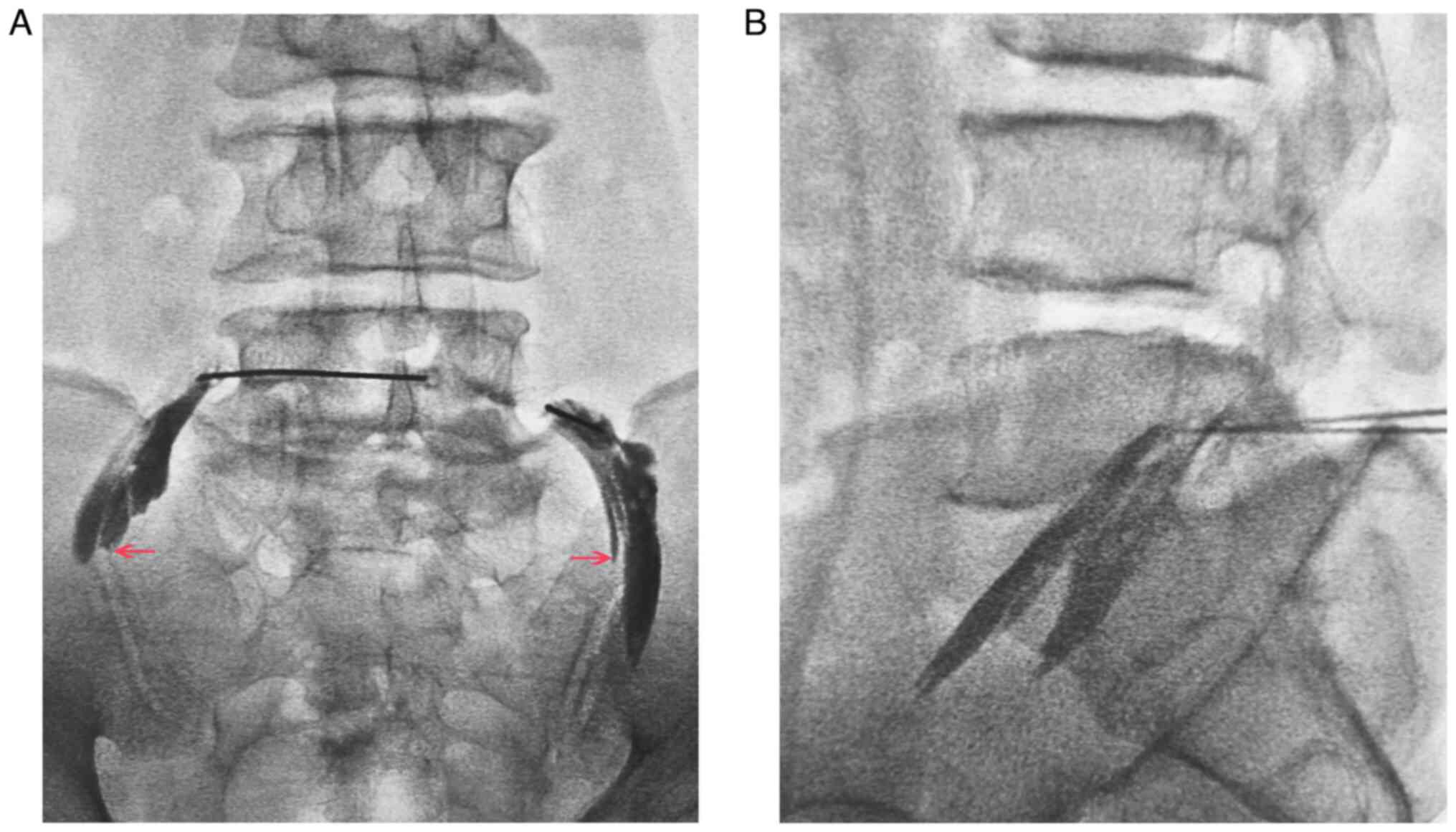
order to confirm the diagnosis considering no significant stenosis
in the vertebral canal and lateral recess, bilateral L5 nerve root
radiculography and blocking were performed (Fig. 5A and B). The sciatic pain was completely
relieved and the bilateral L5/S1 intervertebral foramen was
confirmed to be the major site of stenosis. Due to the iliac
obstruction of the L5/S1 intervertebral foramen, percutaneous
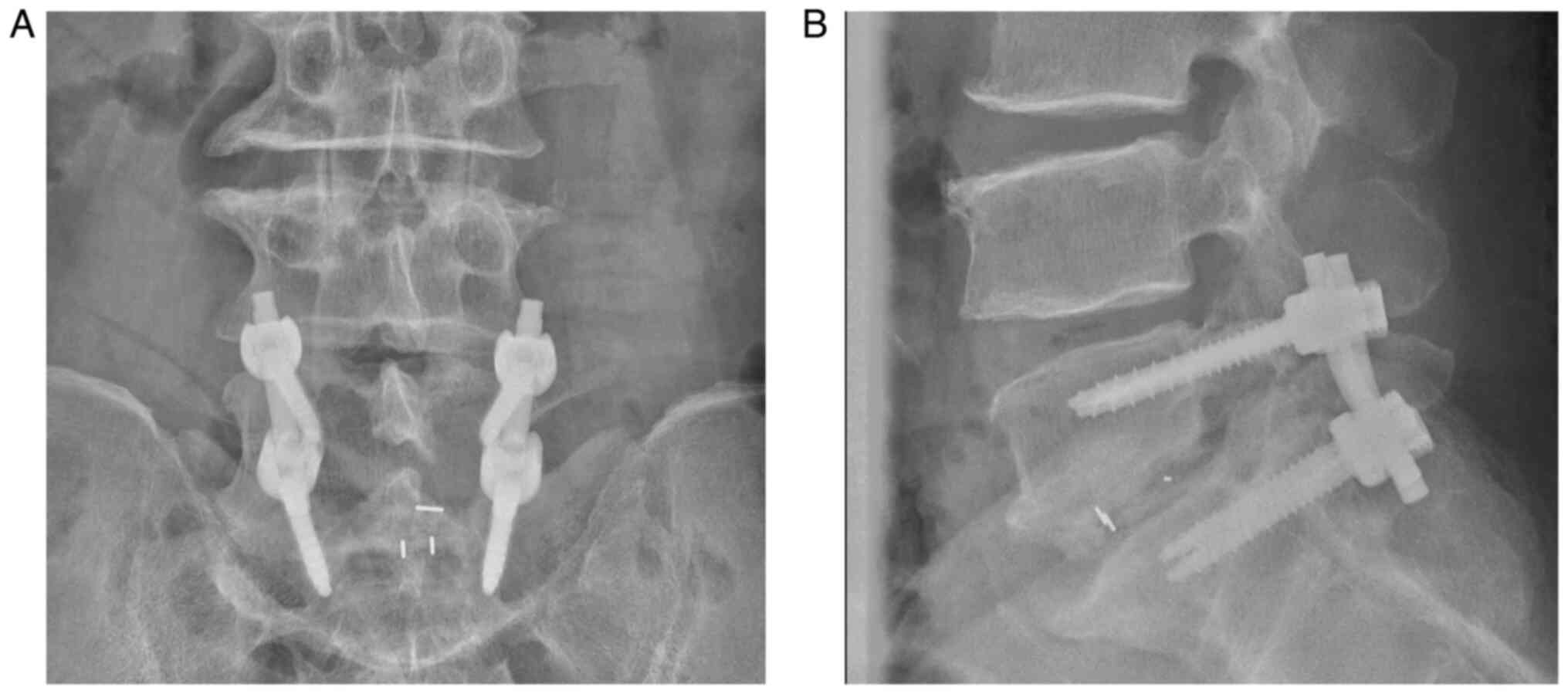
transforaminal endoscopic surgery could not be adopted. Then
posterior surgery of transforaminal lumbar interbody fusion (TLIF)
was performed (post-operational X-ray and CT are presented in
Fig. 6).
During the TLIF operation under general anesthesia,
the inferior articular process, the ligamentum flavum the herniated
disc and part of the posterior longitudinal ligament were removed
to completely decompress the dural sac and exiting nerve. Large
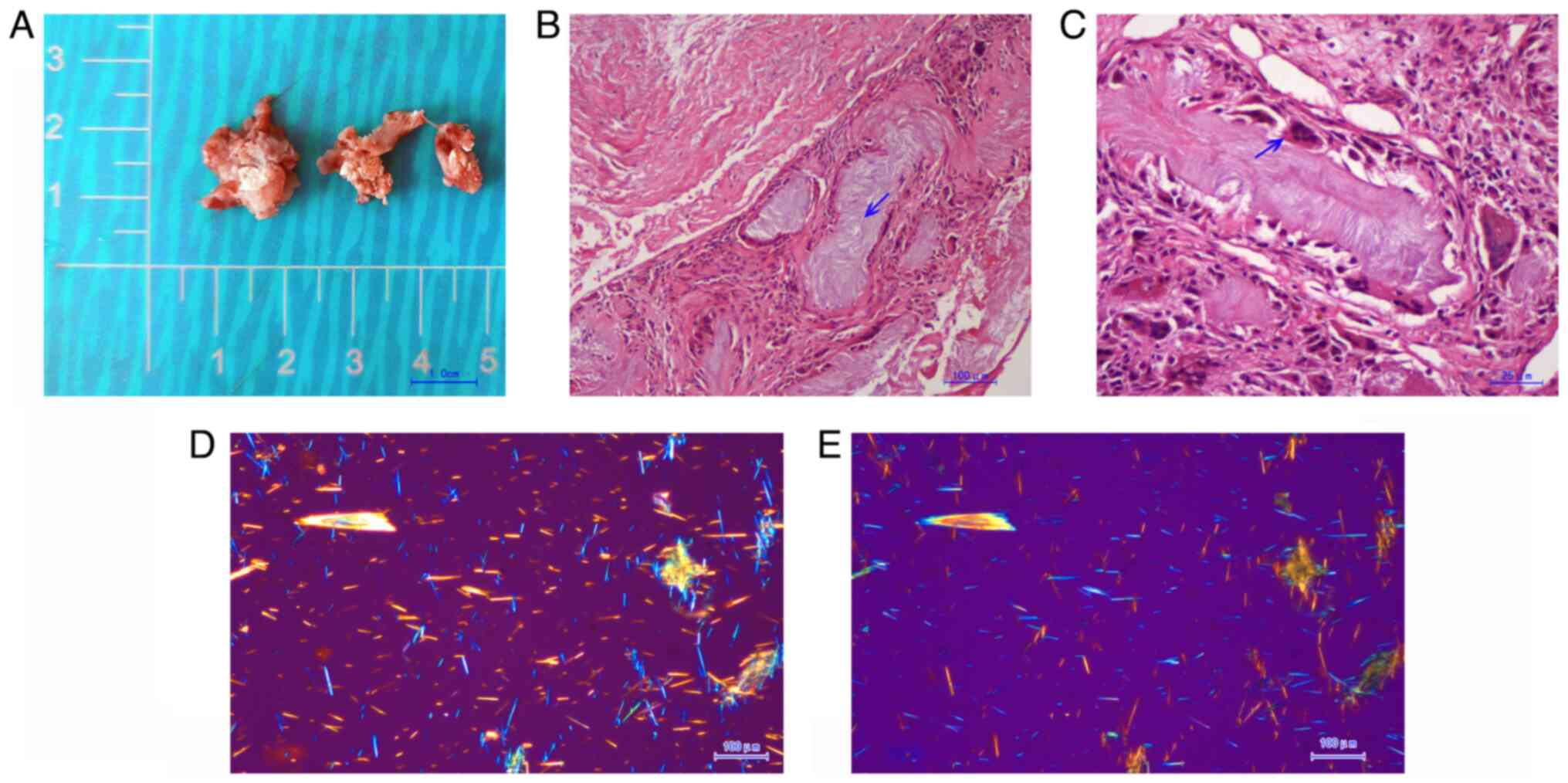
amorphous chalky white lesions were unexpectedly discovered, which
had been compressing the intervertebral foramen peripheral to the
ruptured annulus fibrosis (Fig.
7A). The joint facets were not infiltrated. The white lesions
were partly surrounded by fibrous tissue and partially infiltrated
into the muscle and bone. The extruded nucleus pulpous and all of
the lesions were resected and lumbar fixation and fusion were then
performed. The operative time was 2 h and the blood loss was ~200
ml.
The white chalky material was submitted for H&E
staining (H&E staining procedure: Tissues were immersed in 10%
neutral formalin buffered solution at room temperature for 6 h and
transferred to 70% ethanol. After embedding in paraffin wax blocks,
4 µm-thick tissue sections were stained at room temperature with
hematoxylin for 4 min and eosin for 20 sec). The pathological
result showed that large amounts of amorphous substance containing
urate crystals were encompassed by multinucleate giant cell
granulomas and inflammatory cells (Fig. 7B and C). Polarized light microscopy films of
the positive birefringence (Fig.
7D) vs. negative birefringence (Fig. 7E) of the needle-shaped urate
crystals confirmed the diagnosis of spinal gout.
After surgery, complete alleviation of the
radiculopathy was reported by the patient. No complications
occurred during the perioperative period. The patient was
discharged from the hospital 3 days after surgery. With the
guidance of rheumatologists, colchicine (1 mg per day for 3 months;
Kunyao Pharmaceutical Group Co. Ltd.) and febuxostat (40 mg per day
for 1 year; Wanbang Biopharmaceuticals Co. Ltd.) were prescribed to
alleviate the clinical symptoms as well as to reduce the serum
levels of uric acid. By the 1-year follow-up in August 2023
(follow-ups performed at 3 months, 6 months and 1 year), no
deterioration was found.
Discussion
The present study described the first case of spinal
gout with intervertebral foramen infiltration, which perfectly
mimicked degenerative lumbar disc disorders. For spinal gout, the
most affected locations are the lumbar region and sacroiliac
joints. In the vertebral column, gout tophi can affect almost every
anatomical component, such as vertebral bodies, intervertebral
disc, epidural space, articular facets, laminae, ligamentum flavum,
pedicles and rarely the intradural space (5). It was also reported that the lateral
parts of the vertebra are much more affected than the central areas
(6); however, the sole
infiltration of the intervertebral foramen was never reported.
The incidence of spinal gout may be highly
underestimated and patients with spinal gout may suffer from acute,
subacute or chronic symptoms. It was estimated that 65.4% of
patients had some kind of neurological deficit, such as loss of
sensation, radiculopathy, bowel/bladder dysfunction, motor weakness
or even quadriparesis (2). Owing
to its diverse location in the spine and atypical clinical
symptoms, the differential diagnosis includes epidural abscess,
metastatic disease, spondylodiscitis, rheumatoid arthritis and
other degenerative spinal disorders (3,7). It
was also reported that ~24.6% of patients with spinal gout had no
history of gout or hyperuricemia (2). Although relatively rare, orthopedic
surgeons should take it into consideration as a differential
diagnosis when the patient has chronic lower back pain, even if the
patient has normal serum uric acid levels and no history of gout
(3).
As shown in the present case, clinical clues had
been supplied by the patient, such as history of gout, laboratory
examinations showing high C-reactive protein, spinal CT and MRI
showing no significant stenosis in the vertebral canal and lateral
recess, which was not corresponding to the clinical symptoms. Of
note, initially, spinal gout had not been taken into consideration
as the differential diagnosis for this case.
Although usually nonspecific, imaging technology may
provide useful information for the diagnosis of spinal gout. MR
findings usually have a nonspecific dural tail signal, showing a
homogenous T1 hypointense signal and heterogeneous T2 hypo- to
hyperintense signal, which may mimic epidural abscess or tumor
(8,9). CT findings show periarticular punched
out erosions with overhanging margins and tophi appear as masses
denser than surrounding tissues (10). Compared to normal CT scan,
Dual-energy CT (DECT) displays different chemical substances with
distinct colors on the basis of the different X-ray photon energies
(11,12). A meta-analysis by Ogdie et
al (13) concluded that DECT
had a favor sensitivity of 0.87 and a favor specificity of 0.84 in
the diagnosis of gout tophi. Although DECT is not generally
accessible in most hospitals, DECT is still recommended for its
diagnosis as well as to establish better treatment plans (13).
Once diagnosed as spinal gout, the patient may
receive medications such as allopurinol and colchicine. Most
patients presented with rapid relief of the clinical symptoms;
however, drug-resistant patients or more severe cases may require
further treatment with steroids or even surgical intervention in
order to relieve the neuropathic compression (8). Although no consensus has been
reached, early surgical intervention was still suggested for
patients with severe damage to the joints and progressive
neurological deterioration (14),
and it could probably reduce the possibility of using spinal
instrumentation (15). Laminectomy
through the open approach with or without fusion was suitable for
most of the patients with neurologic deficits (3). In recent years, percutaneous
transforaminal endoscopic discectomy was also reported as an
effective and minimally invasive alternative to identify and treat
spinal gout (3). Due to the iliac
obstruction of the L5/S1 intervertebral foramen, TILF surgery was
performed in the present study.
For most of the cases of spinal gout, histological
examination of the pathological specimen was the only way to make a
definite diagnosis (7). Polarized
light microscopy was reported to reconfirm the diagnosis of gout,
with urate crystals detected as negative birefringent needles
(7). In the present case,
histological examination as well as polarized light microscopy
definitely confirmed the diagnosis of spinal gout. The typical
picture of gout tophus was observed: Large amounts of amorphous
substances containing urate crystals were encompassed by
multinucleate giant cell granulomas and inflammatory cells.
To our knowledge, the present case with lesions of
spinal gout tophus in the intervertebral foramen was the first of
its kind reported in the literature. The initially reported cases
were all massive compressing lesions in the lumbar spinal canal
with definite clinical symptoms, such as in the studies by Chen
et al (3), Abreu Casas
et al (5), Ribeiro da Cunha
et al (7), Brahmbhatt et
al (8) and Kim et al
(15). Compared with the previous
publications, the MRI appearance reported in the present study was
more difficult to differentiate from degenerative spinal disorders.
Due to its rarity, it is seldomly suspected as a differential
diagnosis. However, it should always be considered before an open
surgery is performed. Strict metabolic control of the diet and
pharmacological treatment or anti-inflammatory medications should
be optimized in order to avoid surgery (5,9).
In conclusion, in the present study, the first case
of spinal gout with tophus in the intervertebral foramen, which
perfectly mimicked degenerative lumbar disc disorders, was
presented. Although intraspinal tophaceous gout is relatively rare,
orthopedic surgeons should take it into consideration as a possible
differential diagnosis, especially if the patient has a medical
history of gout. Early diagnosis and timely medical management may
be able to avoid neurological compromise and the need for
surgery.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Tianjin Education
Commission Research Project (grant no. 2022YGYB11).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
FH, LX, DZ and QY made substantial contributions to
the study conception and design. FH, CC and GL contributed to the
study analysis and interpretation of data. FH, LX and QY
contributed to the drafting the manuscript. FH and LX revised the
manuscript. FH and LX confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional research committee and with the 1964 Helsinki
Declaration and its later amendments or comparable ethical
standards.
Patient consent for publication
Written consent for publication of the case data and
the images was obtained from the patient described in this case
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Terkeltaub RA: Clinical practice. Gout. N
Engl J Med. 349:1647–1655. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Toprover M, Krasnokutsky S and Pillinger
MH: Gout in the Spine: Imaging, diagnosis, and outcomes. Curr
Rheumatol Rep. 17(70)2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Chen X, Xu G, Hu Q, Zhao T, Bi Q, Huang Y,
Shao H and Zhang J: Percutaneous transforaminal endoscopic
decompression for the treatment of intraspinal tophaceous gout: A
case report. Medicine (Baltimore). 99(e20125)2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Konatalapalli RM, Lumezanu E, Jelinek JS,
Murphey MD, Wang H and Weinstein A: Correlates of axial gout: A
cross-sectional study. J Rheumatol. 39:1445–1449. 2012.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Abreu Casas D, Lopez-Piloto OR, Rodriguez
de la Paz NJ, Plasencia-Leonardo JM, Iniguez-Avendano D and
Gutierrez JV: spinal cord compression due to tophaceous vertebral
gout: A case report. Cureus. 14(e27101)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Si M, Cong M, Wang D and Ma H: Intraspinal
gouty tophus. Ann Neurol. 88:1048–1049. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ribeiro da Cunha P, Peliz AJ and Barbosa
M: Tophaceous gout of the lumbar spine mimicking a spinal
meningioma. Eur Spine J. 27:815–819. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Brahmbhatt P, Vibhute P, Gupta V, Murray
J, Desai A and Agarwal A: Spinal gout diagnosed by dual-energy CT:
A case report. Radiol Case Rep. 17:4135–4138. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Beier CP, Hartmann A, Woertgen C,
Brawanski A and Rothoerl RD: A large, erosive intraspinal and
paravertebral gout tophus. Case report. J Neurosurg Spine.
3:485–487. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Mogensen MA, DeConde RP and Sarikaya B:
Spinal gout: Imaging and clinical features. PM R. 13:1304–1306.
2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Choi HK, Al-Arfaj AM, Eftekhari A, Munk
PL, Shojania K, Reid G and Nicolaou S: Dual energy computed
tomography in tophaceous gout. Ann Rheum Dis. 68:1609–1612.
2009.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Desai MA, Peterson JJ, Garner HW and
Kransdorf MJ: Clinical utility of dual-energy CT for evaluation of
tophaceous gout. Radiographics. 31:1365–1375; discussion 1376-1367.
2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ogdie A, Taylor WJ, Weatherall M, Fransen
J, Jansen TL, Neogi T, Schumacher HR and Dalbeth N: Imaging
modalities for the classification of gout: Systematic literature
review and meta-analysis. Ann Rheum Dis. 74:1868–1874.
2015.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Elgafy H, Liu X and Herron J: Spinal gout:
A review with case illustration. World J Orthop. 7:766–775.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim T, Kim BJ, Kim SH and Lee SH:
Tophaceous gout in the lumbar spinal canal mimicking epidural
spinal tumor. Korean J Spine. 14:50–52. 2017.PubMed/NCBI View Article : Google Scholar
|