Introduction
Breast cancer represents a leading cause of
cancer-associated mortality in women globally. Despite notable
advancements in the early detection and treatment of breast cancer,
including improved surgical techniques, chemotherapy, radiation
therapy and targeted biological treatments, a subset of patients
still experience recurrence and/or metastasis, resulting in the
failure of conventional therapeutic strategies (1). Thus, there is an urgent need to
understand the etiology of breast cancer and identify innovative
therapeutic approaches to address this critical health concern.
The complex nature of breast cancer, characterized
by its heterogeneity in molecular profiles, pathological features
and response to treatment, underscores the necessity of a deeper
understanding (2). Research is
increasingly focusing on the genetic and molecular underpinnings of
breast cancer, exploring the role of genetic mutations, epigenetic
alterations and the tumor microenvironment in cancer progression
and resistance to treatment (3).
Furthermore, the emerging field of cancer stem cell biology is
shedding light on a subset of cells within tumors that possess the
ability to self-renew, differentiate and potentially drive tumor
growth and metastasis.
In 1983, Mackillop et al (4) first proposed the tumor stem cell
hypothesis, suggesting that tumors contain a small subpopulation of
cells with stem cell-like properties. The significance of the tumor
stem cell hypothesis lies in its implications for cancer treatment
and resistance. Cancer stem cells (CSCs) have been implicated in
the resilience of malignant tumors to chemotherapeutic agents and
radiation therapy (5). Their
stem-like properties allow them to survive traditional therapies,
such as chemotherapy and radiotherapy, that primarily target
rapidly dividing cells. CSCs thrive in hypoxic environments and
exhibit high expression levels of free-radical scavenging
mechanisms. This adaptive response results in the decreased
intracellular accumulation of reactive oxygen species following
exposure to radiation, which consequently gives rise to the
development of a radioresistant phenotype (6). This survival advantage of CSCs is
thought to contribute to the post-treatment recurrence and
metastasis of tumors, as these residual CSCs can regenerate the
tumor mass and facilitate its spread to distant sites (7-9).
CD133, a 5-transmembrane (5_TM) glycoprotein, is a stem cell
surface marker widely used as a biomarker in various solid tumors
including brain (10), lung
(11), gastric (12) and ovarian cancer (13).
The traditional theory of tumor angiogenesis states
that tumor neovascularization primarily stems from two processes:
Angiogenesis and vasculogenesis (14,15).
Angiogenesis involves the sprouting of new blood vessels from
pre-existing ones. In the context of tumors, angiogenic factors are
released by cancer cells, which then stimulate the neighboring
vascular endothelial cells to proliferate and form new vessel
branches (16). Vascular
endothelial growth factor (VEGF) is one of the most potent inducers
of angiogenesis (17). In cancer,
VEGF is produced and secreted by tumor cells, which is associated
with tumor progression, invasiveness, metastasis and tumor
recurrence (18). Fibroblast
growth factor-2 (FGF2) exerts its effects on endothelial cells via
a paracrine signaling after being released by tumor cells (19) Unlike angiogenesis, vasculogenesis
refers to the formation of new blood vessels from endothelial
progenitor cells (EPCs) that originate in the bone marrow. These
EPCs are mobilized to the tumor site, where they differentiate into
endothelial cells and contribute to the neovascular network
(20).
In the absence of vascular endothelial cells, tumor
stem cells within the tumor tissues can differentiate into vascular
endothelial cells, promoting tumor angiogenesis (21,22).
This process, a form of neovascularization distinct from
traditional angiogenesis and vasculogenesis, involves the direct
contribution of tumor stem cells to the tumor vasculature. These
cells undergo endothelial differentiation, integrating into the
developing vascular structure and thereby supporting the angiogenic
process (23). Several studies
have highlighted the link between tumor cell proliferation,
invasion, metastasis, and angiogenesis (24-27).
However, the role of tumor stem cells in inducing the formation of
tumor blood vessels is not fully understood.
The present study utilized CD133 as a stem cell
surface marker to isolate and purify tumor stem cells from breast
cancer cell lines. By enhancing the differentiation of
CD133+ breast CSCs into vascular endothelial cells in
vitro, this study aimed to establish a basis for studying
anti-angiogenic mechanisms.
Materials and methods
Cells and cell culture
MCF-7 (cat. no. SCSP-531) were purchased from the
Cell Bank of the Chinese Academy of Sciences and HUVECs (cat. no.
iCell-h110) were purchased from Cellverse Bioscience Technology
Co., Ltd. The MCF-7 breast cancer cell line was cultured in DMEM
supplemented with 10% FBS, 100 µg/ml streptomycin, and 100 U/ml
penicillin. HUVECs were maintained in an ECM endothelial cell
culture medium supplemented with 5% FBS, 100 µg/ml streptomycin,
and 100 U/ml penicillin. HUVECs are known to exhibit senescence
after several passages, thus they were revived at cell passage 2
and consistently used at low passages, typically below passage 5.
This practice was essential to maintain their physiological
relevance and ensure the consistency of the results. Both cell
types were incubated at 37˚C in a 5% CO2 incubator.
MCF-7 cells exhibiting adherent growth were deemed suitable for
experimental use when adherent growth exceeded 80%. Before
passaging or cryopreservation, cells were digested with
trypsin.
Flow cytometry
Cells were digested using 0.25% trypsin, and
digestion was halted by adding culture media. Subsequently, cells
were washed with 0.01M PBS, resuspended, and centrifuged at 800 x g
for 5 min at room temperature to remove the supernatant. The cells
were prepared at a concentration of 1x106 cells in 1 ml
of 0.01 M PBS. Subsequently, 100 µl cell suspension was added into
5 ml flow tubes with phycoerythrin-labeled CD133 (1:20; cat. no.
12-1338-42; Thermo Fisher Scientific, Inc.), CD31 (1:40; cat. no.
12-0319-42; Thermo Fisher Scientific, Inc.), or CD105 antibodies
(1:40; cat. no. MHCD10504; Thermo Fisher Scientific, Inc.). The
mixture was thoroughly mixed and incubated at 4˚C in the dark for
10 min. Following incubation, cells were washed, resuspended,
centrifuged at 800 x g for 5 min at 4˚C, and the supernatant was
removed. Finally, cells were resuspended in 500 µl 0.01 M PBS, flow
data were collected using BD FACSCanto II (Becton, Dickinson and
Company) and analyzed using FLOWJO version 7.6.2 (flowjo.com/).
Isolation of CD133-positive cells
After culturing MCF-7 cells, they were digested and
washed. Subsequently, cells were resuspended in 300 µl sorting
buffer and combined with 100 µl anti-CD133 immunomagnetic beads
(cat. no. 130-097-049; Miltenyi Biotec GmbH). The mixture was
incubated at 4˚C in the dark for 30 min. After incubation, cells
were washed with 0.01 M PBS and centrifuged at 800 x g for 10 min
at 4˚C. The supernatant was discarded, and the cell pellet was
resuspended in a sorting buffer. A pre-rinsed separation column
with 500 µl sorting buffer was then loaded with the cell suspension
and washed thrice with 0.01 M PBS. CD133+ cells were
eluted by washing the column with 1 ml sorting buffer using a
syringe pump.
MTT assay
The MCF-7 cells were divided into two
subpopulations, MCF-7CD133+ and MCF-7CD133-,
and seeded into 96-well plates. Each well was filled with 0.2 ml
serum-free media supplemented with 20 ng/ml basic fibroblast growth
factor (bFGF; cat. no. AF-100-18B-500UG; Thermo Fisher Scientific,
Inc.) and 20 ng/ml epidermal growth factor (EGF; cat. no.
AF-100-15-500UG; Thermo Fisher Scientific, Inc.). The cells were
cultured for 7 days, with the addition of 20 µl MTT reagent to each
well daily. After 4 h of further incubation, the medium was removed
and replaced with 150 µl DMSO. The absorbance at 490 nm was
measured for each well to determine cell growth rates.
Spheroid formation assay
The MCF-7CD133+ and
MCF-7CD133- subpopulations were seeded at a density of
1,000 cells/ml in 12-well low-adhesion plates, each well contained
1 ml spheroid formation medium. This medium consisted of DMEM/F12
supplemented with 1x B27, 20 ng/ml bFGF, 20 ng/ml EGF, 5 g/ml
insulin, and 1% penicillin-streptomycin. Spheroid formation was
observed under a light microscope (x100 magnification) after 3
days.
Endothelial cell differentiation
culture
The MCF-7CD133+ and
MCF-7CD133- subpopulations were cultured in stem cell
maintenance medium, which was prepared by supplementing StemScale™
PSC medium (Gibco; Thermo Fisher Scientific, Inc.) with bFGF (20
ng/ml), EGF (20 ng/ml), and BMP4 (25 ng/ml; cat. no. 795604;
BioLegend, Inc.). The cells were incubated for 2 days in
low-adhesion dishes, and the media was replaced every 2 days.
Subsequently, the cells were transferred to endothelial cell
differentiation medium, which was composed of ECM medium
(ScienCell) supplemented with VEGF (50 ng/ml; cat. no.
AF-100-20-500UG; Thermo Fisher Scientific, Inc.) and bFGF (20
ng/ml). Cells were maintained in this differentiation culture media
for 6 days, and the media was changed every 2 days.
Endothelial cell tube formation
assay
Matrigel matrix gel was thawed at 4˚C and left
undisturbed for 1 day. To initiate the experiment, refrigerated
pipette tips and µ-Slide angiogenesis culture plates were used. A
total of 10 µl Matrigel was added to each well of the µ-Slide
plate. The µ-Slide plate was placed in an appropriately sized
culture dish containing water-saturated absorbent paper to prevent
moisture evaporation. The culture dishes were incubated for 30 min,
allowing the gel to solidify. Cell suspensions (2x105
cells/ml) were prepared after cell digestion, and 50 µl of this
suspension was added to the µ-Slide plate. The plates were covered
and returned to the incubator for continuous culture. The cells
were monitored using an optical microscope (x200 magnification)
every 4-6 h.
DiI-labeled acetylated low-density
lipoprotein (DiI-Ac-LDL) uptake assay
HUVECs, MCF-7CD133+, or
MCF-7CD133- induced endothelial cells were seeded in
24-well plates and cultured for 48 h. Subsequently, the culture
media was removed and cells were incubated in serum-free ECM
endothelial cell medium for 3 h. DiI-Ac-LDL was prepared in
serum-free EGM medium at a concentration of 10 µg/ml, added to
cells, and incubated at 37˚C for 4 h. Following incubation, the
media was discarded, and cells were washed three times in 0.01M PBS
to eliminate any unbound DiI-Ac-LDL. Finally, supplemented culture
medium was added and the cells were examined under a fluorescence
microscope (x400 magnification).
MDM2/CEN12 fluorescent in situ
hybridization (FISH) fluorescent probe detection
Cell preparation involved washing HUVEC,
MCF-7CD133+, and MCF-7CD133--induced
endothelial cells with 0.01M PBS, dropping them onto a glass slide,
denaturing at 73˚C for 2 min, hybridizing with a MDM2/CEN12 probe
(cat. no. FG0020; Abnova) at 38˚C for 16 h, and washing with 0.3%
NP-40/SSC and 0.1% NP-40/SSC. The slides were sequentially
dehydrated in 70, 90 and 100% ethanol for 2 min each. Finally, the
cell nuclei were counterstained for 5 min at room temperature with
DAPI and observed under a fluorescence microscope (x1,000
magnification).
Statistical analysis
Data were analyzed using SPSS version 18.0 (SPSS
Inc.), all data are presented as the mean ± SD. Differences between
groups were compared using an unpaired Student's t-test. P<0.05
was considered to indicate a statistically significant
difference.
Results
CD133 expression levels in MCF-7 pre-
and post-immunomagnetic bead separation
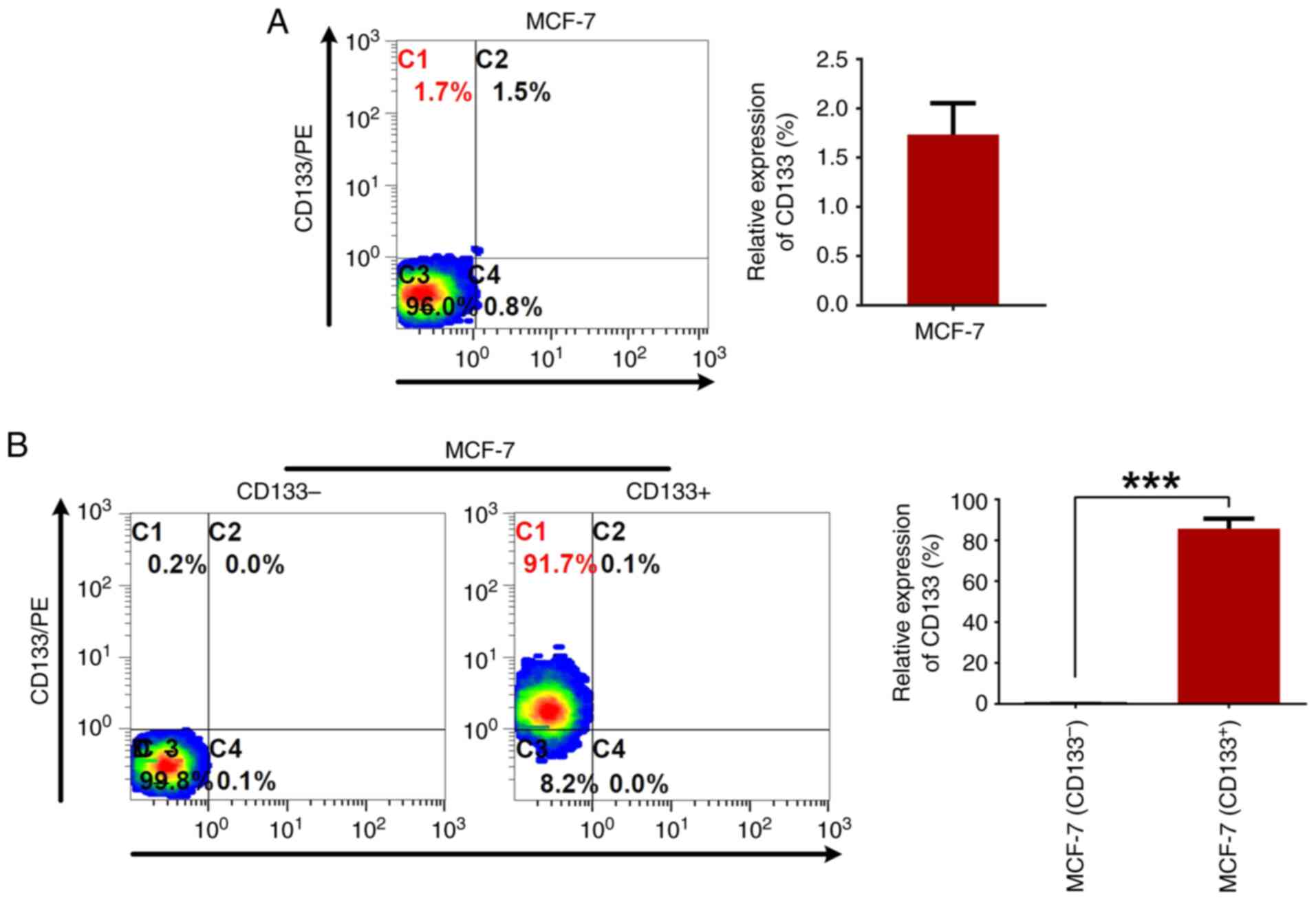
Flow cytometry was used to assess CD133 expression
in the MCF-7 breast cancer cells, and it was found that only
1.7±0.3% of the cells exhibited CD133 expression (Fig. 1A). Post anti-CD133 immunomagnetic
bead sorting, two subpopulations, MCF-7CD133+ and
MCF-7CD133-, were isolated. Flow cytometry was used to
measure the proportions of CD133+ cells in these subpopulations,
revealing expression rates of 85.6±2.8% for MCF-7CD133+
and 0.18±0.08% for MCF-7CD133- (Fig. 1B).
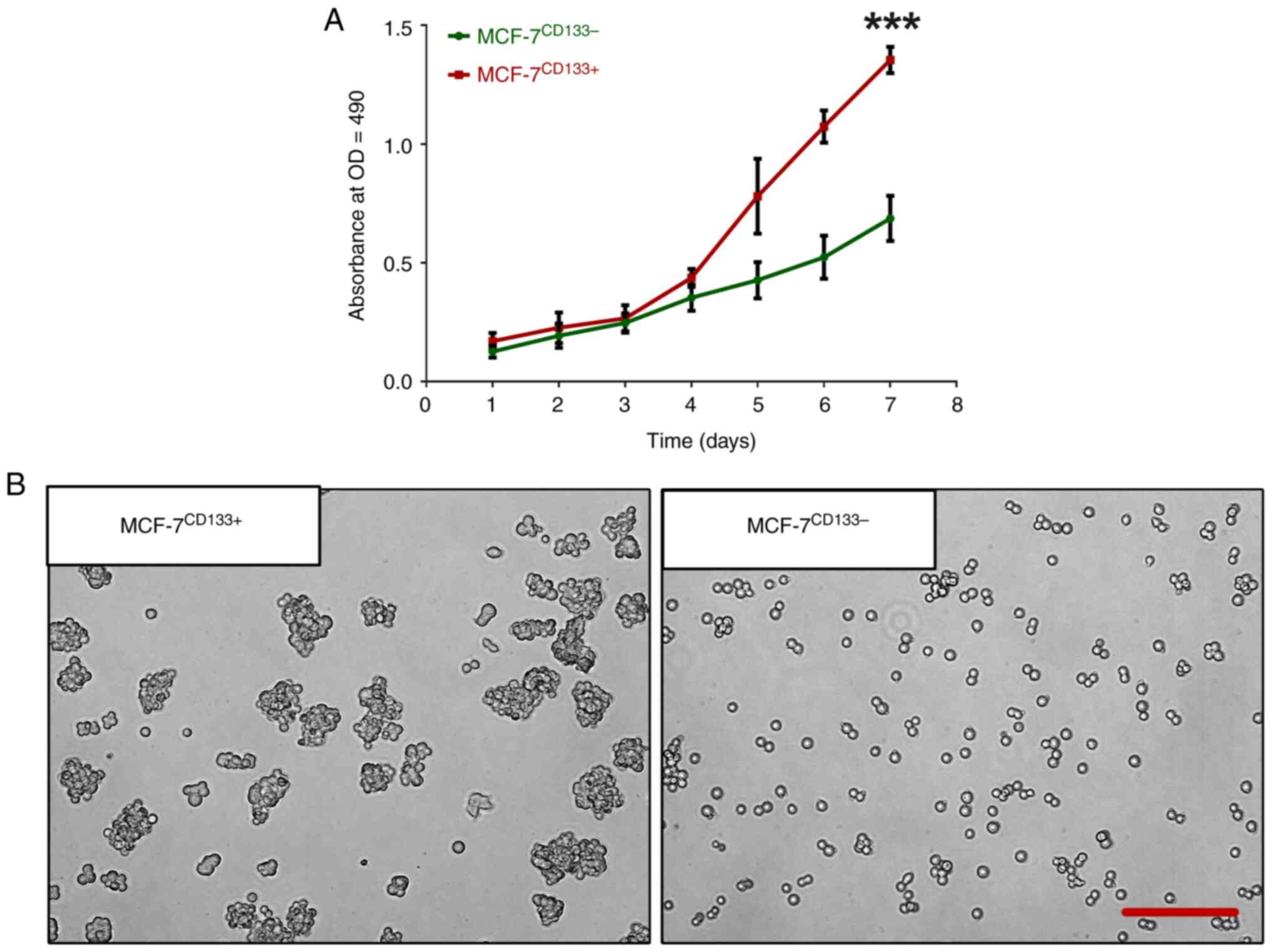
MCF-7 CD133+ cells exhibit
increased in vitro proliferation and spheroid formation
capabilities compared with the MCF-7CD133- cells
Compared with the MCF-7CD133- cells, the
MCF-7CD133+ cells exhibited increased in vitro
proliferation capacity, most notably on day 7 (Fig. 2A). The spheroid formation assay
highlighted the cancer stem cell characteristics of both
MCF-7CD133+ and MCF-7CD133- cells. After 3
days in low-attachment culture plates, MCF-7CD133+ cells
displayed increased differentiation and growth capacity, resulting
in a significantly higher number of spheroids compared with the
MCF-7CD133- (Fig.
2B).
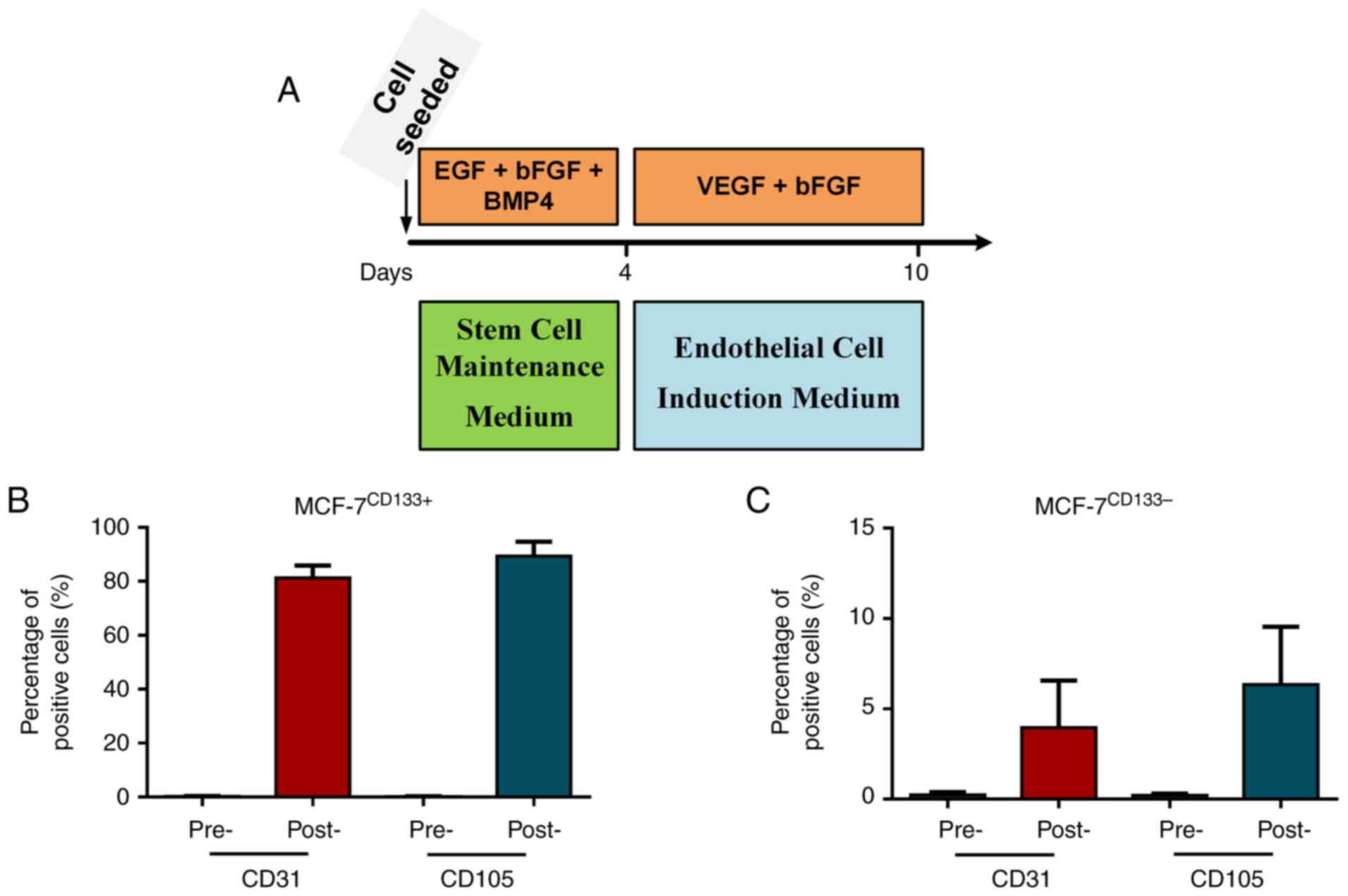
Assessment of endothelial cell marker
expression pre- and post-endothelial cell induction culture
MCF-7CD133+ and MCF-7CD133-
cells were cultured in stem cell maintenance media for 4 days,
followed by 6 days in endothelial cell induction media, to induce
tumor stem cells towards endothelial differentiation (Fig. 3A). Flow cytometry was used to
assess the expression levels of endothelial cell surface markers
CD31 and CD105 before and after induction. In the
MCF-7CD133+ cells, the CD31+ proportions were
0.3±0.16% pre-induction and 81.4±8.37% post-induction, and the
CD105+ proportions were 0.2±0.08% pre-induction and
83.8±7.24% post-induction (Fig.
3B). In MCF-7CD133- cells, the CD31+
proportions were 0.23±0.12% pre-induction and 3.95±2.1%
post-induction, and the CD105+ proportions were
0.26±0.04% pre-induction and 6.3±2.6% post-induction (Fig. 3C).
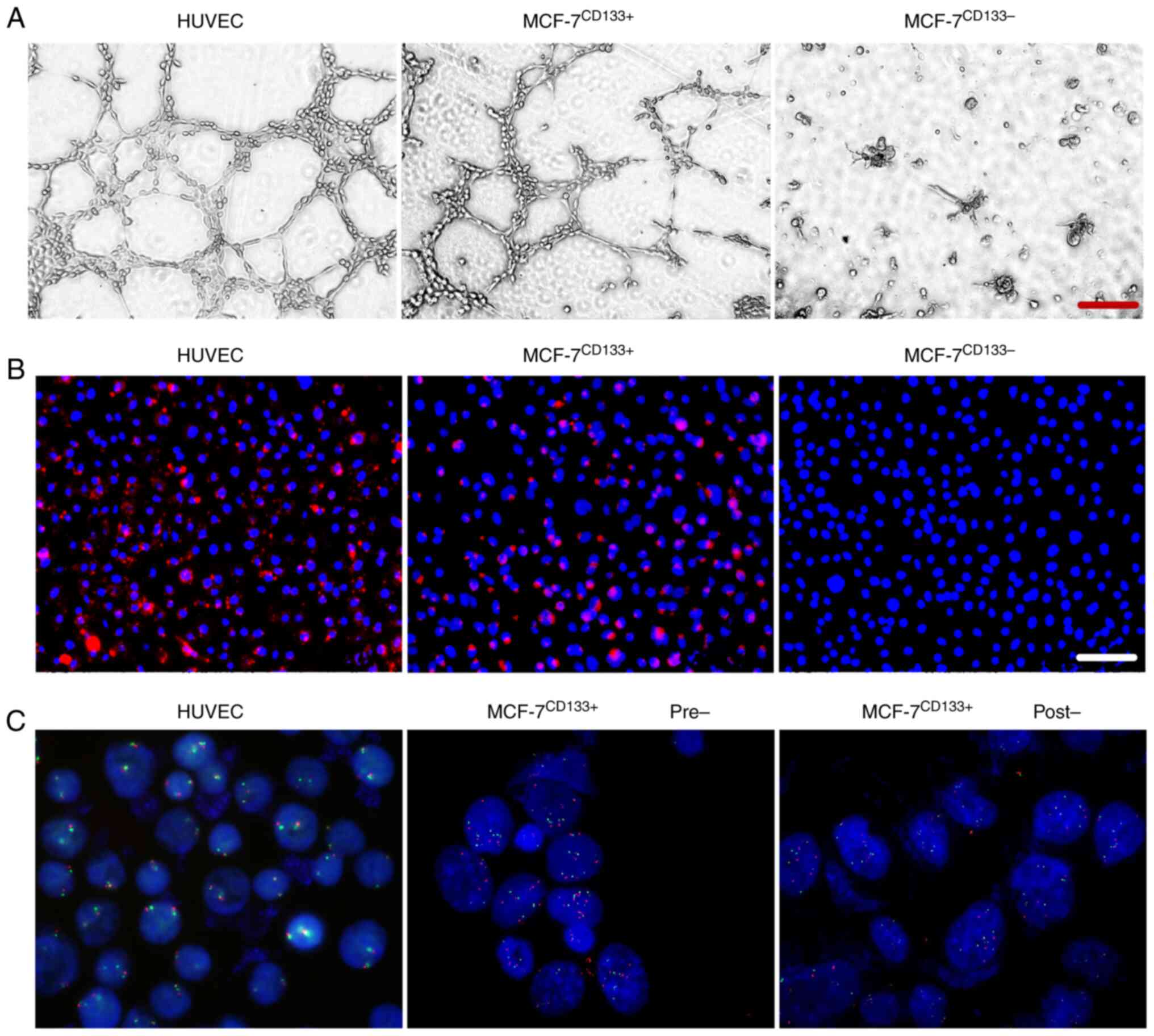
Evaluation of endothelial cell
function and gene amplification
In the endothelial cell tube formation assay, both
the positive control HUVECs and MCF-7CD133+ cells formed
lumen-like structures. In contrast, the MCF-7CD133-
cells did not form these structures (Fig. 4A). In the endothelial cell uptake
assay, both positive control HUVECs and MCF-7CD133+
cells emitted red fluorescence after staining with DiI-Ac-LDL
(Fig. 4B). FISH experiments
indicated that after induction, MCF-7CD133+ cells still
exhibited amplification of the MDM2/CEN12 gene in the cell
chromosomes (Fig. 4C).
Discussion
Breast cancer, a heterogeneous malignant tumor, is
influenced by various risk factors including diet, environment,
genetics, and epigenetics. Current data suggest that the 5-year
survival rates for stage II and III breast cancer patients are 75
and 61%, respectively. However, 20-30% of cases still exhibit
recurrence and/or metastasis (28). Therefore, finding effective
strategies to prevent the recurrence and metastasis of breast
cancer is crucial.
Contemporary theories suggest the presence of tumor
stem cells in tumor patients, cells with characteristics akin to
embryonic stem cells. These cells have unlimited proliferation,
self-renewal, and multilineage differentiation capabilities.
Additionally, they exhibit chemoresistance and radioresistance,
contributing to recurrence and metastasis despite comprehensive
anti-tumor therapies (29-31).
CD133, also known as Prominin-1, a member of the Prominin family,
is a five-transmembrane domain glycoprotein predominantly located
on cell membrane surface protrusions and is recognized as a key
biomarker for CSCs (32,33). In the present study, we initially
identified a minor population of CD133-expressing cells within the
MCF-7 breast cancer cell line. Utilizing anti-CD133 immunomagnetic
bead separation, MCF-7CD133+ cells with a high CD133
positivity rate of approximately 85% were isolated, as determined
by flow cytometry. It is important to note that the proportion of
CSCs within cancer cell lines can indeed change over time and with
continuous passaging. Thus, the proportion of stem cells and cancer
cells within the cell lines across different passages was monitored
to assess this variability. In comparison with
MCF-7CD133- cells, the MCF-7CD133+ cells
exhibited a significantly enhanced proliferative capacity. An
actively proliferating subset of CSCs may play a crucial role in
the growth and progression of tumors. These cells can give rise to
more differentiated tumor cells while maintaining the CSC
population, allowing the tumor to grow and potentially spread. When
cultured in serum-free media enriched with growth factors,
MCF-7CD133+ cells rapidly formed spheroids, exhibiting
growth in suspension, and robust proliferation, while
MCF-7CD133- cells showed a significantly reduced
capacity for spheroid formation. These findings indicate that the
MCF-7CD133+ cells were enriched in stem cells.
Tumor activities such as proliferation, invasion,
and metastasis are closely associated with angiogenesis; however,
the relationship between tumor stem cells and tumor vascular
formation remains unclear and necessitates further investigation.
In the present study, MCF-7CD133+ cells were isolated
using immunomagnetic bead separation, and these cells were
subjected to endothelial cell-inducing and maintenance media to
transform the cells. Subsequently, the MCF-7CD133+
subpopulation cells formed luminal-like structures in the Matrigel
matrix, akin to those formed by HUVECs (the positive control).
Conversely, the MCF-7CD133- cells failed to form similar
structures in endothelial cell transformation culture. In the
endothelial cell phagocytosis assay, both the transformed
MCF-7CD133+ cells and HUVECs internalized DiI-Ac-LDL and
emitted red fluorescence, corroborating related studies (34,35).
FISH was used to confirm that MCF-7CD133+ cells retained
their tumor cell characteristics following endothelial cell culture
conversion. These findings suggest that a distinct subpopulation of
cells with stem cell-like properties, capable of differentiating
into endothelial cells, exists in breast cancer.
The complex molecular mechanisms driving the
differentiation of CSCs into vascular endothelial cells remain
elusive. Previous research has shown that in hypoxic conditions,
CSCs secrete VEGF, a powerful factor stimulating their
transformation into endothelial cells, thus enhancing their
propensity to differentiate under oxygen-deprived conditions
(36). Alvero et al
(37) found that CSCs from ovarian
cancer possess the ability to differentiate into progenitors of
vascular endothelial cells and form vascular-like structures in
xenograft tumor inhibition models. In the present study, by
enriching the culture medium with stimulatory factors such as bFGF,
EGF, BMP4, and VEGF, transformation and maintenance cultivation of
MCF-7CD133+ and MCF-7CD133- cells was
successfully achieved. Subsequent endothelial cell tube formation
and phagocytosis experiments suggested that the transformed
MCF-7CD133+ cells exhibited endothelial cell
functionality. These findings support the notion that CSCs can
transform into vascular endothelial cells.
The tumor microenvironment plays a pivotal role in
the onset and progression of malignant tumors (38). Modulating the tumor
microenvironment can mitigate or inhibit tumor growth (39). Currently, modulating changes in the
tumor microenvironment is challenging, but indirectly delaying or
suppressing the formation of the tumor microenvironment by
adjusting the functions of relevant cells within it, may serve as a
treatment method for malignant tumors. Recent research has
suggested that targeting tumor vascular endothelial cells is a
promising direction for anti-tumor drug development.
Anti-angiogenic drugs target various aspects of the angiogenic
process. This includes inhibiting growth factors such as VEGF and
its receptors (VEGFR), which are key drivers in the formation of
new blood vessels. Drugs such as Bevacizumab (Avastin), an antibody
that binds to VEGF, prevents it from activating VEGFR on
endothelial cells. Tyrosine kinase inhibitors such as Sunitinib
target VEGFR directly. The partial failure of anti-VEGF strategies
in controlling cancer can be attributed to two major factors.
First, the precise molecular mechanisms of cancer neo-angiogenesis
are incompletely understood. Additionally, the abrogation of blood
supply also restricts drug delivery to the tumor, reducing its
effectiveness and promoting drug resistance (40). Correspondingly, a paradox in using
anti-angiogenic drugs has emerged from recent findings. By
inhibiting new blood vessel formation, anti-angiogenic drugs can
increase the level of hypoxia (oxygen deprivation) within the
tumor. This hypoxic environment can lead to the selection of more
aggressive tumor cells that are better adapted to survive in low
oxygen conditions (41). Several
studies suggest that anti-angiogenic therapy might stimulate the
tumor to become more invasive (42-45).
In an attempt to access more blood supply, cancer cells might begin
to invade surrounding tissues or spread to other parts of the body
(46-51).
These suggest that strategies aimed at normalizing tumor vessels,
rather than eradicating blood supply, could enhance the delivery of
therapeutic agents to cancer cells, thereby improving the efficacy
and limiting cancer cell spread (52).
In conclusion, the present study induced the
transformation of breast CSCs, and these transformed cells provided
a more representative model for studying anti-angiogenesis in
vitro than HUVECs. This approach addresses the challenges of
the low availability and difficulty of separation of tumor vascular
endothelial cells in vivo. It also lays the groundwork for
more comprehensive and targeted research for understanding and
combating tumor vascularization.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by grants from the
National Natural Science Foundation of Ningbo (grant nos.
202003N4022 and 2019A610313) and the Zhejiang Provincial Medical
and Health Science and Technology Plan (grant nos. 2021KY312 and
2023KY1048).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QQM and XCJ contributed to the conception and design
of the research. JNZ, WFT, QQM and SCZ performed the experiments
and collected and interpreted the data. The first draft of the
manuscript was written by QQM. SCZ and XCJ confirm the authenticity
of all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Arnold M, Morgan E, Rumgay H, Mafra A,
Singh D, Laversanne M, Vignat J, Gralow JR, Cardoso F, Siesling S
and Soerjomataram I: Current and future burden of breast cancer:
Global statistics for 2020 and 2040. Breast. 66:15–23.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Rivenbark AG, O'Connor SM and Coleman WB:
Molecular and cellular heterogeneity in breast cancer. Am J Pathol.
183:1113–1124. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Szczepanek J, Skorupa M, Jarkiewicz-Tretyn
J, Cybulski C and Tretyn A: Harnessing epigenetics for breast
cancer therapy: The role of DNA methylation, histone modifications,
and microRNA. Int J Mol Sci. 24(7235)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Mackillop WJ, Ciampi A, Till JE and Buick
RN: A stem cell model of human tumor growth: implications for tumor
cell clonogenic assays. J Natl Cancer Inst. 70:9–16.
1983.PubMed/NCBI
|
|
5
|
Llaguno SA and Parada LF: Cancer stem
cells in gliomas: Evolving concepts and therapeutic implications.
Curr Opin Neurol. 34:868–874. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Diehn M, Cho RW, Lobo NA, Kalisky T, Dorie
MJ, Kulp AN, Qian D, Lam JS, Ailles LE, Wong MZ, et al: Association
of reactive oxygen species levels and radioresistance in cancer
stem cells. Nature. 458:780–783. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hu Y and Fu L: Targeting cancer stem
cells: A new therapy to cure cancer patients. Am J Cancer Res.
2:340–356. 2012.PubMed/NCBI
|
|
8
|
Loureiro R, Mesquita KA, Oliveira PJ and
Vega-Naredo I: Mitochondria in cancer stem cells: A target for
therapy. Recent Pat Endocr Metab Immune Drug Discov. 7:102–114.
2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Phi LH, Sari IN, Yang YG, Lee SH, Jun NY,
Kim KS, Lee YK and Kwon HY: Cancer stem cells (CSCs) in drug
resistance and their therapeutic implications in cancer treatment.
Stem Cells Int. 28(5416923)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Griguer CE, Oliva CR, Gobin E, Marcorelles
P, Benos DJ, Lancaster JR and Gillespie GY: CD133 is a marker of
bioenergetic stress in human glioma. PLoS One.
3(e3655)2008.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kunihiro AG, Sarrett SM, Lastwika KJ,
Solan JL, Pisarenko T, Keinänen O, Rodriguez O, Taverne LR,
Fitzpatrick AL, Li CI, et al: CD133 as a biomarker for an
autoantibody-to-immunoPET paradigm for the early detection of small
cell lung cancer. J Nucl Med. 63:1701–1707. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Howard R, Diffalha SA, Pimiento J, Mejia
J, Enderling H, Giuliano A and Coppola D: CD133 expression as a
helicobacter pylori-independent biomarker of gastric cancer
progression. Anticancer Res. 38:4443–4448. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ikram D, Masadah R, Nelwan BJ, Zainuddin
AA, Ghaznawie M and Wahid S: CD133 act as an essential marker in
ovarian carcinogenesis. Asian Pac J Cancer Prev. 22:3525–3531.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Lugano R, Ramachandran M and Dimberg A:
Tumor angiogenesis: Causes, consequences, challenges and
opportunities. Cell Mol Life Sci. 77:1745–1770. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu ZL, Chen HH, Zheng LL, Sun LP and Shi
L: Angiogenic signaling pathways and anti-angiogenic therapy for
cancer. Signal Transduct Target Ther. 8(198)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Viallard C and Larrivée B: Tumor
angiogenesis and vascular normalization: alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ferrara N, Gerber HP and LeCouter J: The
biology of VEGF and its receptors. Nat Med. 9:669–676.
2003.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Apte RS, Chen DS and Ferrara N: VEGF in
signaling and disease: Beyond discovery and development. Cell.
176:1248–1264. 2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Turner N and Grose R: Fibroblast growth
factor signaling: from development to cancer. Nat Rev Cancer.
10:116–129. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Johnson KE and Wilgus TA: Vascular
endothelial growth factor and angiogenesis in the regulation of
cutaneous wound repair. Adv Wound Care (New Rochelle). 3:647–661.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Phillips TM, McBride WH and Pajonk F: The
response of CD24(-/low)/CD44+ breast cancer-initiating cells to
radiation. J Natl Cancer Inst. 98:1777–1785. 2006.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Puca F, Fedele M, Rasio D and Battista S:
Role of diet in stem and cancer stem cells. Int J Mol Sci.
23(8108)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Katayama Y, Uchino J, Chihara Y, Tamiya N,
Kaneko Y, Yamada T and Takayama K: Tumor neovascularization and
developments in therapeutics. Cancers (Basel).
11(316)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Aomatsu N, Yashiro M, Kashiwagi S,
Takashima T, Ishikawa T, Ohsawa M, Wakasa K and Hirakawa K: CD133
is a useful surrogate marker for predicting chemosensitivity to
neoadjuvant chemotherapy in breast cancer. PLoS One.
7(e45865)2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sun C, Li JM, Wang B, Shangguan JJ, Figini
M, Shang N, Pan L and Zhang ZL: Tumor angiogenesis and bone
metastasis-correlation in invasive breast carcinoma. J Immunol
Methods. 452:46–52. 2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lv XQ, Wang YZ, Song YM, Pang X and Li HX:
Association between ALDH1+/CD133+ stem-like
cells and tumor angiogenesis in invasive ductal breast carcinoma.
Oncol Lett. 11:1750–1756. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Sudhan DR, Rabaglino MB, Wood CE and
Siemann DW: Cathepsin L in tumor angiogenesis and its therapeutic
intervention by the small molecule inhibitor KGP94. Clin Exp
Metastasis. 33:461–73. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim W, Kim KS and Park RW: Nomogram of
naive bayesian model for recurrence prediction of breast cancer.
Healthc Inform Res. 22:89–94. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Panigoro SS, Kurnia D, Kurnia A, Haryono
SJ and Albar ZA: ALDH1 cancer stem cell marker as a prognostic
factor in triple-negative breast cancer. Int J Surg Oncol.
2020(7863243)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Yu JG, Liao XH, Li YL, Lv L, Zhi XL, Yu J
and Zhou P: A preliminary study of the role of
extracellular-5'-nucleotidase in breast cancer stem cells and
epithelial-mesenchymal transition. In Vitro Cell Dev Biol Anim.
53:132–140. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang LQ, Shi PF and Zhao GC: Targeting
cancer stem cell pathways for cancer therapy. Signal Transduct
Target Ther. 5(8)2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Irollo E and Pirozzi G: CD133: To be or
not to be, is this the real question? Am J Transl Res. 5:563–581.
2013.PubMed/NCBI
|
|
33
|
Bauer N, Fonseca AV, Florek M, Freund D,
Jászai J, Bornhäuser M, Fargeas CA and Corbeil D: New insights into
the cell biology of hematopoietic progenitors by studying
prominin-1 (CD133). Cells Tissues Organs. 188:127–138.
2008.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Zou L, Liu XW, Li JJ, Li W, Zhang LL, Li J
and Zhang JM: Tetramethylpyrazine enhances the antitumor effect of
paclitaxel by inhibiting angiogenesis and inducing apoptosis. Front
Pharmacol. 10(707)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ahmed N, Abubaker K, Findlay J and Quinn
M: Epithelial mesenchymal transition and cancer stem cell-like
phenotypes facilitate chemoresistance in recurrent ovarian cancer.
Curr Cancer Drug Targets. 10:268–278. 2010.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liu XM, Zhang QP, Mu YG, Zhang XH, Sai K,
Pang JC, Ng HK and Chen ZP: Clinical significance of vasculogenic
mimicry in human gliomas. J Neurooncol. 105:173–179.
2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Alvero AB, Chen R, Fu HH, Montagna M,
Schwart PE, Rutherford T, Silasi DA, Steffensen KD, Waldstrom M,
Visintin I and Mor G: Molecular phenotyping of human ovarian cancer
stem cells unravels the mechanisms for repair and chemoresistance.
Cell Cycle. 8:158–166. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yuan Y, Jiang YC, Sun CK and Chen QM: Role
of the tumor microenvironment in tumor progression and the clinical
applications (review). Oncol Rep. 35:2499–2515. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Hinshaw DC and Shevde LA: The tumor
microenvironment innately modulates cancer progression. Cancer Res.
79:4557–4566. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Rohlenova K, Veys K, Miranda-Santos I,
Bock KD and Carmeliet P: Endothelial cell metabolism in health and
disease. Trends Cell Biol. 28:224–236. 2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Muz B, Puente PD, Azab F and Azab AK: The
role of hypoxia in cancer progression, angiogenesis, metastasis,
and resistance to therapy. Hypoxia (Auckl). 3:83–92.
2015.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Vasudev NS and Reynolds AR:
Anti-angiogenic therapy for cancer: current progress, unresolved
questions and future directions. Angiogenesis. 17:471–494.
2014.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Alexandre J, Batteux F, Nicco C, Chéreau
C, Laurent A, Guillevin L, Weill B and Goldwasser F: . Accumulation
of hydrogen peroxide is an early and crucial step for
paclitaxel-induced cancer cell death both in vitro and in vivo. Int
J Cancer. 119:41–48. 2006.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Fukui M, Yamabe N and Zhu BT: .
Resveratrol attenuates the anticancer efficacy of paclitaxel in
human breast cancer cells in vitro and in vivo. Eur J Cancer.
46:1882–1891. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Haibe Y, Kreidieh M, El Hajj H, Khalifeh
I, Mukherji D, Temraz S and Shamseddine A: Resistance mechanisms to
anti-angiogenic therapies in cancer. Front Oncol.
10(221)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ansari MJ, Bokov D, Markov A, Jalil AT,
Shalaby MN, Suksatan W, Chupradit S, Al-Ghamdi HS, Shomali N,
Zamani A, et al: Cancer combination therapies by angiogenesis
inhibitors; a comprehensive review. Cell Commun Signal.
20(49)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Eelen G, Treps L, Li XR and Carmeliet P:
Basic and therapeutic aspects of angiogenesis updated. Circ Res.
127:310–329. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Lee WS, Yang H, Chon HJ and Kim C:
Combination of anti-angiogenic therapy and immune checkpoint
blockade normalizes vascular-immune crosstalk to potentiate cancer
immunity. Exp Mol Med. 52:1475–1485. 2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Huinen ZR, Huijbers EJ, Beijnum JR,
Nowak-Sliwinska P and Griffioen AW: Anti-angiogenic
agents-overcoming tumour endothelial cell anergy and improving
immunotherapy outcomes. Nat Rev Clin Oncol. 18:527–540.
2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Wehland M, Bauer J, Infanger M and Grimm
M: Target-based anti-angiogenic therapy in breast cancer. Curr
Pharm Des. 18:4244–4257. 2012.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Yetkin-Arik B, Kastelein AW, Klaassen I,
Jansen CH, Latul YP, Vittori M, Biri A, Kahraman K, Griffioen AW,
Amant F, et al: Angiogenesis in gynecological cancers and the
options for anti-angiogenesis therapy. Biochim Biophys Acta Rev
Cancer. 1875(188446)2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Fukumura D and Jain RK: Tumor
microvasculature and microenvironment: targets for
anti-angiogenesis and normalization. Microvasc Res. 74:72–84.
2007.PubMed/NCBI View Article : Google Scholar
|