Introduction
Pilon fractures are lower segment injuries of the
tibia that usually occur due to high-energy impact and affect the
inferior articular surface. These fractures may be accompanied by
additional fractures to the medial, lateral or posterior malleoli
(1). A distal tibial plafond
fracture is also known as a pilon fracture, which describes the
high-energy axial compression force of the tibia acting as a
pestle, driving vertically into the talus. These fractures
constitute 1-10% of the lower leg or tibial fractures and are
frequently accompanied by severe bone comminution and soft-tissue
compromise (2,3). These fractures may also extend to the
metaphyseal extension and be associated with fibular fractures
(4). At present, high-energy pilon
fractures are primarily caused by traffic accidents and high falls
(5).
The two most common classification systems used to
describe pilon fractures are the Ruedi-Allgower classification and
the Arbeitsgemeinschaftfür Osteosynthesefragen
Foundation/Orthopedic Trauma Association classification. Currently,
the Ruedi-Allgower classification is the most commonly used
clinical classification for pilon fractures, which is mainly
evaluated according to the degree of articular surface involvement
and bone block displacement (6).
Type II and III pilon fractures are mostly caused by high-energy
traumas (5,7). When soft-tissue damage is severe and
the articular surface is broken, surgical reduction and fixation
become challenging, and the overall prognosis for the patient is
unfavorable (8). Pilon fractures
can be some of the most difficult fractures to treat; as they are
often associated with high-energy trauma, both soft-tissue
involvement and comminuted fracture patterns pose challenges to
fixation (9). Traditional clinical
treatment for high-energy pilon fractures typically involves
external fixation combined with limited internal fixation (10) and delayed open reduction and
internal fixation (11-13).
With advancements in instruments and the refinement of surgical
techniques, arthroscopy is now also being utilized for the
treatment of high-energy pilon fractures (14-17).
Fractures can result in a variety of cellular
responses, including pyroptosis (18). Pyroptosis is a form of programmed
cell death that occurs when cells are exposed to inflammatory
signals or pathogens, and caspase-1 serves as a significant
biomarker associated with pyroptosis (19). The molecular mechanism of
pyroptosis involves the activation of a protein complex called the
inflammasome, which leads to the cleavage and activation of
caspase-1(20). Once caspase-1 is
activated, it can then cleave several downstream substrates,
including gasdermin D. Cleavage of gasdermin D leads to the
formation of membrane pores, which allow the release of cytoplasmic
contents and ultimately result in cell death (21). Additionally, the activation of
caspase-1 can also lead to the release of pro-inflammatory
cytokines, such as interleukin (IL)-1β and IL-18, which can further
exacerbate inflammation and tissue damage (22). The latest research has revealed the
value of the NLRP3/caspase-1/gasdermin D pyroptosis pathway in
fracture repair (23). However, to
the best of our knowledge, no studies have addressed the clinical
significance of serum caspase-1 in patients with fractures.
The present prospective observational study was
conducted from July 2015 until July 2020, with 136 cases of
high-energy pilon fracture being treated using an
arthroscopic-assisted locking plate. The objective was to
investigate serum levels of caspase-1 in patients with high-energy
pilon fractures and their correlation with clinical results and
prognosis.
Patients and methods
Patients
The present prospective observational study included
136 patients (mean age, 47; age range, 24-66; 52.9% female) with
high-energy pilon fractures who were treated with locking plates
combined with ankle arthroscopy in Ordos Central Hospital (Ordos,
China) from July 2015 to July 2020. The entire study was conducted
according to the Declaration of Helsinki. The study was approved by
the Ethics Committee of Ordos Central Hospital (approval no.
IRB2015-OCHOS-116). Written informed consent was obtained from all
participants.
The inclusion criteria were: i) Meeting the
diagnostic criteria of a pilon fracture, with a history of
high-energy trauma with soft tissue injury; ii) admission
immediately after trauma, with the time from trauma to admission
<360 min; and iii) complete clinical data. The following
patients were excluded: i) Bilateral pilon fracture; ii)
osteofascial compartment syndrome; iii) severe injury to other
organs; and iv) shock.
Rüedi and Allgower classifies pilon fractures into
three types. Type I fractures involve column displacement of the
articular surface. Type II fractures involve displacement of the
articular surface without comminution. Type III fractures are
comminuted, involving the metaphysis and articular surface
(24). The present study combined
type I and II fractures into type II.
Surgery
All patient treatments and surgeries were performed
by the same team at Ordos Central Hospital. At admission, the
affected ankle joints of all patients were reconstructed with
anteroposterior and lateral axial computed tomography (CT)
three-dimensional reconstruction to accurately reconstruct the
fracture morphology and understand the fracture pattern. The doctor
determined the operation time based on the extent of soft tissue
damage of each patient. Notably, the three-dimensional
reconstruction of the CT was performed prior to the selective
operation to ensure that the fracture block remained unchanged
after traction. The CT scan was performed after calcaneal traction
in all cases that required traction.
General anesthesia was administered to all patients
who were positioned supine. A lower limb tourniquet was used under
the premise that pressure and time were controlled. The fibula was
internally fixed to restore the anatomical length of the fibula as
much as possible. Any osteochondral debris and blood clots present
in the joint were removed, and the displacement of the articular
fracture was observed. The fracture was reduced through prying and
pulling under arthroscopy and secured with Kirschner wire.
Following confirmation of a level articular surface on the distal
tibia, the arthroscopy was withdrawn. A C-arm X-ray machine was
used to check the reduction. The steel plate was positioned based
on the structural characteristics of the fracture and the condition
of the skin surrounding the ankle joint. A small incision was made
locally to minimize the excessive stripping of the periosteum and
protect the soft tissue as much as possible. With the assistance of
the tibial tunnel locator, the contralateral fracture block was
secured using the appropriate tibial multiaxial locking plate. The
reduction of the articular surface was examined. If the reduction
was successful, holes were drilled and nails were placed to
complete tibial fixation. Microscopically, the articular cartilage
defect was treated with microfracture to repair the damaged
ligament structure around the ankle joint. After washing and
suturing, a drainage tube was placed.
For postoperative treatment, the affected limb was
fixed with a brace and then raised. The prophylactic use of
antibiotics is recommended for open high-energy pilon fractures to
prevent infection. The drainage tube was removed in a timely manner
according to the drainage condition. The patient was instructed to
conduct exercises so long as no obvious pain was experienced in the
affected area to ensure a step-by-step progression during
postoperative functional rehabilitation. All patients underwent
postoperative radiographs with time intervals, as well as
postoperative CT scans.
Blood sampling measurement
The enzyme-linked immunosorbent assay (ELISA) was
utilized to measure the serum levels of caspase-1, IL-6, IL-1β and
C-reactive protein (CRP). All cases had their blood samples
collected from the median cubital vein (5 ml) after fasting for 8 h
but within 24 h after admission for all cases. These blood samples
were centrifuged at 2,000 x g and a temperature of 4˚C for 15 min,
and the serum (supernatant) was analyzed by ELISA testing utilizing
commercially available kits [caspase-1 (cat. no. MBS084975); IL-6
(cat. no. MBS175877); CRP (cat. no. MBS177184); IL-1β (cat. no.
MBS175901); all from MyBioSource, Inc.]. Serum biomarker levels
were measured at 0, 1, 2, 3, 4 weeks and 3, 6, 12 and 24 months
after treatment initiation.
Reverse transcription-quantitative PCR
(RT-qPCR)
All 136 patients in the two groups were measured for
their mRNA expression of caspase-1 using RT-qPCR. For total RNA
extraction from serum samples, the present study used the RNAiso
Plus kit (Takara Bio, Inc.), and subsequently transcribed it into
cDNA using the Prime-Script™ one-step RT-qPCR kit
(Takara Bio, Inc.) according to the manufacturer's protocols. The
RT-qPCR process was performed on the ABI PRISM7300 Sequence
Detection System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using the SYBR Premix ExTaq (Takara Bio, Inc.). The
thermocycling conditions used for qPCR were as follows: An initial
activation step at 95˚C for 15 min, followed by 35 cycles of
denaturation at 94˚C for 15 sec, annealing at 55˚C for 25 sec and
extension at 70˚C for 30 sec. Primer sequences for caspase-1 were
as follows: Forward, 5'-CACACCGCCCAGAGCACAAG-3', and reverse,
5'-TCCCACAAATGCCTTCCCGAATAC-3'. Primer sequences for GAPDH were as
follows: Forward, 5'-ACACCATGTATTCCGGGTCAAT-3', and reverse,
5'-CCACCACCCTGTTGCTGTAG-3'. GAPDH was utilized as an internal
control. Expression levels of caspase-1 were calculated using the
2-ΔΔCq method (25).
Data collection and scale scoring
Demographic and clinical statistics, such as age,
BMI, sex, intraoperative blood loss, fracture healing time,
Ruedi-Allgower grade, injury causes (falling or traffic accidents),
fracture types (closed or open) and post-surgery complications,
were collected. In addition, all patients were followed up for at
least 24 months, and data from the 24-month period were used with
follow-up through to the end of July 2022, and patients received
the last follow-up in July 2022. The Mazur ankle joint evaluation
system was used to assess the effectiveness of the treatment
(26). Patients with Mazur scores
≥87 were defined as having a good prognosis, while patients with
Mazur scores ≤86 were defined as having a poor prognosis.
Statistical analysis
The database was established using the statistical
software SPSS 25.0 (IBM Corp.). The normal distribution of data was
confirmed by the Kolmogorov-Smirnov analysis. Mean ± standard
deviation was used to express normal distribution data, while
non-normal distribution data were expressed as median (range). The
comparison between two groups was carried out using the
Mann-Whitney test or unpaired Student's t-test. Rates were
determined using χ2 test, and correlation analysis was
conducted using Spearman's rank correlation. Receiver operating
characteristic (ROC) curve analysis was conducted to diagnose the
ability of caspase-1 for predicting poor prognosis in patients with
high-energy pilon fractures. Logistic regression was performed to
identify risk factors for poor prognosis in these patients, with
high-energy pilon fractures. All data were analyzed with three
replicates to ensure statistical robustness. P<0.05 was
considered to indicate a statistically significant difference.
Results
Basic characteristics of all
patients
The present research enrolled a total of 136
patients with high-energy pilon fractures. All patients measured
Mazur ankle joint evaluation system after 24 months of surgery. The
participants were categorized into two groups: The good prognosis
group (Mazur score ≥87; n=107) and the bad prognosis group (Mazur
score ≤86; n=29). Comparing the demographic and clinical data of
two groups when the patients hospitalized, the present study
observed significant differences. The poor prognosis group
exhibited markedly higher levels of intraoperative blood loss,
proportion of Ruedi-Allgower III and the serum levels of caspase-1
compared with the good prognosis group (P<0.05; Table I). The Mazur scores in patients
with good prognoses were markedly higher than those in patients
with bad prognoses (P<0.05; Table
I). Additionally, the fracture healing time in patients with
bad prognoses was significantly longer compared with the patients
with poor prognoses (P<0.05; Table
I). Subsequently, the present study conducted a subgroup
analysis based on sex and observed no significant differences in
intraoperative blood loss, postoperative healing time, baseline
serum cytokine levels and Mazur scores between male and female
patients (Table II).
 | Table IBasic characteristics of all
patients. |
Table I
Basic characteristics of all
patients.
| Variable | Good prognosis
group (n=107) | Poor prognosis
group (n=29) | P-value |
|---|
| Age, years | 46 (24-66) | 51 (29-65) | 0.112 |
| Female sex, n
(%) | 58 (54.2) | 14 (48.2) | 0.480 |
| BMI | 25.11±2.46 | 24.71±2.40 | 0.890 |
| Intraoperative
blood loss, ml | 106.17±8.91 | 117.06±6.82 | <0.001 |
| Fracture healing
time, weeks | 13 (6-22) | 17 (8-23) | 0.037 |
| Injury causes, n
(%) | | | |
|
Falling | 58 (54.21) | 13 (44.83) | 0.663 |
|
Traffic
accidents | 49 (45.79) | 16 (45.17) | 0.663 |
| Ruedi-Allgower, n
(%) | | | |
|
II | 66 (61.68) | 5 (17.24) | <0.001 |
|
III | 41 (39.32) | 24 (82.76) | <0.001 |
| Fracture types, n
(%) | | | |
|
Closed | 64 (59.81) | 18 (62.07) | 0.885 |
|
Open | 43 (40.19) | 11 (37.93) | 0.885 |
| Complications, n
(%) | | | |
|
Infection | 5 (4.67) | 2 (6.90) | 0.767 |
|
Non-union | 3 (2.80) | 1 (3.45) | 0.999 |
|
Other | 5 (4.67) | 2 (6.90) | 0.767 |
| Caspase-1,
pg/ml | 54.07±2.68 | 56.13±3.37 | 0.001 |
| CRP, pg/ml |
1,986.02±162.13 |
1,969.80±150.73 | 0.629 |
| IL-1β, pg/ml | 20.95±3.15 | 20.58±3.39 | 0.577 |
| IL-6, pg/ml | 23.13±3.88 | 22.79±4.51 | 0.683 |
| Mazur score | 93 (87-99) | 75 (64-85) | <0.001 |
 | Table IIComparison of baseline data for sex
subgroup analysis. |
Table II
Comparison of baseline data for sex
subgroup analysis.
| Variable | Male (n=64) | Female (n=72) | P-value |
|---|
| Age, years | 44 (24-64) | 49 (24-66) | 0.370 |
| BMI | 25.33±2.40 | 24.78±2.49 | 0.280 |
| Intraoperative
blood loss, ml | 109.35±9.77 | 107.72±9.43 | 0.325 |
| Fracture healing
time, weeks | 13 (6-22) | 16 (6-23) | 0.362 |
| Caspase-1,
pg/ml | 54.75±2.92 | 54.30±2.98 | 0.376 |
| CRP, pg/ml |
1,958.18±156.90 |
2,004.23±159.48 | 0.093 |
| IL-1β, pg/ml | 20.79±3.04 | 20.94±3.35 | 0.783 |
| IL-6, pg/ml | 23.42±3.59 | 22.74±4.35 | 0.326 |
| Mazur score | 91 (64-99) | 92 (64-99) | 0.906 |
| Poor prognosis, n
(%) | 15 (22.7) | 14 (19.4) | 0.603 |
Comparisons of caspase-1 and
inflammatory factors between the good and bad prognosis groups
To further investigate the connection between
caspase-1 and inflammation in patients with high-energy pilon
fractures, the present study plotted line graphs for all
participants. As the levels of these cytokines had already returned
to normal levels from the 3rd month after surgery, and there was no
significant difference between the two groups, the present study
used serum levels of the cytokines in the first month
postoperatively for analysis. These graphs depict the fluctuations
of caspase-1 and inflammatory biomarkers over the course of the
study. The levels of serum biomarkers (caspase-1, CRP, IL-1β and
IL-6) increased in both groups within the first week, but gradually
decreased during the subsequent treatment and follow-up periods
(Fig. 1A-D). Furthermore,
significant differences in the levels of serum caspase-1 and IL-1β
levels were observed among patients with high-energy pilon
fractures in the poor prognosis group compared with those in the
good prognosis group at all time points (P<0.05). Conversely, no
significant differences were found in serum CRP and IL-6 levels
were found between the two groups (Fig. 1B and D). The serum levels of the cytokines at
the 3rd, 6th, 12th and 24th months after surgery for all patients
are presented in Table SI. There
were no significant differences in any serum cytokine levels
between the good and poor groups from 3-24 months after
surgery.
As cytokines levels during the first postoperative
week were the highest of all time points during the study, based on
the methodology of previous studies (27-29),
serum biomarkers levels in the first week after surgery were used
for further analysis. To verify the high expression of caspase-1 in
patients with high-energy pilon fractures who have a poor
prognosis, the present study analyzed the mRNA expression of
caspase-1 in the serum of all patients during the first week after
surgery using PCR. The results indicated that the mRNA expression
of caspase-1 was significantly higher in patients with a poor
prognosis compared with those with a good prognosis (P<0.05;
Fig. 1E). Spearman's analysis
demonstrated a significant association between caspase-1 and IL-1β
levels and Mazur scores (P<0.05; Table III). The results of the
correlation analyses at the other time points are presented in
Table SII, Table SIII, Table SIV and Table SV, and starting from the third
week after surgery, serum caspase-1 levels were not significantly
associated with other inflammatory factors and Mazur scores.
 | Table IIICorrelation analysis between
caspase-1 and clinical factors. |
Table III
Correlation analysis between
caspase-1 and clinical factors.
| | Caspase-1 |
|---|
| Variables | Spearman's
correlation | P-value |
|---|
| Intraoperative
blood loss | 0.111 | 0.200 |
| Fracture healing
time | 0.130 | 0.132 |
| CRP | 0.117 | 0.175 |
| IL-1β | 0.183 | 0.033 |
| IL-6 | -0.015 | 0.876 |
| Mazur scores | -0.294 | 0.001 |
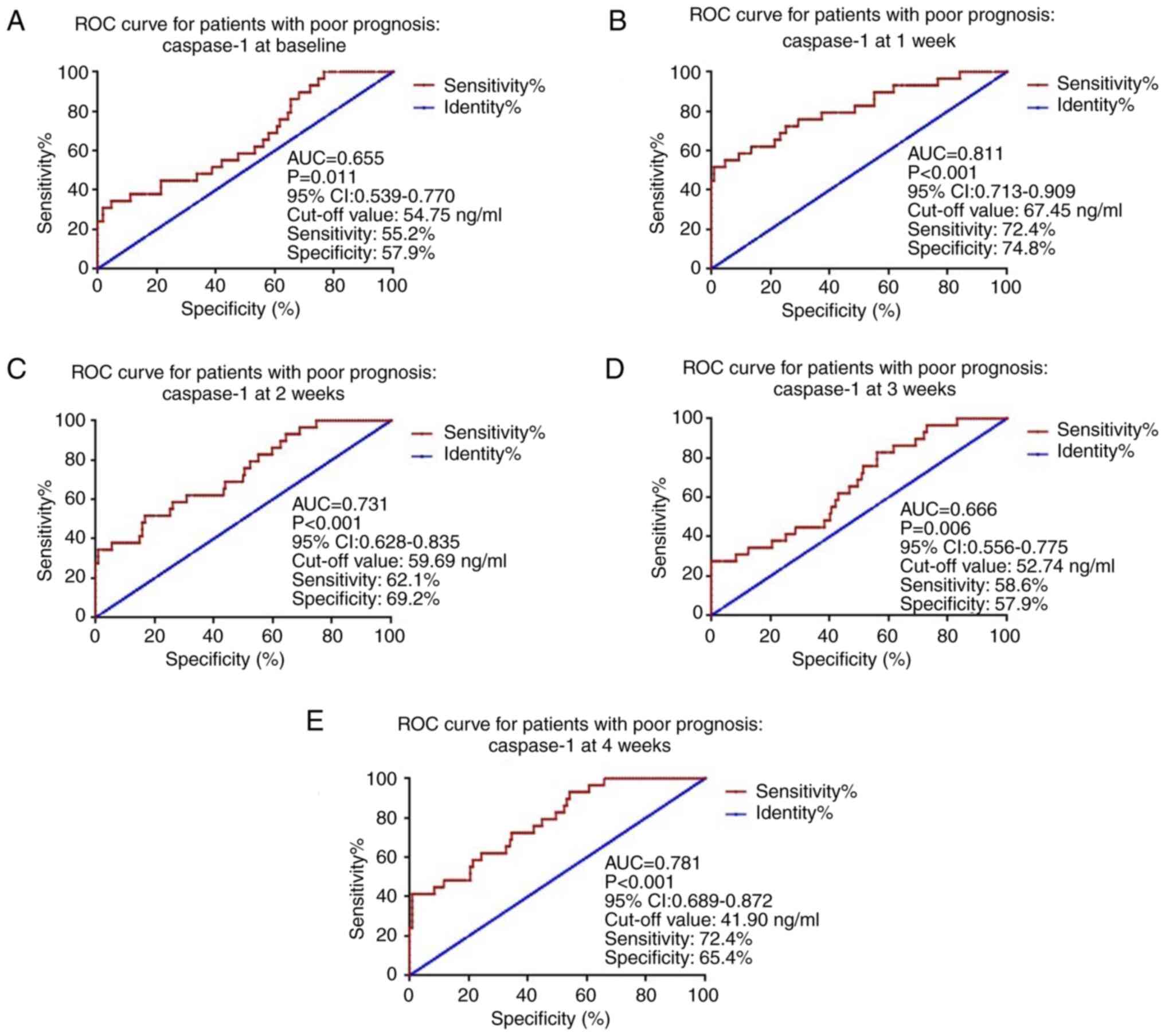
Predictive value of caspase-1 for poor
prognosis of patients with high-energy pilon fractures
The present used the serum caspase-1 levels at
baseline and at 1, 2, 3 and 4 weeks after surgery to draw ROC
curves to evaluate the predictive value of caspase-1 for poor
prognosis of patients with high-energy pilon fractures. The results
showed that caspase-1 had the greatest predictive value for poor
prognosis in patients with high-energy pilon fractures during the
first week after surgery (Fig. 2),
the AUC for caspase-1 was 0.811, with a cutoff value of 67.45
ng/ml; the sensitivity and specificity were 72.4 and 74.8%,
respectively.
Logistic regression for risk factors
of poor prognosis of patients with high-energy pilon fractures
Finally, a binary regression analysis was conducted
for the risk factors associated with poor prognosis in patients
with high-energy pilon fractures. It was revealed that caspase-1
(95% CI, 1.120-1.928; P=0.005), IL-1β (95% CI, 1.069-1.668;
P=0.011), intraoperative blood loss (95% CI 1.069-1.424; P=0.001)
and Ruedi-Allgower grade (95% CI 2.085-85.501; P=0.006) at 1 week
after surgery were the risk factors for poor prognosis of patients
with high-energy pilon fractures (Table IV). The results of the
multivariate logistic regression of serum biomarkers at the other
time points are presented in Table
SVI, Table SVII, Table SVIII and Table SIX, and serum caspase-1 level at
other time points within 1 month of surgery was a risk factor for a
poor prognosis in patients with high-energy pilon fractures.
 | Table IVLogistic regression for risk factors
of poor prognosis of patients with high-energy pilon fractures at 1
week after surgery. |
Table IV
Logistic regression for risk factors
of poor prognosis of patients with high-energy pilon fractures at 1
week after surgery.
| Variables | Wald | Odds ratio | 95% CI | P-value |
|---|
| Age | 3.706 | 1.074 | 0.999-1.154 | 0.054 |
| Sex | 0.003 | 0.954 | 0.181-5.019 | 0.955 |
| BMI | 0.884 | 1.174 | 0.840-1.641 | 0.347 |
| Intraoperative
blood loss | 11.056 | 1.249 | 1.069-1.424 | 0.001 |
| Fracture healing
time | 0.441 | 1.061 | 0.891-1.262 | 0.506 |
| Ruedi-Allgower | 7.482 | 13.351 | 2.085-85.501 | 0.006 |
| CRP | 0.091 | 0.999 | 0.994-1.004 | 0.763 |
| IL-1β | 6.499 | 1.335 | 1.069-1.668 | 0.011 |
| IL-6 | 0.272 | 0.940 | 0.744-1.187 | 0.602 |
| Caspase-1 | 7.733 | 1.470 | 1.120-1.928 | 0.005 |
Discussion
The levels of pilon fracture (30) can be divided into three, as
follows: i) Slight fracture on the articular surface of the distal
tibia; ii) obvious joint displacement with a small degree of
comminution; and iii) severe comminuted fracture on the articular
surface of the distal tibia. Regardless of the severity of the
fracture, the patient experiences considerable physical and
psychological pain, thereby impacting their quality of life
(31). If the treatment method is
not appropriate, it not only fails to alleviate the fracture but
also exacerbates the condition, resulting in complications such as
irregularity of the joint surface, arthritis, malunion and
infection (32,33). Therefore, it is urgent to develop
new biomarkers and comprehensive techniques to prompt diagnosis and
promptly treatment of the patients who may experience a poor
prognosis. The present study revealed that the serum levels of
caspase-1 had the potential to serve as a diagnostic biomarker for
identifying poor prognosis in patients with high-energy pilon
fractures.
Combining a locking plate with ankle arthroscopy
offers several advantages: Firstly, it leads to minimal trauma
during the operation, which in turn reduces blood loss; secondly,
since the joint remains unexposed, the probability of infection is
thus significantly reduced; thirdly, with the use of fluoroscopy,
the amount of radiation from fluoroscopy is lower; fourthly, the
fracture can be observed inside the joint, resulting in a more
accurate treatment position. Disadvantages are threefold. Firstly,
it results in a larger open fracture site, which aggravates the
fracture; secondly, proper attention should be paid to the
classification of the joint and the severity of fracture when
reducing and fixing it; thirdly, when using arthroscopy, sodium
chloride solution should be continuously injected into the joint
(34,35). This can penetrate into every
compartment of the leg, leading to an increase in the frequency of
membranous space syndrome (36).
The severity of the trauma, the extent of the initial soft tissue
injury and the accuracy of the articular surface reconstructions
are key to treating high-energy pilon fractures.
In the present study, according to the fracture
classification, the postoperative recovery effect of Rüedi-Allgower
type II fractures was significantly improved compared with that of
type III fractures. Thus, a greater severity of fracture injury
resulted in a lower final recovery effect. This finding may be
closely related to the severe injury or displacement observed in
type III fractures, complications and high incidence rates. In the
present paper, the total effective rate of locking plate internal
fixation combined with ankle arthroscopy intreating of high-energy
pilon fracture patients was 80.15%, which is comparable to the
effectiveness of other treatments (26,37,38).
In the context of fractures, pyroptosis can be
triggered by the release of damage-associated molecular patterns
(DAMPs) from injured cells (39).
These DAMPs can activate the inflammasome and trigger pyroptosis in
nearby cells, resulting in a localized inflammatory response. This
response can contribute to the progression of tissue damage and
impair the healing process (40).
At present, several cell and animal studies have investigated the
link between cell pyroptosis and fractures. Yang et al
demonstrated that high glucose concentrations may activate
pyroptosis through the caspase-1/gasdermin D/IL-1β pathway, which
inhibits the proliferation and differentiation of osteoblasts in
the alveolar bone (41). Zhu et
al revealed that pyroptosis is a crucial pathway in
osteomyelitis, and inhibition of the pyroptosis pathway can
alleviate the bone destruction caused by Staphylococcus
aureus-induced osteomyelitis, providing a potential therapeutic
target for osteomyelitis (18). In
addition, Zhang et al observed that inhibiting the
pyroptosis pathway of NLRP3/caspase-1/gasdermin D promotes the
proliferation and differentiation of osteoblasts, thereby improving
osteoblast function in fracture repair (23). The present study also found that
the level of the key factor of pyroptosis, caspase-1, gradually
decreased during the treatment of patients with high-energy pilon
fractures, and decreased more quickly in patients with a better
prognosis. In addition, the serum level of caspase-1 was
significant associated with the serum level of IL-1β.
The present research also had some limitations.
First, the present study only included a small size of the study
population. Secondly, only a small number of inflammatory factors
were investigated. Thirdly, the observed correlations, although
statistically significant, are weak, and further in-depth research
is needed to understand the clinical significance and molecular
mechanism of caspase-1 in pilon fractures.
In conclusion, the current study illustrated that
the serum caspase-1 levels were gradually decreased during the
treatment of patients experiencing high-energy pilon fractures, and
decreased more rapidly in patients with a better prognosis. There
was a significant association between caspase-1 and IL-1β levels
and Mazur scores. Furthermore, serum levels of Caspase-1 could
potentially serve as a potential diagnostic biomarker for poor
prognosis in patients with high-energy pilon fractures. The current
study may provide novel targets and a comprehensive approach to
protecting patients with high-energy pilon fractures.
Supplementary Material
Serum cytokines levels at 3, 6, 12 and
24 months during the follow up time.
Results of correlation analysis of
cytokines levels at baseline.
Results of correlation analysis of
cytokines levels at 2 weeks.
Results of correlation analysis of
cytokines levels at 3 weeks.
Results of correlation analysis of
cytokines levels at 4 weeks.
Results of logistic regression
analysis of cytokines levels at baseline.
Results of logistic regression
analysis of cytokines levels at 2 weeks.
Results of logistic regression
analysis of cytokines levels at 3 weeks.
Results of logistic regression
analysis of cytokines levels at 4 weeks.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XG collected and analyzed the data and wrote the
manuscript. FL, GW and YG interpreted the results and collected the
data. XS designed and supervised the research, interpreted and
discussed the data and wrote and submitted the manuscript. All
authors read and approved the final manuscript. XG and XS confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
The present study was approved by the ethic
committee of Ordos Central Hospital (approval no.
IRB2015-OCHOS-116). Written informed consent was obtained by all
participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zelle BA, Dang KH and Ornell SS:
High-energy tibial pilon fractures: An instructional review. Int J
Orthop. 43:1939–1950. 2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bone LB: Fractures of the tibial plafond.
The pilon fracture. Orthop Clin North Am. 18:95–104.
1987.PubMed/NCBI
|
|
3
|
Sirkin M and Sanders R: The treatment of
pilon fractures. Orthop Clin North Am. 32:91–102. 2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kottmeier SA, Madison RD and Divaris N:
Pilon fracture: Preventing complications. J Am Acad Orthop Surg.
26:640–651. 2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Qiu XS, Li XG, Qi XY, Wang Z and Chen YX:
What is the most reliable classification system to assess tibial
pilon fractures? J Foot Ankle Surg. 59:48–52. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wiebking U: Pilon fractures: Review of
diagnostics and classification. Unfallchirurg. 120:632–639.
2017.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
7
|
Leonetti D and Tigani D: Pilon fractures:
A new classification system based on CT-scan. Injury. 48:2311–2317.
2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Chowdhry M and Porter K: The pilon
fracture. Trauma. 12:89–103. 2010.
|
|
9
|
He X, Hu Y, Ye P, Huang L, Zhang F and
Ruan Y: The operative treatment of complex pilon fractures: A
strategy of soft tissue control. Indian J Orthop. 47:487–492.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dombrowsky A, Abyar E, McGwin G and
Johnson M: Is definitive plate fixation overlap with external
fixator pin sites a risk factor for infection in pilon fractures? J
Orthop Trauma. 35:e7–e12. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hebert-Davies J, Kleweno CP and Nork SE:
Contemporary strategies in pilon fixation. J Orthop Trauma. 34
(Suppl 1):S14–S20. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Osman W, Alaya Z, Kaziz H, Hassini L,
Braiki M, Naouar N and Ben Ayeche ML: Treatment of high-energy
pilon fractures using the ILIZAROV treatment. Pan Afr Med J.
27(199)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Yao ND and Wang FL: Delayed operation on
treatment of high-energy distal tibia Pilon fracture. Zhongguo Gu
Shang. 24:256–258. 2011.PubMed/NCBI(In Chinese).
|
|
14
|
Martin KD: Posterior arthroscopic
reduction and internal fixation for treatment of posterior
malleolus fractures. Foot Ankle Int. 41:115–120. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Barbera J, Selverian S, Courington R,
Mikhail C and Colvin A: The top 50 most influential articles in hip
arthroscopy. Arthroscopy. 36:716–722. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Luo H, Chen L, Liu K, Peng S, Zhang J and
Yi Y: Minimally invasive treatment of tibial pilon fractures
through arthroscopy and external fixator-assisted reduction.
Springerplus. 5(1923)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
El-Mowafi H, El-Hawary A and Kandil Y: The
management of tibial pilon fractures with the Ilizarov fixator: The
role of ankle arthroscopy. Foot (Edinb). 25:238–243.
2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhu X, Zhang K, Lu K, Shi T, Shen S, Chen
X, Dong J, Gong W, Bao Z, Shi Y, et al: Inhibition of pyroptosis
attenuates Staphylococcus aureus-induced bone injury in
traumatic osteomyelitis. Ann Transl Med. 7(170)2019.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu J, Liu W, Lv P, Wang Y and Ouyang X:
Activation of nucleotide-binding oligomerization domain-like
receptor family pyrin domain containing 6 by Porphyromonas
gingivalis regulates programmed cell death in epithelium. J Dent
Sci. 18:1867–1875. 2023.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Tsuchiya K: Inflammasome-associated cell
death: Pyroptosis, apoptosis, and physiological implications.
Microbiol Immunol. 64:252–269. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Cao H, Liang J, Liu J, He Y, Ke Y, Sun Y,
Jiang S and Lin J: Novel effects of combination therapy through
inhibition of caspase-1/gasdermin D induced-pyroptosis in lupus
nephritis. Front Immunol. 12(720877)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhou Y, Chen Z, Yang X, Cao X, Liang Z, Ma
H and Zhao J: Morin attenuates pyroptosis of nucleus pulposus cells
and ameliorates intervertebral disc degeneration via inhibition of
the TXNIP/NLRP3/Caspase-1/IL-1β signaling pathway. Biochem Biophys
Res Commun. 559:106–112. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhang J and Wei K: Necrosulfonamide
reverses pyroptosis-induced inhibition of proliferation and
differentiation of osteoblasts through the NLRP3/caspase-1/GSDMD
pathway. Exp Cell Res. 405(112648)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Luo TD, Eady JM, Aneja A and Miller AN:
Classifications in brief: Rüedi-Allgöwer classification of tibial
plafond fractures. Clin Orthop Relat Res. 475:1923–1928.
2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu D, Peng C, Ren G, Yuan B and Liu H:
Novel anterior curved incision combined with MIPO for Pilon
fracture treatment. BMC Musculoskelet Disord.
21(176)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhao L, Li H, Zhang S, Dong Z and Cui Q:
Serum HMGB1 levels and its clinical significance in elderly
patients with intertrochanteric fractures after intramedullary
fixation surgery. Medicine (Baltimore). 102(e32873)2023.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Meyer U, de Jong JJ, Bours SGP, Keszei AP,
Arts JJ, Brink PRG, Menheere P, van Geel TA, van Rietbergen B, van
den Bergh JP, et al: Early changes in bone density,
microarchitecture, bone resorption, and inflammation predict the
clinical outcome 12 weeks after conservatively treated distal
radius fractures: An exploratory study. J Bone Miner Res.
29:2065–2073. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Saribal D, Hocaoglu-Emre FS, Erdogan S,
Bahtiyar N, Caglar Okur S and Mert M: Inflammatory cytokines IL-6
and TNF-α in patients with hip fracture. Osteoporos Int.
30:1025–1031. 2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rüedi TP and Allgöwer M: Fractures of the
lower end of the tibia into the ankle-joint. Injury. 1:92–99.
1969.
|
|
31
|
Tarkin IS, Clare MP, Marcantonio A and
Pape HC: An update on the management of high-energy pilon
fractures. Injury. 39:142–154. 2008.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sirkin MS: Plating of tibial pilon
fractures. Am J Orthop (Belle Mead NJ). 36 (12 Suppl 2):S13–S17.
2007.PubMed/NCBI
|
|
33
|
Crist BD, Khazzam M, Murtha YM and Della
Rocca G: Pilon fractures: Advances in surgical management. J Am
Acad Orthop Surg. 19:612–622. 2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Dial DM and Ryan M: Locking plate
technology and its use in foot and ankle surgery. Clin Podiatr Med
Surg. 28:619–631. 2011.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wang C, Xu C, Li M, Li H, Wang L, Zhong D
and Liu H: Arthroscopic ankle fusion only has a limited advantage
over the open operation if osseous operation type is the same: A
retrospective comparative study. J Orthop Surg Res.
15(80)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Atesok K, Doral MN, Whipple T, Mann G,
Mei-Dan O, Atay OA, Beer Y, Lowe J, Soudry M and Schemitsch EH:
Arthroscopy-assisted fracture fixation. Knee Surg Sports Traumatol
Arthrosc. 19:320–329. 2011.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liangjun J, Qiang Z, Hang L and Zhijun P:
Injury mechanism, fracture characteristics and clinical treatment
of pilon fracture with intact fibula-A retrospective study of 23
pilon fractures. J Clin Orthop Trauma. 8 (Suppl 2):S9–S15.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Galante V, Vicenti G, Corina G, Mori C,
Abate A, Picca G, Conserva V, Speciale D, Scialpi L, Tartaglia N,
et al: Hybrid external fixation in the treatment of tibial pilon
fractures: A retrospective analysis of 162 fractures. Injury. 16
(Suppl 4):S131–S137. 2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Son S, Yoon SH, Chae BJ, Hwang I, Shim DW,
Choe YH, Hyun YM and Yu JW: Neutrophils facilitate prolonged
inflammasome response in the DAMP-rich inflammatory milieu. Front
Immunol. 12(746032)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wright SS, Vasudevan SO and Rathinam VA:
Mechanisms and consequences of noncanonical inflammasome-mediated
pyroptosis. J Mol Biol. 434(167245)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang L, Liu J, Shan Q, Geng G and Shao P:
High glucose inhibits proliferation and differentiation of
osteoblast in alveolar bone by inducing pyroptosis. Biochem Biophys
Res Commun. 522:471–478. 2020.PubMed/NCBI View Article : Google Scholar
|