Introduction
Primary neoplasms of the vertebral column are rare
pathological entities. The most prevalent types of primary osseous
tumors that manifest in the vertebral column include osteosarcomas,
giant cell tumors, osteoblastomas, chondrosarcomas and osseous
cysts. These tumors predominantly exhibit proclivity for
localization within the anterior and posterior vertebral bodies,
pedicles and laminae of the spinal architecture. The incidence of
primary neoplasms originating from the facet joint is particularly
low, owing primarily to its cartilaginous composition (1). Documented instances of primary
neoplasms in this region are sporadic and principally comprise of
giant cell tumors, osteoblastomas and chondrosarcomas (2).
Chondromyxoid fibroma (CMF) is an uncommon benign
osseous neoplasm, which accounts for only a small percentage
(<5%) of all diagnosed bone tumors recorded worldwide (3). Notably, CMF demonstrates a
predilection for pediatric and young adult populations and is
customarily localized within the metaphyseal regions of elongated
bones, notably in the femur and tibia. The characteristic
symptomatology associated with CMF includes pain, localized edema,
and restricted joint mobility, with pain often exacerbating either
nocturnally or after physical exertion (3). The neoplasm characteristically
follows an indolent growth trajectory (4) (Table
I).
 | Table IAn introduction to chondromyxoid
fibroma. |
Table I
An introduction to chondromyxoid
fibroma.
| Aspect | Information | (Refs.) |
|---|
| Epidemiology | - Chondromyxoid
fibroma is an extraordinarily uncommon benign osseous neoplasm | (3) |
| | - It chiefly afflicts
individuals within the second to third decades of life; however,
instances have been cited across a broader age spectrum, including
pediatric and geriatric populations | (3) |
| | - There is no
significant sex predilection | (2) |
| | - Commonly,
chondromyxoid fibroma localizes to the metaphyseal region of
elongated bones such as the femur and tibia, although
manifestations in axial skeletal structures including the
vertebrae, pelvis and cranium have been documented | (4) |
| Diagnostic
Methods | - Radiological
evaluations predominantly rely on computed tomography and magnetic
resonance imaging to elucidate the anatomical locale, dimensional
attributes and morphological characteristics of the neoplasm | (5) |
| | - Immunohistochemical
investigations represent an indispensable diagnostic technique,
consistently revealing positive immunoreactivity for S-100 protein
in neoplastic cells | (6) |
| | - Histopathological
scrutiny via tissue biopsy stands as the gold standard for
definitively corroborating a diagnosis of chondromyxoid
fibroma | (6) |
| Pathological
Features | - Histologically,
chondromyxoid fibroma frequently exhibits a heterogenous
architecture, encompassing both mucinous and fibrous compartments;
the former replete with mucoid substrate and the latter
characterized by fibrous stroma | (6) |
| | - Neoplastic cells
manifest an irregular morphology, frequently assuming either a
fibroblastic or spindle-shaped appearance, with mitotic figures
remaining an uncommon feature | (5) |
| | - Immunohistochemical
assays corroborate the frequent expression of S-100 protein within
chondromyxoid fibroma, thereby facilitating its differential
diagnosis from other neoplastic entities | (4) |
| Treatment
Strategies | - Surgical
extirpation remains the primary therapeutic paradigm, and the ideal
approach encompasses complete neoplastic resection; postoperative
histological analysis substantiates the extent of excision | (7) |
| | - Curettage warrants
consideration in scenarios where comprehensive resection is
unfeasible, albeit at the expense of an elevated risk for
neoplastic recurrence | (8) |
| | - Spinal arthrodesis
may become requisite when the surgical intervention compromises
vertebral stability, thereby ensuring biomechanical integrity | (7) |
| | - Radiation and
chemotherapeutic modalities are generally precluded from the
treatment regimen due to the tumor's benign proclivity | (8) |
The incidence of CMF within the spinal architecture
is relatively atypical. Among the few documented cases, the
predominant manifestation is in the thoracic spine, rather than the
cervical or lumbar spine, and it is almost universally confined to
the vertebrae (5,6). Characteristic radiographic features
include: i) Demonstrable osteolytic degeneration accompanied by
‘moth-eaten’ alterations in the vertebral body, potentially with
concomitant soft tissue masses, as revealed by computed tomography
(CT); and ii) hypointense lesions sharply demarcated from the
surrounding physiological tissues, as well as intratumoral cystic
compartments and hemorrhagic foci, as revealed by magnetic
resonance imaging (MRI) (2)
(Table I).
Pathologically, CMF is characterized by distinct
histological and cytological features. Histomorphologically, CMF
predominantly features mucoid regions interspersed with fibrous
structures, wherein the mucoid compartments comprise of cystic
formations laden with mucin and fibrous compartments consisting of
bundled fibrous connective tissue. Cytomorphologically, CMF is
predominantly characterized by atypical fibroblasts with a
relatively uniform cellular architecture, and infrequent presence
of mitotic figures. Although categorically benign, CMF induces
considerable osseous erosion and degradation under specific
conditions, such as tumor location, growth rate and size. In
addition, inflammation or other biological responses can influence
the pathological process of tumor induction. This destructive
potential is principally attributable to the inherent growth
characteristics of the tumor, which include intraosseous
dissolution and localized erosion, particularly in the surrounding
osseous structures. Such degradative processes are principally
mediated by the secretion of mucinous substances and other
bioactive factors from the tumor, culminating in the attenuation of
neighboring osseous tissues (5,6). In
addition, the structural composition of the tumor, replete with
cystic regions and fibrous compartments, may contribute to its
predilection for invasive propagation into adjacent anatomical
structures, especially within the osseous framework (5,6).
Immunohistochemically, cells inherent to this neoplasm frequently
exhibit positive immunoreactivity for cartilage-specific molecular
markers, notably S-100 protein (5,6).
Therefore, distinguishing CMF from other osseous pathologies such
as chondrosarcoma, giant cell tumor, fibrous dysplasia,
osteochondroma and aneurysmal bone cyst is imperative for an
accurate diagnosis (7). Surgical
intervention remains the principal therapeutic modality. In
alignment with therapeutic protocols established for long-bone
neoplasms, complete surgical resection is mandatory for vertebral
CMFs. In scenarios where complete excision is contraindicated or
impracticable, curettage is an acceptable alternative (8). After complete resection,
perturbations in spinal stability should occur, and vertebral
fusion procedures should be indicated (8) (Table
I).
Case report
A 57-year-old female patient was admitted for
clinical intervention pertaining to chronic lumbosacral radicular
pain for 3 years that was exacerbated over the preceding month.
Since 2019, the patient had manifested inexplicable neuropathic
symptoms in the right lower extremity. In November 2022, acute
exacerbation of neuropathic discomfort and ambulatory impediments
were reported. Initial clinical examination revealed an antalgic
gait pattern, a mild levoscoliosis of the lumbar spine, palpable
tenderness over the spinous processes and paraspinal musculature
adjacent to the L4 and L5 vertebrae, heightened tenderness inferior
to the lumbar region and restricted spinal mobility in both the
sagittal and coronal planes. Forward flexion exacerbated the
radicular symptoms in the right lower extremity. Quantitative
muscle strength evaluation revealed the following distinctions
between the left and right lower limbs: Tibialis anterior (4/3),
extensor hallucis longus (3/4) and extensor digitorum longus (3/4).
Hypoesthesia was identified in the right hip region, posterolateral
thigh, lateral calf, dorsolateral aspect of the foot and plantar
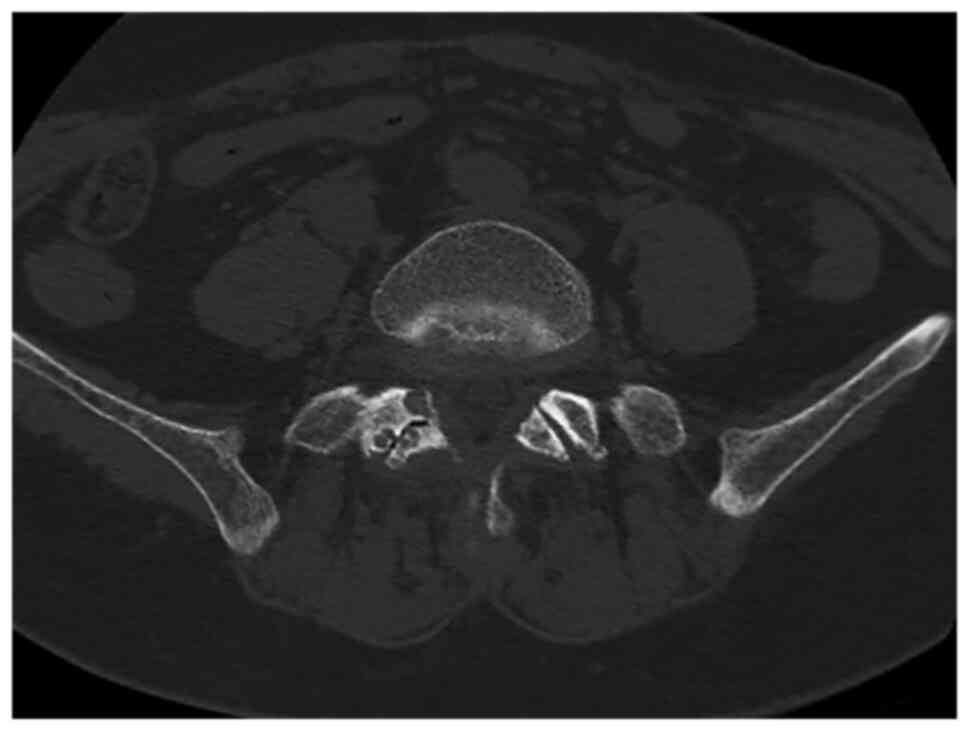
surface. CT demonstrated localized osseous cystic degradation in
the right inferior facet of the L5 vertebra, accompanied by a
nodular lesion of slightly higher density extending into the right
intervertebral foramen between L5 and S1, raising suspicions of
tumor pathologies such as osteoblastoma (Fig. 1). MRI delineated an irregular
nodular formation in the aforementioned intervertebral foramen,
characterized by low signal intensity on T1-weighted images and
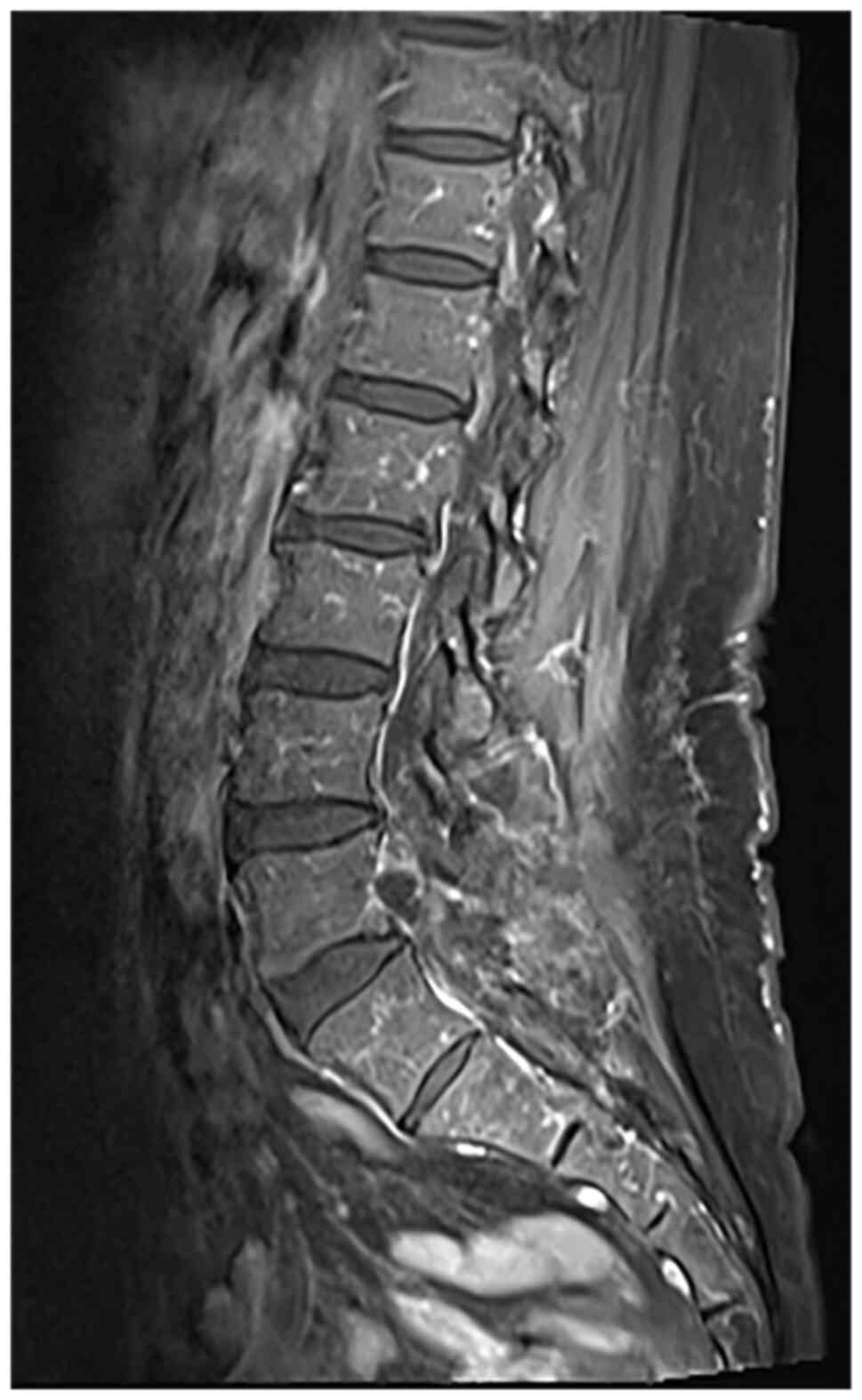
demarcated boundaries, measuring ~0.8x1.6 cm (Fig. 2). Neural impingement of the right
nerve root was also observed. The differential diagnoses included:
i) A lesion in the right inferior facet joint of L5 (possibly
osteoblastoma); ii) intraspinal joint tissue protrusion; and iii)
lumbar spinal canal stenosis.
After induction of general anesthesia, a posterior
lumbar surgical approach was implemented, including the excision of
the appendicular lesion, resection of the intraspinal pathology,
posterolateral bone graft fusion involving the facet joint and
internal stabilization utilizing pedicle screw-rod fixation.
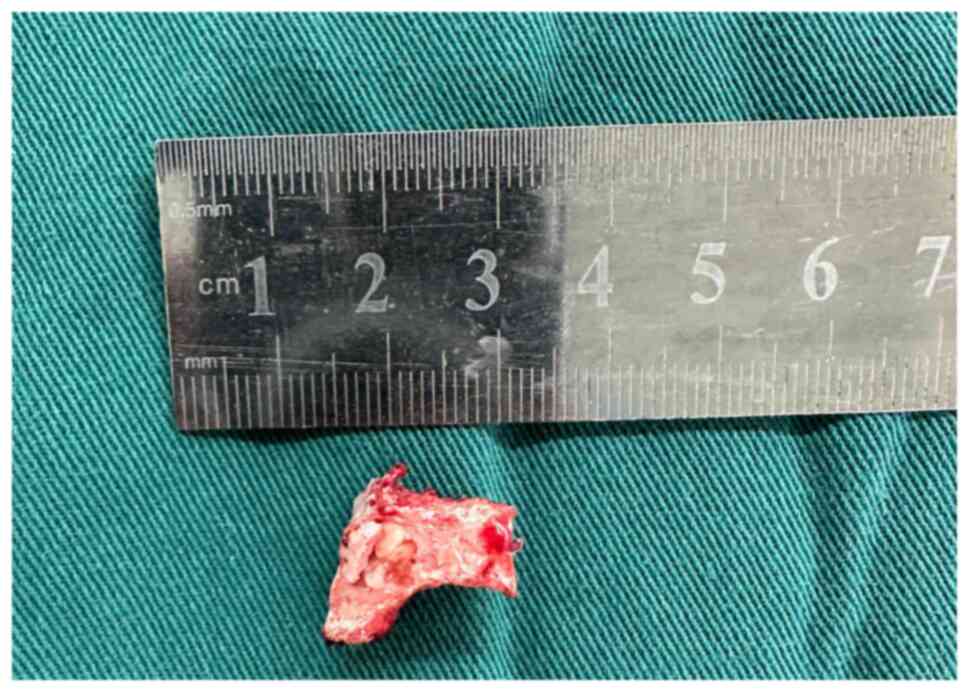
Intraoperatively, an ultrasonic osteotome facilitated complete
resection of the right inferior facet joint of L5, revealing
localized cystic osseous degeneration and an adjacent soft tissue
nodule extending into the right intervertebral foramen between L5
and S1 with dimensions of ~1.0x1.5 cm (Fig. 3). The soft tissue nodule exerted
compressive forces on the right dural sac and nerve root,
necessitating its complete removal.
The postoperative course was devoid of
complications, and the limb kinematics and sensory functions were
within normal limits. Follow-up CT imaging validated the surgical
resection of the right inferior facet joint and adjacent vertebral
lamina of L5, while confirming the secure placement of the
posterior pedicle screw fixation from L5 to S1, devoid of notable
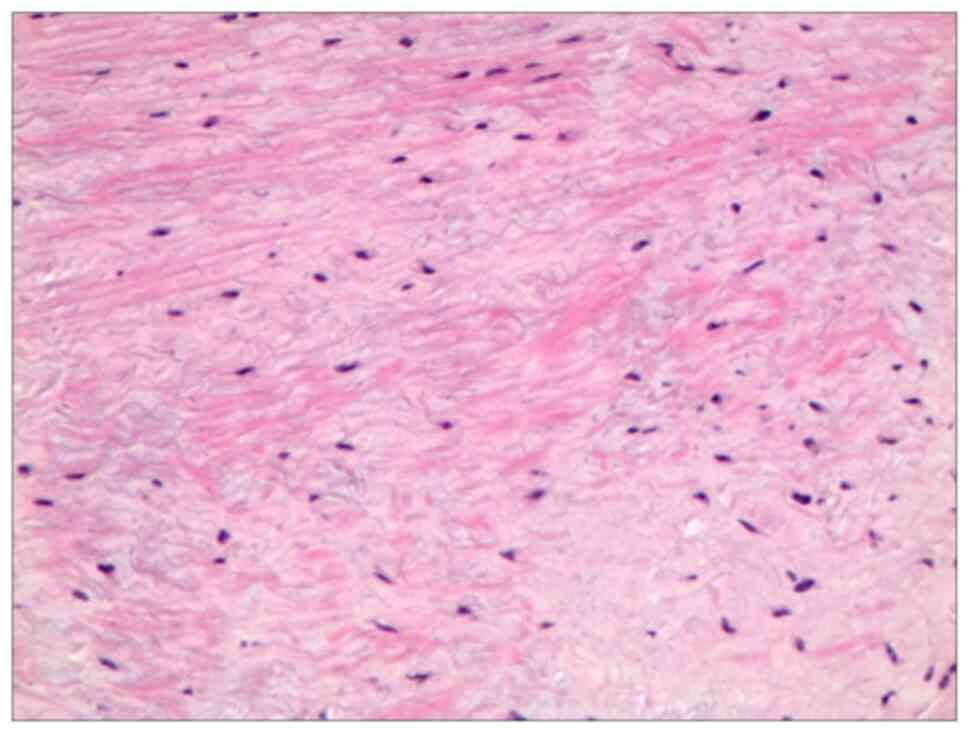
malalignment or hardware failure. Histopathological examination
confirmed the diagnosis of CMF, which was characterized by
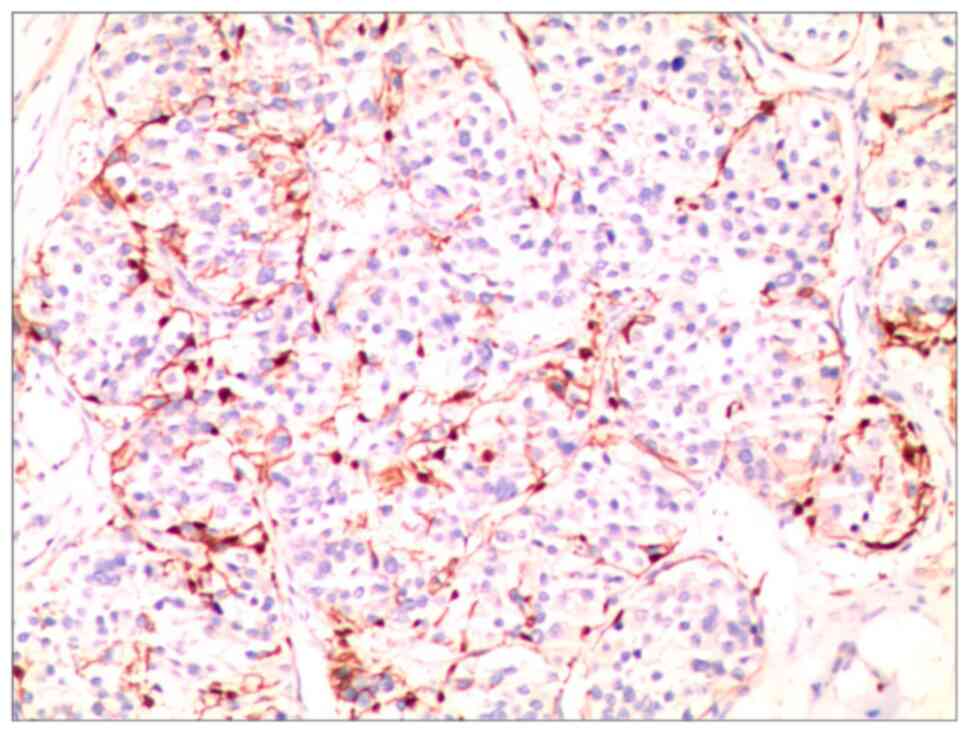
ambiguous tumor margins and local infiltrative patterns (Fig. 4). Additionally, immunohistochemical
assays revealed the expression of the S100 protein (Fig. 5).
During the 6 month postoperative review, the patient
remained asymptomatic, with no clinical or radiographic evidence
suggestive of tumor recurrence or spinal biomechanical
instability.
The following is a description of the H&E
staining method. Initially, tissue samples from the patient's
surgery were obtained and immediately fixed in 10% buffered
formalin for 12 to 24 h at room temperature. After fixation, the
tissues were dehydrated by immersion in gradually increasing
concentrations of ethanol and then cleared and embedded in molten
paraffin wax. This was achieved using an automated tissue
processor. The paraffin-embedded tissue was sliced into thin
sections, typically ranging from 3-5 µm in thickness. These
sections were placed on glass slides. Using the standard procedure
for H&E staining, the sections were first immersed in
hematoxylin dye at room temperature for 2-5 min, which stained cell
nuclei blue or purple. Subsequently, the sections were immersed in
eosin dye for 1-2 min at room temperature, which stains cytoplasm
and collagen fibers pink to red. After staining, the sections
required multiple rinses to remove excess dye. The sections were
then covered with clear glass to protect the sample and examined
using a light microscope.
The following is a description of
immunohistochemistry staining method. Tissue samples of
chondromyxoid fibroma were obtained from the surgical specimen of
the patient and were fixed in 10% buffered formalin, typically for
12 to 24 h at room temperature. Similar to H&E staining, the
fixed tissues were dehydrated, cleared and embedded in molten
paraffin wax. Paraffin-embedded tissue was sliced into thin
sections, typically ranging from 3-5 µm in thickness. The EliVision
two-step method (Fuzhou Maixin Biotech Co., Ltd.) was used for
immunohistochemistry staining. The tissue sections were placed in a
90˚C dry oven for 15 min for deparaffinization and were then
transferred to an automated immunohistochemistry staining
instrument (Roche Benchmark XT; Roche Diagnostics) for automatic
staining. After removing the sections from the automated
immunohistochemistry staining instrument, they were rinsed with
running water for 10 min. Subsequently, the sections were
sequentially placed in 75, 85, 95 and 100% ethanol for 3, 3, 5 and
5 min, respectively. After air-drying naturally, the sections were
briefly placed in xylene for 3 sec, removed and neutral resin was
added to the sections. Cover slips were placed on top, and after
the neutral resin on the sections dried they were observed under an
optical microscope (Fig. 5). The
primary antibody used was S-100 ready-to-use [1:500; cat. nos. (01)
06970905811800; Fuzhou Maixin Biotech. Co., Ltd.], which was used
at 4˚C for 10-12 h. The secondary antibody used was enzyme-labeled
anti-mouse/rabbit IgG polymer ready-to-use [cat. no. (01)
06931988317979; Fuzhou Maixin Biotech. Co., Ltd.], which was used
for 30 min at room temperature. The immunohistochemistry staining
steps were strictly performed following the instructions provided
in the reagent kit.
Discussion
The current report described a case of CMF
originating from the right inferior articular process of the L5
vertebra, which subsequently extended through the corresponding
intervertebral foramen and culminated in lumbar spinal canal
stenosis and neural impingement. A review of extant case studies
allowed us to ascertain that, to the best of our knowledge, the
present case was the second documented occurrence of CMF localized
to the lumbar facet joint. The first case was primarily associated
with lumbosacral discomfort confined to the tumorous lesion site
within the facet joint (9). Hence,
the present report reports a rare manifestation of CMF within the
lumbar facet joint, with clinical ramifications extending to neural
compression and resultant neuropathic symptomatology due to
neoplastic invasion of the intervertebral foramen.
Based on the clinical history of the patient, the
anatomical location of the lesion and pertinent medical literature,
differential diagnosis of the present study tended initially
towards the potential existence of an osseous cystic lesion within
the facet joint. Additional diagnostic considerations included
intervertebral disc herniation and ligamentum flavum calcification.
The accurate diagnosis of CMF within the facet joint presents
considerable hurdles for clinicians and radiologists, and
pathological examination is not exempt from similar challenges.
Therefore, documenting cases such as the current one is imperative
and unequivocal, and will substantially contribute to the body of
literature instrumental in refining the diagnostic precision of
CMF.
To manage this uncommon clinical entity, the present
study implemented a surgical strategy involving comprehensive
lesion resection coupled with articular process fusion and
autologous bone graft implantation. Notably, the patient showed
significant symptom amelioration as early as the immediate
postoperative period, and follow-up assessments revealed no
indications of neoplastic recurrence or spinal biomechanical
destabilization.
Given the limited literature pertaining to CMF,
particularly within the spinal facet joints, our intent to
disseminate this case was dualistic. First, the present study
endeavored to augment the diagnostic acumen of clinicians for
neoplastic entities localized in this specific anatomical domain.
Second, the present study aimed to act as a catalyst for the
initiation of robust research trajectories encompassing molecular
pathogenesis, targeted therapeutic interventions, immunomodulatory
approaches and comprehensive treatment algorithms tailored for CMF.
Such concerted academic efforts are expected to facilitate the
evolution of improved diagnostic methodologies and efficacious
therapeutic regimens for patients presenting with CMFs at disparate
anatomical loci.
Acknowledgements
Not applicable.
Funding
Funding: This case report was supported by the Natural Science
Foundation of Gansu Province (grant nos. 21YF1FA179, 23JRRA538 and
21JR7RA007) and the Army Special Training Program (grant no.
2022YXKY003).
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
BK, CY and SL conceived the study, participated in
the study design and drafted the manuscript. BK, CY, SL, HW and WY
contributed to the image collection. XL, QD, GC, HW and WY
participated in the study design and oversaw the manuscript
drafting process. All authors read and approved the final
manuscript. BK, CY and SL confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The study was conducted with the consent of the
patient and their relatives, who signed an informed consent form
and agreed to publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kelley SP, Ashford RU, Rao AS and Dickson
RA: Primary bone tumours of the spine: A 42-year survey from the
Leeds Regional Bone Tumour Registry. Eur Spine J. 16:405–409.
2007.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Erlemann R: Imaging and differential
diagnosis of primary bone tumors and tumor-like lesions of the
spine. Eur J Radiol. 58:48–67. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jaffe HL and Lichtenstein L: Chondromyxoid
fibroma of bone; a distinctive benign tumor likely to be mistaken
especially for chondrosarcoma. Arch Pathol (Chic). 45:541–551.
1948.PubMed/NCBI
|
|
4
|
Zou MX, Lv GH, Li J and Wang XB: Acute
cauda equina syndrome secondary to chondromyxoid fibroma of the
lumbar spine. Spine J. 16:e587–e588. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Schajowicz F and Gallardo H: Chondromyxoid
fibroma (fibromyxoid chondroma) of bone. A clinico-pathological
study of thirty-two cases. J Bone Joint Surg Br. 53:198–216.
1971.PubMed/NCBI
|
|
6
|
Rahimi A, Beabout JW, Ivins JC and Dahlin
DC: Chondromyxoid fibroma: A clinicopathologic study of 76 cases.
Cancer. 30:726–736. 1972.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wu CT, Inwards CY, O'Laughlin S, Rock MG,
Beabout JW and Unni KK: Chondromyxoid fibroma of bone: A
clinicopathologic review of 278 cases. Hum Pathol. 29:438–446.
1998.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gherlinzoni F, Rock M and Picci P:
Chondromyxoid fibroma. The experience at the Istituto Ortopedico
Rizzoli. J Bone Joint Surg Am. 65:198–204. 1983.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gutiérrez-González R, De Reina L, Saab A,
Jiménez-Heffernan J and García-Uría J: Chondromyxoid fibroma of the
lumbar spine: Case report and literature review. Eur Spine J. 21
(Suppl 4):S458–S462. 2012.PubMed/NCBI View Article : Google Scholar
|