Introduction
In 2019, there were 12 million cases of strokes and
6.55 million stroke-related deaths worldwide, with ischemic stroke
(IS) accounting for 62.4% of all stroke events, jeopardizing the
health and lives of patients (1).
IS constitutes the majority of stroke cases (2) and is primarily caused by cerebral
ischemia and hypoxia due to decreased cerebral blood flow or
insufficient oxygen supply to brain tissue. At present, the most
effective clinical treatment for cerebral ischemia is to
re-establish effective blood supply and restore cerebral perfusion
in the ischemic area by mechanical thrombectomy and intravenous
thrombolysis with drugs such as anticoagulants and, antiplatelet
and thrombolytic drugs (3).
However, inflammation and oxidative stress during reperfusion can
cause secondary injury to brain tissue, resulting in brain
dysfunction (4). This
pathophysiological process is known as cerebral
ischemia/reperfusion injury (CIRI).
A previous study has shown that inhibition of
bromodomain-containing 4 can alleviate apoptosis and endoplasmic
reperfusion injury induced by renal IRI by blocking forkhead box
protein O4 (FOXO4)-mediated oxidative stress (5). FOXO4, a member of the FOXO family, is
involved in numerous biological behaviors including cell energy
metabolism regulation, cell proliferation, differentiation,
apoptosis, cell senescence, homeostasis and oxidative stress
(6,7). However, it is unclear whether FOXO4
serves a regulatory role in CIRI. A literature review showed that
protein levels of FOXO1, FOXO3a and FOXO4 are significantly
increased in the brain following traumatic brain injury (8). In addition, sevoflurane has been
shown to alleviate liver IRI by promoting expression of microRNA-96
and inhibiting expression of FOXO4(9). Moreover, hypoxia/reoxygenation (H/R)
induces FOXO4 upregulation in rat H9C2 cardiomyocytes, and FOXO4
overexpression can reverse the protective effects of
ubiquitin-specific peptidase 10 (USP10) overexpression on
H/R-induced H9C2 cells by regulating the Hippo/yes-associated
protein 1 signaling pathway (10).
To the best of our knowledge, however, the role of FOXO4 in
cerebral ischemia has not been reported.
In the present study, the role of FOXO4 in cerebral
microvascular endothelial cell injury in cerebral ischemia was
investigated and its regulatory mechanisms were explored. The
present study aimed to provide a theoretical basis for clinical
treatment of cerebral ischemia with FOXO4.
Materials and methods
Database
HDOCK SERVER (hdock.phys.hust.edu.cn/) database (11) and JASPAR database (https://jaspar.elixir.no/) were used to predict the
binding between FOXO4 and the CTRP6 promoter.
Cell culture
The human brain microvascular endothelial cell
(BMEC) line HCMEC/D3 (cat. no. BNCC337728; BeNa Culture Collection)
was cultured in standard DMEM with 10% FBS (both Gibco; Thermo
Fisher Scientific, Inc.) at 37˚C with 5% CO2.
Induction of the oxygen-glucose
deprivation/reoxygenation (OGD/R) model
HCMEC/D3 cells were incubated in glucose-free
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) in a hypoxic incubator (5% CO2, 95%
N2) at 37˚C for 2 h. Cells were removed from the anoxic
atmosphere and transferred to a normal environment for 12 h. The
cells cultured in serum-free medium at 37˚C with 5% CO2
were defined as the control group.
Cell transfection
siRNAs specific to FOXO4 (si-FOXO4#1 and si-FOXO4#2)
or CTRP6 (si-CTRP6#1 and si-CTRP6#2), corresponding negative
control (si-NC), pc-DNA3.1 vectors containing the complete sequence
of FOXO4 [overexpression (Ov-)FOXO4] and empty vector (Ov-NC) were
synthesized by Shanghai GenePharma Co., Ltd. The sequences of
si-FOXO4 and si-CTRP6 were as follows: si-FOXO4#1,
5'-CCGTACTGTACCCTACTTCAAGG-3'; si-FOXO4#2,
5'-AGGATCTAGATCTTGATATGTAT-3'; si-CTRP6#1,
5'-CAACGACTTCGACACCTACAT-3'; si-CTRP6#2,
5'-GAAAGAGGCTGTCATCCTGTA-3' and si-NC, 5'-UUCUCCGAACGUGUCACGUTT-3'.
Using Lipofectamine® 3000 reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), 100 nM recombinants were transfected into
HCMEC/D3 cells for 48 h at 37˚C. The transfection efficacy was
examined with western blotting and RT-qPCR and OGD/R induction was
performed 48 h after transfection.
Reverse transcription-quantitative
(RT-q)PCR
RT-qPCR was applied to assess the transfection
efficiency of small interfering RNAs 48 h post-transfection. Total
RNA was isolated from cells using Trizolâ reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) in accordance with the
manufacturer's protocol. A total of 500 ng total RNA was used as a
template to synthesize cDNA using iScript Reverse Transcription
Supermix (Bio-Rad Laboratories, Inc.) according to the
manufacturer's protocol. SYBR-based qPCR was performed to detect
the total mRNA transcripts of the target genes on an ABI 7500
platform (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
relative expression of target genes was assessed using the
2-ΔΔCq method and normalized to the housekeeping gene
GAPDH (12). The following primers
were used: FOXO4 forward, 5'-GGCTGCCGCGATCATAGAC-3' and reverse,
5'-GGCTGGTTAGCGATCTCTGG-3'; C1q/tumor necrosis factor-related
protein 6 (CTRP6) forward, 5'-TGCCTGAGATCAGACCCTACA-3' and reverse,
5'-GCCCACTGAGAAGGCGAAG-3' and GAPDH forward,
5'-AATGGGCAGCCGTTAGGAAA-3' and reverse,
5'-GCGCCCAATACGACCAAATC-3'.
Western blotting
Cell lysates were collected using RIPA (Beijing
Solarbio Science & Technology Co., Ltd.). The protein
concentration of cell lysates were measured with a BCA Protein
Assay Kit (Sangon Biotech Co., Ltd.). Equal protein samples (30 µg
per lane) were separated by 10% SDS-PAGE and transferred to PVDF
membrane. The membranes, blocked using 5% BSA (Beijing Solarbio
Science & Technology Co., Ltd.) for 1 h at room temperature,
were incubated with primary antibodies including FOXO4 (1:1,000;
cat. no. ab128908), Bcl-2 (1:2,000; cat. no. ab182858), Bax
(1:1,000; cat. no. ab32503), cleaved caspase 3 (1:500; cat. no.
ab32042), caspase 3 (1:5,000; cat. no. ab32351), ZO-1 (1:1,000;
cat. no. ab276131), Occludin (1:1,000; cat. no. ab216327),
Claudin-5 (1:1,000; cat. no. ab131259), CTRP6 (1:1,000; cat. no.
ab300583) and β-actin (1:1,000; cat. no. ab8227), all from Abcam,
overnight at 4˚C and then incubated with goat anti-rabbit
horseradish peroxidase-conjugated IgG (1:2,000; cat. no. ab6721;
Abcam) for 2 h at room temperature. Protein bands were visualized
using ECL Prime Western Blotting Detection Reagent (Amersham;
Cytiva) and the density of the bands was determined using ImageJ
software (version 1.8.0; National Institutes of Health).
Cell viability
Cell viability was detected using a Cell Counting
Kit-8 (CCK-8) assay. HCMEC/D3 cells were cultured in a 96-well
plate for 24 h at 37˚C. Then, 10 µl CCK-8 solution (Dojindo
Laboratories, Inc.) was added to each well and cells were incubated
for 2 h. Absorbance was detected at 490 nm with a microplate reader
(Bio-Rad Laboratories, Inc.).
TUNEL assay
To determine apoptosis of HCMEC/D3 cells, a TUNEL
assay with an In Situ Cell Death Detection kit, POD (Roche
Diagnostics GmbH) was performed. Apoptotic cells were fixed with 4%
formaldehyde for 25 min at 4˚C and then permeabilized by 0.2%
TritonX-100 for 5 min at 4˚C. The cells were equilibrated with 100
µl equilibration buffer for 10 min at room temperature. Cells were
labeled with 50 µl TdT reaction mix at 37˚C for 1 h. Saline-sodium
citrate (SSC) buffer was used to stop the reaction and cell nuclei
were mounted with mounting medium containing 1 mg/ml DAPI for 5 min
at room temperature. The images in five random fields were obtained
by fluorescence microscopy (magnification, x100).
Endothelial permeability
FITC-Dextran assay kit (cat. no. ECM644;
MilliporeSigma) was used to determine endothelial permeability in
accordance with the manufacturer's instructions. HCMEC/D3 cells
were seeded into a Transwell chamber (1x104 cells/well;
8-µm pore size; Costar; Corning, Inc.) for 72 h at 37˚C. Then, the
DMEM (Gibco; Thermo Fisher Scientific, Inc.) was discarded and
cells in the upper chamber were incubated with 10 kDa FITC-dextran
(10 mg/ml; 10 µl) for 1 h at 37˚C, and 50 µl of DMEM was added to
the lower compartment. The plates were incubated in the dark for 60
min at 37˚C, after which the fluorescence intensity in the upper
chamber was determined with a fluorescence microscope
(magnification, x200) and was measured using ImageJ software
(version 1.8.0; National Institutes of Health).
Immunofluorescence (IF) staining
HCMEC/D3 cells were fixed with 4% formaldehyde for
15 min at room temperature and subsequently penetrated with 0.5%
Triton X-100 (Sangon Biotech Co., Ltd.) at room temperature for 20
min. Following blocking with 5% normal goat serum (Beijing Solarbio
Science & Technology Co., Ltd.) for 1 h at room temperature,
HCMEC/D3 cells were incubated with primary antibodies against
zonula occludens-1 (ZO-1; cat. no. ab221547; 1:100; Abcam)
overnight at 4˚C. The Alexa Fluor® 488-conjugated goat
anti-rabbit IgG secondary antibodies (cat. no. ab150077; 1:400;
Abcam) were then added to the slides at 37˚C for 1 h. Cells were
incubated with DAPI for 5 min at room temperature in the dark. The
cell slides were finally analyzed under a fluorescent microscope
(magnification, x200; Olympus Corporation). Integrated optical
density or positive cell numbers in each image were assessed using
ImageJ software (version 1.8.0; National Institutes of Health).
ELISA
The levels of IL-1β, IL-10 and tumor necrosis
factor-α (TNF-α) in the supernatants of HCMEC/D3 cells were
examined using commercial IL-1β (cat. no. H002-1-2), IL-10 (cat.
no. H009-1-2) and TNF-α (cat. no. H052-1-2) ELISA kits (all from
Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's instructions. Absorbance was detected at 450 nm.
Cellular reactive oxygen species (ROS) assay (cat. no. E004-1-1),
superoxide dismutase (SOD) activity assay (cat. no. A001-2-2) and
glutathione peroxidase (GSH-Px) assay kits (cat. no. A005-1-2) all
from Nanjing Jiancheng Bioengineering Institute were used to
determine ROS, SOD and GSH-Px activity, respectively, according to
the manufacturer's instructions.
Luciferase reporter assay
The CTRP6 promoter reporter vector [CTRP6-mutant
(MUT) or wild-type (WT)] was designed and synthesized by Sangon
Biotech Co., Ltd. The reporter construct was transiently
transfected along with a Renilla control plasmid and either
Ov-FOXO4 or Ov-NC using Lipofectamine® 3000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Following transfection for 6 h at
37˚C, the DMEM (Gibco; Thermo Fisher Scientific, Inc.) was replaced
with DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.) supplemented
with 0.2% FBS (Gibco; Thermo Fisher Scientific, Inc.). At 48 h
post-transfection, the luciferase activity was detected using a
dual-luciferase reporter assay system (Promega Corporation) and
normalized to Renilla luciferase activities.
Chromatin immunoprecipitation (ChIP)
assay
HCMEC/D3 cells were sonicated at 150 Hz and sheared
with four sets of 10 sec pulses on wet ice to generate 200-500 bp
DNA fragments. The lysate (100 µl) was immunoprecipitated with
anti-FOXO4 (1:100; cat. no. ab128908; Abcam) or IgG antibodies
(negative control; 1:100; cat. no. ab205718; Abcam) overnight at
4˚C. Immunoprecipitated DNAs were obtained by phenol/chloroform
extraction and analyzed by RT-qPCR according to the aforementioned
protocol.
Statistical analysis
All data were analyzed with GraphPad Prism 6.0
(Dotmatics) and are presented as the mean ± SD. All experiments
were performed in triplicate. One-way analysis of variance followed
by Tukey's post hoc test was used to assess the differences and
P<0.05 was considered to indicate a statistically significant
difference.
Results
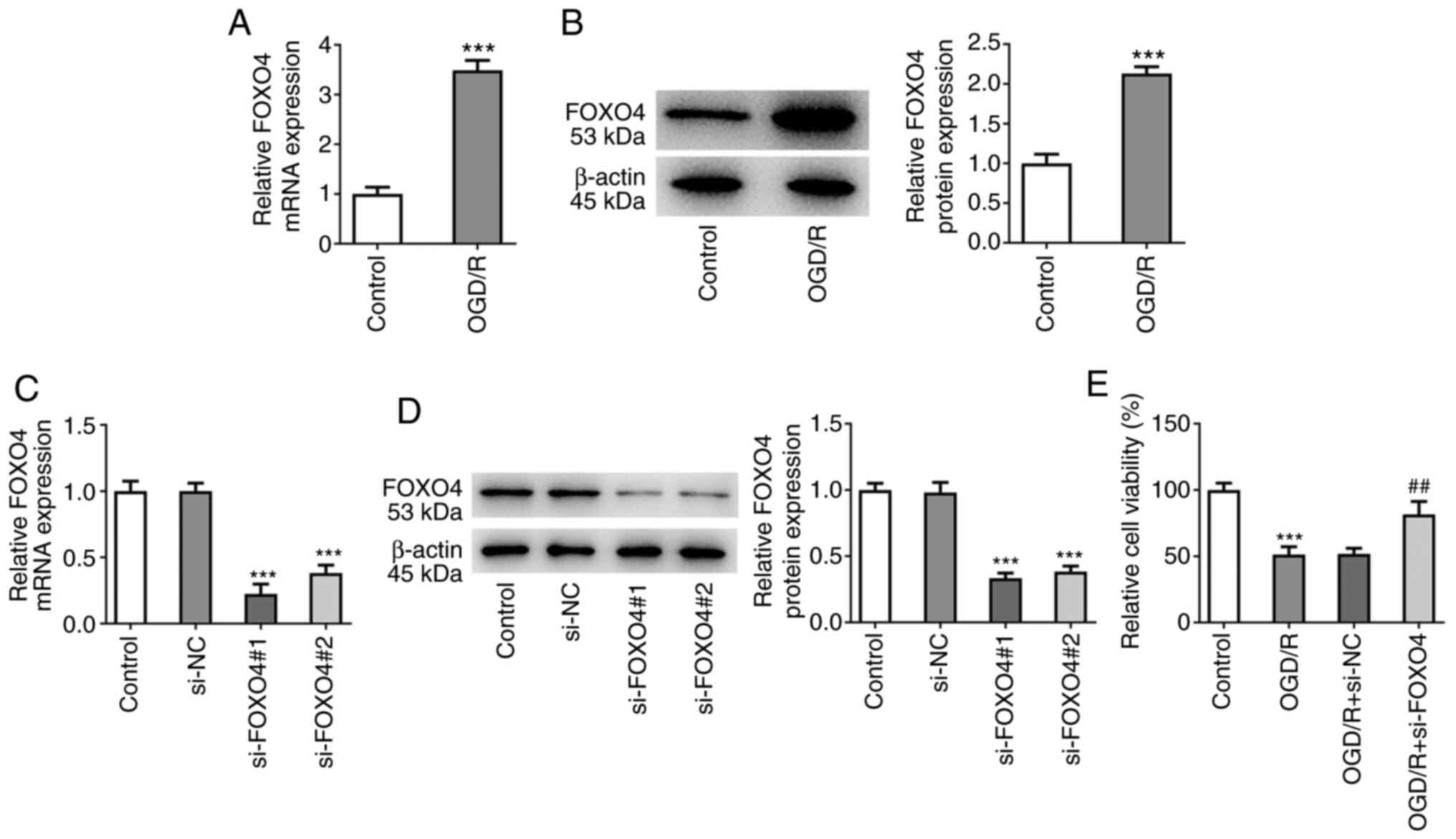
Knockdown of FOXO4 enhances
OGD/R-induced HCMEC/D3 cell viability
Following OGD/R induction, the expression of FOXO4
was detected by RT-qPCR and western blotting. mRNA and protein
expressions of FOXO4 were significantly increased in HCMEC/D3 cells
induced by OGD/R (Fig. 1A and
B). FOXO4 interference plasmid was
constructed and RT-qPCR and western blotting showed successful cell
transfection (Fig. 1C and D). si-FOXO4#1 was selected for follow-up
experiments because it exhibited the highest interference efficacy.
CCK-8 assay showed that the cell viability of OGD/R group was
significantly decreased compared with the control group. Compared
with the OGD/R + si-NC group, viability of the OGD/R + si-FOXO4
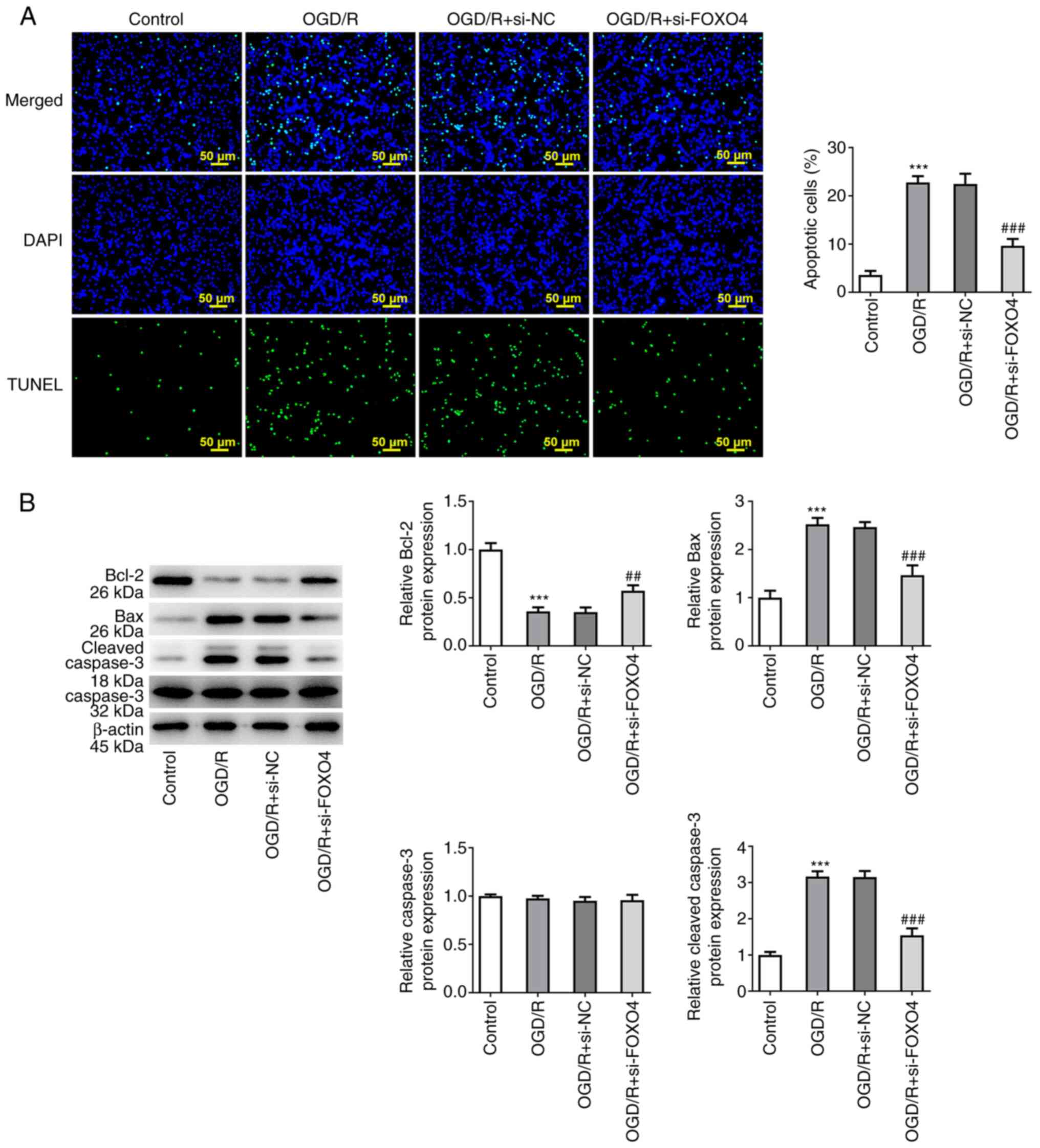
group was significantly increased (Fig. 1E). Apoptosis was detected by TUNEL
assay; cell apoptosis was significantly increased after OGD/R
induction compared with the control group and significantly
inhibited by FOXO4 interference (Fig.
2A). Western blotting of apoptosis-associated proteins showed
that after OGD/R induction, the expression of Bcl-2 was
significantly decreased, while expression of Bax and cleaved
caspase 3 was significantly increased. These effects were all
significantly reversed by si-FOXO4 in comparison with the OGD/R +
si-NC group (Fig. 2B).
Knockdown of FOXO4 improves
OGD/R-induced HCMEC/D3 cell barrier dysfunction
The effect of FOXO4 on cellular barrier dysfunction
was examined. FITC-Dextran kit was used to detect endothelial
permeability; endothelial permeability was significantly increased
after OGD/R induction. Compared with the OGD/R + si-NC group, FOXO4
depletion significantly decreased endothelial permeability
(Fig. 3A). IF assay detected the
expression of tight junction protein ZO-1; expression of ZO-1 was
decreased after OGD/R induction, while FOXO4 interference increased
the expression of ZO-1 in the OGD/R + si-FOXO4 group (Fig. 3B). Western blotting detected the
expression of tight junction proteins ZO-1, occludin and claudin-5;
compared with the control group, the expression of ZO-1, occludin
and claudin-5 was decreased significantly following OGD/R
induction. Compared with the OGD/R + si-NC group, the expression of
ZO-1, occludin and claudin-5 was significantly increased in the
OGD/R + si-FOXO4 group (Fig.
3C).
Knockdown of FOXO4 alleviates
OGD/R-induced oxidative stress and inflammation in HCMEC/D3
cells
Levels of cellular oxidative stress and inflammation
were measured. After OGD/R induction, the activities of SOD and
GSH-Px in HCMEC/D3 cells were significantly decreased, while the
activity of ROS increased. Compared with the OGD/R + si-NC group,
the activities of SOD and GSH-Px in the OGD/R + si-FOXO4 group were
significantly increased, while activity of ROS was decreased
(Fig. 4A). ELISA was used to
detect levels of inflammatory cytokines; levels of IL-6, IL-1β and
TNF-α were significantly increased after OGD/R induction.
Interference with FOXO4 significantly reversed the increase in
IL-6, IL-1β and TNF-α (Fig. 4B).
These results suggested that knockdown of FOXO4 alleviated
OGD/R-induced oxidative stress and inflammation in HCMEC/D3
cells.
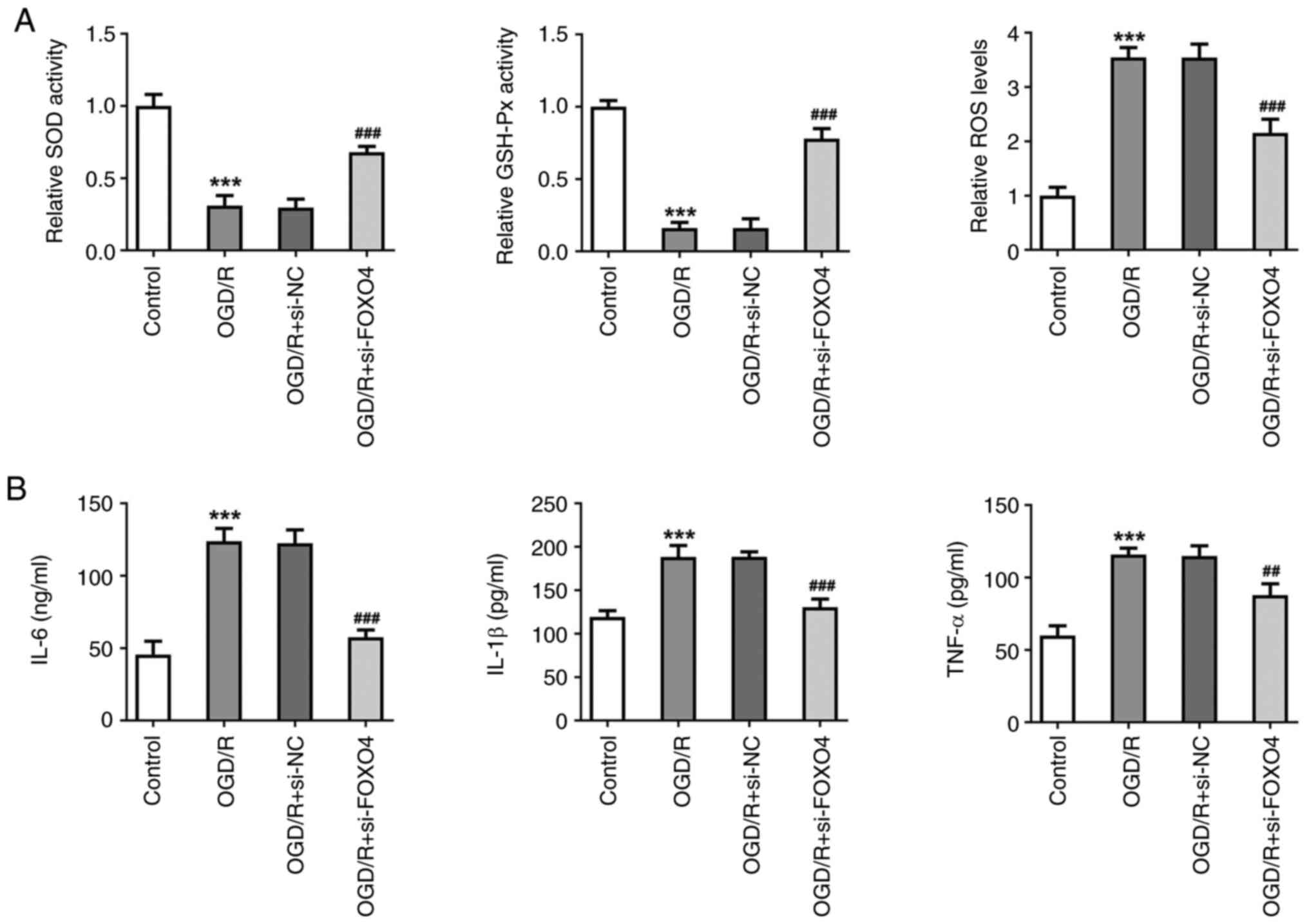 | Figure 4Knockdown of FOXO4 alleviates
OGD/R-induced oxidative stress and inflammation in HCMEC/D3 cells.
(A) Levels of oxidative stress-associated indicators SOD, GSH-Px
and ROS. (B) ELISA was used to detect levels of inflammatory
cytokines in cells. ***P<0.001 vs. control;
##P<0.01, ###P<0.001 vs. OGD/R + si-NC.
OGD/R, oxygen-glucose deprivation/reoxygenation; FOXO4, forkhead
box protein O4; NC, negative control; SOD, superoxide dismutase,
GSH-Px, glutathione peroxidase; ROS, reactive oxygen species; si,
small interfering. |
Knockdown of FOXO4 regulates CTRP6
transcription in HCMEC/D3 cells
The HDOCK SERVER database showed the binding of
FOXO4 to CTRP6 with a -257.66 docking score and 0.8960 confidence
score (Fig. 5A). Potential binding
sequences between FOXO4 and CTRP6 promoter were also predicted
using the JASPAR database (Fig.
5B). Moreover, mRNA and protein expression of CTRP6 was
significantly decreased in OGD/R-induced HCMEC/D3 cells (Fig. 5C and D). FOXO4 was overexpressed in HCMEC/D3
cells and its transfection efficiency was assessed (Fig. 5E and F). RT-qPCR and western blotting results
showed that the mRNA and protein expression of CTRP6 in Ov-FOXO4
group was significantly decreased compared with the Ov-NC group.
Following interference with FOXO4, CTRP6 expression in si-FOXO4
group was significantly increased compared with the si-NC group
(Fig. 5G and H). Moreover, luciferase detection and
ChIP assay both demonstrated the binding ability of FOXO4 and CTRP6
promoter (Fig. 5I and J). These results indicated that FOXO4
inhibited expression of CTRP6.
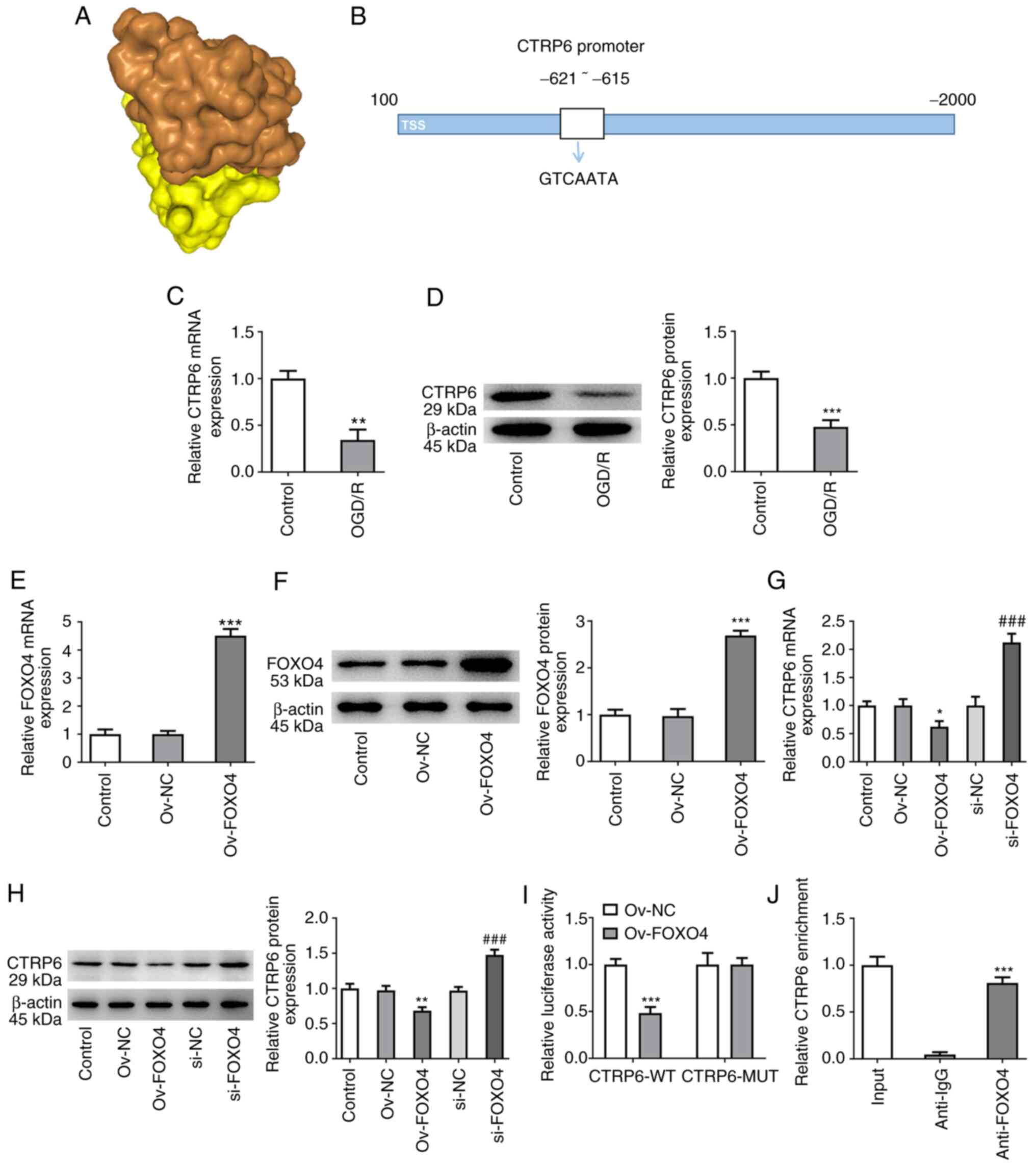 | Figure 5Knockdown of FOXO4 regulates CTRP6
transcription in HCMEC/D3 cells. (A) HDOCK SERVER database
predicted the binding of FOXO4 to CTRP6. (B) Potential binding
sequences between FOXO4 and CTRP6 promoter were predicted by JASPAR
database. Expression of CTRP6 was detected by (C) RT-qPCR and (D)
western blotting. **P<0.01, ***P<0.001
vs. control. FOXO4 overexpression plasmid was transfected. (E)
RT-qPCR and (F) western blotting detected the cell transfection
efficiency. (G) RT-qPCR and (H) western blotting were used to
detect the expression of FOXO4. *P<0.05,
**P<0.01, ***P<0.001 vs. Ov-NC;
###P<0.001 vs. si-NC. (I) Luciferase detection and
(J) ChIP assay demonstrated the binding ability of FOXO4 and CTRP6
promoter. ***P<0.001 vs. Ov-NC. OGD/R, oxygen-glucose
deprivation/reoxygenation; FOXO4, forkhead box protein O4; NC,
negative control; CTRP6, C1q/tumor necrosis factor-related protein
6; ChIP, chromatin immunoprecipitation; si, small interfering; ov,
overexpression; RT-q, reverse transcription-quantitative; WT,
wild-type; MUT, mutant; TSS, Transcription start site. |
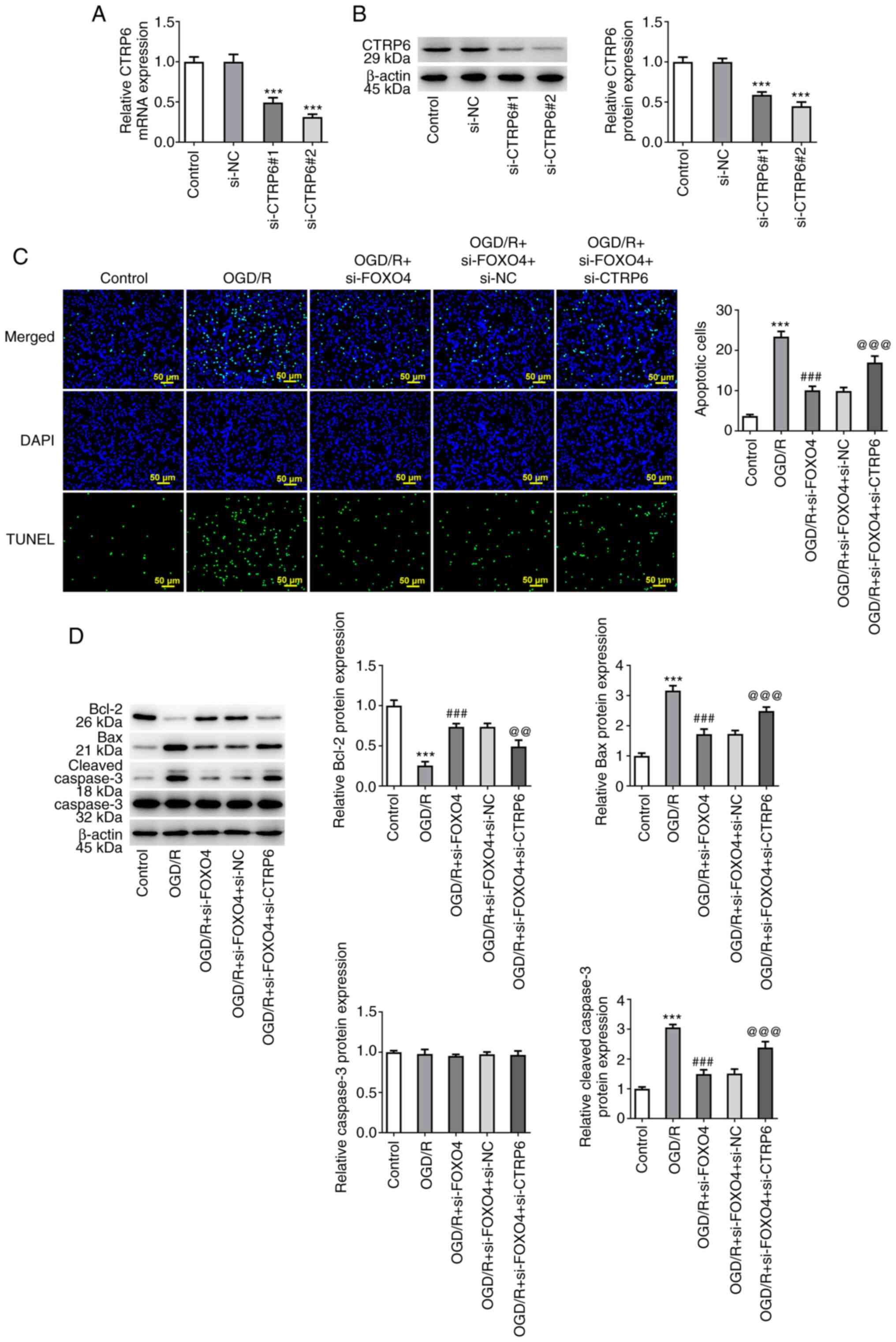
Knockdown of FOXO4 regulates
expression of CTRP6 to protect against OGD/R-induced HCMEC/D3 cell
damage
CTRP6 interference plasmid was constructed and
si-CTRP6#2 was selected for subsequent experiments due to its
strong interference efficiency (Fig.
6A and B). TUNEL assay and
western blotting showed that, compared with the OGD/R + si-FOXO4 +
si-NC group, cell apoptosis in OGD/R + si-FOXO4 + si-CTRP6 group
was significantly increased, accompanied by decreased expression of
Bcl-2 and increased expression of Bax and cleaved caspase 3
(Fig. 6C and D). FITC-Dextran assay results showed that
interference with CTRP6 significantly reversed the inhibitory
effect of FOXO4 interference on OGD/R-induced endothelial
permeability (Fig. 7A). IF assay
showed that compared with the OGD/R + si-FOXO4 + si-NC group,
expression of ZO-1 in the OGD/R + si-FOXO4 + si-CTRP6 group was
significantly decreased (Fig. 7B).
Moreover, western blotting showed that compared with the OGD/R +
si-FOXO4 + si-NC group, expression of ZO-1, occludin and claudin-5
in the OGD/R + si-FOXO4 + si-CTRP6 group was significantly
decreased (Fig. 7C). In addition,
compared with the OGD/R + si-FOXO4 + si-NC group, the activities of
oxidative stress indicators SOD and GSH-Px were decreased in the
OGD/R + si-FOXO4 + si-CTRP6 group, while ROS levels increased
(Fig. 7D). Moreover, decreased
levels of inflammatory cytokines IL-6, IL-1β and TNF-a in the OGD/R
+ si-FOXO4 + si-NC group were significantly increased in the OGD/R
+ si-FOXO4 + si-CTRP6 group (Fig.
7E).
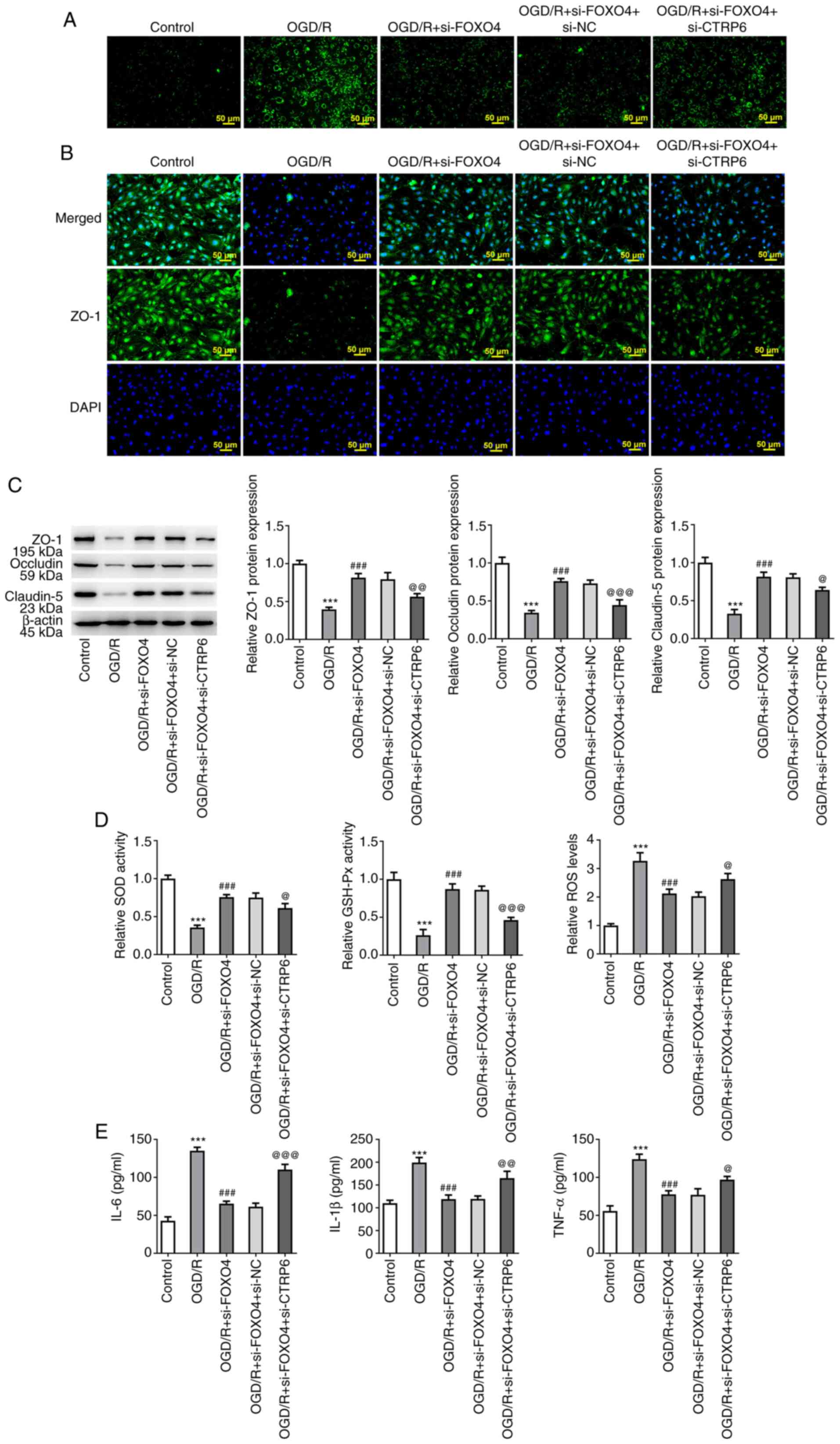 | Figure 7Knockdown of FOXO4 regulates
expression of CTRP6 to protect against OGD/R-induced HCMEC/D3 cell
damage. (A) FITC-Dextran kit was used to detect endothelial
permeability. (B) Immunofluorescence assay detected expression of
tight junction protein ZO-1. (C) Western blotting detected
expression of tight junction proteins ZO-1, occludin and claudin-5.
(D) Levels of oxidative stress-associated indicators SOD, GSH-Px
and ROS. (E) ELISA was used to detect levels of inflammatory
cytokines in cells. ***P<0.001 vs. control;
###P<0.001 vs. OGD/R; @P<0.05,
@@P<0.01, @@@P<0.001 vs. OGD/R + si-FOXO4 + si-NC.
OGD/R, oxygen-glucose deprivation/reoxygenation; FOXO4, forkhead
box protein O4; NC, negative control; CTRP6, C1q/tumor necrosis
factor-related protein 6; ZO-1, zonula occludens-1; si, small
interfering; SOD, superoxide dismutase; GSH-Px, glutathione
peroxidase; ROS, reactive oxygen species. |
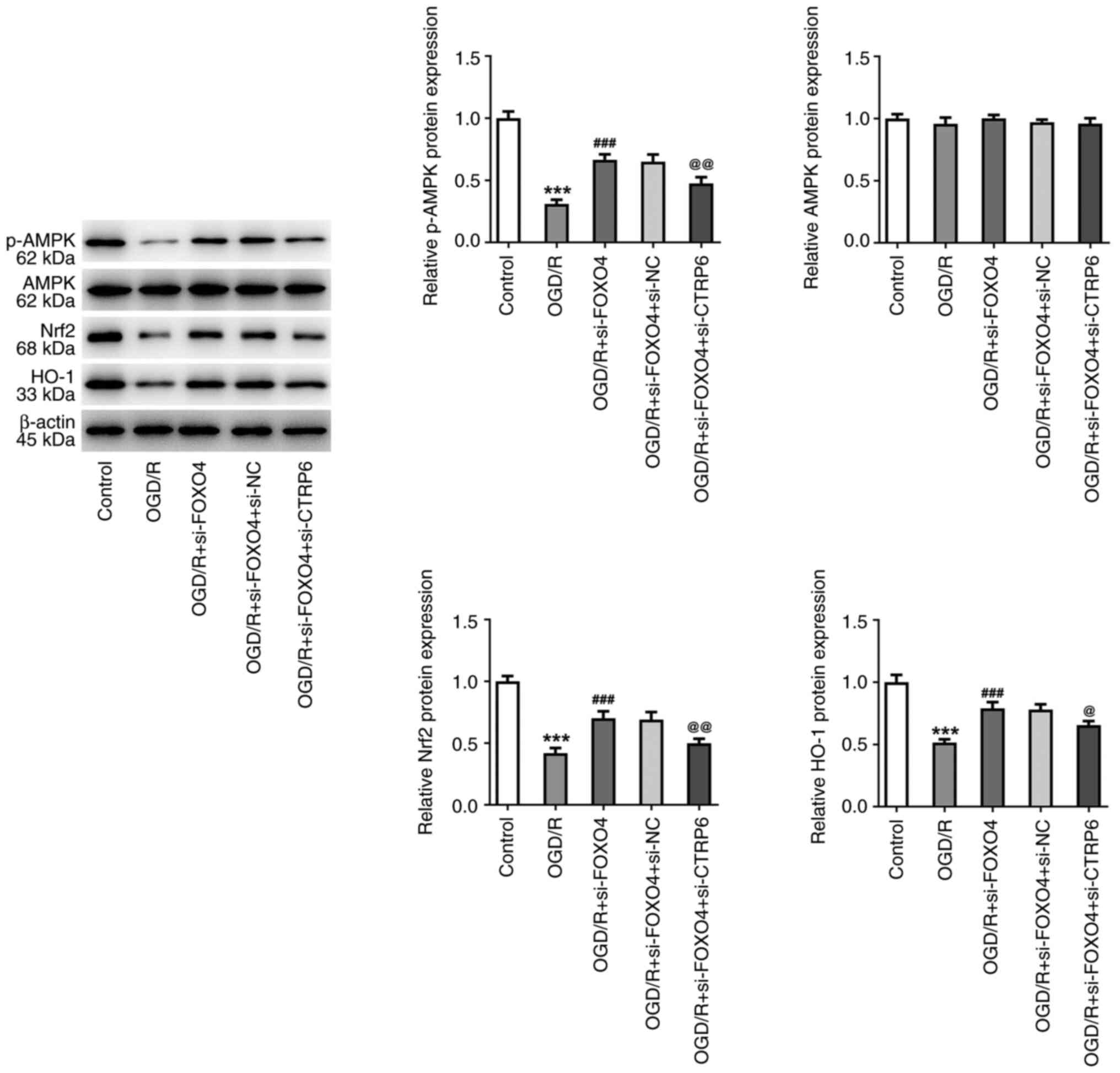
Knockdown of FOXO4 regulates CTRP6
expression to protect against OGD/R-induced HCMEC/D3 cell injury
via the AMPK/Nrf2/heme oxygenase-1 (HO-1) pathway
Expression of AMPK/Nrf2/HO-1 pathway-associated
proteins phosphorylated (p-)AMPK, Nrf2 and HO-1 were significantly
decreased following OGD/R induction. After interfering with the
expression of FOXO4, expression of p-AMPK, Nrf2 and HO-1 in
OGD/R-induced HCMEC/D3 cells was increased. Compared with the OGD/R
+ si-FOXO4 + si-NC group, the expression of p-AMPK, Nrf2 and HO-1
in the OGD/R + si-FOXO4 + SI-NC group was significantly decreased
(Fig. 8).
Discussion
IS exerts damaging effects on cerebral
microcirculation such as oxidative stress, excessive secretion of
inflammatory mediators, leukocyte infiltration, increased
permeability of microvessels, destruction of the blood-brain
barrier (BBB) and calcium overload (13). BMECs, highly specialized
endothelial cells, are a core component of the BBB and play an
important role in maintaining the function of the BBB, dynamic
balance of the cerebral microvascular system and normal cerebral
blood flow (14). BMECs are key
targets affected by cerebral ischemic injury (15,16).
BMECs are sensitive to ischemia and hypoxia (17). Multiple studies have used OGD/R to
induce BMEC ischemic injury in vitro (18,19).
After cerebral ischemic injury, BMECs shed and denature from the
vascular wall, leading to release of inflammatory factors TNF-α,
IL-1β and IL-6(20). The
permeability of the BBB is increased, and the structural and
functional integrity of cells is damaged, resulting in endothelial
cell dysfunction and brain parenchymal injury (21). In the present study, following
OGD/R induction, cell viability was decreased and apoptosis,
inflammatory factor release and oxidative stress levels were
increased. Moreover, HCMEC/D3 cell barrier function was impaired
following OGD/R induction. These results indicated that a BMEC
injury model was successfully constructed.
Previous studies have shown that FOXO4 is highly
expressed during IRI in the liver (9), kidney (5) and heart (22). In addition, FOXO4 is expressed in
OGD/R-treated human cortical neurons, suggesting that FOXO4 may
participate in cerebral stroke (23). To the best of our knowledge, the
present study is the first to demonstrate that FOXO4 expression is
abnormally elevated in OGD/R-induced HCMEC/D3 cells. Apoptosis is a
process of programmed cell death that is involved in the
pathogenesis of CIRI (24,25). FOXO4 can contribute to apoptosis
during hepatic (9), myocardial
(22) and renal IRI (5). In the present study, it was observed
that interference with FOXO4 expression significantly inhibited
OGD/R-induced HCMEC/D3 cell apoptosis, accompanied by elevated
anti-apoptotic Bcl-2 expression and decreased pro-apoptotic Bax and
cleaved caspase 3 expression. The destruction of the BBB is a key
factor in the occurrence and development of IS (26,27).
The BBB is mainly composed of BMECs, tight junction structures,
pericytes, astrocytes, foot processes and basement membranes
(14). These structures and
biological properties enable it to selectively control the exchange
of substances between blood and brain tissue, serving a key role in
maintaining the homeostasis of the central nervous system
environment (14). Tight junction
proteins, including ZO-1, claudin-5 and occludin, are key in
regulating the integrity and permeability of BBB and are disrupted
and redistributed following IS (28). In the present study, FOXO4
silencing promoted ZO-1, claudin-5 and occludin expression,
indicating that FOXO4 downregulation improved the barrier
dysfunction in OGD/R-exposed HCMEC/D3 cells. Oxidative stress and
inflammatory response are common events responsible for CIRI
(29). Oxidative stress-activated
FOXO proteins (30) regulate the
expression of oxidative stress-related genes (31). Moreover, FOXO4 knockdown decreases
ROS generation and increases SOD and GSH-Px activities following
IRI (10,22). Here, in OGD/R-exposed HCMEC/D3
cells, interference with FOXO4 served a protective role in
oxidative stress, demonstrated by improved SOD and GSH-Px
activities and decreased ROS levels. Furthermore, downregulation of
FOXO4 decreased levels of proinflammatory cytokines including IL-6,
IL-1β and TNF-α. These results suggested that interference with
FOXO4 expression might inhibit oxidative stress, inflammation and
apoptosis in cell damage in CIRI.
FOXO4 is a transcription factor that binds to the
promoters of a broad variety of target genes and controls several
cellular processes (32). For
example, FOXO4 aggravates apoptosis and oxidative stress of
H/R-induced cardiomyocytes through negative modulation of USP10
transcription (10). In the
present study, the binding sites of transcription factor FOXO4 and
CTRP6 promoter were predicted using the HDOCK SERVER database. The
binding between FOXO4 and the CTRP6 promoter was further verified
by mechanism assays and CTRP6 expression was demonstrated to be
depleted after FOXO4 was overexpressed and to be raised when FOXO4
was down-regulated, suggesting that FOXO4 could transcriptionally
inhibit expression of CTRP6. Furthermore, it was hypothesized that
knockdown of FOXO4 could upregulate the expression of CTRP6,
thereby protecting against OGD/R-induced HCMEC/D3 cell damage. CTRP
is a highly conserved family of adiponectin-like proteins involved
in a variety of physiological processes, such as cell
proliferation, lipid metabolism, insulin sensitivity, energy
balance and cardiac protection (33). CTRP6 decreases damage of the
central nervous system induced by sevoflurane by promoting
expression of p-Akt (34). CTRP6
protects against CIRI by reducing inflammation, oxidative stress
and apoptosis of rat pheochromocytoma (PC12) cells (35). CTRP6 improves peroxisome
proliferator-activated receptor γ activation to relieve vascular
endothelial dysfunction in angiotensin II-induced hypertension and
spontaneously hypertensive rats (36). These results indicate that CTRP6
serves an important role in the occurrence and development of
cerebral ischemia. In the present study, inhibition of CTRP6
partially reversed the suppressive effect of FOXO4 knockdown on
apoptosis, BBB dysfunction and oxidative stress in OGD/R-exposed
HCMEC/D3 cells.
The downstream pathway of CTRP6 was investigated.
CTRP6 regulates microRNA-34a-5p expression via the AMPK/sirtuin 1
pathway to inhibit TNF-α-induced apoptosis of salivary gland cells
(37). In renal fibrosis, CTRP6
inhibits extracellular matrix deposition and promotes AMPK
phosphorylation by promoting fatty acid oxidation (38). Palmatine prevents CIRI by
activating the AMPK/Nrf2 pathway (39). Salvinorin A alleviates BBB and
brain microvascular endothelial cell injury following IRI and
alleviates endoplasmic reticulum stress of endothelial cells via
the AMPK pathway (40). MitoQ
protects against BMEC damage induced by high glucose levels via the
Nrf2/HO-1 pathway (41).
Therefore, it was hypothesized that FOXO4 could regulate CTRP6 and
downstream AMPK/Nrf2/HO-1 signaling, thus serving a role in
OGD/R-induced BMEC injury. In the present study, FOXO4 depletion
regulated expression of CTRP6, thereby modulating the
AMPK/Nrf2/HO-1 signaling pathway. However, inhibitors or activators
of the AMPK/Nrf2/HO-1 pathway were not used to explore the
mechanism, which is a limitation of the present study and requires
further exploration in future experiments. Furthermore, middle
cerebral artery occlusion is the most widely used IS model
(42). Hence, animal experiments
should be performed to focus on the effects of FOXO4 and CTRP6 on
CIRI using middle cerebral artery occlusion models to corroborate
the findings of the present study. Moreover, the specific role of
FOXO4 and CTRP6 in the infarction area in the brain needs to be
investigated in in vivo models.
Overall, FOXO4 knockdown activated expression of
CTRP6 to protect against cerebral microvascular endothelial cell
injury induced by OGD/R via the AMPK/Nrf2/HO-1 pathway. The present
study suggested that FOXO4 and CTRP6 might serve as promising
biomarkers for IS.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
XC, ZL and YY designed the study and wrote and
revised the manuscript. XC and YY analyzed the data and performed
the literature review. All authors performed the experiments. XC
and YY confirm the authenticity of all the raw data. All authors
have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
GBD 2019 Stroke Collaborators. Global,
regional, and national burden of stroke and its risk factors,
1990-2019: A systematic analysis for the global burden of disease
study 2019. Lancet Neurol. 20:795–820. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Gittler M and Davis AM: Guidelines for
adult stroke rehabilitation and recovery. JAMA. 319:820–821.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Derex L and Cho TH: Mechanical
thrombectomy in acute ischemic stroke. Rev Neurol (Paris).
173:106–113. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kapanova G, Tashenova G, Akhenbekova A,
Tokpınar A and Yılmaz S: Cerebral ischemia reperfusion injury: From
public health perspectives to mechanisms. Folia Neuropathol.
60:384–389. 2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Liu H, Wang L, Weng X, Chen H, Du Y, Diao
C, Chen Z and Liu X: Inhibition of Brd4 alleviates renal
ischemia/reperfusion injury-induced apoptosis and endoplasmic
reticulum stress by blocking FoxO4-mediated oxidative stress. Redox
Biol. 24(101195)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Liu W, Li Y and Luo B: Current perspective
on the regulation of FOXO4 and its role in disease progression.
Cell Mol Life Sci. 77:651–663. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Orea-Soufi A, Paik J, Bragança J, Donlon
TA, Willcox BJ and Link W: FOXO transcription factors as
therapeutic targets in human diseases. Trends Pharmacol Sci.
43:1070–1084. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Liu XL, Gao CC, Qi M, Han YL, Zhou ML and
Zheng LR: Expression of FOXO transcription factors in the brain
following traumatic brain injury. Neurosci Lett.
753(135882)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
He B, Yang F, Ning Y and Li Y: Sevoflurane
alleviates hepatic ischaemia/reperfusion injury by up-regulating
miR-96 and down-regulating FOXO4. J Cell Mol Med. 25:5899–5911.
2021.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
10
|
Huang J, Liu Y, Wang M, Wang R, Ling H and
Yang Y: FoxO4 negatively modulates USP10 transcription to aggravate
the apoptosis and oxidative stress of hypoxia/reoxygenation-induced
cardiomyocytes by regulating the Hippo/YAP pathway. J Bioenerg
Biomembr. 53:541–551. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yan Y, Tao H, He J and Huang SY: The HDOCK
server for integrated protein-protein docking. Nat Protoc.
15:1829–1852. 2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhu H, Wang Z, Xing Y, Gao Y, Ma T, Lou L,
Lou J, Gao Y, Wang S and Wang Y: Baicalin reduces the permeability
of the blood-brain barrier during hypoxia in vitro by increasing
the expression of tight junction proteins in brain microvascular
endothelial cells. J Ethnopharmacol. 141:714–720. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ferro MP, Heilshorn SC and Owens RM:
Materials for blood brain barrier modeling in vitro. Mater Sci Eng
R Rep. 140(100522)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
del Zoppo GJ and Hallenbeck JM: Advances
in the vascular pathophysiology of ischemic stroke. Thromb Res.
98:73–81. 2000.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ishikawa M, Zhang JH, Nanda A and Granger
DN: Inflammatory responses to ischemia and reperfusion in the
cerebral microcirculation. Front Biosci. 9:1339–1347.
2004.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Engelhardt S, Huang SF, Patkar S, Gassmann
M and Ogunshola OO: Differential responses of blood-brain barrier
associated cells to hypoxia and ischemia: A comparative study.
Fluids Barriers CNS. 12(4)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Fan XD, Yao MJ, Yang B, Han X, Zhang YH,
Wang GR, Li P, Xu L and Liu JX: Chinese herbal preparation
sailuotong alleviates brain ischemia via Nrf2 antioxidation
pathway-dependent cerebral microvascular protection. Front
Pharmacol. 12(748568)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jiang W, Li J, Cai Y, Liu W, Chen M, Xu X,
Deng M, Sun J, Zhou L, Huang Y, et al: The novel lncRNA
ENST00000530525 affects ANO1, contributing to blood-brain barrier
injury in cultured hCMEC/D3 cells under OGD/R conditions. Front
Genet. 13(873230)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li F, Li W, Li X, Li F, Zhang L, Wang B,
Huang G, Guo X, Wan L, Liu Y, et al: Geniposide attenuates
inflammatory response by suppressing P2Y14 receptor and downstream
ERK1/2 signaling pathway in oxygen and glucose deprivation-induced
brain microvascular endothelial cells. J Ethnopharmacol. 185:77–86.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lin Q, Wang W, Yang L and Duan X:
4-Methoxybenzylalcohol protects brain microvascular endothelial
cells against oxygen-glucose deprivation/reperfusion-induced injury
via activation of the PI3K/AKT signaling pathway. Exp Ther Med.
21(252)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Yu L, Zhang W, Huang C, Liang Q, Bao H,
Gong Z, Xu M, Wang Z, Wen M and Cheng X: FoxO4 promotes myocardial
ischemia-reperfusion injury: The role of oxidative stress-induced
apoptosis. Am J Transl Res. 10:2890–2900. 2018.PubMed/NCBI
|
|
23
|
Yan B, Jin Y, Mao S, Zhang Y, Yang D, Du M
and Yin Y: Smurf2-mediated ubiquitination of FOXO4 regulates
oxygen-glucose deprivation/reperfusion-induced pyroptosis of
cortical neurons. Curr Neurovasc Res: Oct 12, 2023 (Epub ahead of
print).
|
|
24
|
Li K, Ding D and Zhang M: Neuroprotection
of osthole against cerebral ischemia/reperfusion injury through an
anti-apoptotic pathway in rats. Biol Pharm Bull. 39:336–342.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wu T, Yin F, Kong H and Peng J: Germacrone
attenuates cerebral ischemia/reperfusion injury in rats via
antioxidative and antiapoptotic mechanisms. J Cell Biochem.
120:18901–18909. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shah K and Abbruscato T: The role of
blood-brain barrier transporters in pathophysiology and
pharmacotherapy of stroke. Curr Pharm Des. 20:1510–1522.
2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yin KJ, Hamblin M and Chen YE: Non-coding
RNAs in cerebral endothelial pathophysiology: Emerging roles in
stroke. Neurochem Int. 77:9–16. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Abdullahi W, Tripathi D and Ronaldson PT:
Blood-brain barrier dysfunction in ischemic stroke: Targeting tight
junctions and transporters for vascular protection. Am J Physiol
Cell Physiol. 315:C343–C356. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li M, Tang H, Li Z and Tang W: Emerging
treatment strategies for cerebral ischemia-reperfusion injury.
Neuroscience. 507:112–124. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo
P, Hu LS, Anderson MJ, Arden KC, Blenis J and Greenberg ME: Akt
promotes cell survival by phosphorylating and inhibiting a Forkhead
transcription factor. Cell. 96:857–868. 1999.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Storz P: Forkhead homeobox type O
transcription factors in the responses to oxidative stress.
Antioxid Redox Signal. 14:593–605. 2011.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Link W: Introduction to FOXO biology.
Methods Mol Biol. 1890:1–9. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dong X, Hu H, Fang Z, Cui J and Liu F:
CTRP6 inhibits PDGF-BB-induced vascular smooth muscle cell
proliferation and migration. Biomed Pharmacother. 103:844–850.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Liu Z and Yang B: CTRP6[C1q/tumor necrosis
factor (TNF)-related protein-6] alleviated the sevoflurane induced
injury of mice central nervous system by promoting the expression
of p-Akt (phosphorylated Akt). Bioengineered. 12:5716–5726.
2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Li Y, Sun J, Gu L and Gao X: Protective
effect of CTRP6 on cerebral ischemia/reperfusion injury by
attenuating inflammation, oxidative stress and apoptosis in PC12
cells. Mol Med Rep. 22:344–352. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Chi L, Hu X, Zhang W, Bai T, Zhang L, Zeng
H, Guo R, Zhang Y and Tian H: Adipokine CTRP6 improves PPARγ
activation to alleviate angiotensin II-induced hypertension and
vascular endothelial dysfunction in spontaneously hypertensive
rats. Biochem Biophys Res Commun. 482:727–734. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Qu LH, Hong X, Zhang Y, Cong X, Xiang RL,
Mei M, Su JZ, Wu LL and Yu GY: C1q/tumor necrosis factor-related
protein-6 attenuates TNF-α-induced apoptosis in salivary acinar
cells via AMPK/SIRT1-modulated miR-34a-5p expression. J Cell
Physiol. 236:5785–5800. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Xie YH, Xiao Y, Huang Q, Hu XF, Gong ZC
and Du J: Role of the CTRP6/AMPK pathway in kidney fibrosis through
the promotion of fatty acid oxidation. Eur J Pharmacol.
892(173755)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Tang C, Hong J, Hu C, Huang C, Gao J,
Huang J, Wang D, Geng Q and Dong Y: Palmatine protects against
cerebral ischemia/reperfusion injury by activation of the AMPK/Nrf2
pathway. Oxid Med Cell Longev. 2021(6660193)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xin J, Ma X, Chen W, Zhou W, Dong H, Wang
Z and Ji F: Regulation of blood-brain barrier permeability by
Salvinorin A via alleviating endoplasmic reticulum stress in brain
endothelial cell after ischemia stroke. Neurochem Int.
149(105093)2021.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Yang MY, Fan Z, Zhang Z and Fan J: MitoQ
protects against high glucose-induced brain microvascular
endothelial cells injury via the Nrf2/HO-1 pathway. J Pharmacol
Sci. 145:105–114. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Chaparro-Cabanillas N, Arbaizar-Rovirosa
M, Salas-Perdomo A, Gallizioli M, Planas AM and Justicia C:
Transient middle cerebral artery occlusion model of stroke. J Vis
Exp: Aug 11, 2023 (Epub ahead of print). doi: 10.3791/65857.
2023.
|