Introduction
Hepatocellular carcinoma (HCC) is a common primary
malignancy of the liver and represents a major public health
concern worldwide (1).
Approximately 830,000 individuals succumb to HCC each year, making
it the third leading cause of cancer-associated mortality globally.
This is due to an insidious nature and late clinical presentation,
which lead to a poor prognosis at the time of diagnosis (2). Identifying and addressing the
underlying etiological factors of HCC is essential to promote early
detection and develop effective preventive strategies.
Chronic hepatitis B (CHB) infection has been
recognized as a primary driver in the progression to HCC (3). However, despite extensive vaccination
campaigns and antiviral therapies, the global CHB burden remains
high, with an estimated 296 million individuals affected worldwide
as per the World Health Organization (4). Amongst individuals with CHB, the
lifetime risk of developing HCC can be as high as 15-25% (5). The molecular and cellular
pathophysiological mechanisms underlying this transition involve an
interplay between viral replication, chronic inflammation and
repeated hepatic injury, all of which can contribute to malignant
transformation (6,7).
Metabolic dysfunction-associated fatty liver disease
(MAFLD) has been previously studied in the context of liver
pathologies. Formerly known as non-alcoholic fatty liver disease
(NAFLD), MAFLD encompasses a spectrum of liver abnormalities
ranging from simple steatosis to non-alcoholic steatohepatitis and
it can progress to cirrhosis and even HCC (8). The prevalence of MAFLD has increased
along with the global rise in obesity and type 2 diabetes,
heralding an impending epidemic of MAFLD-associated complications,
including HCC (9).
Consequently, the coexistence of MAFLD and CHB in a
patient presents a complex clinical scenario (10). Preliminary evidence suggests that
this convergence may have a multiplicative effect on the HCC risk
(11). The metabolic derangements
(insulin resistance and dyslipidemia) and inflammatory milieu of
MAFLD (cytokine imbalance and oxidative stress) could exacerbate
the hepatocarcinogenic potential of CHB (12). However, the precise nature and
magnitude of the effects of combining these conditions remains
sparsely documented and unclear. Thus, evaluating the cumulative
HCC risk that MAFLD may impart on patients with CHB is important. A
clear understanding would help to elucidate the clinical prognosis
of these patients and would facilitate stringent surveillance,
early interventions and tailored management plans.
Systematic reviews and meta-analyses are powerful
evidence synthesis tools, especially when the existing literature
provides conflicting or inconclusive results (13). A rigorous, methodical consolidation
of the available evidence may help clarify the scarce and
heterogeneous data on the combined roles of MAFLD and CHB in HCC
pathogenesis.
In the present systematic review and meta-analysis,
the available literature on the topic was comprehensively evaluated
and the risk of HCC in patients with CHB with concomitant MAFLD was
assessed. The findings of the present study may potentially reduce
the knowledge gap and pave the way for future focused research,
refined clinical guidelines and targeted public health measures in
this emergent field of hepatology.
Materials and methods
Study guidelines and registration
The methodology for the present systematic review
and meta-analysis was planned according to the Preferred Reporting
Items for Systematic Reviews and Meta-Analyses 2020 guidelines
(13). The meta-analysis was
registered at PROSPERO (registration no. CRD42023453979).
Eligibility criteria
The eligibility criteria for the present study were
as follows: i) Population: Studies involving patients diagnosed
with CHB were included and no restrictions were applied regarding
age, sex, geographic location or ethnicity; ii) exposure and
comparison: Studies on patients with CHB with or without MAFLD were
included; iii) outcomes: The principal outcome of interest was the
incidence of HCC; and iv) study design: All types of study designs
that were published in English from the inception of the databases
until July 2023 were included. To minimize publication bias, both
published literature and grey literature were included in the
literature search.
Information sources
Strategic searches were conducted across electronic
databases including PubMed (https://pubmed.ncbi.nlm.nih.gov), Embase (https://www.embase.com/), Cochrane Central Register of
Controlled Trials (https://www.cochranelibrary.com/central) and
Cumulative Index to Nursing & Allied Health Literature
(https://www.ebsco.com/products/research-databases/cinahl-database).
In addition, manual searches were performed within references of
pinpointed studies and pertinent reviews. To ensure exhaustive and
comprehensive information retrieval, the authors of primary studies
were contacted as needed to gather unpublished data or clarify
study specifics. String searches were formulated using the
following terms: ‘Metabolic-associated fatty liver disease’,
‘MAFLD’, ‘non-alcoholic fatty liver disease’, ‘NAFLD’, ‘hepatic
steatosis’, ‘viral hepatitis’, ‘chronic hepatitis B’,
‘hepatocellular carcinoma’ and ‘HCC’ and both Medical Subject
Headings and associated keywords were used. Appendix S1 delineates the detailed
search algorithm used.
Study records. Data management
EndNote X9 (Clarivate) citation management software
was used to systematically retrieve and manage studies. Duplicate
entries were identified and excluded and the remaining articles
were subjected to eligibility screening.
Selection process. A total of two independent
individuals screened the titles and abstracts of the retrieved
studies and then performed full-text evaluations to ensure
relevance and fit for inclusion into the present study.
Discrepancies between reviewers were reconciled through
dialogue.
Data collection process. Data were extracted
from the selected studies using a standardized extraction template
by two reviewers. The harvested data included study attributes
(authors, year of publication, design and setting), participant
specifics (count, age, sex and MAFLD and CHB status), risk factor
details and outcomes.
Risk of bias in individual
studies
The risk of bias in observational studies was
calculated using Newcastle Ottawa scale (NOS) (14). A score of ≥7 on NOS was classed as
indicative of a high-quality study. A total of two individuals
undertook the evaluations settling any disagreements via
discussions.
Statistical analysis
STATA software (version 17; StataCorp LP) was used
to consolidate the meta-analysis data. A random-effects model with
the inverse variance technique was used to account for potential
study variability. Heterogeneity variance was estimated using the
DerSimonian-Laird method (15).
Effect measures encompassed pooled hazard ratios (HRs; for studies
reporting the estimates as HRs) and odds ratios (ORs; for
dichotomous outcomes) (15).
Forest plots were produced to visualize findings with 95% CIs.
Subgroup analyses were conducted on geographical regions, study
designs and follow-up lengths. Heterogeneity was assessed using the
I2 and τ2 statistics and χ2 tests
(15). Funnel plot and Egger's
regression test were used to detect publication bias. Sensitivity
analysis was performed by excluding the included studies one-by-one
and checking for the single study effects and the consistent nature
of the effect size. The quality of evidence for every outcome was
assessed by applying the Grading of Recommendations Assessment,
Development and Evaluation (GRADE) approach, which considered bias
risk, result consistency, evidence directness, estimate precision
and publication bias susceptibility (16). P<0.05 was considered to indicate
a statistically significant difference.
Results
Search results
Through primary screening, a total of 1,903
citations were identified across the databases. Following the
removal of duplicates, 275 full-text articles were retrieved. After
a secondary screening, 18 studies were included that fully
satisfied the eligibility criteria (Fig. 1) (11,17-33).
Characteristics of the included
studies
For the present meta-analysis, data were obtained
from a diverse range of studies from across the globe (Hong Kong,
Korea, Canada, Taiwan, Singapore, China, Israel and Thailand). Most
studies had retrospective cohort designs, but three studies were
based on prospective cohorts (22,29,31)
and one on a nested case-control approach (25). The sample sizes varied from
270-63,273 participants. The follow-up periods lasted from 3.0-28.1
years. The participants' profiles also were varied, with some
studies focusing on male participants only and others including
participants of both sexes. A mixed risk of bias was found across
studies, with 11 studies were designated as having a ‘high’ risk of
bias (Table I).
 | Table ICharacteristics of the 18 included
studies. |
Table I
Characteristics of the 18 included
studies.
| First author,
year | Geographical
location | Study design | Sample size, n | Follow-up duration,
months | Study participant
description | Mean patient age,
years | Male to female
ratio, % | Risk of bias | (Refs.) |
|---|
| Chan et al,
2017 | Hong Kong | Retrospective
cohort | 270 | 79.9 | Patients with
consecutive HBV infection undergoing liver biopsy between January
2006 and December 2009 | 43.6 | 75.2:24.8 | High | (18) |
| Chang et al,
2021 | Korea | Retrospective
cohort | 720 | 36.0 | Treatment-naïve
patients >18 years old with virologically (HBV DNA <2,000
IU/ml) and biochemically (alanine aminotransferase level <40
IU/l) quiescent chronic hepatitis B who underwent TE | 52 | 58.2:41.8 | High | (26) |
| Cho et al,
2020 | Korea | Retrospective
cohort | 826 | 43.1 | Patients whose
serum HBV DNA levels were continuously suppressed <2,000 IU/ml
by treatment | 53.5 | 61.0:39.0 | High | (30) |
| Choi et al,
2020 | Canada | Retrospective
cohort | 1,089 | 120.0 | Patients with CHB
from electronic medical records who underwent a liver biopsy | 38 | 65.9:34.1 | Low | (27) |
| Hsueh et al,
2022 | Taiwan | Prospective
cohort | 2,385 | 337.0 | Male,
HBsAg-positive civil servants aged ≥30 years at baseline who were
recruited from the Government Employees Central Clinics during
routine free physical examination | 43.2 | 100.0:0.0 | Low | (22) |
| Huang et al,
2023 | Taiwan | Retrospective
cohort | 10,546 | 61.0 | Patients aged ≥20
years with CHB | 52 | 51.0:49.0 | High | (21) |
| Kim et al,
2019 | Korea | Retrospective
cohort | 334 | 60.0 | Patients with
treatment-naïve CHB with available TE results who were initiated on
entecavir or tenofovir | 51 | 62.9:37.1 | High | (23) |
| Kim et al,
2023 | Korea | Retrospective
cohort | 63,273 | 104.0 | Patients with
CHB | NR | NR | High | (20) |
| Lee et al,
2019 | Korea | Retrospective
cohort | 321 | 63.0 | Patients with
consecutive CHB who underwent liver biopsy | 41 | 61.1:38.9 | High | (17) |
| Li et al,
2021 | Taiwan | Retrospective
cohort | 2,158 | 132.0 | Patients aged ≥18
years with CHB verified by individual chart review who were
Asian | 49.6 | 70.9:29.1 | Low | (33) |
| Lim et al,
2020 | Singapore | Retrospective
cohort | 289 | 111.1 | Patients with CHB
who underwent liver biopsy | 46.4 | 72.3:27.7 | High | (28) |
| Mak et al,
2021 | China | Prospective
cohort | 2,403 | 46.4 | Patients with CHB,
defined as persistent seropositivity for HBsAg for ≥6 months, aged
≥18 years, who were treatment-naïve or not currently receiving
treatment and were consecutively recruited for TE assessment | 55.6 | 55.6:44.4 | Low | (31) |
| Oh et al,
2021 | Korea | Retrospective
cohort | 1,823 | 60.0 | Adults aged ≥40
years with CHB who underwent Fibroscan evaluations | 56 | 67.0:33.0 | High | (19) |
| Peleg et al,
2019 | Israel | Retrospective
cohort | 524 | 72.0 | Patients with
treatment-naïve CHB | 50.5 | 60.1:38.9 | High | (24) |
| Rugivarodom et
al, 2023 | Thailand | Prospective
cohort | 408 | 164.0 | Patients with
consecutive chronic HBV infection who underwent a liver biopsy to
determine the need for antiviral treatment | 44 | 65.4:34.6 | Low | (29) |
| van Kleef et
al, 2021 | Canada | Retrospective
cohort | ,076 | 116.0 | Patients with CHB
who underwent 1liver biopsy | 38.6 | 65.7:34.3 | High | (11) |
| Wang et al,
2023 | China | Retrospective
cohort | 332 | 47.0 | Patients with
hepatitis B-related cirrhosis | 50.4 | 74.9:25.1 | Low | (32) |
| Yu et al,
2022 | Taiwan | Nested case control
study | 1,453 | 231.0 | Male,
HBsAg-positive civil servants aged >30 years | 49.2 | 100.0:0.0 | Low | (25) |
Association between MAFLD and HCC in
patients with CHB
Data from 18 studies comprising 23,927 participants
were included for the analysis of the number of events and
participants. The pooled OR for the association between the
presence of MAFLD and an increased risk of HCC in patients with CHB
was 1.053 (95% CI, 0.704-1.576), with no statistical significance
obtained from the test of overall effects (z=0.252; P=0.801;
Fig. 2). A high degree of
heterogeneity was found among the included studies, with a
Cochran's Q value of 71.78 [degrees of freedom (df)=12;
P<0.001]. The I² test result was 83.3% (95% CI, 37.5-92.4),
which indicated that a substantial proportion of the total
variation in effect estimates was due to between-study
heterogeneity. The estimated heterogeneity variance using the
DerSimonian-Laird method was 0.4076.
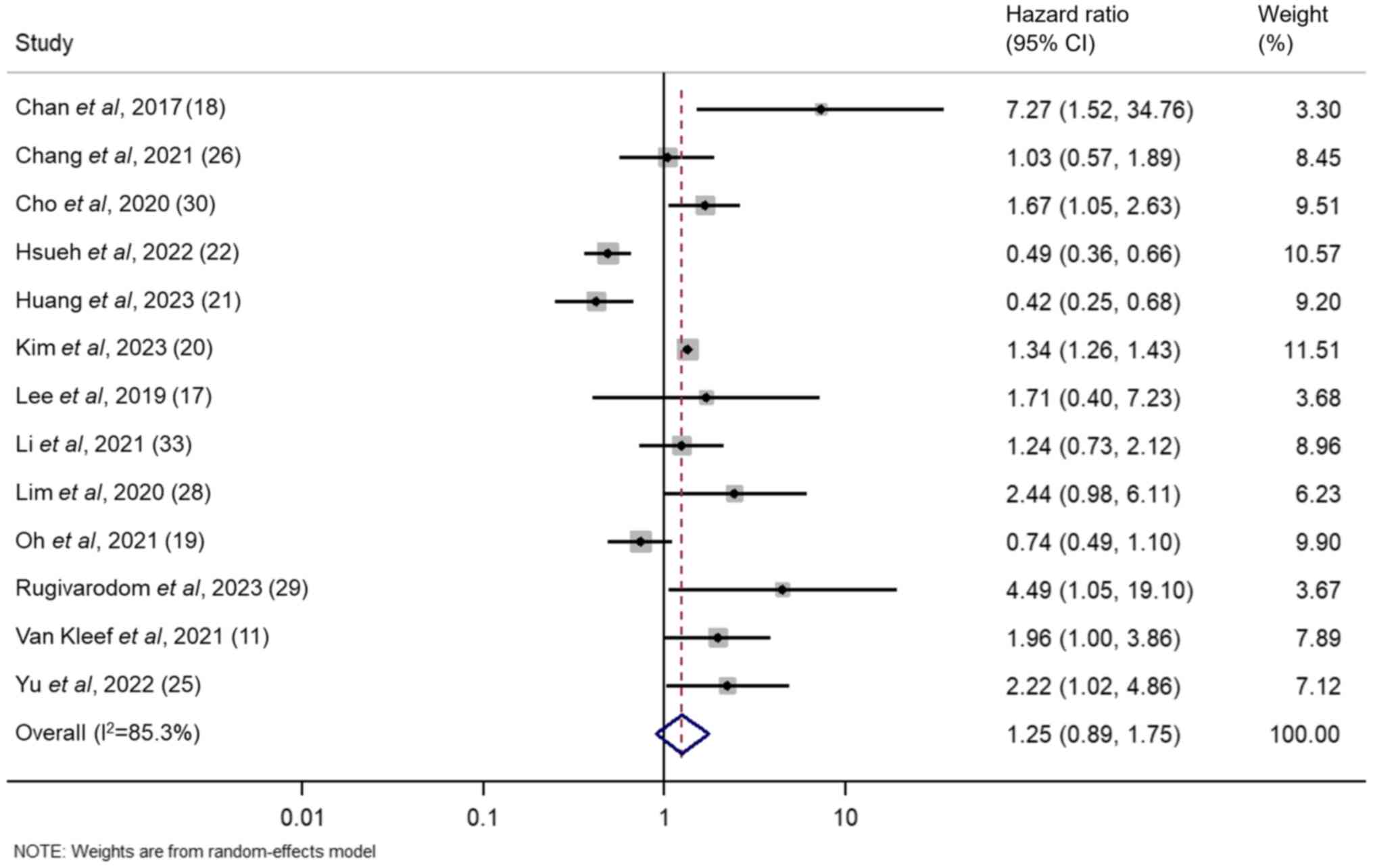
A random-effects inverse-variance model with the
DerSimonian-Laird estimate of τ2 was used to pool the
HRs from individual studies. The HRs for the effect of MAFLD on the
risk of HCC in patients with CHB from the included studies ranged
from 0.420-7.270. The summary HR derived from the overall pooled
data suggested that MAFLD was associated with a 1.253-fold
increased risk of HCC in patients with CHB. However, this
association was not statistically significant (95% CI, 0.895-1.754;
z=1.313; P=0.189). In addition, significant heterogeneity was
demonstrated among the included studies, as evidenced by a
Cochran's Q value of 81.52 (P<0.001) and an I² statistic of
85.3%, which indicated substantial variations in study outcomes.
The modified H² value was 5.793 and τ2 was 0.2550
(Fig. 3).
Subgroup analyses
The association between MAFLD and the risk of HCC in
patients with CHB was assessed based on the geographical location
of the study (Fig. S1). Data from
11 of the studies included were from Asian countries (17-19,21-23,25,28,31-33).
The pooled OR for the aforementioned studies was 0.783 (95% CI,
0.568-1.080), which accounted for 86.29% of the overall weight. The
Cochran's Q value was 34.27 (df=10; P<0.001) with an I² of
70.8%, which indicated moderate heterogeneity. Data were also
analysed from two studies from other geographical regions (Canada
and Israel) (24,27). The pooled OR of the aforementioned
studies was 4.380 (95% CI, 2.440-7.864), which represented 13.71%
of the overall weight. No evidence of heterogeneity was found with
a Cochran's Q value of 0.00 (df=1; P=0.971) and an I² of 0.0%. The
test for the subgroup effect size demonstrated no significant
difference for Asian countries (z=-1.490; P=0.136), while the
subgroup effect of the other geographical location analysis was
statistically significant (z=4.948; P<0.001). The heterogeneity
between the subgroups was also statistically significant with a Q
value of 25.54 (df=1; P<0.001).
Based on the study design, two prospective cohort
studies were identified (Fig. S2)
(22,31). The pooled OR of the aforementioned
analysis was 0.479 (95% CI, 0.365-0.629), which contributed to
17.93% of the total weight. Heterogeneity measurement demonstrated
no evidence of variation, with a Cochran's Q value of 1.00 (df=1;
P=0.318) and an I² of 0.0%. A total of 11 retrospective cohort
studies were identified (17-19,21,23-25,27,28,32,33).
The combined OR of the aforementioned studies was 1.294 (95% CI,
0.813-2.059), which accounted for 82.07% of the total weight.
Significant heterogeneity between these studies was demonstrated,
as indicated by a Cochran's Q value of 55.95 (df=10, P<0.001)
and an I² of 82.1%. The tests for subgroup effect sizes
demonstrated a significant difference for the prospective cohort
studies (z=-5.303; P<0.001), but not for the retrospective
studies subgroup (z=1.086; P=0.277). Significant heterogeneity
between the subgroups was also observed, with a Q value of 13.09
(df=1; P<0.001).
Data from nine studies were used for follow-up
length (<10 years) subgroup analyses (Fig. S3) (17-19,21,23,24,28,31,32).
The combined OR was 1.147 (95% CI, 0.670-1.965), which contributed
to 63.94% of the total weight. Significant heterogeneity among
these studies was found with a Cochran's Q value of 38.58 (df=8;
P<0.001) and an I² of 79.3%. A total of four studies had a
follow-up period of ≥10 years (22,25,27,33).
The pooled OR of the aforementioned studies was 0.944 (95% CI,
0.459-1.939), which accounted for 36.06% of the overall weight.
These studies demonstrated substantial heterogeneity with a
Cochran's Q value of 32.19 (df=3; P<0.001) and an I² of 90.7%.
The tests for subgroup effect sizes did not reveal a statistically
significant result for either follow-up group (<10 years,
z=0.500; P=0.617; ≥10 years, z=-0.158; P=0.874). The heterogeneity
between the two subgroups was not significant (Q=0.18; df=1;
P=0.670).
Publication bias assessment
Egger's regression asymmetry test demonstrated
evidence of publication bias. The slope coefficient was 0.2585 (95%
CI, 0.0396-0.4774; t=2.60; P=0.025). However, the intercept (bias)
term was-0.3096 and was not statistically significant (95% CI,
-2.3297-1.7105; t=-0.34; P=0.742), which suggested that the funnel
plot asymmetry may be due to factors other than publication bias
(Fig. 4).
Meta-regression analysis results
To identify potential sources of heterogeneity and
evaluate the potential influence of study-level characteristics on
the reported effect sizes, meta-regression analyses were conducted.
The following covariates were considered: Mean age, follow-up
duration, study design and geographical location of the study.
Mean age
The regression coefficient suggested that for every
1-year increase in the mean age, the effect size decreased by
0.0514; however, this association was not statistically significant
(coefficient=-0.0514; P=0.253). A between-study variance
(τ2) of 0.4398 was demonstrated, which suggested that
~83.58% of the total variation in effect sizes was due to
heterogeneity.
Follow-up duration. Similar effect sizes were
found in the follow-up groups [coefficient for follow-up group 2
(≥10 years)=-0.0303; P=0.949]. The τ2 value was 0.4117
with 82.20% of the total variation in effect sizes attributed to
heterogeneity.
Study design. Similar effect sizes were
demonstrated in the two study design groups [coefficient for design
group 2 (≥10 years)=0.3617; P=0.557]. The between-study variance
(τ2) was 0.3627 and ~77.05% of the total variation in
effect sizes resulted from heterogeneity.
Geographical location of study. The effect
sizes were similar among the country groups [coefficient for
country group 2 (countries outside Asia)=0.4470; P=0.574]. The
τ2 value was 0.3816 with 86.24% of the total variation
in effect sizes attributed to heterogeneity.
Sensitivity analysis
Sensitivity analysis was performed to check the
robustness of the estimates (Fig.
S4). The findings of the present study were not unduly
influenced by any single study and the results remained consistent
across the analysis, as there was no change in direction or
magnitude of the overall pooled estimate after removal of any
single study, which affirmed the reliability of the overall
conclusions of the present study.
GRADE evidence
The quality of evidence was initially graded as low,
because the review included observational studies. However, the
presence of studies with high risk of bias, imprecision and
non-significant associations between MAFLD and HCC risk caused a
further downgrading in the quality of evidence rating to very
low-quality.
Discussion
The concomitant presence of MAFLD and HCC in
patients with CHB has emerged as an area of notable clinical
interest. The present comprehensive meta-analysis, which included
data from 18 studies and 23,927 participants, aimed to explore a
possible association between MAFLD and HCC with depth and rigor.
The findings of the present study generated further questions and
underscored the complexity of the topic.
The principal finding of the present meta-analysis
was the non-significant association between MAFLD and the HCC risk
in patients with CHB, with a pooled OR of 1.053. This finding
diverges from several previous primary investigations which have
proposed MAFLD as a significant risk factor for HCC (17,18,24,27).
The wide CI value suggested that MAFLD may confer a modest risk,
but it could also be protective against HCC. Thus, clinicians and
researchers need to be cautious in their interpretation of the
findings of the present study, considering the study's design and
the populations analysed.
Further analysis of the potential mechanisms linking
MAFLD to HCC in patients with CHB should clarify this issue and
potentially reveal the processes involved in this association.
Chronic inflammation is central to the progression of MAFLD
(34). Hepatic steatosis, a
hallmark of MAFLD, can activate Kupffer cells, the resident
macrophages of the liver, which leads to the secretion of
pro-inflammatory cytokines, such as TNF-α and IL-6(35). Such inflammatory markers can
promote hepatocarcinogenesis, especially if the liver is already
compromised by CHB infection (36). Insulin resistance is a key feature
of metabolic syndrome and MAFLD. Elevated insulin levels and a
consequentially increased insulin-like growth factor can activate
cellular pathways that stimulate hepatocyte proliferation and
inhibit apoptosis, which fosters an environment conducive to
neoplastic transformation (36).
Fatty acid accumulation in hepatocytes can cause
mitochondrial dysfunction, which leads to elevated reactive oxygen
species levels. Oxidative stress damages DNA and can initiate and
promote carcinogenesis. In patients with CHB, the added
viral-induced cellular stress may synergize with the oxidative
stress from MAFLD and amplify the risk for malignant transformation
(37). Adipose tissues, especially
in the context of obesity and MAFLD, actively secrete adipokines,
such as leptin and adiponectin. Leptin, which is increased in
individuals with obesity, promotes cell proliferation and reduces
apoptosis, whilst adiponectin serves an anti-inflammatory role. An
imbalance in these adipokine contents, as observed in MAFLD, can
alter hepatic homeostasis and promote oncogenesis (38). The role of the gut microbiota in
liver diseases is being investigated, as dysbiosis, a disruption in
the gut microbial equilibrium observed in MAFLD, can lead to
increased gut permeability, which allows bacterial endotoxins to
enter the liver via the portal circulation. These endotoxins can
activate hepatic stellate and Kupffer cells, stimulating
inflammation and fibrosis, which are both precursors for HCC,
especially in the vulnerable milieu of a CHB-affected liver
(39).
The geographical subgroup analysis performed in the
present study provided some noteworthy observations. The studies
from Asian countries, which represented a considerable proportion
of the studies in the present meta-analysis, demonstrated a
non-significant decreased risk of HCC in patients with MAFLD and
CHB. By contrast, the pooled OR from studies from other
geographical regions indicated a significantly higher risk of HCC
in the patients with MAFLD and CHB. For hepatologists practicing in
Asia, this information could be important for risk stratification
and patient counselling.
Differing study designs also yielded varying
results. Notably, prospective cohort studies demonstrated a
significant protective effect of MAFLD on the HCC risk in patients
with CHB, while retrospective cohort studies did not. This
highlighted the inherent challenges of observational studies, in
which confounding factors and biases can significantly impact study
outcomes.
The apparent protective association between MAFLD
and HCC in patients with CHB infection observed in certain studies,
although seemingly counterintuitive, may occur due to a number of
factors. The presence of MAFLD may modulate the immune response in
a manner that could be protective against HCC. For example, certain
immune cells that are prevalent in MAFLD, such as regulatory T
cells, have previously been reported to suppress liver
inflammation. This could potentially mitigate the inflammatory
cascades that drive carcinogenesis in patients with CHB infection
(40). A liver with MAFLD
undergoes a high rate of hepatocyte turnover due to recurrent minor
injury and repair. This constant cell renewal could prevent the
long-term survival and accumulation of cells with oncogenic
mutations induced by CHB infection (41). It has been suggested that lipid
accumulation in hepatocytes, known as steatosis, may represent a
cellular defense mechanism. Lipids could sequester harmful agents,
such as viral proteins or other potential carcinogens, reducing
their bioavailability and the harm they would otherwise cause to
DNA and the cellular machinery (42). Genetic factors serve a significant
role in the susceptibility to, and progression of, liver diseases.
Certain genetic polymorphisms [GCLC promoter region
polymorphism (c. c-129t, rs17883901, single nucleotide polymorphism
rs4880)] associated with a higher risk of MAFLD may paradoxically
confer a protective effect against HCC development in patients with
CHB (43).
One of the major features of the present
meta-analysis was the high degree of heterogeneity among the
included studies. Several factors may be responsible for this
heterogeneity. First, the definition and diagnostic criteria for
MAFLD varied across studies, which led to potential
misclassifications and introduced variability. Second, there are
inherent challenges in collating data from studies spanning diverse
populations conducted on the basis of diverse methodologies and
time frames. Meta-regression was used to attempt to identify the
sources of the heterogeneity and the influence of certain factors,
such as the mean age, follow-up length, study design and
geographical location of the study, but none of these factors
provided a satisfactory explanation for the observed
heterogeneity.
The presence of publication bias, as suggested by
Egger's regression asymmetry test results, was observed in the
present study. This bias could imply a tendency towards publishing
studies with significant findings, thereby possibly artificially
enhancing the observed association. However, the non-significant
intercept from Egger's test suggested that there may be other
contributing factors to the funnel plot asymmetry such as
methodological quality variations, artefacts or by chance.
Moreover, the quality of evidence was downgraded to very
low-quality according to the GRADE criteria, indicating that the
certainty in the findings of the present study is limited.
The strengths of the present study lie in its
comprehensive approach, rigorous statistical methodologies and
subgroup analyses, which add depth to the findings. The inclusion
of a diverse set of studies also adds to the generalizability of
the present results. However, there were a number of limitations.
First, the retrospective nature of most studies posed an inherent
challenge with potential confounders. Second, individual
patient-level data were unavailable, which restricted the ability
to control for other potential confounders such as sociodemographic
profile, behavioural risk factors and comorbidities. Moreover, the
diagnosis of MAFLD and HCC was not uniform across studies and
probably introduced a certain degree of bias. It is important to
have uniformity in diagnostic criteria for producing consistent
results. However, the criteria for MAFLD diagnosis have evolved
over time and a number of the older studies included in the present
meta-analysis used previous definitions (17,19),
while newer studies adopted more recent criteria (20-22).
Establishing a single standardized criterion would exclude a
significant portion of available literature, potentially leading to
loss of valuable insights. In addition, some studies directly
reported the presence of MAFLD without explicitly detailing the
diagnostic criteria used (19,30).
Excluding these studies based on the absence of a specified
criterion would further reduce the number of studies included,
potentially compromising the comprehensiveness and depth of the
present analysis. However, this heterogeneity was addressed by
utilizing the random-effects model in the present meta-analysis,
which takes into account the variability among studies. This
provided a more conservative estimate of the association and
reflected the diversity of included studies.
Potential variability introduced by different
follow-up times was also demonstrated across the included articles.
The duration of follow-up and interventional treatments received
during this period may significantly influence outcomes and
introduce heterogeneity among studies. To account for this, a
subgroup analysis was conducted based on follow-up duration,
segregating studies into categories, such as short-term,
medium-term and long-term follow-up. This allowed the assessment to
determine if the association between MAFLD and HCC risk in CHB
patients was consistent across these subgroups or if
duration-specific patterns emerged. Additionally, follow-up
duration was included as a covariate in the present meta-regression
analysis. This aided in quantifying the potential impact of varying
follow-up durations on the observed effect sizes and ensured that
the results of the present study considered this important aspect
of study design.
There are several avenues for potential future
research. Prospective cohort studies, with a standardized
diagnostic criterion for MAFLD and adjusted for potential
confounders, should provide a more definitive understanding of this
possible association between MAFLD and HCC in patients with CHB.
Moreover, molecular and genetic studies should elucidate any
pathophysiological mechanisms linking MAFLD and HCC in patients
with CHB to potentially reveal future therapeutic targets.
The present meta-analysis results were inconclusive
for an association between MAFLD and the HCC risk in patients with
CHB. These results highlighted the need for more rigorous studies
on this complex topic. Currently, clinicians should keep in mind
the nuanced nature of risk and the importance of individualized
patient care. Continued research in required in this domain, given
its profound clinical implications for a vast population of
patients with CHB worldwide.
Supplementary Material
Search strategy used in the present
study.
Forest plot showing the geographical
regions subgroup analysis results for the association between
metabolic dysfunction-associated fatty liver disease and
hepatocellular carcinoma. DL, DerSimonian and Laird approach.
Forest plot showing the study design
subgroup analysis results for the association between metabolic
dysfunctionassociated fatty liver disease and hepatocellular
carcinoma. DL, DerSimonian and Laird approach.
Forest plot showing the follow-up
length subgroup analysis results for the association between
metabolic dysfunction-associated fatty liver disease and
hepatocellular carcinoma. DL, DerSimonian and Laird approach.
Sensitivity analysis.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SS conceived and designed the study. SS and LP
collected the data and performed the literature search. SS wrote
the manuscript. Both authors have read and approved the final
manuscript. SS and LP confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chidambaranathan-Reghupaty S, Fisher PB
and Sarkar D: Hepatocellular carcinoma (HCC): Epidemiology,
etiology and molecular classification. Adv Cancer Res. 149:1–61.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kulik L and El-Serag HB: Epidemiology and
management of hepatocellular carcinoma. Gastroenterology.
156:477–491.e1. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rapti I and Hadziyannis S: Risk for
hepatocellular carcinoma in the course of chronic hepatitis B virus
infection and the protective effect of therapy with nucleos(t)ide
analogu. World J Hepatol. 7:1064–1073. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Brody H: Hepatitis B. Nature.
603(S45)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
El-Serag HB: Epidemiology of viral
hepatitis and hepatocellular carcinoma. Gastroenterology.
142:1264–1273.e1. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Krump NA and You J: Molecular mechanisms
of viral oncogenesis in humans. Nat Rev Microbiol. 16:684–698.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Mui UN, Haley CT and Tyring SK: Viral
oncology: Molecular biology and pathogenesis. J Clin Med.
6(111)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pouwels S, Sakran N, Graham Y, Leal A,
Pintar T, Yang W, Kassir R, Singhal R, Mahawar K and Ramnarain D:
Non-alcoholic fatty liver disease (NAFLD): A review of
pathophysiology, clinical management and effects of weight loss.
BMC Endocr Disord. 22(63)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pipitone RM, Ciccioli C, Infantino G, La
Mantia C, Parisi S, Tulone A, Pennisi G, Grimaudo S and Petta S:
MAFLD: A multisystem disease. Ther Adv Endocrinol Metab.
14(20420188221145549)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang X and Xie Q: Metabolic
dysfunction-associated fatty liver disease (MAFLD) and viral
hepatitis. J Clin Transl Hepatol. 10:128–133. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
van Kleef LA, Choi HSJ, Brouwer WP, Hansen
BE, Patel K, de Man RA, Janssen HLA, de Knegt RJ and Sonneveld MJ:
Metabolic dysfunction-associated fatty liver disease increases risk
of adverse outcomes in patients with chronic hepatitis B. JHEP Rep.
3(100350)2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chen X, Zhou J, Wu L, Zhu X and Deng H:
MAFLD is associated with the risk of liver fibrosis and
inflammatory activity in HBeAg-negative CHB patients. Diabetes
Metab Syndr Obes. 15:673–683. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Lo CKL, Mertz D and Loeb M:
Newcastle-Ottawa Scale: Comparing reviewers' to authors'
assessments. BMC Med Res Methodol. 14(45)2014.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Cumpston M, Li T, Page MJ, Chandler J,
Welch VA, Higgins JP and Thomas J: Updated guidance for trusted
systematic reviews: A new edition of the cochrane handbook for
systematic reviews of interventions. Cochrane Database Syst Rev.
10(ED000142)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kirmayr M, Quilodrán C, Valente B, Loezar
C, Garegnani L and Franco JVA: The GRADE approach, Part 1: How to
assess the certainty of the evidence. Medwave.
21(e8109)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Lee YB, Ha Y, Chon YE, Kim MN, Lee JH,
Park H, Kim KI, Kim SH, Rim KS and Hwang SG: Association between
hepatic steatosis and the development of hepatocellular carcinoma
in patients with chronic hepatitis B. Clin Mol Hepatol. 25:52–64.
2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Chan AWH, Wong GLH, Chan HY, Tong JHM, Yu
YH, Choi PCL, Chan HLY, To KF and Wong VWS: Concurrent fatty liver
increases risk of hepatocellular carcinoma among patients with
chronic hepatitis B. J Gastroenterol Hepatol. 32:667–676.
2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Oh JH, Lee HW, Sinn DH, Park JY, Kim BK,
Kim SU, Kim DY, Ahn SH, Kang W, Gwak GY, et al: Controlled
attenuation parameter value and the risk of hepatocellular
carcinoma in chronic hepatitis B patients under antiviral therapy.
Hepatol Int. 15:892–900. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Kim MN, Han K, Yoo J, Hwang SG, Zhang X
and Ahn SH: Diabetic MAFLD is associated with increased risk of
hepatocellular carcinoma and mortality in chronic viral hepatitis
patients. Int J Cancer. 153:1448–1458. 2023.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Huang SC, Su TH, Tseng TC, Chen CL, Hsu
SJ, Liao SH, Hong CM, Liu CH, Lan TY, Yang HC, et al: Distinct
effects of hepatic steatosis and metabolic dysfunction on the risk
of hepatocellular carcinoma in chronic hepatitis B. Hepatol Int.
17:1139–1149. 2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hsueh RC, Wu WJ, Lin CL, Liu CJ, Huang YW,
Hu JT, Wu CF, Sung FY, Liu WJ and Yu MW: Impact of PNPLA3 p. I148M
and hepatic steatosis on long-term outcomes for hepatocellular
carcinoma and HBsAg seroclearance in chronic hepatitis B. J
Hepatocell Carcinoma. 9:301–313. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kim DS, Jeon MY, Lee HW, Kim BK, Park JY,
Kim DY, Ahn SH, Han KH and Kim SU: Influence of hepatic steatosis
on the outcomes of patients with chronic hepatitis B treated with
entecavir and tenofovir. Clin Mol Hepatol. 25:283–293.
2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Peleg N, Issachar A, Sneh Arbib O,
Cohen-Naftaly M, Braun M, Leshno M, Barsheshet A and Shlomai A:
Liver steatosis is a strong predictor of mortality and cancer in
chronic hepatitis B regardless of viral load. JHEP Rep. 1:9–16.
2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yu MW, Lin CL, Liu CJ, Wu WJ, Hu JT and
Huang YW: Metabolic-associated fatty liver disease, hepatitis B
surface antigen seroclearance, and long-term risk of hepatocellular
carcinoma in chronic hepatitis B. Cancers (Basel).
14(6012)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chang JW, Lee JS, Lee HW, Kim BK, Park JY,
Kim DY, Ahn SH and Kim SU: No influence of hepatic steatosis on the
3-year outcomes of patients with quiescent chronic hepatitis B. J
Viral Hepat. 28:1545–1553. 2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Choi HSJ, Brouwer WP, Zanjir WMR, de Man
RA, Feld JJ, Hansen BE, Janssen HLA and Patel K: Nonalcoholic
steatohepatitis is associated with liver-related outcomes and
all-cause mortality in chronic hepatitis B. Hepatology. 71:539–548.
2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lim CT, Goh GBB, Li H, Lim TK, Leow WQ,
Wan WK, Azhar R, Chow WC and Kumar R: Presence of hepatic steatosis
does not increase the risk of hepatocellular carcinoma in patients
with chronic hepatitis b over long follow-Up. Microbiol Insights.
13(1178636120918878)2020.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Rugivarodom M, Pongpaibul A, Chainuvati S,
Nimanong S, Chotiyaputta W, Tanwandee T and Charatcharoenwitthaya
P: Prognostic relevance of metabolic dysfunction-associated
steatohepatitis for patients with chronic hepatitis B. J Clin
Transl Hepatol. 11:76–87. 2023.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Cho H, Chang Y, Lee JH, Cho YY, Nam JY,
Lee YB, Lee DH, Cho EJ, Yu SJ, Kim YJ, et al: Radiologic
nonalcoholic fatty liver disease increases the risk of
hepatocellular carcinoma in patients with suppressed chronic
hepatitis B. J Clin Gastroenterol. 54:633–641. 2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mak LY, Hui RWH, Fung J, Liu F, Wong DK,
Li B, Cheung KS, Yuen MF and Seto WK: Reduced hepatic steatosis is
associated with higher risk of hepatocellular carcinoma in chronic
hepatitis B infection. Hepatol Int. 15:901–911. 2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang X, Wei S, Wei Y, Wang X, Xiao F, Feng
Y and Zhu Q: The impact of concomitant metabolic
dysfunction-associated fatty liver disease on adverse outcomes in
patients with hepatitis B cirrhosis: A propensity score matching
study. Eur J Gastroenterol Hepatol. 35:889–898. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Li J, Yang HI, Yeh ML, Le MH, Le AK, Yeo
YH, Dai CY, Barnett S, Zhang JQ, Huang JF, et al: Association
between fatty liver and cirrhosis, hepatocellular carcinoma, and
hepatitis b surface antigen seroclearance in chronic hepatitis B. J
Infect Dis. 224:294–302. 2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Petrescu M, Vlaicu SI, Ciumărnean L,
Milaciu MV, Mărginean C, Florea M, Vesa ȘC and Popa M: Chronic
inflammation-A link between nonalcoholic fatty liver disease
(NAFLD) and dysfunctional adipose tissue. Medicina (Kaunas).
58(641)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Chen J, Deng X, Liu Y, Tan Q, Huang G, Che
Q, Guo J and Su Z: Kupffer cells in non-alcoholic fatty liver
disease: Friend or foe? Int J Biol Sci. 16:2367–2378.
2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sakurai Y, Kubota N, Yamauchi T and
Kadowaki T: Role of insulin resistance in MAFLD. Int J Mol Sci.
22(4156)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Ma Y, Lee G, Heo SY and Roh YS: Oxidative
stress is a key modulator in the development of nonalcoholic fatty
liver disease. Antioxidants (Basel). 11(91)2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Zorena K, Jachimowicz-Duda O, Ślęzak D,
Robakowska M and Mrugacz M: Adipokines and obesity. Potential link
to metabolic disorders and chronic complications. Int J Mol Sci.
21(3570)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Brenner DA, Paik YH and Schnabl B: Role of
gut microbiota in liver disease. J Clin Gastroenterol. 49 (Suppl
1):S25–S27. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Kountouras J, Kazakos E, Kyrailidi F,
Polyzos SA, Zavos C, Arapoglou S, Boziki M, Mouratidou MC,
Tzitiridou-Chatzopoulou M, Chatzopoulos D, et al: Innate immunity
and nonalcoholic fatty liver disease. Ann Gastroenterol.
36:244–256. 2023.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Duncan AW, Dorrell C and Grompe M: Stem
cells and liver regeneration. Gastroenterology. 137:466–481.
2009.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Ipsen DH, Lykkesfeldt J and Tveden-Nyborg
P: Molecular mechanisms of hepatic lipid accumulation in
non-alcoholic fatty liver disease. Cell Mol Life Sci. 75:3313–3327.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Severson TJ, Besur S and Bonkovsky HL:
Genetic factors that affect nonalcoholic fatty liver disease: A
systematic clinical review. World J Gastroenterol. 22:6742–6756.
2016.PubMed/NCBI View Article : Google Scholar
|