Introduction
Ovarian cancer is one of the most common
gynecological malignancies and exhibits the highest mortality rate
worldwide among all malignant tumors affecting the reproductive
organs (1). Epithelial ovarian
cancer (EOC) constitutes ~95% of ovarian malignancies worldwide
(2) and typically lacks specific
clinical symptoms during its early stages, leading to the majority
of patients being diagnosed at an advanced disease stage.
Currently, the first-line treatment for ovarian cancer involves
surgical intervention followed by platinum-based chemotherapy
(3). However, ~70% of patients
with advanced stages of EOC worldwide will experience relapse
within 2 years (4,5). Furthermore, the majority of patients
with recurrent cancer exhibit resistance to platinum-based
chemotherapy, resulting in a low 5-year survival rate (6). Therefore, comprehending the
resistance mechanisms of platinum-based chemotherapy is
advantageous in enhancing the prognosis of patients with EOC.
Cisplatin, the first platinum chemotherapy analogue
approved for use in the treatment of ovarian cancer, induces DNA
damage and subsequent cell death by crosslinking with DNA chains to
inhibit the DNA replication process (7). Cisplatin and paclitaxel combination
chemotherapy significantly enhances the outcome of patients with
EOC (8,9). However, the majority of patients
experience relapse due to the aforementioned development of
platinum resistance (10). Growing
evidence has suggested that the process of epithelial to
mesenchymal transition (EMT) can contribute to the development of
chemotherapy resistance (11,12).
For instance, the presence of EMT has been associated with
cisplatin resistance in EOC (13,14).
Additionally, evidence suggests that the interplay between STAT3
and p53/RAS signaling pathways regulates both metastasis and
cisplatin resistance in ovarian cancer cells through EMT (15).
High mobility group box 1 (HMGB1) is a highly
conserved nuclear protein that binds to DNA and influences
chromatin regulation and transcription (16), and is upregulated in various types
of solid tumors, including breast cancer, sarcomas, pancreatic
cancer, head and neck/oral squamous cell carcinomas, melanoma,
hepatoblastoma and ovarian and non-small cell lung cancer, in
humans (17). HMGB1 plays a
crucial role in the chemoresistance of tumor cells, including those
from osteosarcoma and lung cancer (18,19).
A previous study has shown that expression of HMGB1 is associated
with chemotherapy sensitivity in patients with ovarian cancer
(20). The upregulation of HMGB1
in carboplatin-resistant SKOV3 cells suggests its potential
involvement in the development of resistance to carboplatin in this
cell line (21). Furthermore,
tumor cells can secrete HMGB1, which plays a crucial role in tumor
growth, migration and invasion (22-24).
As previously reported, elevated expression of HMGB1 promotes EMT
in liver cancer and glioblastoma cells through the GSK-3β/Snail and
STAT3/microRNA-34a/NF-κB signaling pathways (25,26).
Furthermore, HMGB1 has been found to be upregulated in ovarian
cancer cells and is associated with the growth and metastasis of
ovarian cancer (27). However, the
precise mechanism by which HMGB1 induces metastasis in ovarian
cancer cells remains unclear.
In the present study, the expression and function of
HMGB1 in cisplatin-resistant A2780/DDP cells, its regulatory role
in the migratory and invasive abilities of these cells and its
associated mechanisms were investigated.
Materials and methods
Cell culture
The human ovarian cancer cell line, A2780, and the
cisplatin-resistant cell line, A2780/DDP, were purchased from The
Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. A2780 cells were cultured in RIPA 1640 medium containing
10% fetal bovine serum, both purchased from Gibco (Thermo Fisher
Scientific, Inc.), and 1% penicillin and streptomycin. A2780/DDP
cells were cultured in RIPA 1640 medium containing 1 µg/ml
cisplatin, 10% fetal bovine serum and 1% penicillin and
streptomycin. Cells were incubated at 37˚C in an atmosphere of 5%
CO2 and 95% air.
Cell Counting Kit-8 (CCK-8) assay
Cell viability and the half maximal inhibitory
concentration (IC50) of cisplatin were assessed using
the CCK-8 assay. Briefly, A2780/DDP and A2780 cells
(2x104 cells/well) were seeded into 96-well plates and
incubated overnight. Then, concentration gradients of
cisplatin-containing medium (0, 5, 10, 20, 40 and 80 µg/ml) were
added to the plates with eight replicate wells/concentration. After
48 h, 10 µl CCK-8 solution (MedChemExpress), was added to each well
and incubated for 1 h. Then, the absorbance was measured at 450 nm
and the cell viability was calculated as follows: Cell viability
(%)=(drug group A-blank group A)/(no cisplatin group A-blank group
A) x100. The resistance index was calculated as follows: Resistance
index=IC50 of A2780/DDP cells/IC50 of A2780
cells.
Western blotting analysis
Cells were lysed to extract total protein for
western blotting as previously described (28). The RIPA Lysis buffer
(MedChemExpress) was utilized for the extraction of total protein.
The protein concentration was determined using Bicinchoninic acid.
The protein samples (30 mg/lane) were evenly loaded onto 8-12%
sodium dodecyl sulfate polyacrylamide gels for electrophoresis.
Then, proteins were subsequently transferred onto a polyvinylidene
difluoride membrane. After blocking with 5% skimmed milk powder
(Wuhan Servicebio Technology Co., Ltd.) at room temperature for 1
h. The membranes were incubated with primary antibodies overnight
at 4˚C and with secondary antibodies for 1 h at room temperature.
The primary and secondary antibodies were diluted in a universally
purchased antibody dilution buffer from Beyotime Institute of
Biotechnology. The concentration of the primary antibody was
1:1,000, while that of the secondary antibody was 1:5,000. Primary
antibodies used for immunodetection were anti-HMGB1,
anti-phosphorylated (p)-GSK-3β, anti-GSK-3β, anti-E-cadherin,
anti-vimentin and anti-glyceraldehyde-3-phosphate dehydrogenase
(GAPDH), and all primary antibodies were acquired from Cell
Signaling Technology, Inc. Secondary antibodies were anti-rabbit
and anti-mouse IgG peroxidase conjugate (OriGene Technologies,
Inc.). The antigen-antibody immunocomplexes were detected using
enhanced chemiluminescent substrate purchased from Thermo Fisher
Scientific, Inc. The results were semi-quantified by densitometry,
using Image J software (V1.8, NIH). Results were expressed as a
ratio of the protein of interest and GAPDH. GAPDH was the loading
control for each sample. The relative expression of p-GSK-3β was
normalized to the level of (unphosphorylated) GSK-3β.
Transwell migration and invasion
assays
Upon reaching 80-90% confluency, cells were digested
and Transwell migration and invasion assays were performed as
described in a previous study (28). Images (magnification, x200) of
stained cells were captured by the Olympus IX51 inverted microscope
(Olympus Corporation). Cells were counted manually in four random
fields of each chamber.
Knockdown of the HMGB1 gene in
A2780/DDP cells
The small interfering RNA sequences targeting human
HMGB1 (siRNA-HMGB1; Table I) were
designed by Shanghai GenePharma Co., Ltd. A2780/DDP cells were
seeded in six-well plates without antibiotics for 12 h. When a
50-60% confluency was reached, cells were transfected with nothing
(Con group), 20 nM of negative control (N.C.; scrambled sequence)
or 20 nM of siRNA-HMGB1 (50 nmol/l) using Lipofectamine®
2000 (Invitrogen; Thermo Fisher Scientific, Inc.) at 37˚C for 6 h.
After 48 h of transfection, cells were digested for subsequent
experiments.
 | Table IsiRNA-HMGB1 sequences. |
Table I
siRNA-HMGB1 sequences.
| Name | Sense (5'-3') | Antisense
(5'-3') |
|---|
|
HMGB1-siRNA-197 |
CCUAAGAAGCCGAGAGGCATT |
UGCCUCUCGGCUUCUUAGGTT |
|
HMGB1-siRNA-252 |
GGGAGGAGCAUAAGAAGAATT |
UUCUUCUUAUGCUCCUCCCTT |
|
HMGB1-siRNA-624 |
CUGCGAAGCUGAAGGAAAATT |
UUUUCCUUCAGCUUCGCAGTT |
| N.C. |
UUCUCCGAACGUGUCACGUTT |
ACGUGACACGUUCGGAGAATT |
Statistical analysis
GraphPad Prism version 5.01 (Dotmatics) was used for
statistical analysis. All experiments were repeated three times and
data are presented as the mean ± SEM. Paired Student's t-test was
used to analyze the difference between two groups. One-way analysis
of variance (ANOVA) followed by Dunnett's multiple comparisons test
was conducted to analyze the difference among groups. Two-way ANOVA
followed by Tukey's multiple comparisons test was used to analyze
the difference in cell viability between the A2780 and A2780/DDP
cell lines. P<0.05 was considered to indicate a statistically
significant difference.
Results
Determination of drug resistance in
the A2780 and A2780/DDP ovarian cancer cell lines
Following treatment with a concentration gradient of
cisplatin for 48 h, the CCK-8 assay revealed that the cell
viability of A2780/DDP cells was significantly higher than that of
A2780 cells at lower cisplatin concentrations (Fig. 1). The IC50 values for
A2780/DDP and A2780 were 17.66 and 2.24 µg/ml, respectively,
meaning that the drug resistance index of A2780/DDP was 7.898.
Migratory and invasive abilities of
A2780 cells and cisplatin-resistant A2780/DDP cells
The results of the Transwell migration assay
demonstrated that the migratory ability of the cisplatin-resistant
A2780/DDP cells was significantly higher than that of non-resistant
A2780 cells (Fig. 2; P<0.001).
Moreover, the invasive ability of A2780/DDP cells was significantly
higher than that of A2780 cells, as shown in representative images
from the Transwell invasion assay (Fig. 2; P<0.001).
Expression levels of HMGB1, p-GSK-3β
and EMT-associated proteins in A2780 and A2780/DDP cells
As shown in Fig. 3,
the expression levels of HMGB1 were significantly higher in the
cisplatin-resistant A2780/DDP cells than in the A2780 cells
(P=0.0003). Similarly, the expression levels of p-GSK-3β (P=0.0206)
and the mesenchymal phenotype marker, vimentin, (P=0.001) were
higher in A2780/DDP cells than in A2780 cells, while the levels of
the epithelial phenotype marker, E-cadherin, were lower in
A2780/DDP cells (P=0.005).
 | Figure 3Expression levels of HMGB1, p-GSK-3β
and EMT-associated proteins in A2780 and A2780/DDP cells. (A)
Representative western blots showing the expression levels of
HMGB1, p-GSK-3β, vimentin and E-cadherin in A2780 and A2780/DDP
cells (the relative expression of p-GSK-3β was normalized to the
level of GSK-3β). (B) Semi-quantitative analysis of the HMGB1,
p-GSK-3β, vimentin and E-cadherin expression levels in A2780 and
A2780/DDP cells. *P<0.05, **P<0.005,
***P<0.001. HMGB1, high mobility group box 1; EMT,
epithelial to mesenchymal transition; p-, phosphorylated; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase. Paired Student's t-test
was used to analyze the difference between the two groups. |
HMGB1 enhances the migratory and
invasive abilities of A2780/DDP cells by facilitating EMT
To investigate whether HMGB1 increased the migratory
and invasive abilities of A2780/DDP cells, the expression of HMGB1
in A2780/DDP cells was knocked down. The expression of HMGB1 was
significantly decreased following the transfection of three
siRNA-HMGB1 sequences in A2780/DDP cells (Fig. 4), particularly following the
transfection of siRNA-HMGB1-197 (P=0.0082). Thus, siRNA-HMGB1-197
was used in all subsequent experiments. As shown in Fig. 5, the expression levels of HMGB1
(P=0.0028), p-GSK-3β (P=0.0242) and vimentin (P=0.0345) were
significantly decreased in A2780/DDP cells following transfection
with siRNA-HMGB1-197, whereas the expression of E-cadherin was
significantly increased (P=0.0101). Moreover, Transwell migration
assay demonstrated that the migratory ability of A2780/DDP cells
was significantly decreased following transfection with
siRNA-HMGB1-197 (Fig. 6A and
B; P<0.0001). Similarly, the
invasive ability of A2780/DDP cells was significantly decreased
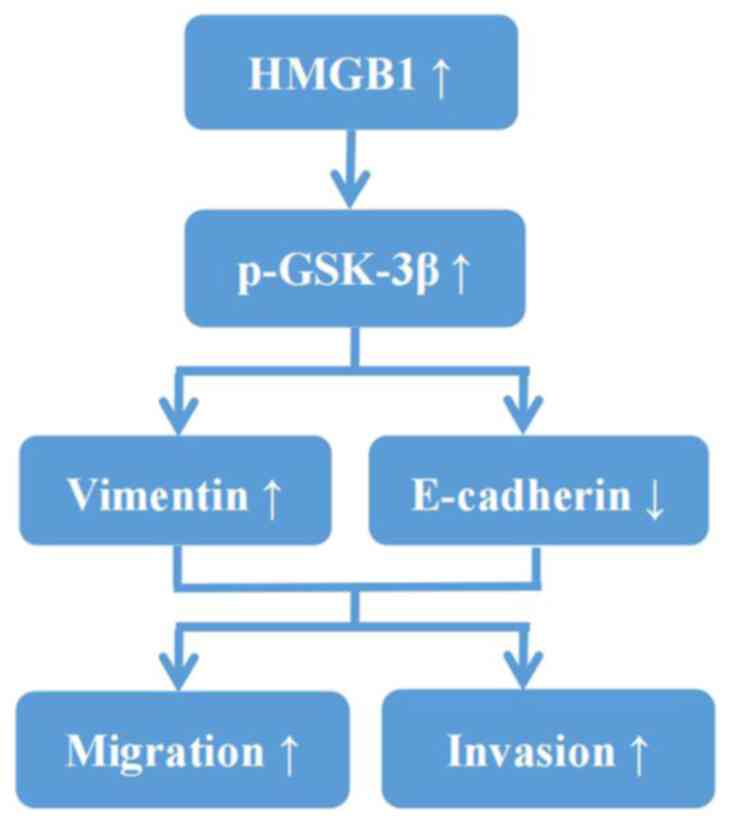
following transfection with siRNA-HMGB1-197 (Fig. 6A and C; P<0.0001). Therefore, the high
expression levels of HMGB1 in cisplatin-resistant A2780/DDP cells
may have enhanced cell migration and invasion by facilitating EMT
via GSK-3β (Fig. 7).
 | Figure 5Expression levels of HMGB1, p-GSK-3β
and EMT-associated proteins in A2780/DDP cells following
transfection with siRNA-HMGB1. (A) Representative western blots
showing the expression level of HMGB1, p-GSK-3β, vimentin and
E-cadherin in A2780/DDP cells following transfection with
siRNA-HMGB1 (the relative expression of p-GSK-3β was normalized to
the level of GSK-3β). (B) Semi-quantitative analysis of HMGB1,
p-GSK-3β, Vimentin and E-cadherin expression levels in A2780/DDP
cells following transfection with siRNA-HMGB1
*P<0.05, **P<0.005,
***P<0.001. N.C., negative control; ns, not
significant; HMGB1, high mobility group box 1; p-, phosphorylated;
EMT, epithelial to mesenchymal transition; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase; si(RNA), small
interfering RNA. One-way analysis of variance followed by Dunnett's
multiple comparisons test were conducted to analyze the difference
among groups. |
Discussion
Platinum resistance can lead to the recurrence and
metastasis of ovarian cancer (1).
Understanding the underlying mechanisms behind recurrence is
crucial in enhancing the prognosis of patients with ovarian cancer.
The present study confirmed that the migratory and invasive
abilities of cisplatin-resistant A2780/DDP cells were significantly
higher than those of A2780 cells. In addition, the expression
levels of HMGB1, p-GSK-3β and the mesenchymal phenotype marker,
vimentin, were found to be significantly elevated in A2780/DDP
cells, whereas the expression level of the epithelial phenotype
marker, E-cadherin, was significantly reduced. After interference
of HMGB1 expression in A2780/DDP cells, the migratory and invasive
abilities of A2780/DDP cells were significantly reduced.
Additionally, the expression levels of HMGB1, p-GSK-3β and vimentin
were significantly downregulated, while the expression level of
E-cadherin was significantly upregulated. Therefore, it was
hypothesized that the high expression of HMGB1 in A2780/DDP cells
promotes cell migration and invasion by facilitating EMT via
GSK-3β.
EMT refers to the process in which epithelial
phenotype-associated genes (for example E-cadherin and cytokeratin)
are downregulated, while mesenchymal phenotype-associated genes
(for example N-cadherin and vimentin) are upregulated under certain
conditions, including wound healing, fibrosis and cancer
metastasis, resulting in structural transformation of the cell into
the mesenchymal form (29). This
process can significantly upregulate the migratory and invasive
abilities of cells (29). In
addition, EMT also increases the proliferation and metastatic
abilities and resistance to chemotherapy of cancer cells (30). A previous study by Li et al
(31) reported that the EMT
process contributed to cell proliferation, migration and invasion
of nasopharyngeal carcinoma cells. Cui et al (32) similarly concluded that EMT promoted
pancreatic cancer cell motility and metastasis. Furthermore, Liang
et al (33) demonstrated
that upregulation of ZEB1, an EMT transcription factor, promoted
EMT, invasion and metastasis in serous ovarian cancer. Kim et
al (34) demonstrated that
long non-coding RNA steroid receptor RNA activator mediated cell
migration, invasion and the progression of ovarian cancer via EMT.
In the present study, Transwell migration and invasion assays
indicated that the migratory and invasive abilities of
cisplatin-resistant A2780/DDP cells were significantly elevated
compared with A2780 cells. Additionally, expression of the
mesenchymal phenotype marker, vimentin, was significantly
upregulated in A2780/DDP cells, while expression of the epithelial
phenotype marker, E-cadherin, was significantly downregulated.
These results supported the hypothesis that EMT plays a crucial
role in the migration and invasion of cisplatin-resistant A2780/DDP
cells.
HMGB1 plays a prominent role in tumor development
and metastasis through its ability to promote cell migration and
angiogenesis (35,36). Overexpression of HMGB1 in gastric
cancer cells promoted EMT activation, cell invasion and tumor
growth (37). Moreover, a study by
Liu et al (38) indicated
that downregulation of HMGB1 inhibited EMT, invasion and migration
of lung cancer cells. As demonstrated by Li et al (39), serum HMGB1 levels were
significantly upregulated in patients with ovarian cancer. Paek
et al (40) demonstrated
that positive staining of HMGB1 in ovarian cancer tissue was
detected in 80% of patients with EOC, and high HMGB1 expression was
an independent predictor for progression-free survival of patients.
Moreover, Jiang et al (41)
found that downregulation of HMGB1 expression inhibited ovarian
cancer migration, invasion and angiogenesis. In addition, another
study demonstrated that HMGB1 promoted Snail-mediated EMT in
glioblastoma cells via the degradation of GSK-3β (25). However, the present study did not
investigate whether forced overexpression of HMGB1 in A2780 cells
could enhance their migratory and invasive capabilities. This
limitation highlights the need for further research in this
area.
In conclusion, the results of the present study
demonstrated that high expression of HMGB1 in cisplatin-resistant
A2780/DDP cells may enhance cell migration and invasion by
facilitating EMT via GSK-3β. Consequently, targeting HMGB1
represents a promising therapeutic strategy for ovarian cancer
treatment in the future. As such, the present study offers novel
insights into the clinical management of platinum-resistant ovarian
cancer.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by The Fujian Provincial
Health Technology Project (grant nos. 2018-CX-28 and 2018-ZQN-42),
The Natural Science Foundation of Fujian Province (grant no.
2020J01967) and The Joint Funds for the Innovation of Science and
Technology, Fujian (grant no. 2019Y9130).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors contributed to the study conception and
design. QZ also contributed to protocol development and manuscript
writing; JW also contributed to project development and data
collection; YZ also contributed to data collection and analysis; SC
also contributed to data analysis; LC also contributed to data
analysis and manuscript editing. QZ, JW and LC confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethical approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Havasi A, Cainap SS, Havasi AT and Cainap
C: Ovarian cancer-insights into platinum resistance and overcoming
it. Medicina (Kaunas). 59(544)2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lheureux S, Braunstein M and Oza AM:
Epithelial ovarian cancer: Evolution of management in the era of
precision medicine. CA Cancer J Clin. 69:280–304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Pignata S, C Cecere S, Du Bois A, Harter P
and Heitz F: Treatment of recurrent ovarian cancer. Ann Oncol. 28
(suppl_8):viii51–viii56. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yang L, Xie HJ, Li YY, Wang X, Liu XX and
Mai J: Molecular mechanisms of platinum-based chemotherapy
resistance in ovarian cancer (Review). Oncol Rep.
47(82)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Arnaoutoglou C, Dampala K, Anthoulakis C,
Papanikolaou EG, Tentas I, Dragoutsos G, Machairiotis N,
Zarogoulidis P, Ioannidis A, Matthaios D, et al: Epithelial ovarian
cancer: A five year review. Medicina (Kaunas).
59(1183)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Song M, Cui M and Liu K: Therapeutic
strategies to overcome cisplatin resistance in ovarian cancer. Eur
J Med Chem. 232(114205)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Goldberg JM, Piver MS, Hempling RE and
Recio FO: Paclitaxel and cisplatin combination chemotherapy in
recurrent epithelial ovarian cancer. Gynecol Oncol. 63:312–317.
1996.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ozols RF: Paclitaxel (Taxol)/carboplatin
combination chemotherapy in the treatment of advanced ovarian
cancer. Semin Oncol. 27 (3 Suppl 7):S3–S7. 2000.PubMed/NCBI
|
|
10
|
van Zyl B, Tang D and Bowden NA:
Biomarkers of platinum resistance in ovarian cancer: What can we
use to improve treatment. Endocr Relat Cancer. 25:R303–R318.
2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Loret N, Denys H, Tummers P and Berx G:
The role of epithelial-to-mesenchymal plasticity in ovarian cancer
progression and therapy resistance. Cancers (Basel).
11(838)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ahmed N, Abubaker K, Findlay J and Quinn
M: Epithelial mesenchymal transition and cancer stem cell-like
phenotypes facilitate chemoresistance in recurrent ovarian cancer.
Curr Cancer Drug Targets. 10:268–278. 2010.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Rosanò L, Cianfrocca R, Spinella F, Di
Castro V, Nicotra MR, Lucidi A, Ferrandina G, Natali PG and Bagnato
A: Acquisition of chemoresistance and EMT phenotype is linked with
activation of the endothelin A receptor pathway in ovarian
carcinoma cells. Clin Cancer Res. 17:2350–2360. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cao L, Wan Q, Li F and Tang CE: MiR-363
inhibits cisplatin chemoresistance of epithelial ovarian cancer by
regulating snail-induced epithelial-mesenchymal transition. BMB
Rep. 51:456–461. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liang F, Ren C, Wang J, Wang S, Yang L,
Han X, Chen Y, Tong G and Yang G: The crosstalk between STAT3 and
p53/RAS signaling controls cancer cell metastasis and cisplatin
resistance via the Slug/MAPK/PI3K/AKT-mediated regulation of EMT
and autophagy. Oncogenesis. 8(59)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Qiu Y, Chen Y, Zeng T, Guo W, Zhou W and
Yang X: High-mobility group box-B1 (HMGB1) mediates the
hypoxia-induced mesenchymal transition of osteoblast cells via
activating ERK/JNK signaling. Cell Biol Int. 40:1152–1161.
2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kang R, Chen R, Zhang Q, Hou W, Wu S, Cao
L, Huang J, Yu Y, Fan XG, Yan Z, et al: HMGB1 in health and
disease. Mol Aspects Med. 40:1–116. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Huang J, Ni J, Liu K, Yu Y, Xie M, Kang R,
Vernon P, Cao L and Tang D: HMGB1 promotes drug resistance in
osteosarcoma. Cancer Res. 72:230–238. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Zheng H, Chen JN, Yu X, Jiang P, Yuan L,
Shen HS, Zhao LH, Chen PF and Yang M: HMGB1 enhances drug
resistance and promotes in vivo tumor growth of lung cancer cells.
DNA Cell Biol. 35:622–627. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Li S and Wei Y: Association of HMGB1,
BRCA1 and P62 expression in ovarian cancer and chemotherapy
sensitivity. Oncol Lett. 15:9572–9576. 2018.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shu W: Downregulation of high mobility
group protein box-1 resensitizes ovarian cancer cells to
carboplatin. Oncol Lett. 16:4586–4592. 2018.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Curtin JF, Liu N, Candolfi M, Xiong W,
Assi H, Yagiz K, Edwards MR, Michelsen KS, Kroeger KM, Liu C, et
al: HMGB1 mediates endogenous TLR2 activation and brain tumor
regression. PLoS Med. 6(e10)2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Candolfi M, Yagiz K, Foulad D, Alzadeh GE,
Tesarfreund M, Muhammad AK, Puntel M, Kroeger KM, Liu C, Lee S, et
al: Release of HMGB1 in response to proapoptotic glioma killing
strategies: Efficacy and neurotoxicity. Clin Cancer Res.
15:4401–4414. 2009.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Chen Y, Cai L, Guo X, Li Z, Liao X, Zhang
X, Huang L and He J: HMGB1-activated fibroblasts promote breast
cancer cells metastasis via RAGE/aerobic glycolysis. Neoplasma.
68:71–78. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Li H, Li J, Zhang G, Da Q, Chen L, Yu S,
Zhou Q, Weng Z, Xin Z, Shi L, et al: HMGB1-Induced p62
overexpression promotes snail-mediated epithelial-mesenchymal
transition in glioblastoma cells via the degradation of GSK-3β.
Theranostics. 9:1909–1922. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Zhang Y, Ren H, Li J, Xue R, Liu H, Zhu Z,
Pan C, Lin Y, Hu A, Gou P, et al: Elevated HMGB1 expression induced
by hepatitis B virus X protein promotes epithelial-mesenchymal
transition and angiogenesis through STAT3/miR-34a/NF-κB in primary
liver cancer. Am J Cancer Res. 11:479–494. 2021.PubMed/NCBI
|
|
27
|
Chen J, Liu X, Zhang J and Zhao Y:
Targeting HMGB1 inhibits ovarian cancer growth and metastasis by
lentivirus-mediated RNA interference. J Cell Physiol.
227:3629–3638. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zheng Q, Xu Y, Lu J, Zhao J, Wei X and Liu
P: Emodin Inhibits Migration and Invasion of Human Endometrial
Stromal Cells by Facilitating the Mesenchymal-Epithelial Transition
Through Targeting ILK. Reprod Sci. 23:1526–1535. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yeung KT and Yang J:
Epithelial-mesenchymal transition in tumor metastasis. Mol Oncol.
11:28–39. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mittal V: Epithelial mesenchymal
transition in tumor metastasis. Annu Rev Pathol. 13:395–412.
2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Li L, Li P, Zhang W, Zhou H, Guo E, Hu G
and Zhang L: FERMT1 contributes to the migration and invasion of
nasopharyngeal carcinoma through epithelial-mesenchymal transition
and cell cycle arrest. Cancer Cell Int. 22(70)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Cui X, Wang Y, Lan W, Wang S, Cui Y, Zhang
X, Lin Z and Piao J: SPOCK1 promotes metastasis in pancreatic
cancer via NF-κB-dependent epithelial-mesenchymal transition by
interacting with IκB-α. Cell Oncol (Dordr). 45:69–84.
2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Liang H, Yu T, Han Y, Jiang H, Wang C, You
T, Zhao X, Shan H, Yang R, Yang L, et al: LncRNA PTAR promotes EMT
and invasion-metastasis in serous ovarian cancer by competitively
binding miR-101-3p to regulate ZEB1 expression. Mol Cancer.
17(119)2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Kim LK, Park SA, Yang Y, Kim YT, Heo TH
and Kim HJ: LncRNA SRA mediates cell migration, invasion, and
progression of ovarian cancer via NOTCH signaling and
epithelial-mesenchymal transition. Biosci Rep.
41(BSR20210565)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
He H, Wang X, Chen J, Sun L, Sun H and Xie
K: High-Mobility Group Box 1 (HMGB1) promotes angiogenesis and
tumor migration by regulating hypoxia-inducible factor 1 (HIF-1α)
expression via the phosphatidylinositol 3-kinase (PI3K)/AKT
signaling pathway in breast cancer cells. Med Sci Monit.
25:2352–2360. 2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
van Beijnum JR, Nowak-Sliwinska P, van den
Boezem E, Hautvast P, Buurman WA and Griffioen AW: Tumor
angiogenesis is enforced by autocrine regulation of high-mobility
group box 1. Oncogene. 32:363–374. 2013.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chung HW, Jang S, Kim H and Lim JB:
Combined targeting of high-mobility group box-1 and interleukin-8
to control micrometastasis potential in gastric cancer. Int J
Cancer. 137:1598–1609. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Liu PL, Liu WL, Chang JM, Chen YH, Liu YP,
Kuo HF, Hsieh CC, Ding YS, Chen WW and Chong IW: MicroRNA-200c
inhibits epithelial-mesenchymal transition, invasion, and migration
of lung cancer by targeting HMGB1. PLoS One.
12(e0180844)2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Li Y, Tian J, Fu X, Chen Y, Zhang W, Yao H
and Hao Q: Serum high mobility group box protein 1 as a clinical
marker for ovarian cancer. Neoplasma. 61:579–584. 2014.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Paek J, Lee M, Nam EJ, Kim SW and Kim YT:
Clinical impact of high mobility group box 1 protein in epithelial
ovarian cancer. Arch Gynecol Obstet. 293:645–650. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jiang W, Jiang P, Yang R and Liu DF:
Functional role of SIRT1-induced HMGB1 expression and acetylation
in migration, invasion and angiogenesis of ovarian cancer. Eur Rev
Med Pharmacol Sci. 22:4431–4439. 2018.PubMed/NCBI View Article : Google Scholar
|