Introduction
Allergic asthma, a complex chronic allergy-related
inflammatory disease, is characterized by airway
hyperresponsiveness, inflammation and remodeling. The clinical
manifestations of allergic asthma include cough, chest tightness
and croup (1). Currently, allergic
asthma is one of the most prevalent diseases, affecting more than
300 million individuals worldwide. The incidence of allergic asthma
is projected to reach 400 million by 2025(2). Asthma is frequently characterized by
the upregulation of systemic allergen-specific immunoglobulin E
(IgE) levels and pulmonary eosinophil infiltration. Various
hypotheses have been proposed to explain the pathophysiology of
asthma. According to one hypothesis, asthma onset is associated
with the disruption of the intestinal milieu. The ecological
dysregulation of colonic bacteria (such as thick-walled bacteria
and mycobacteria) promotes their transfer from the intestine to the
lungs via the lymph and blood, promoting the development of
respiratory diseases, such as lung cancer, asthma, tuberculosis and
cystic fibrosis (3). Usage of
antibiotics (ABX), which markedly modifies the abundance and
composition of the bacterial population, is among the major factors
that influence gut microbiota composition (4). In particular, ABX usage alters the
microbiome and decreases microbial diversity. Previous studies have
demonstrated that ABX usage exerts adverse effects on health
(5,6). ABX usage during early pregnancy
determines the gut microbial composition of the mother and fetus
and increases the risk of atopic rhinitis and asthma in the fetus
(7).
An aberrant intestinal milieu increases the
susceptibility of the intestine to foreign bacterial colonization
and consequently disrupts gut microbiota homeostasis, promotes
intestinal immune dysfunction, and modifies host immunity.
Intestinal mucosal barrier function is essential for maintaining a
balanced response between the host and its microbiome (8). The intestinal mucosal barrier
comprises chemical, biological, immune and mechanical barriers,
which are essential for its function (9). Occludin (OCLN) and claudin 1 (CLDN1)
are transmembrane proteins predominantly expressed in the
intercellular space. The band closure protein Tight Junction
Protein 1 (TJP1), which is essential for the aggregation of CLDN1,
is a crucial TJP. Additionally, TJP1 is an adaptor protein that
promotes the binding between OCLN and CLDN1 at the cell junctions,
as well as the binding of transmembrane proteins with the actin
cytoskeleton (10,11).
Although the correlation between ABXs, gut
microbiota and asthma has been identified, the underlying
mechanisms have not been completely elucidated (12). Based on a previous study (13), the present study examined the
long-term effects of ABXs on the gut microbiota and the
pathogenesis of allergic inflammation using the ovalbumin
(OVA)-induced asthma mouse model.
Materials and methods
Experimental materials and
reagents
Materials and reagents used in the present study
were as follows: OVA (cat. no. A5503; Sigma-Aldrich; Merck KGaA),
aluminum hydroxide (cat. no. 77161; Thermo Fisher Scientific,
Inc.), methacholine (MCh; cat. no. A2251; Sigma-Aldrich; Merck
KGaA), enzyme-linked immunosorbent assay (ELISA) kits for TGF-β
(cat. no. MB-6138A) and OVA-sIgE (cat. no. MB-6148A) (both from
Jiangsu Enzyme Biological Co., Ltd.), total protein extraction kit
(RIPA Lysis Buffer:Phenylmethanesulfonyl fluoride:Protease
inhibitor cocktail for general use, 100X=100:1:1; cat. nos. P0013B,
ST506 and P1005; Beyotime Institute of Biotechnology),
bicinchoninic acid (BCA) kit (cat. no. P0010; Beyotime Institute of
Biotechnology), rabbit polyclonal antibodies for TJP1 (cat. no.
AF5145; Affinity Biosciences), rabbit polyclonal antibodies for
CLDN1 (cat. no. AF0127; Affinity Biosciences), rabbit polyclonal
antibodies for OCLN (cat. no. DF7504; Affinity Biosciences), rabbit
anti-actin antibodies (cat. no. 10966R; BIOSS), and horseradish
peroxidase (HRP)-conjugated Affinipure goat anti-Rabbit IgG (H + L)
(cat. no. SA00001-2; Proteintech Group, Inc.). 2X Taq plus master
kit (cat. no. P211-02; Nanjing Novozan Biotechnology Co., Ltd.),
TruSeq DNA Library Preparation Kit v2 (cat. no. FC-121-2001;
Illumina Inc.), primer synthesis [Sangong Bioengineering (Shanghai)
Co., Ltd.] and ChamQ SYBR Color qPCR Master Mix (2X) (cat. no.
Q421-02; Vazyme Biotech Co, Ltd.).
Experimental animals
A total of 40 female BALB/c mice (age: 6-8 weeks;
body weight: 20 g) of specific pathogen-free grade were purchased
from Chengdu Dashuo Co. [Production license: SCXK (chuan)
2020-030]. The mice were reared under the following conditions:
Temperature, 22-25˚C; humidity, ~60%; 12-h light/dark cycle; access
to food and water, ad libitum. Mice treated with ABXs for 1
week were assigned to the control, model, OVA-ABX, and ABX-OVA
groups, while those treated with ABXs for 2 weeks were assigned to
the control 2, model 2, OVA-ABX 2 and ABX-OVA 2 groups. The number
of mice in each group was 6. All animal experiments were approved
by the Animal Ethics Committee of Yunnan University of Traditional
Chinese Medicine (approval no. R-06202085; Kunming, China). After
the detection of enhanced pause (Penh), the mice were anesthetized
using an instrument via isoflurane inhalation under the following
conditions: Exposure rate, 300-500 ml/min; isoflurane
concentration, 4-5%; duration, 3-4 min. The orbital plexus blood
sample (1 ml) was collected from the anesthetized mice. After blood
sample collection, mice were euthanized via decapitation. The
heartbeat in the chest cavity, the nerve reaction, and the body
temperature were examined to confirm the death of mice. The
remaining tissues were extracted and immediately transferred to the
animal room of Yunnan University of Traditional Chinese Medicine
for centralized treatment.
Drug preparation
Based on previous studies (14,15),
mice were administered with the following ABX cocktail in
physiological saline: Amoxicillin (100 mg/kg bodyweight) (to
eliminate gram-negative bacteria); neomycin sulfate (100 mg/kg
bodyweight) (to eliminate gram-positive and gram-negative
bacteria); metronidazole (100 mg/kg bodyweight) (to eliminate
gram-positive anaerobic and gram-negative anaerobic bacteria);
amphotericin (1 mg/kg bodyweight) (to eliminate fungi); vancomycin
(50 mg/kg bodyweight) (to eliminate gram-positive bacteria)
(Beijing Solarbio Science & Technology Co., Ltd.).
Establishment of animal models
The asthma mouse model was established using OVA and
aluminum hydroxide suspension. The model group was
intraperitoneally administered with 50 µg OVA + 50 µl aluminum
hydroxide (volume: 0.2 ml) during the first and second weeks of the
experiment. Asthma was stimulated through nebulization with 2% OVA
(6 ml/12 animals) for 30 min every other day beginning from the
third week (for the model 2 group, the time of stimulation was at
weeks 3 and 4). The control group was nebulized with saline.
Mice in the OVA-ABX groups were gavaged with an ABX
cocktail at the beginning of the third week of the experiment (the
third to the fourth week for the OVA-ABX 2 group) twice a day (0.2
ml/dose) and nebulized every second day.
Meanwhile, mice in the ABX-OVA groups were gavaged
with the ABX cocktail at week 1, twice per day (weeks 1-2 for the
ABX-OVA 2 group), intraperitoneally administered with OVA at weeks
2-3 (weeks 3-4 for the ABX-OVA 2 group), and nebulized on alternate
days between day 21 and 29. The precise modeling timing and
methodology is illustrated in Fig.
1.
Whole Body Plethysmography (WBP) of
Penh levels in the respiratory system of mice
The room temperature and tranquility were maintained
throughout the experiment. The experiment was performed in a WBP
(cat. no. 27389; EMMS; http://www.electromedsys.com/wbp.html). A nebulizer
was used to administer different concentrations of MCh (0, 6.25,
12.5, 25, 50 and 100 mg/ml) for 2 min. The change in the
respiratory movement after inhalation of the nebulized gas caused
corresponding changes in the pressure and flow rate outputs in the
box. The pressure sensors (flow rate sensors) connected to the body
descriptor box transmitted the collected signals to the signal
processing system. The signal amplifiers converted these signals to
values of various respiratory dynamic indices. The Penh values were
recorded for 3 min. The duration between the administration of two
concentrations was 4 min.
Hematoxylin and eosin (H&E)
staining of pathological samples
The right lower lung lobe and colon tissue 1 cm from
the rectum were rapidly excised after blood sampling. The samples
were placed in EP tubes, fixed with 4% paraformaldehyde for 24 h at
room temperature, dehydrated, embedded, sectioned (~5-µm thick),
stained with H&E, and sealed. The pathological morphological
changes in the lung and intestinal tissues were evaluated under a
light microscope (Nikon Eclipse 2000 equipped with Nikon DS-Fi2;
Nikon Corporation).
ELISA
The levels of OVA-specific IgE in the mouse serum
and TGF-β in the lung tissue homogenates were examined using ELISA.
At the end of the experiment, the blood sample (~1 ml per mouse)
was obtained from mice at the time of euthanasia. The blood sample
was allowed to naturally clot at room temperature for 1 h and
centrifuged at 4˚C and 2,000 g x for 15 min. The supernatant was
collected and stored at -80˚C. The lung tissue samples (50 mg) were
homogenized with 450 ml phosphate-buffered saline. The homogenate
was centrifuged for 15 min at 626 x g. The supernatant was
collected and stored at -80˚C until analysis.
The samples were spiked, incubated and washed,
following the ELISA kit instructions. The optical density at 450 nm
of the reaction mixture was measured using an enzyme marker. The
levels of TGF-β expression in the lung homogenates and OVA-specific
IgE in the serum were measured based on enzyme standardization and
a standard curve using a microplate reader (SYNERGY H1; BioTek
Instruments, Inc.).
Western blotting
The protein expression levels of TJP1, CLDN1 and
OCLN in the colon were analyzed using western blotting. Briefly,
total proteins were extracted using the total tissue protein
extraction kit, following the manufacturer's instructions, and
quantified using the BCA kit. The extracted proteins (20 µg/lane)
were subjected to sodium dodecyl sulfate-polyacrylamide gel
electrophoresis with a 10% gel. The resolved proteins were
electro-transferred onto a polyvinyl difluoride membrane (0.45 µm)
at 300 mA for 45 min. The membrane was then incubated with anti-
TJP1, anti-CLDN1, anti-OCLN and anti-β-actin (1:1,000 for all
antibodies) antibodies at 4˚C for 12 h. After blocking with 5%
skimmed milk for 1 h at room temperature, the membrane was washed
several times with Tris-buffered saline containing 0.1% Tween 20.
After washing, the membrane was incubated with secondary antibodies
(1:6,000) for 1 h at room temperature. Immunoreactive signals were
developed and visualized using a digital imaging and analysis
system Panasonic WV-CP230 (Panasonic Corp). The expression levels
of target proteins were normalized to those of β-actin using ImageJ
V1.8.0 (National Institutes of Health).
Quantification of mouse gut microbiota
using 16S rDNA-specific primers
Genomic DNA was extracted from the fecal samples
using the fast DNA® spin kit (cat. no. 116570200; MP
Biomedicals), following the manufacturer's instructions. The
quality of the isolated DNA was evaluated using agarose gel
electrophoresis with a 1% gel (SYBR Green). The DNA concentration
and purity were assessed using NanoDrop 2000 (Thermo Fisher
Scientific, Inc.). DNA concentration ≥10 was ng/µl; while DNA
purity was assessed on the A260/A280 nm ratio being 1.8-2.0.
Amplification was performed using ABI GeneAmp®, 9700 with
TransStart® FastPfu DNA Polymerase (cat. no. AP221-02;
TransGen Biotech Co., Ltd.), following the manufacturer's
instructions. The amplicons from the same sample were pooled and
subjected to agarose gel electrophoresis with a 2% gel. High
fidelity PCR was utilized to amplify bacterial 16S rDNA
hypervariable region 3 (V3) and 4 (V4) with the primers 338F,
5'ACTCCTACGGGAGGCAGCAG-3' and 806R, 5'-GGACTACHVGGGTWTCTAAT-3'.
Next, the amplicons were recovered from the gel using the
AxyPrepDNA gel recovery kit (cat. no. AP-GX-500; Axygen; Corning,
Inc.), eluted with Tris-HCl, and confirmed using agarose
electrophoresis with a 2% gel. Based on the preliminary
quantitative results of electrophoresis, the amplicons were
quantified using Qubit 4.0 (Thermo Fisher Scientific, Inc.).
Determination of loading concentration was conducted using Quantus™
Fluorometer (Promega Corporation); the range of the loading
concentration of the final library was 54.13 nmol and sequencing
libraries were generated with TruSeqTM DNA Sample Prep Kit
(Illumina Inc.). Purified amplicons were pooled in equimolar and
paired-end sequenced (2x300) on an PE300 platform (Illumina, Inc.)
according to the standard protocols by Majorbio Bio-Pharm
Technology Co. Ltd. The average sequence length was 420 bp.
The relative abundance of bacterial taxa was used to
rank the taxa. Operational taxonomic units were analyzed using
Uparse (16) (version 7.0.1090;
http://drive5.com/uparse/). The species
composition of the community was determined for each sample and
compared with the Silva database (Release138 http://www.arb-silva.de) with confidence threshold of
70%. Principal coordinate analysis (PCoA) was performed using R
(version 3.3.1) to generate PCoA statistics and plots. Community
composition at the phylum and genus levels was analyzed using R
(version 3.3.1) tools to obtain the statistical data and plots.
Comparative analysis of colonies between the groups was performed
using the stats package in R (version 3.3.1) and the Scipy package
in Python. Environmental factors were compared using the heatmap
package in R (version 3.3.1) to generate the correlation heatmaps.
Linear discriminant analysis effect size (LEfSe) analysis was
performed using the LEfSe software (http://huttenhower.sph.harvard.edu/galaxy/root?tool_id=lefse_upload).
Absolute fluorescence quantification
of mouse colonic contents
DNA extraction and primer sequences were consistent
with the aforementioned. The OD260 values of the constructed
plasmids were determined by UV spectrophotometer (NanoDrop2000;
Thermo Fisher Scientific, Inc.), and converted to copy number
(copies/µl) by the following formula: Plasmid Name: 16S rDNA,
Concentration (ng/µl): 103.61, Number of copies/µl:
2.99x1010; Plasmid vector name: pMD18-T; Plasmid vector
size: 2692 bp; Plasmid starting copy number conversion formula
(copies/µl)=concentration (ng/µl) x10-9
x6.02x1023/(molecular weight x660). It should be noted
that molecular weight refers to the size of the vector plus the
fragment size of the target gene.
Standard curve samples were prepared as follows:
10-fold gradient dilutions of each constructed plasmid (90 µl
dilution + 10 µl plasmid) was used to prepare a standard curve
comprising 4-6 points. Based on pre-experiments, 10-2 to 10-6
dilutions of 16S standard were selected for the preparation of the
standard curve.
Regarding the fluorescence quantitative PCR assay,
the amplification was performed in a fluorescent quantitative PCR
instrument Model ABI7300 (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the instruction manual of ChamQ SYBR
Color qPCR Master Mix 2X (cat. no. Q421-02; Vazyme Biotech Co,
Ltd.). The copy values were calculated based on the number of
cycles after obtaining the following data: 16S gene fluorescence
PCR amplification curve and melting curve, amplification curve and
melting curve of 16S gene quantitative standard, slash diagram of
16S gene quantification standards and hybridization efficiency of
16S standard [Slope: -3.4601; Y-Inter: 42.231; R2: 0.9996;
Efficiency (%): 94.54].
Statistical analysis
Data are presented as mean ± standard deviation. The
experiment was repeated three times. The mean between groups was
compared using one-way analysis of variance (ANOVA), followed by
Tukey's post hoc test. The relative levels of OVA-IgE and microbial
community species were analyzed using the Multivariate Association
with Linear Models (MaAslin). The correlation between OVA-IgE and
the relative abundance of microbial community species (data) was
analyzed using MaAslin (R version 3.3.1; MaAsLin2 package; R Core
Team), which constitutes a multivariate linear model. The
correlation coefficients were calculated and analyzed using one-way
ANOVA. Heatmap analysis was performed to calculate Spearman rank
correlation coefficients between TJPs and microorganisms, as well
as to obtain the R and P values of the correlations. The heatmap
was plotted with R values distinguished by different color shades.
*P<0.05 was considered to indicate a statistically
significant difference. Statistical analyses were performed using
SPSS 26.0 software (IBM Corp.). Graphing was performed using
GraphPad Prism 8.0.2 software (Dotmatics).
Results
Effect of ABXs on the bodyweight of the
OVA-induced asthma mouse model. The bodyweight of mice was not
significantly different between the control, model and model 2
groups. However, the bodyweight of mice in the OVA-ABX, ABX-OVA,
OVA-ABX 2, and ABX-OVA 2 groups significantly decreased after ABX
administration (Fig. 2A). These
findings indicated that ABXs decrease the bodyweight of the
OVA-induced asthma mouse model.
Effect of ABX administration on the
Penh values in the OVA-induced asthma mouse model
Compared with those in the control group, the Penh
values were higher in the model, OVA-ABX, ABX-OVA, model 2, OVA-ABX
2 and ABX-OVA 2 groups. Furthermore, MCh dose-dependently increased
the Penh value (Fig. 2B). Thus,
ABXs increased the Penh values in the OVA-induced asthma mouse
model.
Histopathological pulmonary changes in
different groups
Mice in the control and control 2 groups did not
exhibit marked inflammatory cell infiltration in the lung tissue.
Additionally, the tracheal lumen was unobstructed with no marked
thickening of the tracheal basement membrane. Furthermore,
congestion and edema in the tracheal mucosa epithelium and
epithelial damage were not observed, and the alveolar structure was
intact. By contrast, the other groups exhibited an increased
incidence of inflammatory cell infiltration, bronchial mucosa
congestion and edema, smooth muscle and basement membrane
thickening, alveolar collapse, widening of intervals, partial
alveolar expansion, detached epithelial cells in the alveolar lumen
and mucus in the tracheal lumen (Fig.
3A). These observations suggested the successful establishment
of the asthma model.
 | Figure 3Antibiotic administration exacerbates
inflammation and upregulates IgE expression in the asthma mouse
model. (A) H&E staining of the lung sections of mice in
different groups. Blue arrow, alveolar wall thickening; green
arrow, neutrophils; black arrow, bronchial epithelial cells that
are shed; (B) H&E staining of the colon sections of mice in
different groups. Green arrow, lymphocytic infiltration; red arrow,
granulocyte infiltration; black arrow, mucosal epithelial cells of
intestinal tissue that are shed. (C) The levels of TGF-β in the
lung tissue homogenate of mice in different groups. (D) The
expression of OVA-induced IgE in the serum of mice in different
groups (n=5). *P<0.05 and **P<0.01,
control group vs. model group; ##P<0.01 control 2
group vs. control group; ^P<0.05 and ^^P<0.01,
model group vs. OVA-ABX and ABX-OVA group; ∆P<0.05
and ∆∆P<0.01, model 2 group vs. OVA-ABX 2 and ABX-OVA
2 group. IgE, immunoglobulin E; H&E, Hematoxylin and eosin OVA,
ovalbumin; ABX, antibiotics. |
As shown in Fig.
3B, mice in the control and control 2 groups exhibited an
intact colon mucosal tissue structure with no inflammatory
infiltration in the mucosal, submucosal and muscle layers. In the
other groups, the colonic tissue within the lamina propria
exhibited atrophy of large intestinal glands that were loosely
arranged, which was accompanied by inflammatory cell infiltration.
The levels of inflammatory cell infiltration were upregulated in
the model 2 and OVA-ABX 2 groups, while the lamina propria was
severely damaged in the ABX-OVA 2 group (Fig. 3B). These observations indicated
that inflammatory lesions are induced in the colon of the
OVA-induced asthma mouse model and that ABXs may differentially
affect the development of asthma.
Pulmonary TGF-β and serum OVA-induced
IgE levels in different groups
The lung tissue homogenate levels of TGF-β in all
experimental groups were significantly upregulated when compared
with those in the control and control 2 groups (P<0.05 and
P<0.01) (Fig. 3C). The
expression of TGF-β in the OVA-ABX, OVA-ABX 2, ABX-OVA and ABX-OVA
2 groups was significantly upregulated when compared with that in
the model and model 2 groups (P<0.05 and P<0.01).
The serum levels of OVA-induced IgE in the model,
OVA-ABX and ABX-OVA groups were higher than those in the control
group (P<0.01). Similarly, the serum levels of OVA-induced IgE
in the model 2, OVA-ABX 2 and ABX-OVA 2 groups were upregulated
when compared with those in the control 2 group (P<0.01).
Compared with those in the model and model 2 groups, the increased
IgE levels were significantly elevated in the OVA-ABX, OVA-ABX 2,
ABX-OVA and ABX-OVA 2 groups (P<0.05 and P<0.01). Thus, ABX
administration upregulated OVA-induced IgE expression (Fig. 3D). These results demonstrated that
long-term treatment of ABX can upregulate the expression of
inflammatory markers and the production of immunoglobulins in the
OVA-induced asthma mouse model.
Effect of ABXs on the expression of
TJPs in the asthma mouse model
The colonic expression levels of TJP1, CLDN1 and
OCLN in the OVA-ABX, OVA-ABX 2, ABX-OVA and ABX-OVA 2 groups were
downregulated when compared with those in the control and control 2
groups (P<0.05 and P<0.01) (Fig.
4A and B). Compared with those
in the model and model 2 groups, the expression levels of TJPs were
significantly downregulated in the OVA-ABX and OVA-ABX 2 groups but
the downregulation was less significant in the ABX-OVA and ABX-OVA
2 groups. Furthermore, the downregulation of TJPs in the group
treated with ABX for 2 weeks was lower than that in the group
treated with ABX for 1 week. These findings suggested that the
expression of TJPs is downregulated in the OVA-induced asthma model
and that ABX administration further downregulates their
expression.
 | Figure 4Antibiotic administration
downregulates the expression of tight junction proteins and
suppresses gut microbial colonization. (A) The protein expression
levels of TJP1, CLDN1 and OCLN in different groups. (B) The ratio
of TJP1, CLDN1, or OCLN grayscale values to β-actin grayscale
values in different groups (n=3). (C) Bacterial load in the colonic
contents of different groups (n=5). *P<0.05 and
**P<0.01, compared with the model group;
^P<0.05 and ^^P<0.01, compared with the
control 2 group; #P<0.05 and ##P<0.01,
compared with the model 2 group; IP<0.05 and
IIP<0.01. CLDN1, claudin-1; OCLN, Occludin; OVA,
ovalbumin; ABX, antibiotics. |
Effect of ABX administration on the
colonic bacterial load
Absolute fluorescence quantification of mouse colon
contents revealed that the fecal bacterial load in the OVA-ABX and
OVA-ABX 2 groups was significantly lower than that in the other
groups (Fig. 4C). Furthermore, the
bacterial load recovered one week and two weeks after ABX
discontinuation with recovery being directly proportional to time.
Thus, the bacterial load of mice was found to be markedly decreased
after ABX administration and can progressively be restored after
the discontinuation of ABX treatment.
Analysis of the diversity, structure
and correlation of gut microbiota in different groups. Effect of
ABXs on the intestinal microbial diversity in the asthma mouse
model
Shannon index in the OVA-ABX and ABX-OVA groups was
lower than that in the control group. Furthermore, the Shannon
index in the ABX-OVA group was significantly lower than that in the
control, model and OVA-ABX groups (P<0.01). ABX treatment after
2 weeks exerted similar effects on the Shannon index (P<0.01)
(Fig. 5A). Chao index in the
OVA-ABX and ABX-OVA groups was significantly lower than that in the
control and model groups. ABX treatment after 2 weeks exerted
similar effects on the Chao index (Fig. 5B). These findings suggested that
ABX administration alters the variety and richness of gut
microbiota in the OVA-induced mouse model, especially when the
asthma model is established after ABX administration.
The similarities and differences in community
composition in different groups were analyzed using beta diversity
analysis and comparison group analysis of intestinal diversity in
various mice (Fig. 5C and Table I). The community composition was
similar in the control and model groups but dissimilar in the
OVA-ABX, ABX-OVA, OVA-ABX 2 and ABX-OVA 2 groups. Analysis of
similarity revealed that the R value was >0, suggesting that the
difference between the groups was more significant than that within
each group (P<0.05). Furthermore, the differences were
significant between the ABX-treated, control and model groups.
 | Table IAnosim analysis results. |
Table I
Anosim analysis results.
| Method | ANOSIM |
|---|
| Statistic | 0.6129 |
| P-value | 0.001 |
| Permutation
number | 999 |
Effect of ABX administration on the abundance of
gut microbiota at the phylum level. The predominant phyla were
Proteobacteria, Firmicutes and Bacteroidota.
Compared with those in the control and control 2 groups, the
abundance of Firmicutes decreased and the abundance of
Proteobacteria increased in the model, model 2, ABX-OVA,
ABX-OVA 2 and OVA-ABX 2 groups. Additionally, the relative
abundance of Bacteroidota in the model group was higher than
that in the control group but was downregulated in the ABX-OVA and
OVA-ABX groups (Fig. 6A and
B). Thus, ABX administration was
revealed to alter the abundance of gut microbiota in the
OVA-induced asthma mouse model.
Effect of ABX administration on the abundance of
gut microbiota at the genus level. Escherichia-Shigella,
Lactobacillus and Lachnospira were the predominant
genera in the mouse intestinal contents. Compared with those in the
control group, the relative abundances of
Escherichia-Shigella were higher and the relative abundances
of Lactobacillus were lower in the model 2, OVA-ABX and
ABX-OVA groups (Fig. 7A and
B). Thus, broad-spectrum ABXs
markedly affected the diversity and composition of healthy mouse
intestinal microbiota.
Major differential genera of gut microbiota in
mice. Based on the initial findings, the distinct species
affected by ABX treatment were identified. Thus, LEfSe analysis was
performed to identify differential species (Fig. 8A and B). The distinctive bacteria in different
groups were as follows: Control group, Lachnospira; model
group, Lactobacillus and Enterorhabdus; OVA-ABX
group, Brevundimonas, Staphylococcus and
Bacillus; control 2 group, Enterorhabdus; model 2
group, Parabacteroides, Prevotellaceae and
Odoribacter; OVA-ABX 2 group, Novosphingobium and
Allorhizobium; ABX-OVA 2 group, Escherichia-Shigella.
These findings suggested that asthma and ABX usage can alter the
composition of the gut microbiota and impair the equilibrium of the
microbiome.
Analysis of the correlation between distinct
mouse species and environmental factors. Correlation heatmap
plots were used to evaluate the correlation between the proportion
of genera and the expression of TJPs. Additionally, MaAslin was
used to determine the linear correlation between environmental
factors and the relative abundance of the differential microbial
species. IgE expression was negatively correlated with
Lactobacillus, Lachnospira NK4A136 group,
Alistipes and Lachnospira UCG-006 abundances and
positively correlated with Escherichia-Shigella and
Staphylococcus abundances in the model and ABX-treated
groups relative to the control group (P<0.05 and P<0.01)
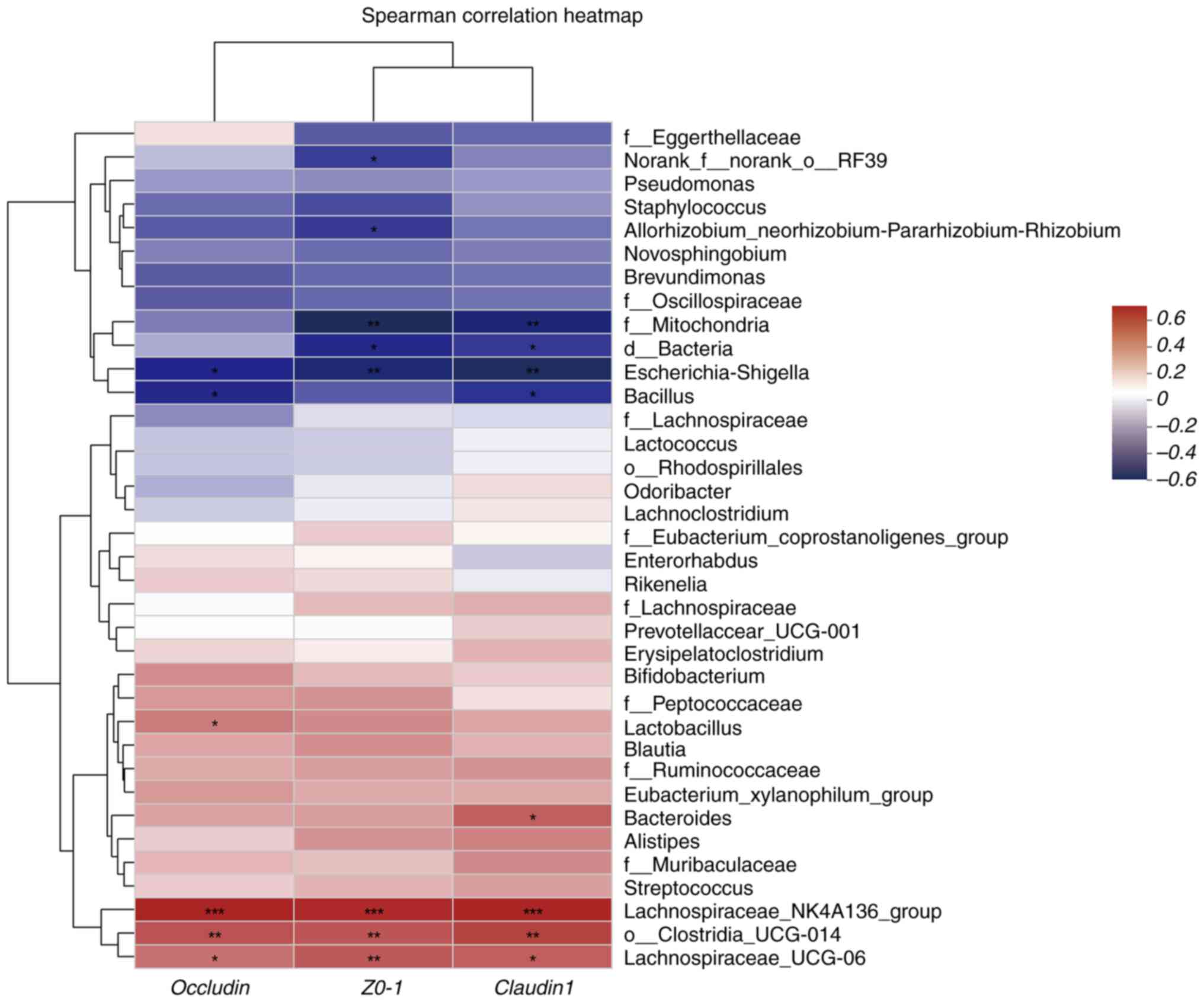
(Fig. 9). TJP expression was
positively correlated with Lachnospira NK4A136 group and
Lachnospira UCG-006 abundances and negatively correlated
with Escherichia-Shigella abundances (P<0.05 and
P<0.01) (Fig. 10). These
findings indicate the correlation between gut microbiota
composition and the production of TJPs and IgE in the model and
ABX-treated groups.
Discussion
Allergic asthma is a chronic inflammatory disease of
the airways involving several inflammatory cells, including
eosinophils, mast cells and macrophages. Recent studies have
demonstrated that the gut microbiota plays a vital role in immune
development, maturation and regulation (17,18).
Genetic factors, delivery methods, living environment and ABX abuse
can disrupt the gut microbiota, leading to the development of
allergic rhinitis and asthma. Gut microbes are involved in
maintaining local (19) and
systemic immune balance (12,20).
Dysbiosis promotes the colonization and proliferation of
potentially pathogenic bacteria, induces the production of
inflammatory factors, and adversely affects the immune barrier and
homeostasis (21), contributing to
the development of immune diseases, such as asthma (22,23).
According to the theory of the lung-gut axis, pulmonary diseases
are associated with gut microorganisms (12). Additionally, a microbe-rich
environment positively affects the maturation of the gut microbiota
of infants during the first year of life, modulating the ability of
gut microbiota to produce the short-chain fatty acid butyric acid
and consequently exert protective effects on childhood asthma
(24). Microorganisms affect the
immune response of intestinal and respiratory epithelial cells.
Patients with chronic obstructive pulmonary disease have been
reported to be associated with an increased prevalence of
inflammatory bowel disease (25).
Various factors affect the gut microbiota, and controlling these
variables in human studies evaluating the correlation between gut
microbiota and asthma is challenging. Thus, the use of experimental
models enables the monitoring of all variables and provides
reliable evidence.
To establish the asthma model with ABX usage, mice
were administered with ABXs for different durations and stacked
sequences. Fluorescence measurement of bacterial load in the
intestinal contents of mice demonstrated that the administration of
ABXs efficiently altered gut microbiota. However, the bacterial
load was restored when the number of ABX was decreased. Next, the
composition and abundance of gut microbiota in different groups
were examined using 16S rDNA sequencing. ABX administration
decreased the diversity and abundance of gut microbiota in mice,
especially in the ABX-OVA and ABX-OVA 2 groups. Furthermore,
Proteobacteria was the dominant phylum, while
Escherichia-Shigella and Lachnospira were the
dominant genera. Escherichia-Shigella and Lachnospira
were not detected in the control group but their abundance
gradually increased in the model, OVA-ABX and ABX-OVA groups.
Proteobacteria is the predominant bacterial phylum,
comprises gram-negative bacteria with lipopolysaccharides in the
outer membrane, exerts pro-inflammatory effects, and dysregulates
immunological reactions (26).
Additionally, Proteobacteria is prevalent in respiratory
illnesses (27), including chronic
obstructive pulmonary disease, asthma (28) and bronchiectasis (29). The abundance of
Proteobacteria was reported to be upregulated in the gut
microbiota of patients with asthma and rhinitis (30). Wu et al (31) demonstrated similar findings in the
OVA-sensitized asthma mouse model and reported a raised abundance
of Proteobacteria. Elevated levels of Proteobacteria
were accompanied by inflammatory cell infiltration in lung and
intestinal tissues and Th2 cell subsets and the levels of
Th2-related cytokines interleukin-4 (IL-4, IL-5 and IL-13) raised
in asthmatic mice. Proteobacteria is likely to exacerbate
asthma by stimulating a th2-type inflammatory response. In the
present study, the abundance of Proteobacteria was
upregulated in the model group and further upregulated upon ABX
administration. Additionally, ABX administration exacerbated airway
inflammation in mice. Dysbiosis and decreased Lachnospira
abundance during the first 100 days of life were reported in
infants at risk of developing asthma. The transplantation of
Lachnospira in germ-free mice alleviated airway inflammation
in adulthood (32).
Lachnospira reduce serum levels of IL-17A, and IL-6 in lung
homogenates and OVA-specific IgG2a, and significantly reduce the
amount of Th17 in the immune response to achieve a lower
inflammatory response (33). In
the present study, the abundance of Lachnospira was
downregulated in the model group and further downregulated upon
treatment with ABX. Additionally, the abundance of
Lachnospira was positively correlated with the expression of
TJPs.
ABXs disrupt the gut microbiota composition by
increasing the number of pathogenic bacteria or decreasing the
number of beneficial bacteria, contributing to the development of
atopic rhinitis, asthma, and other allergic illnesses.
A consensus on the correlation between ABXs, asthma
and the intestinal barrier has not been achieved. In the present
study, ABX administration affected the intestinal mucosa and lamina
propria of mice. Additionally, intestinal tissue disruption was
severe with prolonged ABX treatment. ABXs downregulated the
expression of TJPs (TJP1, CLDN1 and OCLN), especially in the
OVA-ABX and OVA-ABX 2 groups. This suggested that ABXs impair tight
junctions and the protective function of the intestinal barrier
in vivo.
Previous studies have demonstrated that clindamycin
affects the function of the intestinal barrier by modulating the
diversity of the animal intestinal microbiota or through other
mechanisms (34-36).
TJPs are crucial for maintaining the integrity of the intestinal
mucosal barrier. CLDN1 and OCLN proteins play a vital role in
maintaining barrier integrity and permeability by sealing and
tightening the tight junctions. CLDN1-deficient animals do not
survive owing to a compromised mucosal barrier (37). OCLN protein interacts with TJP1
protein to modulate intracellular and extracellular signaling and
intestinal mucosal permeability (36). Inflammatory mediators, intestinal
microbes and cytokines dysregulate the expression of CLDN1, OCLN
and TJP1. Lachnospira is positively correlated with the
expression of TJPs and negatively correlated with the proportion of
Escherichia-Shigella. Electroacupuncture restored TJP
expression and the balance of thick-walled bacteria, such as
Lachnospira and Ehrlichia in rats with drowsy colitis
(35). By contrast,
Escherichia-Shigella, which are adnexal invasive bacteria,
exacerbated the development of colitis in the ulcerative colitis
group, which was accompanied by the downregulation of TJPs
(38). Impaired intestinal barrier
function and intestinal mucosal immunity exacerbate allergic
disorders, such as asthma.
Intestinal mucosal barrier dysfunction promotes the
entry of pathogenic bacteria, endotoxins and metabolic wastes in
the gut wall into the bloodstream, resulting in systemic
inflammatory responses, severe infections and immunological
dysfunction. ABX administration exacerbated the inflammatory cell
infiltration into the lung and colonic tissues in the asthma mouse
model. Additionally, ABX administration upregulated the expression
of OVA-induced IgE and TGF-β in the asthma mouse model. Previous
studies have reported that dysbiosis contributes to the breakdown
of the intestinal mucosal barrier and the enhanced production of
inflammatory markers, aggravating the related illness. One
ulcerative colitis study reported that P2RY13 disrupts the
intestinal mucosal barrier, increases the production of I-6 and
other components, and aggravates the inflammatory response
(39). The enhanced production of
inflammatory factors, including IL6, TNF and TGF-β disrupts the
immunological balance of Th1/Th2 and Th17/Treg cells (40). The disruption of the gut microbiota
structure promotes inflammation and increases the number of
pathogenic microorganisms in the host (41). Additionally, the prolonged use of
ABXs time-dependently exacerbates the inflammatory response during
asthma attacks.
Most recent studies on asthma have focused on
symptom relief during acute asthma exacerbation. Meanwhile, the
present study focused on asthma triggers and mid-stage and
late-stage repair. ABXs are often used to treat infections during
asthma exacerbation. The present study demonstrated that the
administration of ABXs can disrupt the homeostasis of gut
microbiota, adversely affecting the recovery of asthma.
Additionally, the abundance of intestinal bacteria was
significantly downregulated, while that of opportunistic pathogens,
such as Proteobacteria and Lachnospira was
upregulated in the ABX-OVA, ABX-OVA 1, OVA-ABX and OVA-ABX 2
groups. Moreover, the inflammatory response and asthma symptoms
were aggravated upon ABX treatment. Prolonged ABX treatment
disrupted the balance of gut microbiota and the expression of TJP1,
CLDN1 and OCLN as evidenced by the manifestations of the ABX-OVA 2
and OVA-ABX 2 groups. These changes may induce inflammatory
responses and are not conducive to asthma alleviation during the
recovery period. Dysbiosis increases the risk and severity of acute
asthma attacks. The OVA-IgE and TGF-β expression levels and the
pathological scores in the ABX-OVA, ABX-OVA 2, OVA-ABX and OVA-ABX
2 groups were higher than those in the model and model 2 groups.
These findings indicated that the maintenance of gut microbiota
balance can prevent acute asthma attacks.
Acknowledgements
The authors would like to thank Yunnan Provincial
Key Lab of Molecular Biology for Sinomedicine for platform support
and use of their research site for the experiments.
Funding
Funding: The present study was supported by the National Nature
Science Foundations of China (grant nos. 81660765 and 81860778),
the Yunnan Applied Basic Research Projects (grant no.
2018FF001-017), and the Science and Technology Plan of Yunnan
Science and Technology Department (grant no.
202101AZ070001-171).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article and its supplementary
information files. The datasets generated and/or analyzed during
the current study are available in the National Center for
Biotechnology Information repository (https://www.ncbi.nlm.nih.gov/sra/PRJNA946646).
Authors' contributions
CLX, JC and CW designed the experiments and
completed the study. CLX, GBL and TZ established the asthma model.
CLX, CW and GBL were responsible for RT-qPCR and western blotting.
CLX, RLZ and GBL wrote the manuscript. CLX, CW, GBL, TZ and RLZ
made substantial contributions to the acquisition of data and
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
All animal experiments were approved (approval no.
R-06202085) by the Animal Ethics Committee of Yunnan University of
Traditional Chinese Medicine (Kunming, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shipp CL, Gergen PJ, Gern JE, Matsui EC
and Guilbert TW: Asthma management in children. J Allergy Clin
Immunol Prac. 11:9–18. 2023.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Voskamp AL, Kormelink TG, van Wijk RG,
Hiemstra PS, Taube C, de Jong EC and Smits HH: Modulating local
airway immune responses to treat allergic asthma: Lessons from
experimental models and human studies. Semin Immunopathol.
42:95–110. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Trivedi R and Barve K: Gut microbiome a
promising target for management of respiratory diseases. Biochem J.
477:2679–2696. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ye X, Wang A, Lin W, Xu Y, Dong X, Zhou Y,
Tian K and Xu X: The role of intestinal flora in anti-tumor
antibiotic therapy. Front Biosci (Landmark Ed).
27(281)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Francino MP: Antibiotics and the human gut
microbiome: Dysbioses and accumulation of resistances. Front
Microbiol. 6(1543)2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Cryan JF, O'Riordan KJ, Cowan CSM, Sandhu
KV, Bastiaanssen TFS, Boehme M, Codagnone MG, Cussotto S, Fulling
C, Golubeva AV, et al: The microbiota-gut-brain axis. Physiol Rev.
99:1877–2013. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
McAleer JP and Kolls JK: Contributions of
the intestinal microbiome in lung immunity. Eur J Immunol.
48:39–49. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ottman N, Reunanen J, Meijerink M, Pietilä
TE, Kainulainen V, Klievink J, Huuskonen L, Aalvink S, Skurnik M,
Boeren S, et al: Pili-like proteins of Akkermansia muciniphila
modulate host immune responses and gut barrier function. PLoS One.
12(e0173004)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Fan H, Wang A, Wang Y, Sun Y, Han J, Chen
W, Wang S, Wu Y and Lu Y: Innate lymphoid cells: Regulators of gut
barrier function and immune homeostasis. J Immunol Res.
2019(2525984)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Turner JR: Molecular basis of epithelial
barrier regulation: From basic mechanisms to clinical application.
Am J Pathol. 169:1901–1909. 2006.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kuo WT, Odenwald MA, Turner JR and Zuo L:
Tight junction proteins occludin and ZO-1 as regulators of
epithelial proliferation and survival. Ann N Y Acad Sci.
1514:21–33. 2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hufnagl K, Pali-Schöll I, Roth-Walter F
and Jensen-Jarolim E: Dysbiosis of the gut and lung microbiome has
a role in asthma. Semin Immunopathol. 42:75–93. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jia W, Xu C, Zhao T, Fan Q, Qiao B, Wu Y,
Yuan J and Chen J: Integrated network pharmacology and gut
microbiota analysis to explore the mechanism of sijunzi decoction
involved in alleviating airway inflammation in a mouse model of
Asthma. Evid Based Complement Alternat Med.
2023(1130893)2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Guo W, Zhou X, Li X, Zhu Q, Peng J, Zhu B,
Zheng X, Lu Y, Yang D, Wang B and Wang J: Depletion of gut
microbiota impairs gut barrier function and antiviral immune
defense in the liver. Front Immunol. 12(636803)2021.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Reikvam DH, Erofeev A, Sandvik A, Grcic V,
Jahnsen FL, Gaustad P, McCoy KD, Macpherson AJ, Meza-Zepeda LA and
Johansen FE: Depletion of murine intestinal microbiota: Effects on
gut mucosa and epithelial gene expression. PLoS One.
6(e17996)2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Edgar RC: UPARSE: Highly accurate OTU
sequences from microbial amplicon reads. Nat Methods. 10:996–998.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Brodin P: Immune-microbe interactions
early in life: A determinant of health and disease long term.
Science. 376:945–950. 2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Leonardi I, Gao IH, Lin WY, Allen M, Li
XV, Fiers WD, De Celie MB, Putzel GG, Yantiss RK, Johncilla M, et
al: Mucosal fungi promote gut barrier function and social behavior
via Type 17 immunity. Cell. 185:831–846.e814. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Han P, Gu JQ, Li LS, Wang XY, Wang HT,
Wang Y, Chang C and Sun JL: The association between intestinal
bacteria and allergic diseases-cause or consequence? Front Cell
Infect Microbiol. 11(650893)2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Brodin P: Immune-microbe interactions
early in life: A determinant of health and disease long term.
Science. 376:945–950. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Barcik W, Boutin RCT, Sokolowska M and
Finlay BB: The role of lung and gut microbiota in the pathology of
asthma. Immunity. 52:241–255. 2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lambrecht BN, Hammad H and Fahy JV: The
cytokines of asthma. Immunity. 50:975–991. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zou XL, Wu JJ, Ye HX, Feng DY, Meng P,
Yang HL, Wu WB, Li HT, He Z and Zhang TT: Associations between gut
microbiota and asthma endotypes: A cross-sectional study in south
china based on patients with newly diagnosed asthma. J Asthma
Allergy. 14:981–992. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Depner M, Taft DH, Kirjavainen PV,
Kalanetra KM, Karvonen AM, Peschel S, Schmausser-Hechfellner E,
Roduit C, Frei R, Lauener R, et al: Maturation of the gut
microbiome during the first year of life contributes to the
protective farm effect on childhood asthma. Nat Med. 26:1766–1775.
2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Raftery AL, Tsantikos E, Harris NL and
Hibbs ML: Links between inflammatory bowel disease and chronic
obstructive pulmonary disease. Front Immunol.
11(2144)2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Nieuwdorp M, Gilijamse PW, Pai N and
Kaplan LM: Role of the microbiome in energy regulation and
metabolism. Gastroenterology. 146:1525–1533. 2014.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Satokari R: High intake of sugar and the
balance between pro- and anti-inflammatory gut bacteria. Nutrients.
12(1348)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dicker AJ, Huang JTJ, Lonergan M, Keir HR,
Fong CJ, Tan B, Cassidy AJ, Finch S, Mullerova H, Miller BE, et al:
The sputum microbiome, airway inflammation, and mortality in
chronic obstructive pulmonary disease. J Allergy Clin Immunol.
147:158–167. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Guo MY, Chen HK, Ying HZ, Qiu FS and Wu
JQ: The role of respiratory flora in the pathogenesis of chronic
respiratory diseases. Biomed Res Int. 2021(6431862)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Wan J, Song J, Lv Q, Zhang H, Xiang Q, Dai
H, Zheng H, Lin X and Zhang W: Alterations in the gut microbiome of
young children with airway allergic disease revealed by
next-generation sequencing. J Asthma Allergy. 16:961–972.
2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Wu Y, Chen Y, Li Q, Ye X, Guo X, Sun L,
Zou J, Shen Y, Mao Y, Li C and Yang Y: Tetrahydrocurcumin
alleviates allergic airway inflammation in asthmatic mice by
modulating the gut microbiota. Food Funct. 12:6830–6840.
2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Arrieta MC, Stiemsma LT, Dimitriu PA,
Thorson L, Russell S, Yurist-Doutsch S, Kuzeljevic B, Gold MJ,
Britton HM, Lefebvre DL, et al: Early infancy microbial and
metabolic alterations affect risk of childhood asthma. Sci Transl
Med. 7(307ra152)2015.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Arrieta MC, Sadarangani M, Brown EM,
Russell SL, Nimmo M, Dean J, Turvey SE, Chan ES and Finlay BB: A
humanized microbiota mouse model of ovalbumin-induced lung
inflammation. Gut Microbes. 7:342–352. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Yang J, McDowell A, Seo H, Kim S, Min TK,
Jee YK, Choi Y, Park HS, Pyun BY and Kim YK: Diagnostic models for
atopic dermatitis based on serum microbial extracellular vesicle
metagenomic analysis: A pilot study. Allergy Asthma Immunol Res.
12:792–805. 2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tulstrup MV, Christensen EG, Carvalho V,
Linninge C, Ahrné S, Højberg O, Licht TR and Bahl MI: Antibiotic
treatment affects intestinal permeability and gut microbial
composition in wistar rats dependent on antibiotic class. PLoS One.
10(e0144854)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Hoedt EC, Hueston CM, Cash N, Bongers RS,
Keane JM, van Limpt K, Amor KB, Knol J, MacSharry J and van
Sinderen D: A synbiotic mixture of selected oligosaccharides and
bifidobacteria assists murine gut microbiota restoration following
antibiotic challenge. Microbiome. 11(168)2023.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Rouaud F, Vasileva E, Spadaro D, Tsukita S
and Citi S: R40.76 binds to the α domain of ZO-1: Role of ZO-1 (α+)
in epithelial differentiation and mechano-sensing. Tissue Barriers.
7(e1653748)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Fan L, Qi Y, Qu S, Chen X, Li A, Hendi M,
Xu C, Wang L, Hou T, Si J and Chen S: B. adolescentis ameliorates
chronic colitis by regulating Treg/Th2 response and gut microbiota
remodeling. Gut Microbes. 13:1–17. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wu X, Wei S, Chen M, Li J, Wei Y, Zhang J
and Dong W: P2RY13 Exacerbates intestinal inflammation by damaging
the intestinal mucosal barrier via activating IL-6/STAT3 pathway.
Int J Biol Sci. 18:5056–5069. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ahmad R, Sorrell MF, Batra SK, Dhawan P
and Singh AB: Gut permeability and mucosal inflammation: Bad, good
or context dependent. Mucosal Immunol. 10:307–317. 2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Baltazar-Díaz TA, González-Hernández LA,
Aldana-Ledesma JM, Peña-Rodríguez M, Vega-Magaña AN, Zepeda-Morale
ASM, López-Ro RI, Toro-Arreola SD, Martínez-López E, Salazar-Montes
AM and Bueno-Topete MR: Escherichia/Shigella, SCFAs, and metabolic
pathways-the triad that orchestrates intestinal dysbiosis in
patients with decompensated alcoholic cirrhosis from Western
Mexico. Microorganisms. 10(1231)2022.PubMed/NCBI View Article : Google Scholar
|