Introduction
Sepsis is a serious medical condition associated
with a severe systemic inflammation, termed systemic inflammatory
response syndrome, and the presence of a known infection. It can
evolve to septic shock, multiple organ dysfunction syndrome and
mortality (1). Sepsis is regarded
as the immune response of the host to fight the infection, being
characterized by pro- and anti-inflammatory responses (2), resulting in hemodynamic consequences,
metabolic derangement and damage to organs (3,4).
In sepsis, the behavior of the polymorphonuclear
neutrophils (PMN) changes and they become resistant to apoptosis
and, in addition, they induce the apoptosis of other cells, such as
CD4 + lymphocytes (5-7).
In an experimental study, it was demonstrated that PMN are
protective at the onset of sepsis, because they control the
bacteremia, but after the onset of sepsis they become harmful, as
they lose their innate immune functions (8). As activated PMN are nonspecific in
their function, they can harm the ‘innocent bystander’ cells and
induce tissue injury and further organ dysfunction (5,9). The
septic monocytes are resistant to apoptosis (10) and have reduced expression of the
major histocompatibility antigen HLA-DR (5,11,12).
One study found evidence supporting the idea that an early
circulating factor in severe sepsis/shock modulates the apoptosis
of CD4+ lymphocytes and monocytes (10). On the other hand, increased
apoptosis induces the decrease of dendritic cells and lymphocytes:
CD4+ T cells, CD8+ T cells and B cells (13). The T regulatory subcategory appears
to be more resistant to apoptosis in sepsis than the other subsets
of lymphocytes (5,13). One study also indicates the
decrease of NK lymphocytes in a cohort of septic patients with
purulent meningitis (14).
These changes of leukocytes have an effect on the
clinical course and outcome of patients with sepsis. Neutrophilia
in association with lymphopenia are correlated with the severity of
the clinical course (15-17).
The need for quick indicators to predict the evolution of patients
with sepsis has been evident for many years. In line with this,
different ratios were calculated from the cell blood count (CBC)
with promising value for the prediction and prevention of sepsis
mortality: Neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte
ratio (PLR), lymphocyte-monocyte ratio (LMR) (16,18,19).
These ratios are valuable also for the early prediction of neonatal
sepsis (20). Systemic
inflammation response index (SIRI), systemic inflammation index
(SII) and the aggregate index of systemic inflammation (AISI) were
found to predict the outcome in different pathologies, but
especially in COVID-19(21).
Considering these studies focused on finding early,
useful and inexpensive predictors for the outcome of patients with
sepsis, the main goal of the present study was to compare the
changes of the following parameters on day 1 and day 5, in sepsis
compared with septic shock, as well as in survivors compared with
non-survivors: Cell blood count parameters, neutrophil-lymphocyte
ratio (NLR), platelet-lymphocyte ratio (PLR) and systemic
inflammation index (SII), C reactive protein (CRP), ferritin,
procalcitonin (PCT), CD 3+ T cells, CD4+ T cells, CD8+ T cells,
CD16+CD56+/CD3-NK cells and CD19+ B cells. The relationship between
the subcategories of lymphocytes with the inflammatory markers were
also evaluated.
In Romania, these markers have not been evaluated in
sepsis, and the results of the various studies are contradictory.
In addition, the identification of such biomarkers as predictors of
the evolution in sepsis would be of great use in a country with
limited financial resources.
Materials and methods
The present study was a prospective observational
single center study, enrolling 102 patients with sepsis admitted in
the Intensive Care Unit (ICU) of the County Emergency Clinical
Hospital from Târgu Mureș (Mureș, Romania), between July 2021 and
March 2023.
The present study was approved by the Ethics
Committee of the University of Medicine and Pharmacy, Science, and
Technology ‘G.E. Palade’ from Târgu Mureș (Mureș, Romania; approval
no 1425/01.07.2021) and was conducted in accordance with the
Helsinki Declaration.
The current study included patients over 18 years of
age, diagnosed with sepsis according to the Sepsis 3 Consensus
criteria (22). Exclusion criteria
were cancer with current chemotherapy or radiation therapy,
treatment with corticosteroids or immunosuppressive medication, or
evidence of autoimmune disorders.
Informed consent for inclusion in the study was
obtained from each patient or legal guardian of patients, as well
as consent for publication of obtained data.
The studied parameters were: Age, sex, body mass
index (BMI), complete blood count (CBC), CRP, ferritin, PCT, T
cells (CD 3+), Th cells (CD4+), Tc cells (CD8+), NK cells
(CD16+CD56+/CD3-), B cells (CD19+). All these parameters were
evaluated on day 1 and day 5 after admission to the ICU. The
identification of leukocytes subsets was performed using a flow
cytometry (BD FACSCalibur; BD Biosciences) and they were quantified
as percentages (%). CBC was performed using a Sysmex XN-1000
analyzer (Sysmex Europe GmbH). For CBC and immunophenotyping venous
blood samples were collected in K2 EDTA tubes. From patients' serum
CRP (turbidimetry), ferritin (electrochemiluminescence immunoassay
ECLIA) and PCT (chemiluminescent immunoassay, CLIA) were determined
using Cobas c 501 analyzer (Roche Diagnostics).
BMI was calculated based on weight and height
(kg/m2).
The studied ratios and indexes were calculated as
follows: NLR as the neutrophil to lymphocyte ratio, PLR as the
platelet to lymphocyte ratio and SII as neutrophils x
platelets/lymphocytes.
Based on data reported in the literature, the
patients included in the study were divided according to the serum
ferritin concentration in two groups: One with serum ferritin
values <500 µg/l and one with serum ferritin values ≥500
µg/l.
Data was entered into MS Excel. Statistical,
descriptive and inferential processing was performed with the
GraphPad Prism 5 Demo version (Dotmatics). Means or medians with
confidence intervals were calculated for descriptive statistics.
The mean was calculated for data with a normal distribution, and
the median was calculated for those with a non-Gaussian
distribution. To establish the differences in the mean, the
Student's t test or the Mann Whitney test was used, depending on
the Gaussian or non-Gaussian distribution. For binary data the Chi
Square test was used. The regression tests used were Pearson's or
Spearman's. For receiver operating characteristic (ROC) analysis
SPSS 17.0 (SPSS, Inc.) was used. P<0.05 was considered to
indicate a statistically significant difference (23).
Results
The present study included 39 women (38.24%) and 63
men (61.76%). The mean age was 68 years (minimum 37 years old; max
90 years old). A total of 76 patients succumbed (74.51%) and 26
patients survived (25.49%). The mean BMI was 28.57±5.6 (minimum
15.60; maximum 49.40). A total of 40 patients (39.22%) evolved to
septic shock.
The underlying conditions in the study group were as
follows: Cardiovascular disorders (82 patients; 80.4%), renal
disorders (68 patients; 66.7%), respiratory disorders (63 patients;
61.8%), neurological disorders (46 patients; 45.1%), diabetes
mellitus (31 patients; 30.4%) and polytrauma (8 patients;
7.8%).
Table I shows the
values of the studied parameters on day 1 and day 5. Day 1 was
defined as the day on which the patient was clinically diagnosed
with sepsis.
 | Table IThe studied parameters on day 1 and
day 5. |
Table I
The studied parameters on day 1 and
day 5.
| Parameter | Day 1 | Day 5 | P-value |
|---|
| Leukocytes,
x103/µl | 14.42 | 13.50 | 0.80a |
| Neutrophils,
x103/µl | 12.02 | 11.69 | 0.98a |
| Lymphocytes,
x103/µl | 0.85 | 1.00 |
0.02a |
| Thrombocytes,
x103/µl | 213.00 | 223.00 | 0.67a |
| NLR | 15.39 | 11.38 | 0.11a |
| PLR | 272.10 | 214.7 | 0.20a |
| SII | 3224.00 | 3868.00 | 0.88a |
| CRP, mg/l | 179.30 | 131.90 |
0.01b |
| Ferritin, µg/l | 592.50 | 446.50 | 0.27a |
| PCT, ng/ml | 3.08 | 1.06 |
0.01a |
| T cells (CD3+),
% | 76.30 | 73.21 | 0.43a |
| Th cells (CD4+),
% | 63.53 | 65.6 | 0.70a |
| Tc cells (CD8+),
% | 30.59 | 30.74 | 0.74a |
| NK cells
(CD16+56+/CD3-), % | 8.00 | 8.45 | 0.74a |
| B cells (CD19+),
% | 12.80 | 10.30 | 0.25a |
The lymphocytes count was significantly higher, and
the serum concentration of CRP and PCT was significantly lower on
day 5 compared with day 1.
Table II compared
the studied parameters between patients with sepsis and those who
developed septic shock.
 | Table IIThe studied parameters in septic
shock vs. sepsis. |
Table II
The studied parameters in septic
shock vs. sepsis.
| Parameter | Septic shock | Sepsis | P-value |
|---|
| Leukocytes,
x103/µl | 16.22±1.61 | 16.68±1.34 | 0.83a |
| Neutrophils,
x103/µl | 14.26±1.49 | 14.23±1.22 | 0.98a |
| Lymphocytes,
x103/µl | 0.88±0.085 | 1.22±0.16 | 0.12a |
| Thrombocytes,
x103/µl | 239.70±24.46 | 239.10±16.41 | 0.98a |
| NLR | 18.32±1.70 | 16.63±1.43 | 0.45a |
| PLR | 326.20±34.60 | 320.90±35.31 | 0.91a |
| SII | 4415±551.80 | 4003±472.20 | 0.57a |
| CRP, mg/l | 182.00±20.18 | 177.5±13.74 | 0.84b |
| Ferritin, µg/l | 1436±235.50 | 811.60±137.80 |
0.01a |
| PCT, ng/ml | 25.92±17.82 | 8.80±2.77 | 0.19a |
| T cells (CD3+),
% | 63.76±2.68 | 83.11±11.85 | 0.23a |
| Th cells (CD4+),
% | 65.65±2.61 | 62.38±1.93 | 0.31b |
| Tc cells (CD8+),
% | 26.41±1.87 | 32.86±1.83 |
0.02a |
| NK cells
(CD16+56+/CD3-), % | 13.61±2.18 | 9.22±0.87 |
0.03a |
| B cells (CD19+),
% | 20.07±2.46 | 16.05±1.92 | 0.20a |
Among the markers of inflammation, ferritin was
significantly higher in patients with septic shock. The percentage
of cytotoxic T lymphocytes was significantly decreased and the
percentage of NK lymphocytes was significantly increased in
patients with septic shock.
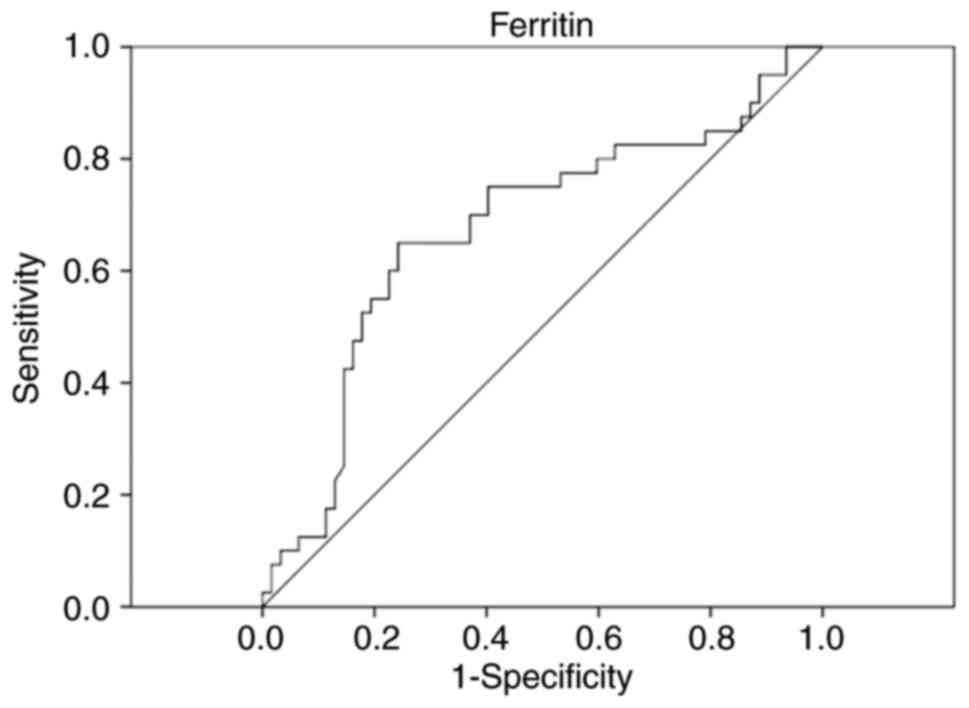
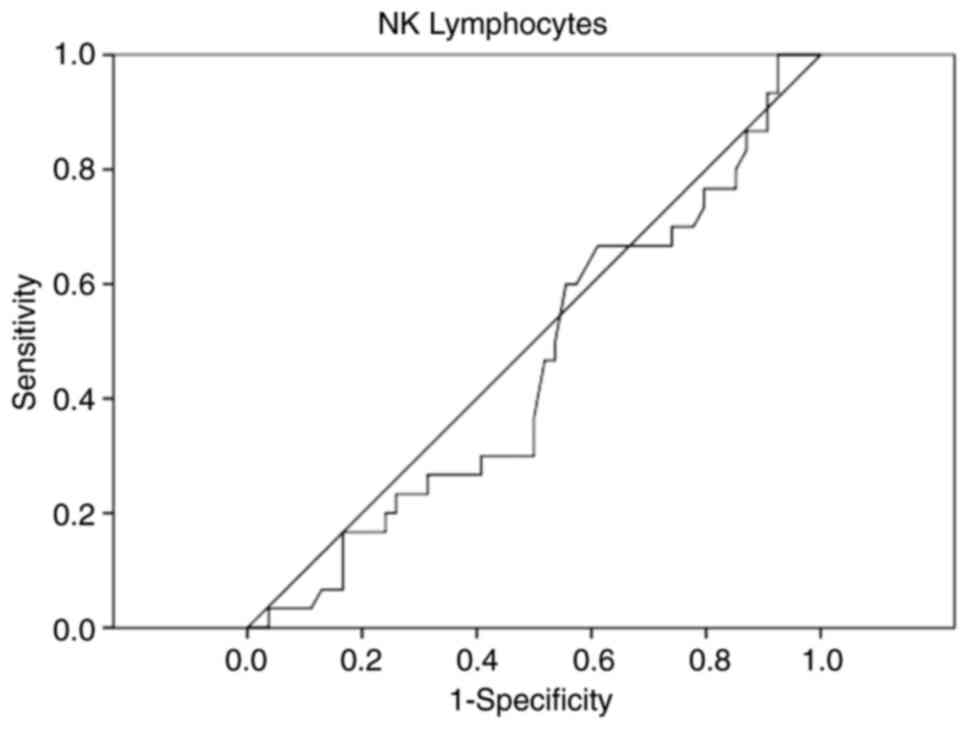
As statistically significant differences were
observed in ferritin and NK lymphocytes, ROC curve analysis was
performed to assess the early diagnostic value of these two markers
for discriminating between septic shock and sepsis, as can be seen
in Figs. 1 and 2.
Analyzing the ROC curves, only ferritin is important
in the early discrimination between sepsis and septic shock.
Among the 40 patients who developed septic shock, 30
patients (75%) had serum ferritin levels ≥500 µg/l (P=0.0005). The
mortality rate was also significantly higher in patients with
ferritin ≥500 µg/l (82.14% of these patients succumbed,
P=0.028).
Comparing the analyzed parameters between survivors
and non-survivors on admission, no significant difference were
obtained, as can be seen in Table
III.
 | Table IIIThe studied parameters in survivors
vs. non-survivors. |
Table III
The studied parameters in survivors
vs. non-survivors.
| Parameter | Survivors | Non-survivors | P-value |
|---|
| Age (years) | 64.57 | 69.33 | 0.08b |
| Sex M/F (no,
%) | 18, 28.57%/8,
20.51% | 45, 71.43%/31,
79.49% | 0.18c |
| BMI
(kg/m2) | 29.33 | 28.31 | 0.55a |
| Leukocytes,
x103/µl | 16.13 | 18.67 | 0.34a |
| Neutrophils,
x103/µl | 13.99 | 16.54 | 0.33a |
| Lymphocytes,
x103/µl | 1.11 | 1.15 | 0.82a |
| Thrombocytes,
x103/µl | 233.3 | 246.9 | 0.73a |
| NLR | 15.65 | 19.29 | 0.40a |
| PLR | 263.2 | 285.9 | 0.71a |
| SII | 3595.00 | 5416.00 | 0.34a |
| CRP, mg/l | 157.7 | 130.8 | 0.26b |
| Ferritin, µg/l | 742.10 | 817.10 | 0.71a |
| PCT, ng/ml | 4.75 | 9.81 | 0.29a |
| T cells (CD3+),
% | 72.18 | 71.77 | 0.91a |
| Th cells (CD4+),
% | 65.25 | 66.63 | 0.72a |
| Tc cells (CD8+),
% | 30.62 | 29.74 | 0.79a |
| NK cells
(CD16+56+/CD3-), % | 6.91 | 8.67 | 0.24a |
| B cells (CD19+),
% | 16.27 | 15.92 | 0.92a |
Table IV evaluated
the correlations between the subcategories of leukocytes and the
inflammation markers.
 | Table IVCorrelations between leukocyte
subcategories and inflammation markers. |
Table IV
Correlations between leukocyte
subcategories and inflammation markers.
| Parameter | CRP (mg/l) | Ferritin
(µg/l) | PCT (ng/ml) |
|---|
| Neutrophils,
x103/µl | ρ=0.10 | ρ=0.12 | ρ=0.17 |
| | (-0.10-0.30) | (-0.08-0.31) | (-0.10-0.43) |
| | aP=0.33 | aP=0.22 | aP=0.19 |
| T cells (CD3+)
% | ρ=-0.09 | ρ=-0.16 | ρ=-0.32 |
| | (-0.31-0.12) | (-0.37-0.04) | (-0.57-0.02) |
| | aP=0.38 | aP=0.11 | aP=0.03 |
| Th cells (CD4+)
% | r=0.007 | ρ=0.09 | ρ=-0.01 |
| | (-0.21-0.22) | (-0.11-0.30) | (-0.32-0.28) |
| | Pb=0.94 | aP=0.36 | aP=0.91 |
| Tc cells (CD8+)
% | ρ=-0.08 | ρ=-0.17 | ρ=-0.09 |
| | (-0.29-0.14) | (-0.37-0.04) | (-0.38-0.22) |
| | aP=0.46 | aP=0.09 | aP=0.55 |
| NK cells
(CD16+56+/CD3-) % | ρ=-0.10 | ρ=0.04 | ρ=-0.25 |
| | (-0.32-0.11) | (-0.16-0.26) | (-0.51-0.05) |
| | aP=0.32 | aP=0.65 | aP=0.09 |
| B cells (CD19+)
% | ρ=0.02 | ρ=0.08 | ρ=0.36 |
| | (-0.20-0.24) | (-0.13-0.29) | (0.06-0.60) |
| | aP=0.84 | aP=0.44 | aP=0.01 |
A negative significant correlation was observed
between the percentage of T lymphocytes and PCT concentration, and
a positive significant correlation between the percentage of B and
PCT concentration.
Discussion
The present study focused on finding early, useful
and inexpensive predictors for patients with sepsis. For this
purpose, the studied parameters were compared in a cohort of
patients admitted in ICU on day 1 and day 5, in sepsis compared
with septic shock, as well as in survivors compared with
non-survivors. Briefly, the results showed that the serum
concentration of CRP and PCT were significantly lower on day 5
compared with day 1 and serum ferritin was significantly higher in
patients with septic shock. The percentage of cytotoxic T
lymphocytes was significantly decreased and the percentage of NK
lymphocytes was significantly increased in patients who developed
septic shock.
The non-survivors were older than the survivors,
even if not significantly, probably due to associated chronic
diseases (e.g. diabetes mellitus, chronic obstructive pulmonary
disease) and altered immune response, which is similar the results
of other studies (24,25).
Despite its involvement in the pathogenesis of many
diseases (26,27), increasing BMI appears to offer an
advantage in the survival of patients with sepsis, as the BMI was
higher in survivors compared with non-survivors. This phenomenon
has been described as the obesity paradox (25,28,29).
The mortality rate in the present study group was
much higher, compared with the values reported in other studies
(30,31). This could be considered as a
consequence of an immune-paralysis due to an immunosuppressive
state that exposes patients to a secondary sepsis with bacteria,
viruses or fungi and might progress with uncontrolled inflammatory
response (32). These results
might suggest the need to improve the management of sepsis and
septic shock according to the patient immunologic profile.
Neutrophilia and lymphopenia are known hematological
changes in sepsis. The increase in neutrophils is due to the
release of immature neutrophils and delayed apoptosis of
circulating neutrophils (33). In
the present study the neutrophil count at admission was similar
between survivors and non-survivors, in accord with other findings
(24,34).
Furthermore, it is considered that some neutrophil
subsets can suppress the immune function of T cells through several
mechanisms: Depletion of L-arginine, release of reactive oxygen
species and interferon γ-induced programmed cell death ligand 1 and
apoptosis of T lymphocytes (33).
This last mechanism is especially important in sepsis (33,35).
On day 5, compared with day 1, an increase was observed in the
number of lymphocytes, as well as a decrease in inflammatory
markers, among which CRP and PCT decreased significantly, probably
because of the compensatory anti-inflammatory response syndrome, or
immune-paralysis (36). Ferritin
also decreased, although not significantly. The number of T helper,
cytotoxic, NK, and B lymphocytes varied very little between day 1
and 5, as it is known that both their number and their function
need several weeks to recover, in those who survive (33,36,37).
Sepsis modifies both the naive T-cell pool, as well as the memory T
cells, increasing the risk of secondary infections (38). When the patients with septic shock
were compared with those with sepsis it was observed that the
number of CD8 cells was significantly lower in septic shock and the
number of NK cells was significantly higher. The reported results
regarding the changes of CD8 cells in septic shock are
controversial. As in the present results, the CD8 cells decrease in
septic shock is the finding of one study (39), but according to another study, the
percentage of CD8+T lymphocytes in the septic shock group was
slightly higher than that in the sepsis group (40).
The number and function of B cells is also affected
in sepsis. According to some studies, although the number of B
cells decreases, the proportion of B cells in total lymphocytes
appears to increase and the circulating B cell number is reduced in
septic shock patients (41,42).
In the present study, the percentage of B cells was lower in day 5
compared with day 1, a consequence of apoptosis, but was not
significantly changed when sepsis was compared with septic
shock.
In terms of survival, in the present study, none of
the analyzed parameters was significantly changed. In one study,
the results indicate that the percentages of CD4+ lymphocytes and
CD19+ lymphocytes were lower in the non-survivor group, the
percentage of NK lymphocytes was higher in the non-survivor group
and there was no difference in the percentage of CD8+ lymphocytes
between the non-survivor and survivor groups (43). The present study obtained just a
mild decrease of CD19+ and a mild increase of NK cells in
non-survivors. Considering the low number of patients included in
the aforementioned study (43),
further testing, using a larger cohort of patients is needed to
evaluate these results. As many studies use a healthy control
group, the present study found limited information regarding the
dynamics of changes in lymphocyte subsets in sepsis/septic
shock.
In the present study, PLR, NLR and SII did not prove
useful for early indication of unfavorable evolution, since
significant differences between day 1 and day 5, between sepsis and
septic shock and between survivors and non-survivors were not
obtained. One study indicates NLR is higher in non-survivors, but
PLR values did not differ significantly between survivors and
non-survivors (24). However, the
results of the present study indicated a higher value of NLR in
non-survivors vs. survivors (19.29 vs. 15.65). In a cohort of 194
patients with sepsis, both NLR and PLR were significantly higher in
the non-survival group than in the survival group (44). The results of another study are
opposite, as NLR was reduced in the non-survivor group (34). One meta-analysis indicates
different changes of NLR and the outcomes in heterogeneous cohorts
of critically ill adults with sepsis and highlights the need to
evaluate NLR in future stratification models (45).
SII was evaluated in cohort of 209 patients with
sepsis and the results showed that it was significantly lower in
patients with sepsis compared with those with septic shock
(46). The results of the present
study were similar, even if not significant. An important
difference in SII value can be observed comparing the group of
survivors vs. that of non-survivors (3,595.00 vs. 5,416.00). As SII
is little investigated, further studies are necessary and SII will
be evaluated in a larger cohort of patients.
Inflammatory markers are used to diagnose and
monitor the evolution of patients with sepsis as well as the
treatment and for prognosis (47-49).
Among the three evaluated inflammatory markers, ferritin was
significantly increased in patients with septic shock, compared
with those with sepsis, as well as the percentage of NK cells,
probably because of the macrophage activation syndrome (MAS)
complicating sepsis, but no significant difference between
survivors and non-survivors was found. When the patients we
compared according to the ferritin threshold of 500 µg/l, ferritin
levels were significantly higher in patients with septic shock and
in non-survivors, similar to the results of one study (50). According to the results of one
study, high-level serum ferritin is an independent prognostic
marker for the prediction of mortality in patients with sepsis
(51).
PCT increased in patients with septic shock, even if
not significantly. The pathogenesis of MAS is not fully understood
and it is associated with increased activation of macrophages and
NK cells (52). Other studies
indicate that both CRP and PCT have poor predictive value referred
to 30-day all-cause mortality in patients admitted with sepsis or
septic shock (53) and that PCT
and CRP threshold values or their kinetics cannot predict
ventilator-associated pneumonia survival or septic shock
development (54). A study on the
administration of antibiotics in patients with COVID-19 indicates
that procalcitonin remains useful for associated bacterial
infection (55).
The present study tested possible correlation
between the three tested inflammatory markers and the changes of
lymphocytes subsets and found that PCT decreases with decreasing
proportion of T cells and increases with the increasing proportion
of B cells, probably related to the pathogenic phases of sepsis and
the functional abnormalities of T and B cells subsets during
sepsis.
The present study has some limitations. The limited
number of patients made it difficult to draw final conclusions. As
it was a single center study, there was some bias regarding the
overview of the pathology. In the future, the authors hope to
increase the study group and continue the evaluation of the tested
parameters on a larger cohort of patients.
In conclusion, the serum concentration of CRP and
PCT was significantly lower on day 5 compared with day 1 and serum
ferritin was significantly higher in patients with septic shock.
The percentage of cytotoxic T lymphocytes was significantly
decreased and the percentage of NK lymphocytes was significantly
increased in patients who developed septic shock. The results
indicated a negative significant correlation between the proportion
of T lymphocytes and PCT concentration, and a positive significant
correlation between the proportion of B and PCT concentration.
Regarding the value of the present study in clinical practice,
among the parameters tested, ferritin is important in predicting
early evolution towards septic shock.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the University of
Medicine, Pharmacy, Sciences and Technology ‘George Emil Palade’ of
Târgu Mureș (grant no. 10126/17.12.2020).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AB wrote the draft of the manuscript and contributed
to conception and design, acquisition of data, analysis and
interpretation of data; OC was responsible for investigation and
read and corrected the manuscript; VB was responsible for the study
design and performed the statistical analysis; AV read and
corrected the manuscript; IS, RF and BG were responsible for
investigation and read and corrected the manuscript. Data
authentication is not applicable. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the University of Medicine and Pharmacy, Science, and
Technology ‘G.E. Palade’ from Târgu Mureș (Mureș, Romania; approval
no 1425/01.07.2021) and was conducted in accordance with the
Helsinki Declaration.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
David S and Brunkhorst FM: Sepsis-3: What
has been confirmed in therapy? Internist (Berl). 58:1264–1271.
2017.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
2
|
Doganyigit Z, Eroglu E and Akyuz E:
Inflammatory mediators of cytokines and chemokines in sepsis: From
bench to bedside. Hum Exp Toxicol.
41(9603271221078871)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Huang M, Cai S and Su J: The pathogenesis
of sepsis and potential therapeutic targets. Int J Mol Sci.
20(5376)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gentile LF, Cuenca AG, Vanzant EL, Efron
PA, McKinley B, Moore F and Moldawer LL: Is there value in plasma
cytokine measurements in patients with severe trauma and sepsis?
Methods. 61:3–9. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rimmelé T, Payen D, Cantaluppi V, Marshall
J, Gomez H, Gomez A, Murray P and Kellum JA: ADQI XIV Workgroup.
Immune cell phenotype and function in sepsis. Shock. 45:282–291.
2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang JF, Li JB, Zhao YJ, Yi WJ, Bian JJ,
Wan XJ, Zhu KM and Deng XM: Up-regulation of programmed cell death
1 ligand 1 on neutrophils may be involved in sepsis-induced
immunosuppression: An animal study and a prospective case-control
study. Anesthesiology. 122:852–863. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Taneja R, Parodo J, Jia SH, Kapus A,
Rotstein OD and Marshall JC: Delayed neutrophil apoptosis in sepsis
is associated with maintenance of mitochondrial transmembrane
potential and reduced caspase-9 activity. Crit Care Med.
32:1460–1469. 2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Hoesel LM, Neff TA, Neff SB, Younger JG,
Olle EW, Gao H, Pianko MJ, Bernacki KD, Sarma JV and Ward PA:
Harmful and protective roles of neutrophils in sepsis. Shock.
24:40–47. 2005.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Pool R, Gomez H and Kellum JA: Mechanisms
of organ dysfunction in sepsis. Crit Care Clin. 34:63–80.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Vaki I, Kranidioti H, Karagianni V,
Spyridaki A, Kotsaki A, Routsi C and Giamarellos-Bourboulis EJ: An
early circulating factor in severe sepsis modulates apoptosis of
monocytes and lymphocytes. J Leukoc Biol. 89:343–349.
2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Venet F, Tissot S, Debard AL, Faudot C,
Crampé C, Pachot A, Ayala A and Monneret G: Decreased monocyte
human leukocyte antigen-DR expression after severe burn injury:
Correlation with severity and secondary septic shock. Crit Care
Med. 35:1910–1917. 2007.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Joshi I, Carney WP and Rock EP: Utility of
monocyte HLA-DR and rationale for therapeutic GM-CSF in sepsis
immunoparalysis. Front Immunol. 14(1130214)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hotchkiss RS, Monneret G and Payen D:
Immunosuppression in sepsis: A novel understanding of the disorder
and a new therapeutic approach. Lancet Infect Dis. 13:260–268.
2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Holub M, Klucková Z, Helcl M, Príhodov J,
Rokyta R and Beran O: Lymphocyte subset numbers depend on the
bacterial origin of sepsis. Clin Microbiol Infect. 9:202–211.
2003.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Liu H, Liu G and Tian Z: Changes in blood
lymphocytes in sepsis patients. Zhonghua Wei Zhong Bing Ji Jiu Yi
Xue. 26:148–152. 2014.PubMed/NCBI(In Chinese).
|
|
16
|
Zahorec R: Ratio of neutrophil to
lymphocyte counts-rapid and simple parameter of systemic
inflammation and stress in critically ill. Bratisl Lek Listy.
102:5–14. 2001.PubMed/NCBI(In English, Slovak).
|
|
17
|
Huang Z, Fu Z, Huang W and Huang K:
Prognostic value of neutrophil-to-lymphocyte ratio in sepsis: A
meta-analysis. Am J Emerg Med. 38:641–647. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kriplani A, Pandit S, Chawla A, de la
Rosette JJMCH, Laguna P, Jayadeva Reddy S and Somani BK:
Neutrophil-lymphocyte ratio (NLR), platelet-lymphocyte ratio (PLR)
and lymphocyte-monocyte ratio (LMR) in predicting systemic
inflammatory response syndrome (SIRS) and sepsis after percutaneous
nephrolithotomy (PNL). Urolithiasis. 50:341–348. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang G, Mivefroshan A, Yaghoobpoor S,
Khanzadeh S, Siri G, Rahmani F and Aleseidi S: Prognostic value of
platelet to lymphocyte ratio in sepsis: A systematic review and
meta-analysis. Biomed Res Int. 2022(9056363)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang S, Luan X, Zhang W and Jin Z:
Platelet-to-lymphocyte and neutrophil-to-lymphocyte ratio as
predictive biomarkers for early-onset neonatal sepsis. J Coll
Physicians Surg Pak. 30:821–824. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fois AG, Paliogiannis P, Scano V, Cau S,
Babudieri S, Perra R, Ruzzittu G, Zinellu E, Pirina P, Carru C, et
al: The systemic inflammation index on admission predicts
in-hospital mortality in COVID-19 patients. Molecules.
25(5725)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (sepsis-3). JAMA.
315:801–810. 2016.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Măruşteri M and Bacârea V: Comparing
groups for statistical differences: How to choose the right
statistical test? Biochem Med. 20:15–32. 2010.
|
|
24
|
Djordjevic D, Rondovic G, Surbatovic M,
Stanojevic I, Udovicic I, Andjelic T, Zeba S, Milosavljevic S,
Stankovic N, Abazovic D, et al: Neutrophil-to-lymphocyte ratio,
monocyte-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and
mean platelet volume-to-platelet count ratio as biomarkers in
critically ill and injured patients: Which ratio to choose to
predict outcome and nature of bacteremia? Mediators Inflamm.
2018(3758068)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kingren MS, Starr ME and Saito H:
Divergent sepsis pathophysiology in older adults. Antioxid Redox
Signal. 35:1358–1375. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bacârea A, Bacârea VC, Buicu F, Crăciun C,
Kosovski B, Guiné R and Tarcea M: Emotional eating sustainability
in Romania-A questionnaire-based study. Sustainability.
15(2895)2023.
|
|
27
|
Bacârea A, Tarcea M, Boţianu PVH, Ruţă F
and Bacârea V: Age cut-off for type 2 diabetes mellitus screening
amongst young adults from Mures District, Romania-A pilot study.
Obes Res Clin Pract. 9:527–530. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Schetz M, De Jong A, Deane AM, Druml W,
Hemelaar P, Pelosi P, Pickkers P, Reintam-Blaser A, Roberts J, Sakr
Y and Jaber S: Obesity in the critically ill: A narrative review.
Intensive Care Med. 45:757–769. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Kalani C, Venigalla T, Bailey J, Udeani G
and Surani S: Sepsis patients in critical care units with obesity:
Is obesity protective? Cureus. 12(e6929)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Bauer M, Gerlach H, Vogelmann T, Preissing
F, Stiefel J and Adam D: Mortality in sepsis and septic shock in
Europe, North America and Australia between 2009 and 2019-results
from a systematic review and meta-analysis. Crit Care.
24(239)2020.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bauer M, Groesdonk HV, Preissing F,
Dickmann P, Vogelmann T and Gerlach H: Mortality in sepsis and
septic shock in Germany. Results of a systematic review and
meta-analysis. Anaesthesist. 70:673–680. 2021.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
32
|
Fattahi F and Ward PA: Understanding
immunosuppression after sepsis. Immunity. 47:3–5. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hotchkiss RS, Monneret G and Payen D:
Sepsis-induced immunosuppression: From cellular dysfunctions to
immunotherapy. Nat Rev Immunol. 13:862–874. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
34
|
Riché F, Gayat E, Barthélémy R, Le Dorze
M, Matéo J and Payen D: Reversal of neutrophil-to-lymphocyte count
ratio in early versus late death from septic shock. Crit Care.
19(439)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Leliefeld PH, Wessels CM, Leenen LP,
Koenderman L and Pillay J: The role of neutrophils in immune
dysfunction during severe inflammation. Crit Care.
20(73)2016.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Boomer JS, Green JM and Hotchkiss RS: The
changing immune system in sepsis: Is individualized
immuno-modulatory therapy the answer? Virulence. 5:45–56.
2014.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Martin MD, Badovinac VP and Griffith TS:
CD4 T cell responses and the sepsis-induced immunoparalysis state.
Front Immunol. 11(1364)2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Danahy DB, Strother RK, Badovinac VP and
Griffith TS: Clinical and experimental sepsis impairs CD8
T-cell-mediated immunity. Crit Rev Immunol. 36:57–74.
2016.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Chen R, Qin S, Zhu H, Chang G, Li M, Lu H,
Shen M, Gao Q and Lin X: Dynamic monitoring of circulating
CD8+ T and NK cell function in patients with septic
shock. Immunol Lett. 243:61–68. 2022.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Peng Y, Wang X, Yin S and Wang M: A new
indicator: The diagnostic value of CD8+T/B lymphocyte ratio in
sepsis progression. Int J Immunopathol Pharmacol.
36(3946320221123164)2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Ma C, Liu H, Yang S, Li H, Liao X and Kang
Y: The emerging roles and therapeutic potential of B cells in
sepsis. Front Pharmacol. 13(1034667)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Gustave CA, Gossez M, Demaret J, Rimmelé
T, Lepape A, Malcus C, Poitevin-Later F, Jallades L, Textoris J,
Monneret G and Venet F: Septic shock shapes B cell response toward
an exhausted-like/immunoregulatory profile in patients. J Immunol.
200:2418–2425. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Chen X and Ye J and Ye J: Analysis of
peripheral blood lymphocyte subsets and prognosis in patients with
septic shock. Microbiol Immunol. 55:736–742. 2011.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Tian T, Wei B and Wang J: Study of
C-reactive protein, procalcitonin, and immunocyte ratios in 194
patients with sepsis. BMC Emerg Med. 21(81)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Russell CD, Parajuli A, Gale HJ, Bulteel
NS, Schuetz P, de Jager CPC, Loonen AJM, Merekoulias GI and Baillie
JK: The utility of peripheral blood leucocyte ratios as biomarkers
in infectious diseases: A systematic review and meta-analysis. J
Infect. 78:339–348. 2019.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Ma K, Zhang Y, Hao J, Zhao J, Qi Y and Liu
C: Correlation analysis of systemic immune inflammatory index,
serum IL-35 and HMGB-1 with the severity and prognosis of sepsis.
Pak J Med Sci. 39:497–501. 2023.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Pierrakos C, Velissaris D, Bisdorff M,
Marshall JC and Vincent JL: Biomarkers of sepsis: Time for a
reappraisal. Crit Care. 24(287)2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Faix JD: Biomarkers of sepsis. Crit Rev
Clin Lab Sci. 50:23–36. 2013.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Ryu JA, Yang JH, Lee D, Park CM, Suh GY,
Jeon K, Cho J, Baek SY, Carriere KC and Chung CR: Clinical
usefulness of procalcitonin and c-reactive protein as outcome
predictors in critically ill patients with severe sepsis and septic
shock. PLoS One. 10(e0138150)2015.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Knaak C, Nyvlt P, Schuster FS, Spies C,
Heeren P, Schenk T, Balzer F, La Rosée P, Janka G, Brunkhorst FM,
et al: Hemophagocytic lymphohistiocytosis in critically ill
patients: Diagnostic reliability of HLH-2004 criteria and HScore.
Crit Care. 24(244)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Fang YP, Zhang HJ, Guo Z, Ren CH, Zhang
YF, Liu Q, Wang Z and Zhang X: Effect of serum ferritin on the
prognosis of patients with sepsis: Data from the MIMIC-IV database.
Emerg Med Int. 2022(2104755)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Karakike E and Giamarellos-Bourboulis EJ:
Macrophage activation-like syndrome: A distinct entity leading to
early death in sepsis. Front Immunol. 10(55)2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Schupp T, Weidner K, Rusnak J, Jawhar S,
Forner J, Dulatahu F, Dudda J, Brück LM, Hoffmann U, Bertsch T, et
al: C-reactive protein and procalcitonin during course of sepsis
and septic shock. Ir J Med Sci. 193:457–468. 2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Hillas G, Vassilakopoulos T, Plantza P,
Rasidakis A and Bakakos P: C-reactive protein and procalcitonin as
predictors of survival and septic shock in ventilator-associated
pneumonia. Eur Respir J. 35:805–811. 2010.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Stoichitoiu LE, Pinte L, Ceasovschih A,
Cernat RC, Vlad ND, Padureanu V, Sorodoc L, Hristea A, Purcarea A,
Badea C and Baicus C: In-hospital antibiotic use for COVID-19:
Facts and rationales assessed through a mixed-methods study. J Clin
Med. 11(3194)2022.PubMed/NCBI View Article : Google Scholar
|