Introduction
For patients with hepatocellular carcinoma and
cirrhosis, venous hypertension can lead to lymph fluid stasis in
the lymphatic plexus. The rupture of thin lymphatic vessel walls in
some areas can lead to a profuse outflow of lymph fluid, eventually
leading to chylothorax (1).
Thoracic duct ligation (TDL) and thoracic duct interventional
embolization are commonly used treatments for traumatic chylothorax
(2). The present study details the
unique case of a patient who developed chylothorax following
cirrhosis, amidst congenital lymphatic system abnormalities. A
chylothorax is the accumulation of chyle in the pleural space. This
is most commonly seen following traumatic disruption of the
thoracic duct and is typically diagnosed based on the milky
appearance of fluid due to high-fat content. Most patients with
chylothoraces require surgical exploration of the thoracic duct.
Where TDLs fail, side-to-end lymphatic venous anastomosis (LVA) can
offer an alternative. LVA reroutes lymphatic fluid directly into
the bloodstream, bypassing the thoracic duct. The success of this
procedure highlights LVA's potential as an effective treatment for
chylothorax, especially in cases where traditional methods like TDL
are unsuccessful. Each case of chylothorax requires an
individualized approach, yet the findings of this study offer
valuable insight into the expanding surgical options for this
complex condition.
Case report
A 33-year-old man presented to Guangxi Medical
University Cancer Hospital in January 2022. The patient presented
with dull pain in the right upper abdomen without any other
discomfort such as abdominal distension or diarrhea. Computed
tomography examination previously performed in a local hospital
indicated that the patient had massive liver cancer with multiple
intrahepatic metastases in the right lobe of the liver.
Furthermore, the examination also indicated that there was a tumor
embolus formation (12.5x10.0x7.5 cm) in the right branch of the
portal vein. The patient presented at Guangxi Medical University
Cancer Hospital with an alpha fetoprotein level of 1,210 ng/ml.
After 3 days, a surgical procedure, right hemihepatectomy with
portal vein thrombectomy, was performed to treat primary liver
cancer located in the right lobe of the liver. The cancer was
complicated by portal vein thrombosis (BCLC Stage C and CNLC IIIB
stage) (3,4). During the surgery, the medical team
discovered that the cancer had invaded the right adrenal gland,
which was then partially removed. A spontaneous chylothorax was
identified in the left chest region postoperatively. In February
2022, the patient underwent a trans-thoracoscopic low TDL; however,
their symptoms did not improve postoperatively, with daily chest
drainage of chyle fluid ranging from 1,500 to 2,000 ml. The patient
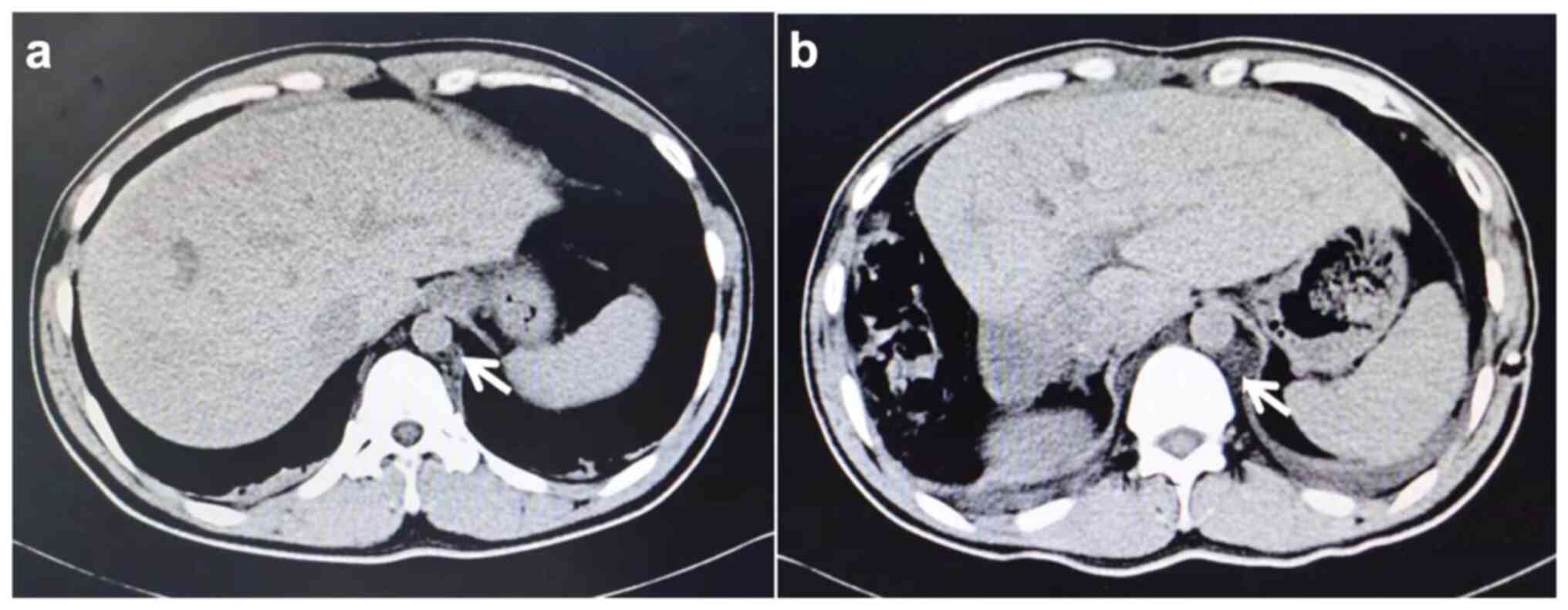
was assessed using computed tomography (CT) and then diagnosed with
massive hepatocellular carcinoma in combination with cirrhosis. CT
findings showed dilated choroidal plexus, and the presence of
multiple cystic foci of vascular origin in the inferior vena cava
and abdominal aorta (Fig. 1).
In April 2022, a high TDL was performed, following
which aggravated the tortuous expansion of the thoracic duct, and
multiple points of lymphatic fluid exudation were observed. A leak
was found below the 8th rib, with exudation of a large amount of
milky white lymphatic fluid. A hemiazygos vein and its bifurcations
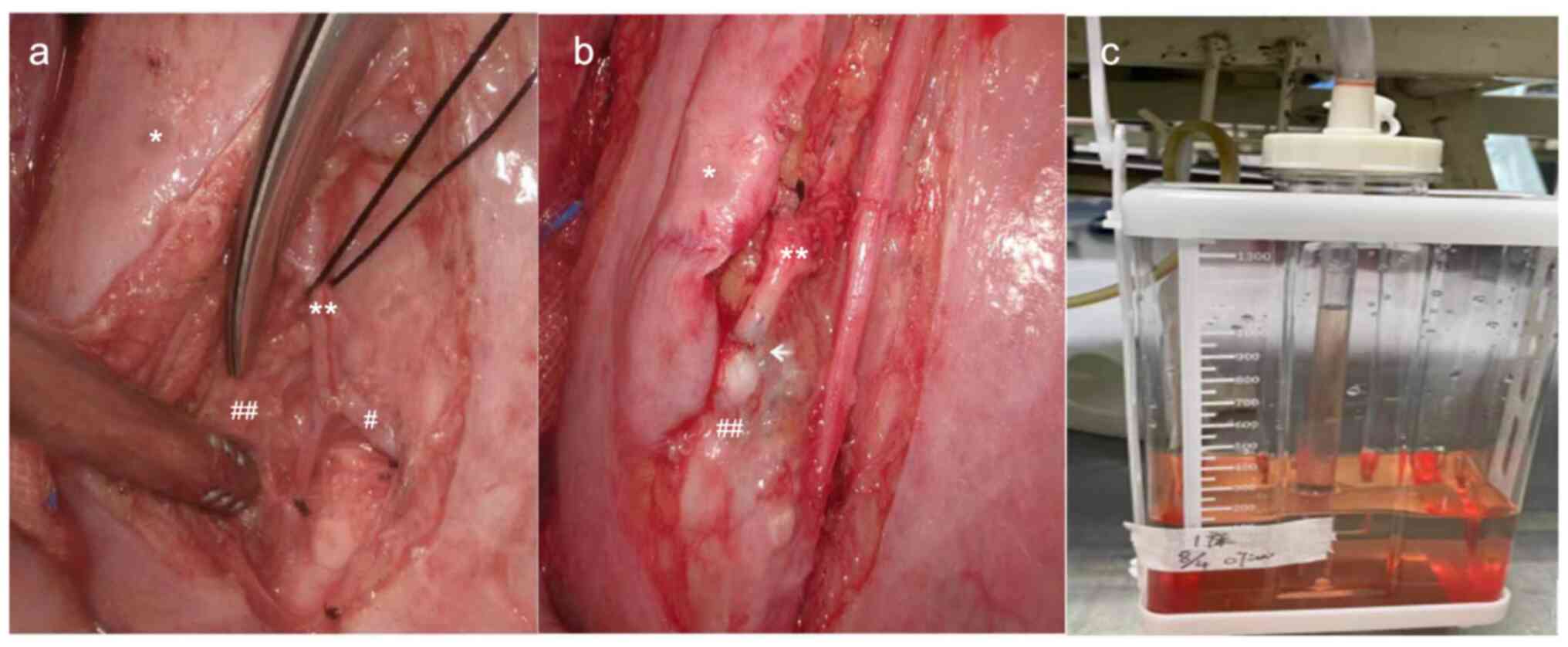
were observed next to the leak. Therefore, a side-to-end LVA at the
location of the leak was performed to re-establish lymphatic
circulation and reduce intra-thoracic pressure in the lymphatic
vessels (Fig. 2A and B). After completion of the anastomosis,
milky white lymphatic fluid was observed entering the vein, and the
diameter of the previously dilated lymphatic plexus was reduced. No
lymphatic fluid leaked from the original multiple exudation points
after ~3 min. On postoperative day 1, the drainage fluid was clear
(volume, 200 ml; Fig. 2C). The
patient was discharged 3 days later and the chest drain was
removed.
Discussion
Chyloperitoneum and chylothorax may result from
surgical trauma or congenital lymphatic system abnormalities
(5). In the present case report,
the surgical procedure for treating liver cancer did not involve
the thoracic region; therefore, chylothorax was not caused by the
operation. To the best of our knowledge, no previous studies have
reported spontaneous chylothorax without abdominal chyle in adults
with cirrhosis. Additional research is required to determine
whether a dilated non-venous plexus exists in patients with a
history of cirrhosis and liver cancer who are undergoing chest and
abdominal CT, as to the best of our knowledge this has not been
previously reported. If so, it may be necessary to clarify whether
the patient has congenital dysplasia of the lymphatic system. When
performing the liver cancer-related treatment, clinicians may need
to consider prophylactic thoracic duct-internal jugular vein
anastomosis. Abnormal congenital development of the lymphatic
system in the patient assessed in the present study was the cause
of the chylothorax in the absence of abdominal chyle and the reason
for the previous TDL failure. The presence of abnormal lymphatic
system development should be suspected in patients with
hepatocellular carcinoma when CT findings indicate abnormal
vascular dilatation. LVA can be performed as a salvage surgery for
patients who have undergone TDL and thoracic catheter
interventional embolization with no postoperative improvement.
Acknowledgements
Not applicable.
Funding
Funding: This case report was supported by the Guangxi Medical
and Health Suitable Technology Development and Popularization
Application Project (grant no. S2020095).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XL and YJ designed and performed the surgical plan.
CL, YH, YJ and NM followed-up the patient, drafted the article and
all authors have read and approved the final version of the
manuscript. XL and YJ confirm the authenticity of all the raw
data.
Ethics approval and consent to
participate
Informed consent was obtained from the patient.
Patient consent for publication
The patient provided written informed consent for
the publication of this case report.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Nomura M, Ohta H, Minegishi K, Akimoto M,
Hamamoto K and Yamaguchi Y: Spouting chylothorax in Gorham-Stout
disease. Am J Respir Crit Care Med. 205:e53–e54. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Seeger M and Bewig B: Images in clinical
medicine. Ultrasound imaging of the thoracic duct. N Engl J Med.
359(e28)2008.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Reig M, Forner A, Rimola J, Ferrer-Fàbrega
J, Burrel M, Garcia-Criado Á, Kelley RK, Galle PR, Mazzaferro V,
Salem R, et al: BCLC strategy for prognosis prediction and
treatment recommendation: The 2022 update. J Hepatol. 76:681–693.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xie DY, Ren ZG, Zhou J, Fan J and Gao Q:
2019 Chinese clinical guidelines for the management of
hepatocellular carcinoma: Updates and insights. Hepatobiliary Surg
Nutr. 9:452–463. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Witte MH and Williams WH: Chylothorax and
chyloperitoneum. N Engl J Med. 354(879)2006.PubMed/NCBI View Article : Google Scholar
|