Introduction
Glucose transporter isoform 1 (GLUT1) is expressed
in a variety of human and animal tissues such as blood vessels,
muscles, liver, and skin (1). It
plays an important role in transporting glucose into the cytoplasm
(1). Various malignant tumors
upregulate the expression of GLUT1 to facilitate cellular glucose
uptake to boost their rapid growth and progression (2-5).
The expression of GLUT1 is associated with
[18F]-2-fluro-2-deoxy-D-glucose uptake in positron
emission tomography in various types of cancers such as
cholangiocellular carcinoma (6)
and ovarian cancer (7).
Furthermore, GLUT1 expression is associated with tumor
proliferation, angiogenesis and survival; GLUT1 is regarded as a
prognostic marker in several types of cancer (2,5,8).
Baer et al (9) investigated
GLUT1 expression in several types of skin tumors. This study showed
positive staining in cutaneous squamous cell carcinoma. By
contrast, GLUT1 staining was negative in benign nevi and seborrheic
keratosis (9). Other studies have
reported that metastatic melanoma lesions have stronger GLUT1
immunostaining compared with primary lesions (10,11).
In addition, GLUT1 suppression significantly inhibits melanoma cell
growth and hepatic metastases in mouse models (10).
Although the association between GLUT1 expression
levels and tumor aggressiveness has been investigated in a variety
of cancers, to the best of our knowledge, it has not been studied
in extramammary Paget's disease (EMPD). Once EMPD tumor cells
invade the dermis, they frequently metastasize to lymph nodes and
other organs, resulting in a worse prognosis (12). Thus, the present study investigated
the relationship between GLUT1 expression levels and the degree of
tumor progression in EMPD.
Materials and methods
Patient samples
All samples used in this study were obtained from
patients who underwent surgery or biopsy in the Department of
Dermatology, Hirosaki University Hospital between 2005 and 2018.
All patients with EMPD whose tumor samples were available were
included in this study. The relationship between GLUT1 expression
levels and the degree of tumor progression was investigated in 51
patients with EMPD, including 23 with only intraepidermal lesions
and 28 with dermal-invasive lesions. Among 51 patients, 27 (52.9%)
were females and 24 (47.1%) were males. Age ranged from 51 to 93
years (median, 73 years) (Table
SI). Of 28 patients with dermal invasion, 13 had lymph node
metastasis. Overall, nine of the patients had available samples of
lymph node metastasis (Table I).
The present study was approved by the institutional review board of
Hirosaki University Graduate School of Medicine (Hirosaki, Japan;
approval no. 2021-116). This study was conducted in accordance with
the ethical principles of the Declaration of Helsinki.
 | Table IOutcomes and GLUT1 staining scores in
patients who have EMPD with lymph node metastasis. |
Table I
Outcomes and GLUT1 staining scores in
patients who have EMPD with lymph node metastasis.
| | GLUT1 staining
score |
|---|
| Patient no. | Age (years) | Sex | Outcome | Intraepidermal
lesion | Invasive lesion | Metastatic
lesion |
|---|
| 3 | 70 | M | Died of EMPD | 1 | 4 | 4 |
| 6 | 66 | F | Died of EMPD | 2 | 3 | 4 |
| 7 | 79 | F | Died of EMPD | 0 | 1 | NA |
| 8 | 70 | F | Survived for 3 years
after surgery without recurrence | 1 | 4 | NA |
| 9 | 78 | M | Completed 5-year
follow-up without recurrence | 1 | 2 | 2 |
| 10 | 51 | F | NA | 0 | 0 | 4 |
| 11 | 76 | M | NA | 1 | 2 | 2 |
| 12 | 63 | F | Died of EMPD | 0 | 0 | 4 |
| 13 | 82 | F | NA | 0 | 1 | 3 |
| 14 | 67 | M | Died of EMPD | 1 | 3 | 4 |
| 20 | 74 | M | Died of EMPD | 0 | 1 | 4 |
| 26 | 66 | M | NA | 1 | 4 | NA |
| 27 | 77 | F | NA | 2 | 2 | NA |
Immunohistochemistry
Tumor tissue samples were fixed in 10% buffered
formalin for 24-48 h at room temperature. GLUT1
immunohistochemistry was performed on formalin-fixed,
paraffin-embedded samples using anti-GLUT1 primary antibody
(ready-to-use solution; cat. no. #760-4526; Roche Diagnostics).
Briefly, tissue samples were cut into 5-µm thick sections and
deparaffinized using xylene and a graded alcohol series. For
antigen retrieval, sections were autoclaved in 10 mM sodium citrate
buffer (pH 6.0) for 10 min at 125˚C. These sections were washed
with distilled water three times and incubated in 0.3%
H2O2 at room temperature for 10 min to block
endogenous peroxidase activity. Sections were incubated with
primary antibody at 4˚C overnight. Next, the sections were
incubated with secondary antibody (anti-rabbit Poly-HRP-IgG,
ready-to-use solution, BOND Polymer Refine Detection Kit, cat. no.
DS9800; Leica Biosystems) at room temperature for 30 min. The sites
of GLUT1 localization were visualized with diaminobenzidine.
Sections were counterstained with hematoxylin for 10-20 sec at room
temperature for microscopic examination. A total of five random
fields of view on each slice were evaluated under high
magnification (magnification, x400) using a light microscope (BX43;
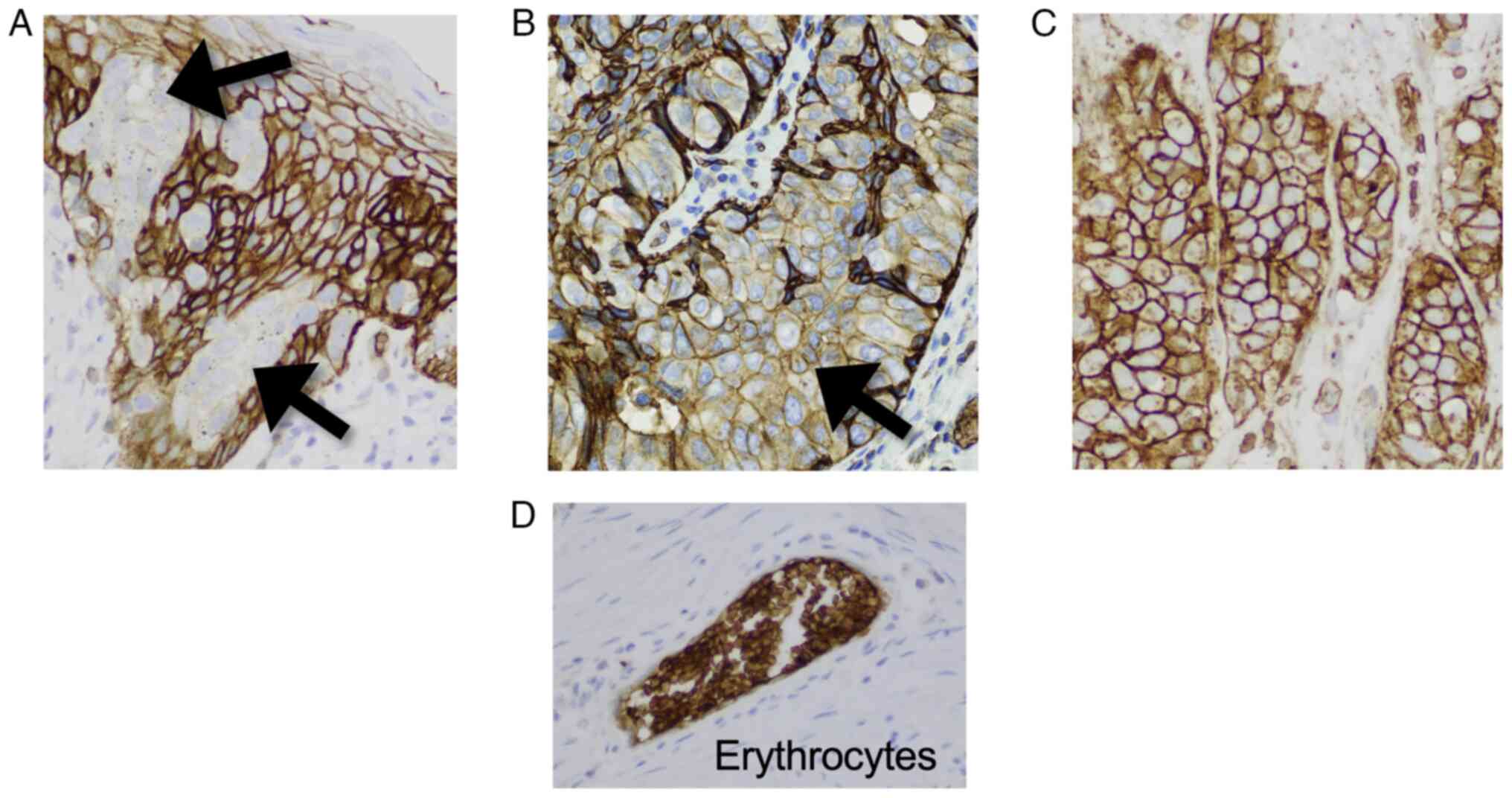
Olympus Corporation). Staining intensity was categorized as
negative, weak or strong with reference to the immunostaining of
erythrocytes, which were scored as strong, as described previously
(Fig. 1) (10). The percentage of positively stained
cells was rated using a semiquantitative scale as 0-10%, 11-50% or
51-100%. Staining results were scored from 0 to 4 according to a
previously reported scoring system based on the intensity and
percentage of positively stained cells (Table II) (6). Quantification was performed by two
independent investigators in a blinded manner. Staining results
were independently scored for intraepidermal, dermal-invasive and
metastatic lesions in each sample.
 | Table IIScoring system for GLUT1
immunohistochemistry. |
Table II
Scoring system for GLUT1
immunohistochemistry.
| | Staining
intensity |
|---|
| Stained cells
(%) | Weak | Strong |
|---|
| 0-10 | 0 | 2 |
| 11-50 | 1 | 3 |
| 51-100 | 2 | 4 |
Statistical analysis
The Wilcoxon matched-pair signed-rank test was used
for comparison between intraepidermal and dermal-invasive lesions.
One-way analysis of variance with Friedman's test and Dunn's
post-hoc multiple comparisons test were used for comparisons of
intraepidermal lesions vs. dermal-invasive lesions vs. metastatic
lesions. The Mann-Whitney U test was used to analyze the
significance of the differences between patients with only
intraepidermal lesions vs. those with both intraepidermal and
dermal-invasive lesions. Multiple logistic regression was performed
to assess the relationship between GLUT1 scores and dermal
invasion. There was adjustment for age and sex as confounding
factors. Statistical analyses were performed with GraphPad Prism
software, version 8.4.3 (Dotmatics). P<0.05 was considered to
indicate a statistically significant difference.
Results
GLUT1 staining scores are higher in
dermal-invasive lesions compared with intraepidermal lesions
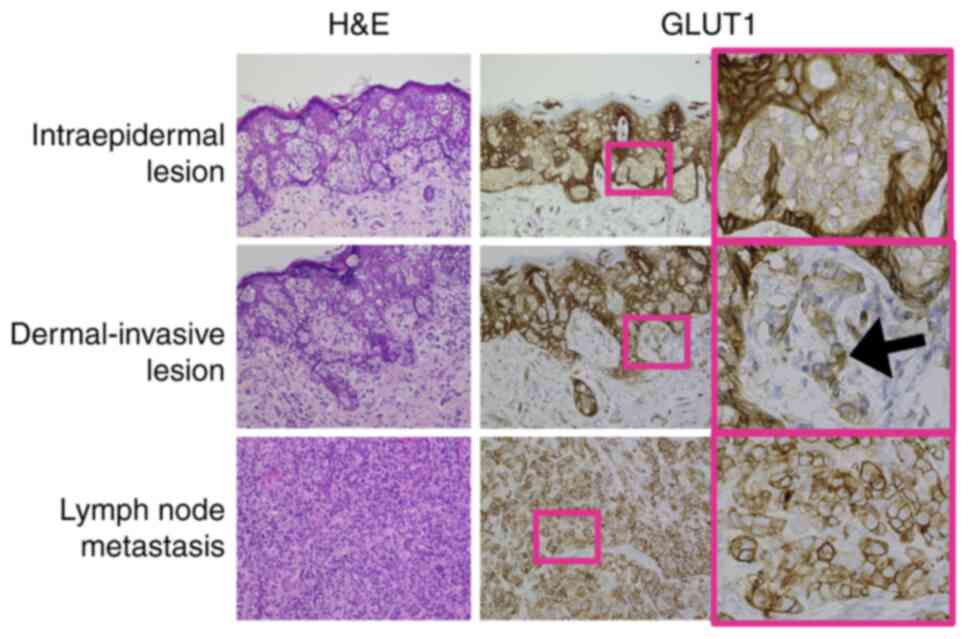
GLUT1 staining in intraepidermal tumor nests was
faint compared with staining in surrounding normal keratinocytes
(Fig. 2, upper panels). GLUT1
staining was stronger in dermal-invasive cells compared with in
intraepidermal tumor cells. GLUT1 staining was positive in both the
plasma membrane and cytoplasm (Fig.
2, middle panels, black arrow). Among patients who had
dermal-invasive EMPD, GLUT1 staining scores were statistically
higher in invasive lesions compared with those in intraepidermal
lesions (n=28; P<0.0001; Fig.
3A). Multiple logistic regression analysis revealed that GLUT1
staining scores were associated with dermal invasion after
adjusting for age and sex (odds ratio, 2.399; 95% confidence
interval, 1.149-5.612; P=0.0282).
GLUT1 staining scores are higher in
metastatic lesions compared with intraepidermal lesions
In most of the current metastatic samples, GLUT1
staining was strong, particularly in the plasma membrane and the
cytoplasm (Fig. 2, lower panels).
Among patients from whom intraepidermal, dermal-invasive and
metastatic samples were all available, GLUT1 staining scores were
significantly higher in the metastatic lesions compared with the
intraepidermal lesions (n=9; P=0.0008; Fig. 3B). Because of the small sample
size, survival analysis was not performed. Among nine patients with
lymph node metastases available for analysis, at least five
patients died of EMPD progression; four of these five patients had
metastatic lesions with GLUT1 staining scores of 4 (Table I). On the other hand, a patient who
achieved 5-year recurrence-free survival after lymph node
dissection had a GLUT1 score of 2, even in metastatic lesions
(patient 9).
Patients with invasive EMPD had higher
intraepidermal tumor cell GLUT1 staining scores compared with
patients with only in situ EMPD
The present study compared GLUT1 staining scores of
intraepidermal lesions between EMPD lesions with vs. without dermal
invasion (i.e., intraepidermal lesion only) to evaluate whether
GLUT1 expression was upregulated in preinvasive lesions. GLUT1
scores were significantly higher in intraepidermal tumor cells of
dermal-invasive EMPD (n=28) compared with tumor cells of only in
situ EMPD (n=23) (P=0.0338; Fig.
3C).
GLUT1 staining scores in EMPD are
independent of glycometabolism
The present study evaluated the association between
diabetes mellitus and GLUT1 staining scores because GLUT1 was
reported to be upregulated by blood glucose and insulin (13). The GLUT1 scores of intraepidermal
tumors were compared between patients with diabetes (n=10) and
without diabetes (n=39) (Table
SI). Two patients were excluded because of missing data. There
were no significant differences in GLUT1 staining scores between
the two groups (P=0.858; Fig. 3D).
This finding suggests that GLUT1 staining scores in EMPD were
independent of glycometabolism.
Discussion
In the present immunohistochemical analyses, GLUT1
was upregulated during the transition from preinvasive tumor to
invasive or metastatic tumor in EMPD. GLUT1 was already upregulated
even during the preinvasive phase in patients with invasive EMPD,
suggesting that GLUT1 immunostaining can predict the risk of dermal
invasion. Collectively, the expression of GLUT1 is upregulated in
EMPD tumor cells that have more aggressive and malignant features.
However, the number of patients was not large enough to perform a
statistical analysis of prognosis. Thus, further studies with
larger cohorts and longer follow-up periods are needed to confirm
that GLUT1 expression levels are associated with prognosis in
patients with EMPD.
Previous studies have reported that metastatic
melanoma lesions have stronger GLUT1 immunostaining than primary
lesions (10,11). In addition, shRNA-suppression of
GLUT1 inhibits melanoma cell growth and hepatic metastases in mouse
models (10). Treatment with a
specific small molecule inhibitor of GLUT1, WZB117, caused a
dose-dependent reduction in glucose consumption, proliferation and
apoptosis resistance in melanoma and lung cancer cell lines
(10,14). Furthermore, GLUT1 plays a role in
the development of resistance to anticancer agents in some types of
cancer such as laryngeal cancer and breast cancer (15,16).
Inhibition of GLUT1 using the natural flavonoid apigenin results in
overcoming chemoresistance to cisplatin (15,17).
Based on these data, GLUT1 is a promising target molecule for
personalized therapeutic approaches to treating patients with
invasive or metastatic EMPD.
To the best of our knowledge, this is the first
report on GLUT1 upregulation in invasive and metastatic EMPD. The
current study has several limitations. This is a retrospective
study. In addition, information on follow-up and comorbidities was
not available for several patients. Thus, the present study was not
able to perform survival analyses. The data provides new evidence
to pursue future in vitro and in vivo studies to
confirm that GLUT1 expression enhances tumor aggressiveness in
EMPD.
Supplementary Material
Clinicopathological parameters of 51
patients with extramammary Paget’s disease.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
MM, HN, EA and DR contributed to the conception and
design of the study. MM, TS and HM acquired the data. MM, YM, DS
and DR interpreted the data and conducted statistical analyses. MM
drafted the original manuscript. DR, HN, EA and DS provided
supervision. DR critically revised the manuscript. MM and DR
confirmed the authenticity of the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the Declaration of Helsinki and was approved by the institutional
review board of Hirosaki University Graduate School of Medicine
(approval no. 2021-116). An opt-out approach was used to obtain
informed consent from study participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Olson AL and Pessin JE: Structure,
function, and regulation of the mammalian facilitative glucose
transporter gene family. Annu Rev Nutr. 16:235–256. 1996.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wang J, Ye C, Chen C, Xiong H, Xie B, Zhou
J, Chen Y, Zheng S and Wang L: Glucose transporter GLUT1 expression
and clinical outcome in solid tumors: A systematic review and
meta-analysis. Oncotarget. 8:16875–16886. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kitamura K, Hatano E, Higashi T, Narita M,
Seo S, Nakamoto Y, Yamanaka K, Nagata H, Taura K, Yasuchika K, et
al: Proliferative activity in hepatocellular carcinoma is closely
correlated with glucose metabolism but not angiogenesis. J Hepatol.
55:846–857. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Macheda ML, Rogers S and Best JD:
Molecular and cellular regulation of glucose transporter (GLUT)
proteins in cancer. J Cell Physiol. 202:654–662. 2005.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Semaan A, Munkarah AR, Arabi H,
Bandyopadhyay S, Seward S, Kumar S, Qazi A, Hussein Y, Morris RT
and Ali-Fehmi R: Expression of GLUT-1 in epithelial ovarian
carcinoma: Correlation with tumor cell proliferation, angiogenesis,
survival and ability to predict optimal cytoreduction. Gynecol
Oncol. 121:181–186. 2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Paudyal B, Oriuchi N, Paudyal P, Higuchi
T, Nakajima T and Endo K: Expression of glucose transporters and
hexokinase II in cholangiocellular carcinoma compared using
[18F]-2-fluro-2-deoxy-D-glucose positron emission tomography.
Cancer Sci. 99:260–266. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kurokawa T, Yoshida Y, Kawahara K,
Tsuchida T, Okazawa H, Fujibayashi Y, Yonekura Y and Kotsuji F:
Expression of GLUT-1 glucose transfer, cellular proliferation
activity and grade of tumor correlate with
[F-18]-fluorodeoxyglucose uptake by positron emission tomography in
epithelial tumors of the ovary. Int J Cancer. 109:926–932.
2004.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim TH, Kwak Y, Song C, Lee HS, Kim DW, Oh
HK, Kim JW, Lee KW, Kang SB and Kim JS: GLUT-1 may predict
metastases and death in patients with locally advanced rectal
cancer. Front Oncol. 13(1094480)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Baer SC, Casaubon L and Younes M:
Expression of the human erythrocyte glucose transporter Glut1 in
cutaneous neoplasia. J Am Acad Dermatol. 37:575–577.
1997.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Koch A, Lang SA, Wild PJ, Gantner S, Mahli
A, Spanier G, Berneburg M, Müller M, Bosserhoff AK and Hellerbrand
C: Glucose transporter isoform 1 expression enhances metastasis of
malignant melanoma cells. Oncotarget. 6:32748–32760.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Wachsberger PR, Gressen EL, Bhala A,
Bobyock SB, Storck C, Coss RA, Berd D and Leeper DB: Variability in
glucose transporter-1 levels and hexokinase activity in human
melanoma. Melanoma Res. 12:35–43. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Fujisawa Y, Yoshino K, Kiyohara Y, Kadono
T, Murata Y, Uhara H, Hatta N, Uchi H, Matsushita S, Takenouchi T,
et al: The role of sentinel lymph node biopsy in the management of
invasive extramammary Paget's disease: Multi-center, retrospective
study of 151 patients. J Dermatol Sci. 79:38–42. 2015.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Laybutt DR, Thompson AL, Cooney GJ and
Kraegen EW: Selective chronic regulation of GLUT1 and GLUT4 content
by insulin, glucose, and lipid in rat cardiac muscle in vivo. Am J
Physiol. 273:1309–1316. 1997.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Liu Y, Cao Y, Zhang W, Bergmeier S, Qian
Y, Akbar H, Colvin R, Ding J, Tong L, Wu S, et al: A small-molecule
inhibitor of glucose transporter 1 downregulates glycolysis,
induces cell-cycle arrest, and inhibits cancer cell growth in vitro
and in vivo. Mol Cancer Ther. 11:1672–1682. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Xu YY, Wu TT, Zhou SH, Bao YY, Wang QY,
Fan J and Huang YP: Apigenin suppresses GLUT-1 and p-AKT expression
to enhance the chemosensitivity to cisplatin of laryngeal carcinoma
Hep-2 cells: An in vitro study. Int J Clin Exp Pathol. 7:3938–3947.
2014.PubMed/NCBI
|
|
16
|
Chen Q, Meng YQ, Xu XF and Gu J: Blockade
of GLUT1 by WZB117 resensitizes breast cancer cells to adriamycin.
Anticancer Drugs. 28:880–887. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kowalczyk A, Bodalska A, Miranowicz M and
Karłowicz-Bodalska K: Insights into novel anticancer applications
for apigenin. Adv Clin Exp Med. 26:1143–1146. 2017.PubMed/NCBI View Article : Google Scholar
|