Introduction
Chronic low back pain (CLBP) is considered the
leading cause of disability and repeated visits for patients with
lumbar degenerative disease, contributing to the global
socioeconomic burden (1-3).
Intervertebral disc tissue is composed of three predominant
structures: Nucleus pulposus (NP), annulus fibrosus (AF) and
cartilage endplate (EP) (4). NP is
a flexible sphere located in the center of the disc, surrounded by
AF in the front, back, left and right directions (5). EP is attached to the superior and
lower NP (6). Disc degeneration is
an age-related biological process characterized by decreased
hydration and extracellular matrix (ECM) deposition, increased
inward growth of neurovascular structures and release of
inflammation-related cytokines within NP tissues, which causes
spinal instability along with CLBP (4,7,8).
Proinflammatory factors can inhibit ECM production in human nucleus
pulposus cells (HNPC) (9). On the
other hand, it can promote the production of degrading enzymes such
as matrix metalloproteinase (MMP) (10). Therefore, inhibiting inflammation
is an effective way to alleviate disc degeneration.
Sphingosine 1-phosphate (S1P) is a bioactive
phospholipid that regulates numerous cellular physiological
processes such as proliferation, survival and cytoskeletal
rearrangement by binding to a family of five G protein-coupled
receptors (GPCRs) (11). S1P
receptor 3, (S1PR3) belonging to the GPCR family, is necessary for
the promotion of bone formation in response to S1P (12). The S1P-S1PR3 axis has been reported
to have anti-inflammatory functions (13). S1P lyase inhibition improves sepsis
by increasing the S1P-S1PR3 signaling axis and reducing production
of cytokines, including tumor necrosis factor (TNF)-α and
interleukin (IL)-6(14). In
addition, S1P3-/- mice showed the lower survival rate of
and the higher levels of TNF-α and IL-6(15).
However, the biological roles of S1PR3 in
intervertebral disc degeneration and the underlying mechanism are
not well understood. The current study focused on the roles of
S1PR3 in disc degeneration and in vitro functional studies
were performed in LPS-induced HNPCs to further elucidate the
mechanism underlying the regulation of S1PR3 in
inflammation-related disc degeneration. The current study provided
novel insights into the functional roles of S1PR3 in the
pathogenesis of disc degeneration.
Materials and methods
Cell culture
Immortalized human NP cell line HNPC (cat. no.
iCell-0028a) provided by Cellverse Bioscience Technology Co., Ltd.
were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.)
supplied with 10% FBS and 1% penicillin/streptomycin (both Gibco;
Thermo Fisher Scientific, Inc.) at 5% CO2 and at 37˚C.
Lipopolysaccharide (LPS; Sigma-Aldrich; Merck KGaA) with
concentration range of 0.01-100 µg/ml was used to treat HNPCs for
24-48 h at 37˚C, aiming to trigger cell inflammation in
vitro.
Cell transfection
To overexpress S1PR3 and Toll-like receptor (TLR) 2,
the pc-DNA3.1 vector containing the whole length of S1PR3
(over-S1PR3) or TLR2 (over-TLR2), and the empty vector
[over-negative control (NC)] were synthesized by Genepharm, Inc.
With the application of Lipofectamine® 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.), 100 nM recombinants were
introduced to HNPCs following the manufacturer's instructions.
After 48 h of transfection, cells were used for follow-up
experiments.
Cell counting kit-8 (CCK-8) assay
The CCK-8 assay was used for the estimation of HNPC
viability. Cells were cultured in DMEM with 10% FBS for 24 h at
37˚C, followed by incubation with 10 µl WST-8 (Beyotime Institute
of Biotechnology) for 2 h. A microplate reader (Bio-Rad
Laboratories, Inc.) was used, and optical density was determined at
450 nm.
ELISA
The levels of IL-1β, IL-6 and TNF-α in cell culture
supernatants were investigated using ELISA kits (cat. nos. #DLB50,
#D6050B, #DTA00D, respectively; R&D Systems, Inc.) following
the manufacturer's instructions. A microplate reader (Bio-Rad
Laboratories, Inc.) was used, and optical density was determined at
450 nm.
Apoptosis assay
Apoptosis was investigated using the FITC Annexin
V/PI Apoptosis Detection Kit I (cat. no. 556547; BD Biosciences)
following the manufacturer's instructions. Briefly, the collection
of cells was carried out following the indicated treatment.
Afterward, PBS-rinsed cells were resuspended in binding buffer.
Subsequently, cells were incubated with Annexin V-FITC for 15 min
and propidium iodide (PI; 10 mg/ml) for 5 min away from the light
using BD FACSVerse™ System (BD Biosciences), all steps at room
temperature. FlowJo_V10 (FlowJo LLC ) was used for analysis.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNAs were extracted from HNPC using TRIzol
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's protocol. Then the RNA was reverse transcribed into
cDNA using PrimeScript RT Master Mix (Perfect Real Time; Takara
Bio, Inc.) following the manufacturer's instructions. The
amplification of cDNA was carried out by qPCR using the SYBR Premix
Ex Taq™ II kit (Takara Bio, Inc.). The PCR program was 95˚C for 3
min, followed by 35 cycles of denaturation at 95˚C for 30 sec,
annealing at 60˚C for 30 sec and extension at 72˚C for 1 min. A
final extension step at 72˚C for 7 min was performed. The primer
sequences for PCR are presented as below: A disintegrin and
metalloproteinase with thrombospondin motifs 4 (Adamts-4) forward,
5'-GGAAATTCAGATGTGGTACTGCC-3', and reverse
5'-GCCACTAGGACTTGCAGTGT-3'; Adamts-5 forward,
5'-CCATGGCAACTGGGGATCTT-3', and reverse,
5'-TCTCCTCCACATACTCCGCA-3'; Aggrecan forward,
5'-AGGGCGAGTGGAATGATGTT-3', and reverse,
5'-GCGTTTGTAGGTGGTGGCTG-3'; Collagen II forward,
5'-CTTCCCCCTCCTGCTCCAAG-3', and reverse,
5'-TCTCCGAAGGGGATCTCAGG-3'; MMP3 forward,
5'-TGAGGACACCAGCATGAACC-3', and reverse,
5'-ACTTCGGGATGCCAGGAAAG-3'; MMP13 forward,
5'-GCACTTCCCACAGTGCCTAT-3', and reverse 5'-AGTTCTTCCCTTGATGGCCG-3';
TLR2 forward, 5'-CCAAGTGAAGGCAGGAAGACA-3', and reverse
5'-GGAAACTCGAGGCAGACCAA-3'; GAPDH forward,
5'-GGGAAACTGTGGCGTGAT-3', and reverse, 5'-GAGTGGGTGTCGCTGTTGA-3'.
The primer sequences were synthesized from Sangon Biotech Co.,
Ltd., China. Relative gene expression was quantified using the
2-ΔΔCq method normalized to GAPDH (16,17).
Western blotting
Total protein from sample cells was isolated using
RIPA buffer (Beyotime Institute of Biotechnology) and
quantification was completed using the BCA Protein Assay kit
(Beijing Dingguo Changsheng Biotechnology Co., Ltd.) following the
manufacturer's instructions. A total of 30 µg protein per well were
separated by 10% SDS-polyacrylamide gels and transferred to PVDF
membranes. Subsequently, membranes were blocked using 5% non-fat
milk for 2 h at room temperature and incubated with the following
primary antibodies: S1PR3 (cat. no. ab126622); Bcl-2 (cat. no.
ab182858); Bax (cat. no. ab32503); Adamts-5 (cat. no. ab41037);
Adamts-4 (cat. no. ab314856); Aggrecan (cat. no. ab315486);
Collagen II (cat. no. ab307674); MMP3 (cat. no. ab52915); MMP13
(cat. no. ab39012); TLR2 (cat. no. ab68159); phosphorylated
(p)-STAT3 (cat. no. ab267373); STAT3 (cat. no. ab68153); p-JNK
(cat. no. ab124956); JNK (cat. no. ab179461); p-ERK (cat. no.
ab201015); ERK (cat. no. ab18469); p-p38 (cat. no. ab308038); p38
(cat. no. ab182453); and GAPDH (cat. no. ab8245) (all dilutions
1:1,000; Abcam) overnight at 4˚C and the HRP-conjugated goat
anti-rabbit or mouse secondary antibodies (cat. nos. sc-2004 or
sc-2005; 1:5,000; Santa Cruz Biotechnology, Inc.) for 1 h at room
temperature. The visualization of protein bands was carried out
using an enhanced chemiluminescence (ECL) reagent kit (Amersham
Biosciences), while protein density was analyzed using ImageJ
(version 1.49; National Institutes of Health).
Statistical analysis
SPSS (version 22.0; IBM Corp.) and GraphPad Prism
(version 6; Dotmatics) were used for data analysis. Data are shown
as mean ± SD of results derived from three independent experiments
performed in triplicate. One-way ANOVA with Bonferroni post hoc
test were used for comparisons among multiple groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of S1PR3 in LPS-induced
HNPCs is decreased
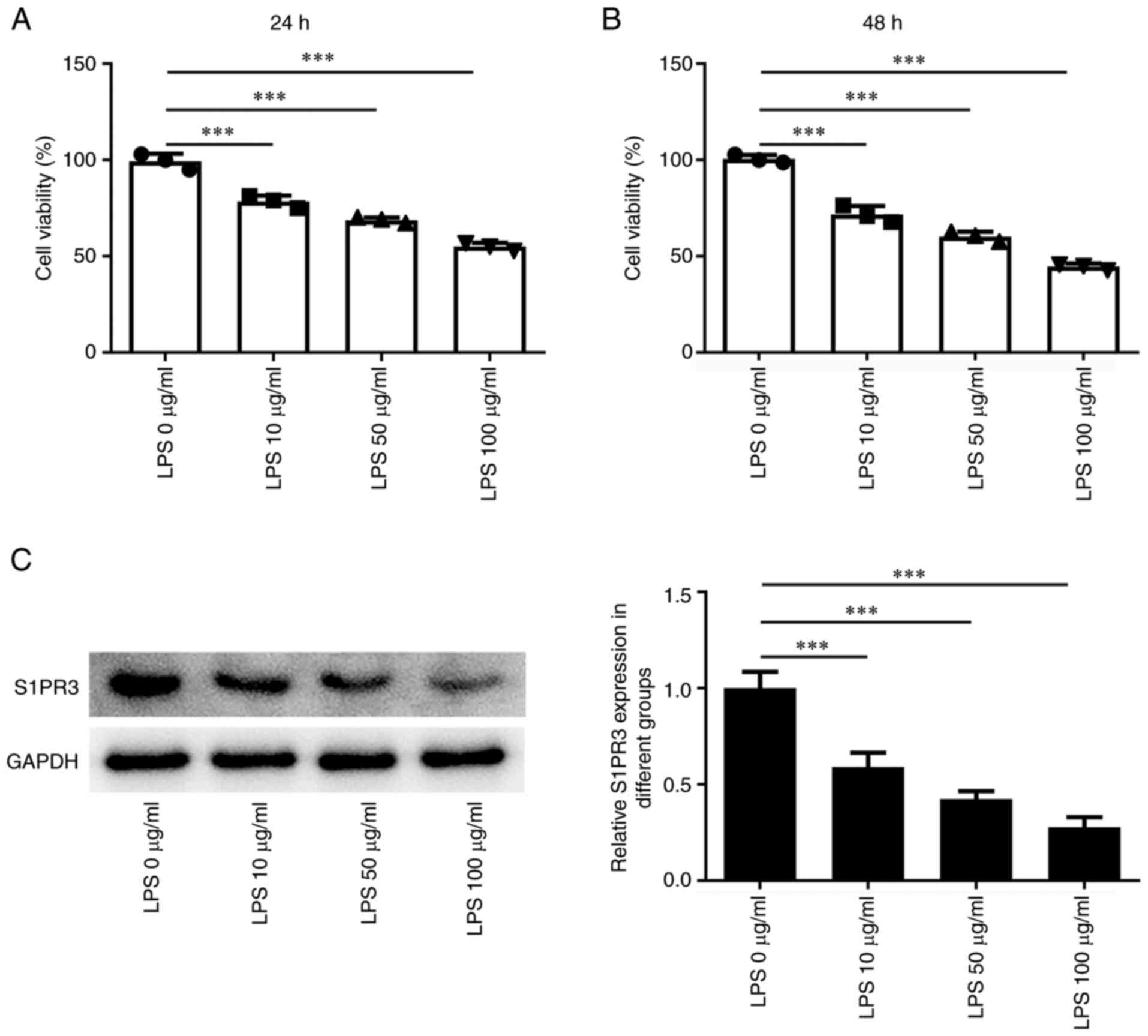
To investigate the biological roles of S1PR3 in
LPS-induced HNPCs, the effects of LPS on the viability of HNPCs
were detected. Treatment with 10-100 µg/ml LPS reduced HNPC
viability compared with the control group (Fig. 1A and B). In addition, the western blotting
results demonstrated that LPS concentration-dependently declined
S1PR3 protein content in HNPCs (Fig.
1C).
Overexpression of S1PR3 inhibits
LPS-induced cell damage, inflammatory release and apoptosis of
HNPCs
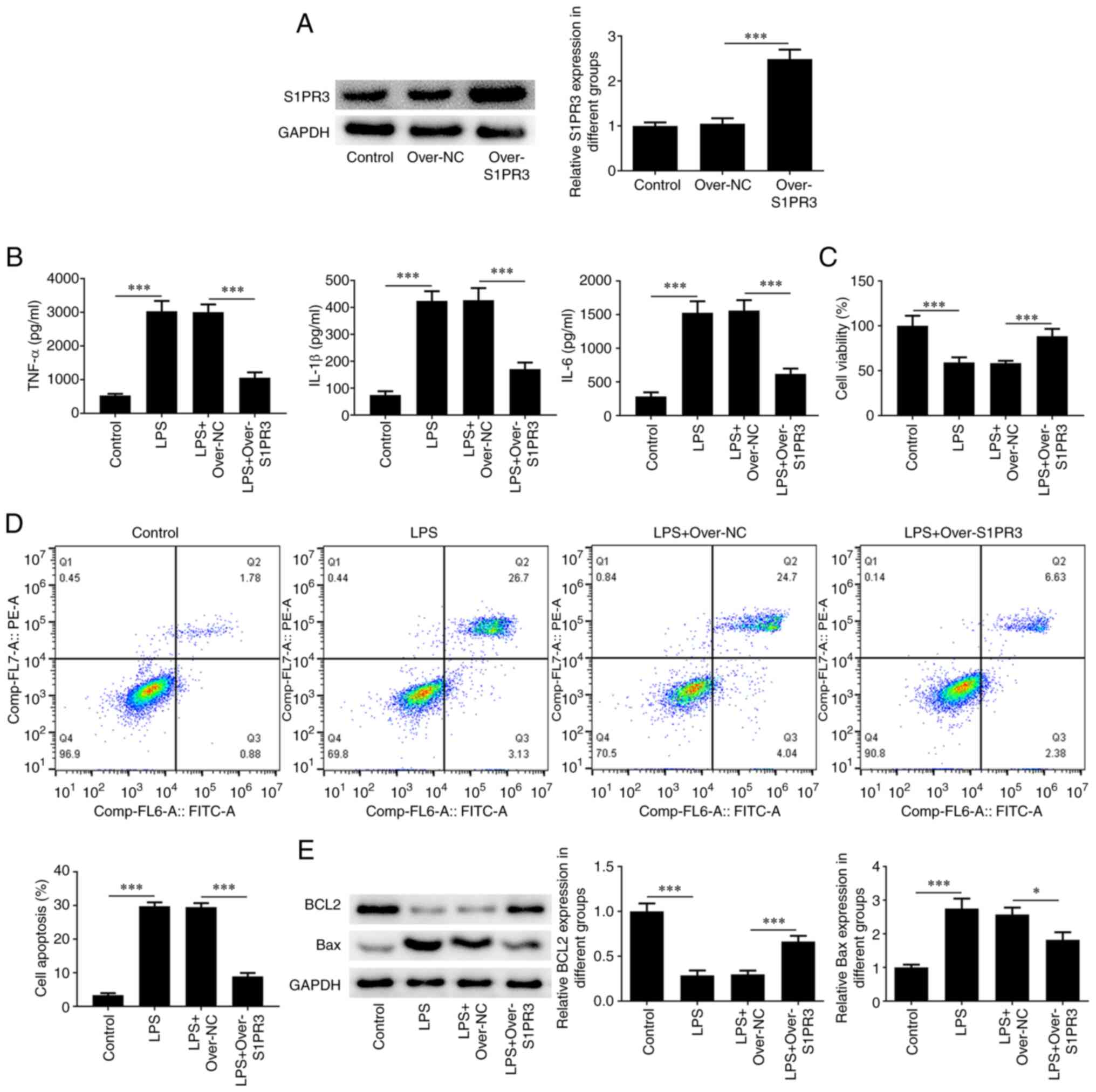
With the aim of investigating the role that S1PR3
plays in intervertebral disc degeneration, S1PR3 was overexpressed
in LPS-induced HNPCs, and transfection efficiency is shown in
Fig. 2A. The production of TNFα,
IL-1β and IL-6 were significantly decreased after transfection with
over-S1PR3 compared with the LPS + over-NC group (Fig. 2B). The CCK-8 assay results revealed
that the viability in HNPCs with S1PR3 overexpression increased 30%
compared with the LPS + over-NC group (Fig. 2C). In addition, flow cytometry
showed a significant reduction of 21% in the rate of apoptosis
after transfection with over-S1PR3 compared with the LPS + over-NC
group (Fig. 2D). Moreover, S1PR3
overexpression increased the level of Bcl-2, while it decreased the
level of Bax in LPS-treated cells (Fig. 2E).
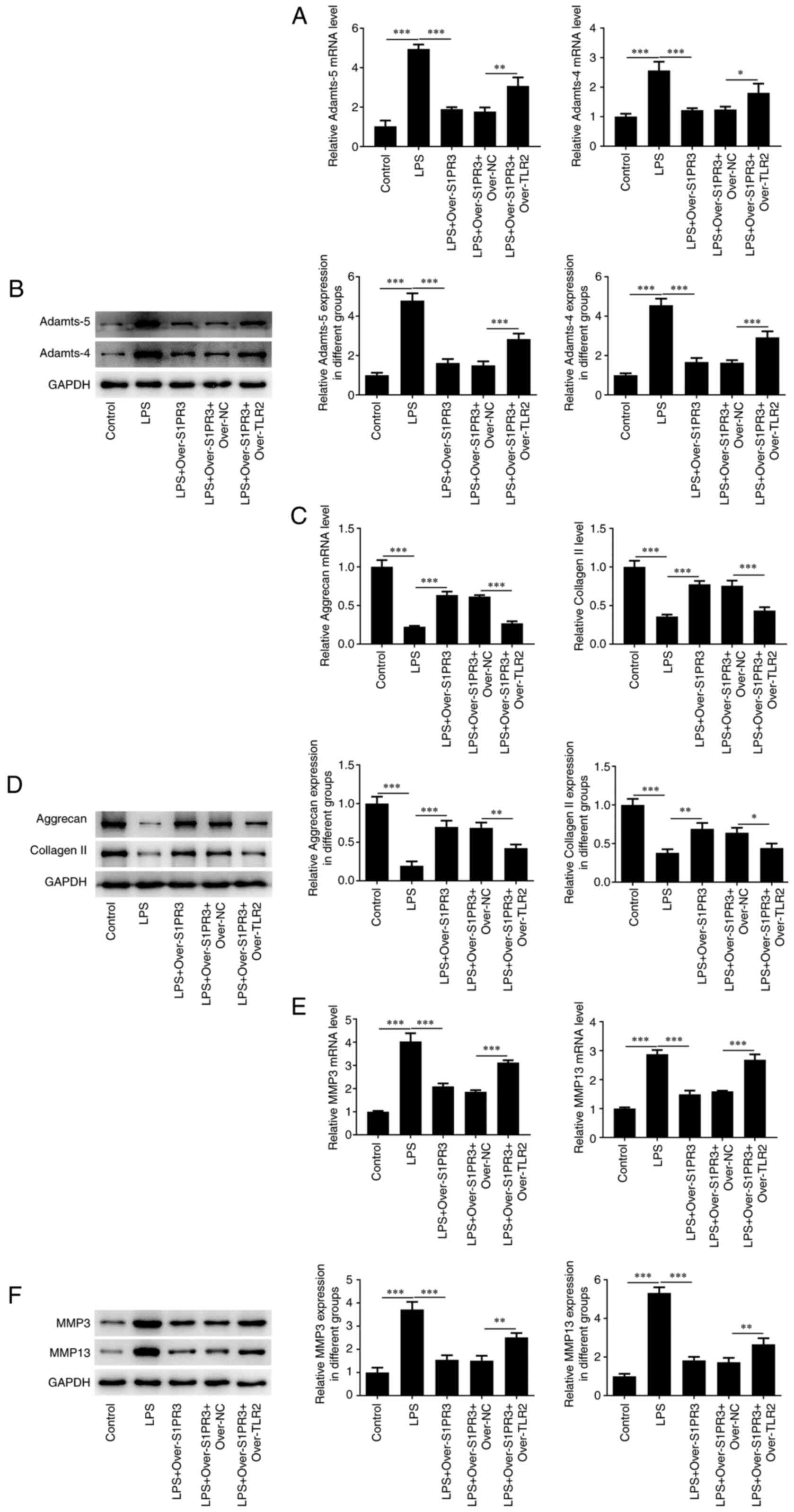
Overexpression of S1PR3 promotes
LPS-induced deposition of ECM proteins in HNPCs
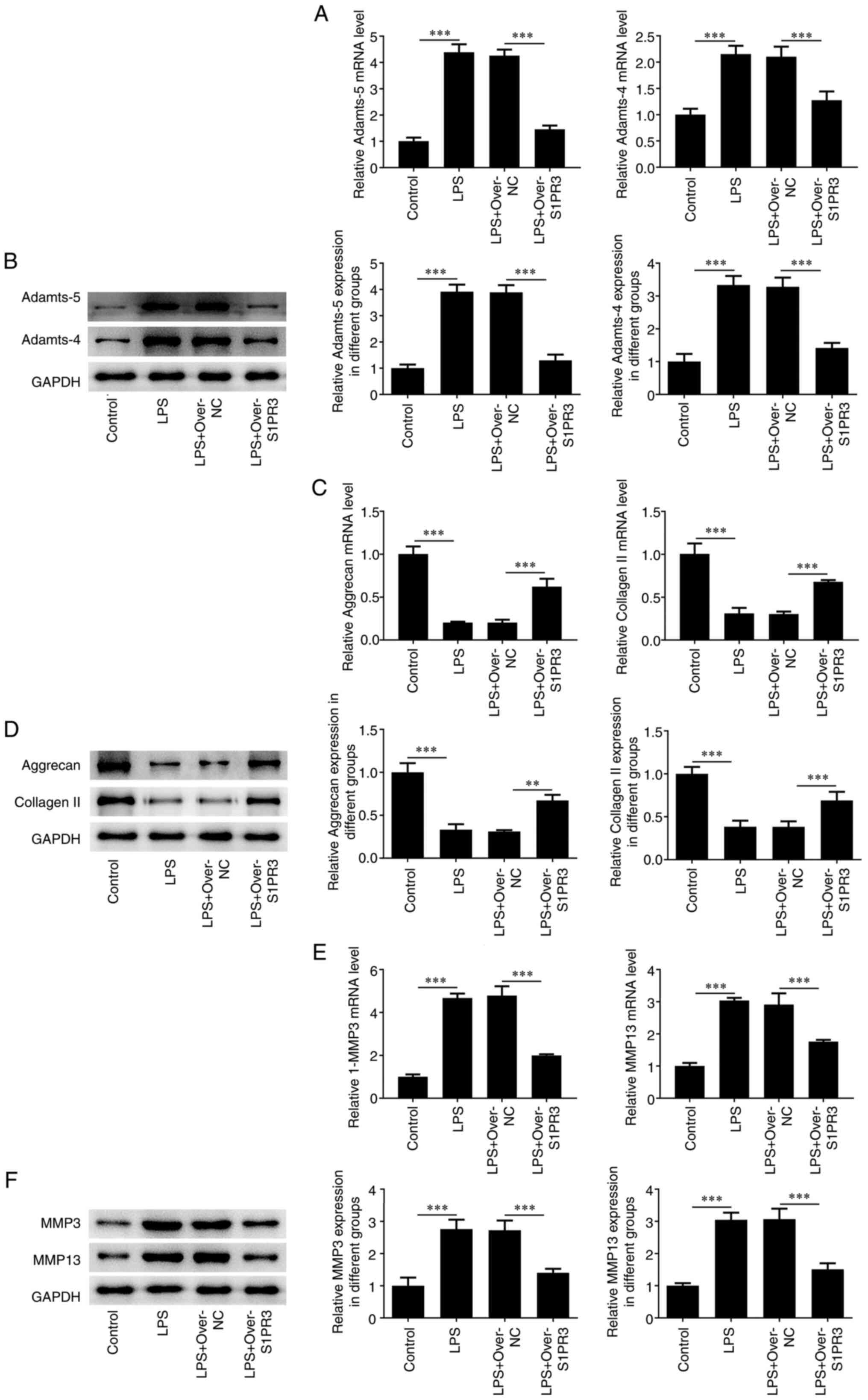
RT-qPCR and western blotting results indicated that
the levels of Adamts-5 and -4 were notably decreased after
transfection with over-S1PR3 compared with those in the LPS +
over-NC group (Fig. 3A and
B). Moreover, the contents of the
polyproteoglycan aggrecan and collagen II were found to be elevated
after overexpressing S1PR3 in LPS-induced HNPCs (Fig. 3C and D). Additionally, the levels of the matrix
degrading enzymes MMP3 and MMP13 were reduced in LPS-induced HNPCs
transfected with over-S1PR3 compared with those in the LPS +
over-NC group (Fig. 3E and
F).
Upregulation of S1PR3 represses the
expression of TLR2-regulated STAT3 and MAPK signaling in
LPS-induced HNPCs
Next, the mechanism involved in the function of
S1PR3 in LPS-induced HNPCs was further explored. LPS elevated the
protein level of TLR2 by 5-fold compared with the control, while
S1PR3 overexpression reduced the production of TLR2 by 1.8-fold
compared with the LPS-over-NC group in HNPCs (Fig. 4A). Upregulation of S1PR3
significantly reduced the phosphorylation of STAT3 induced by LPS
in HNPCs (Fig. 4B). Additionally,
the expression of p-JNK, p-ERK and p-p38 were increased by 2.3-,
2.7- and 3.9-fold in the LPS group, but S1PR3 overexpression
reduced the expression levels of p-JNK, p-ERK and p-p38 by 1.2-,
1.5- and 1.8-fold (Fig. 4C). With
the aim of exploring the biological role that TLR2 plays in HNPCs,
TLR2 was overexpressed and transfection efficiency is shown in
Fig. 4D. The significantly
decreased p-STAT3 and p-JNK expression levels in
S1PR3-overexpressed HNPCs were found to be elevated after
overexpressing TLR2 (Fig. 4E).
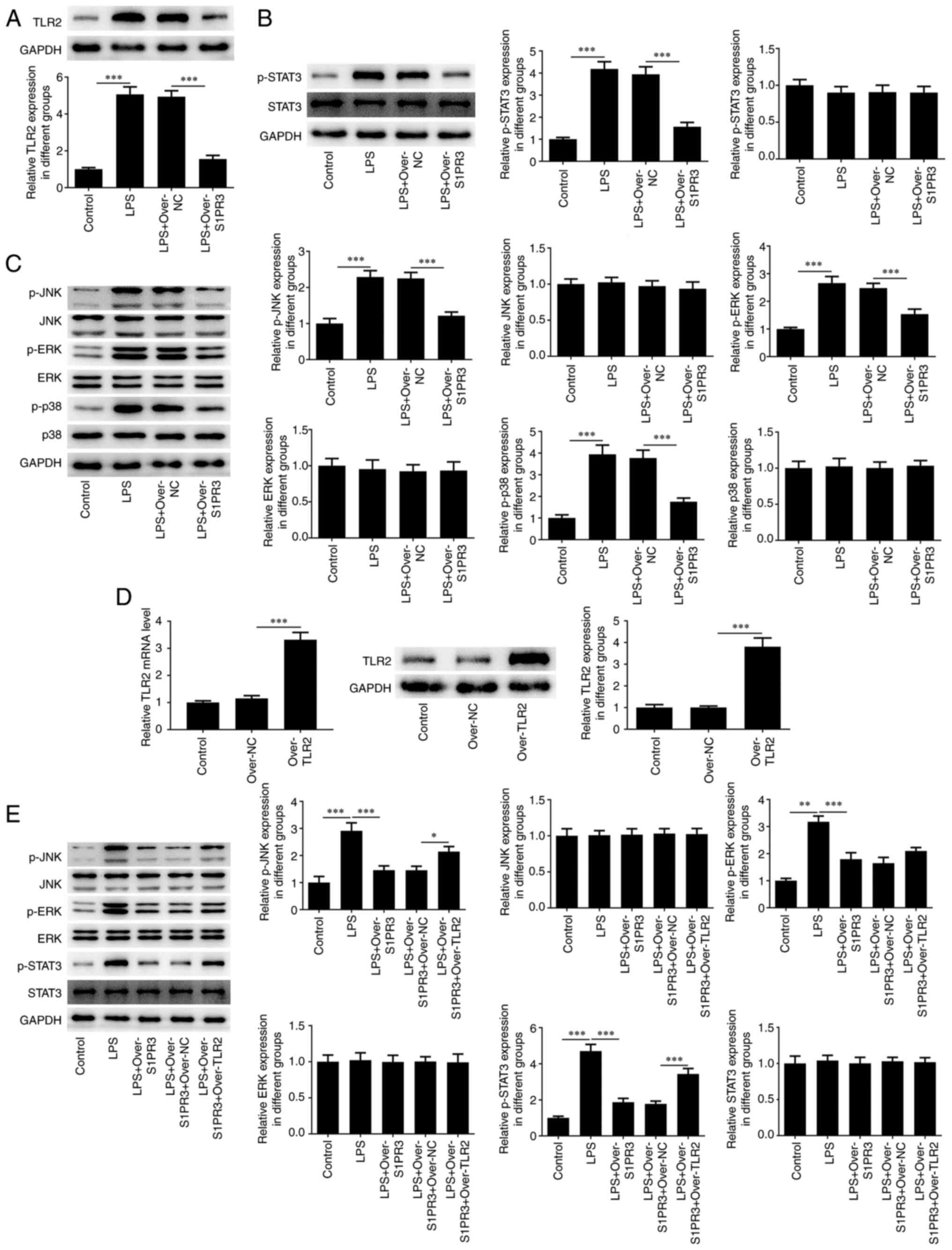 | Figure 4S1PR3 overexpression represses the
expressions of TLR2-regulated STAT3 and MAPK signaling in
LPS-induced HNPCs. (A) The protein level of TLR2 in LPS-induced
HNPCs transfected with Over-S1PR3 or Over-NC was detected by
western blotting. (B) The protein levels of p-STAT3 and STAT3 in
LPS-induced HNPCs transfected with Over-S1PR3 or Over-NC were
detected by western blotting. (C) The protein levels of p-JNK,
p-ERK, p-p38, JNK, ERK and p38 in LPS-induced HNPCs transfected
with Over-S1PR3 or Over-NC were detected by western blotting. (D)
The levels of TLR2 in LPS-induced HNPCs after TLR2 overexpression
were detected by RT-qPCR and western blotting. (E) The protein
levels of p-JNK, p-ERK, p-STAT3, JNK, ERK and STAT3 in LPS-induced
HNPCs transfected with Over-S1PR3 or Over-TLR2 were detected by
western blotting. *P<0.05, **P<0.01,
***P<0.001. S1PR3, sphingosine 1-phosphate receptor
3; HNPC, human neural progenitor cells; LPS, lipopolysaccharide;
over, overexpression; NC, negative control; RT-qPCR, reverse
transcription-quantitative PCR; p-, phosphorylated. |
S1PR3 alleviates LPS-induced
inflammatory release, apoptosis and ECM degradation of HNPCs
through the TLR2/STAT3 and TLR2/MAPK pathways
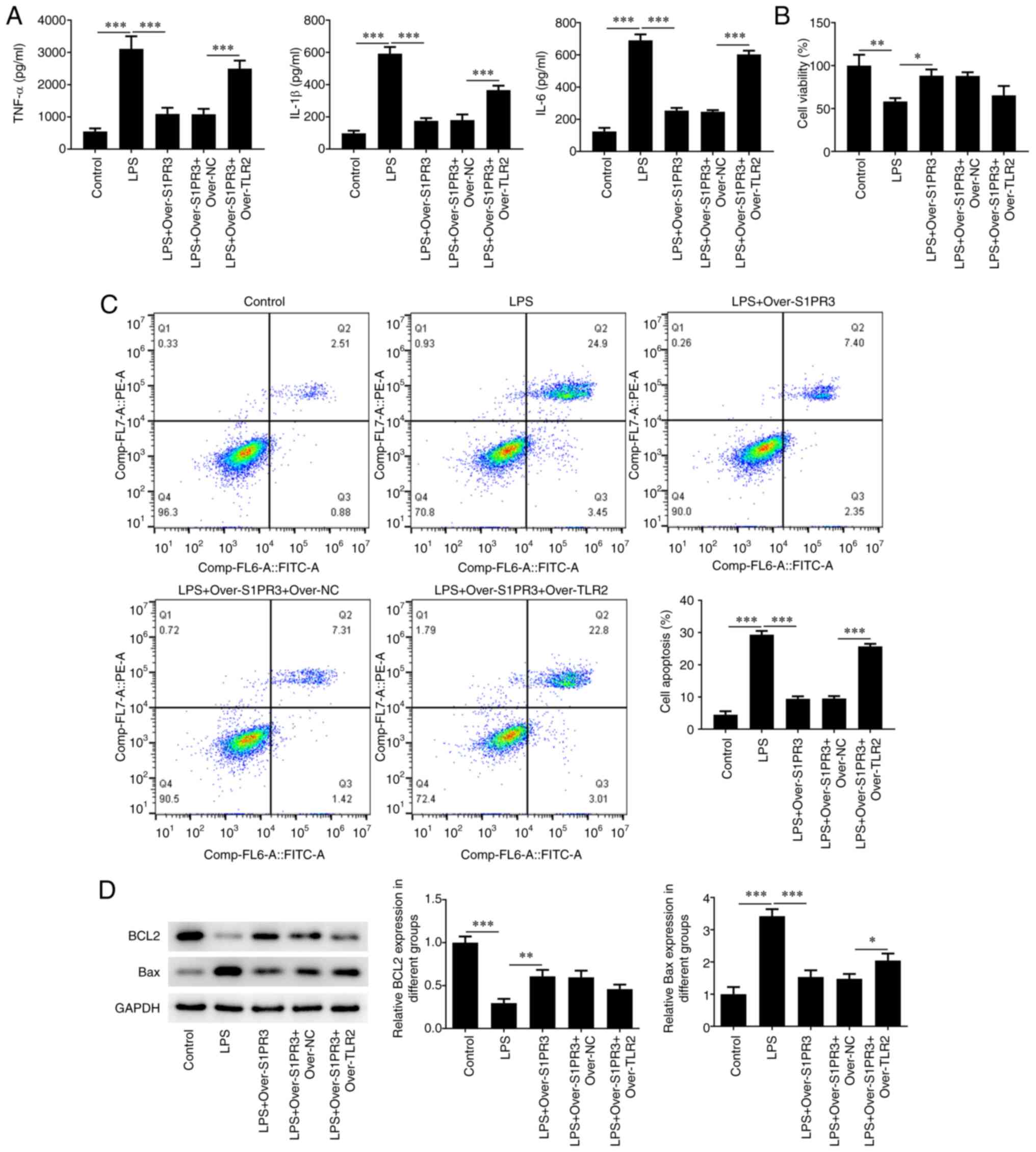
TLR2 overexpression elevated the production of TNFα,
IL-1β and IL-6 in S1PR3-overexpressed HNPCs (Fig. 5A). Moreover, the CCK-8 assay data
showed that TLR2 overexpression decreased the viability of
S1PR3-overexpressed HNPCs (Fig.
5B). In addition, the apoptosis rate in cells co-transfected
with over-S1PR3 + over-TLR2 was increased by 26% compared with that
in cells transfected with over-S1PR3 alone (Fig. 5C), which is in line with western
blotting data, as evidenced by the reduced Bcl-2 and elevated Bax
contents (Fig. 5D). Furthermore,
RT-qPCR and western blotting revealed that TLR2 overexpression
significantly increased the levels of Adamts-5, Adamts-4, MMP3 and
MMP13, while it reduced the levels of aggrecan and collagen II in
LPS-induced HNPCs transfected with over-S1PR3 (Fig. 6).
Discussion
Intervertebral disc degeneration, characterized by
progressive failure of structure and ageing of the intervertebral
disc, leads to pain in the lower back and even global disability
(18). Existing studies have
revealed that aberrant functions in HNPCs, including senescence,
cytokine secretion, apoptosis and ECM degradation, are associated
with IDD pathogenesis (19-21).
HNPCs can generate ECM components, such as aggrecan, type II and X
collagen, playing a crucial function in maintaining the integrity
of intervertebral discs (22).
Additional evidence indicates that the aberrant functions of HNPCs
may be the key to the degeneration pathogenesis of intervertebral
discs (23). In the current study,
the aim was to identify the potential role of S1PR3 in HNPC
viability, inflammation, apoptosis and ECM degradation, as well as
disclose the molecular mechanisms underlying its function.
S1P, a bioactive signaling agent, is derived from
mammalian membrane sphingolipids, and has roles in immune and
inflammatory responses and cardiovascular systems (24). Most of the biological functions of
S1P are regulated by S1PR1-5. Among these receptors, S1PR3 mediates
multiple biological activities such as inflammation and vascular
barrier function (25-27).
It has been reported that pFTY720 activates S1PR3 and inhibits the
TLR2/4-PI3K-NFκB signaling pathway to protect astrocytes from
neuroinflammation induced by oxygen-glucose deprivation (28). S1PR3 expression has also been shown
to be reduced in tissue with intervertebral disc degeneration
(29). In the current study, it
was shown that S1PR3 expression was low in LPS-induced HNPCs. S1PR3
overexpression restrained the cell inflammation response by
reducing the levels of TNFα, IL-1β and IL-6, and repressed the
level of apoptosis in LPS-induced HNPCs. It was also observed that
S1PR3 overexpression repressed the levels of Adamts-5, Adamts-4,
MMP3 and MMP13, while it increased the levels of aggrecan and
collagen II, indicating that S1PR3 overexpression suppressed ECM
degradation of LPS-stimulated HNPCs.
A previous study found that S1PR3 can inhibit the
expression of the TLR family protein TLR2(28). Activation of the
TLR2/JNK/mitochondrial-mediated pathway can promote apoptosis of
nucleus pulpocytes and induce intervertebral disc degeneration
(30). Nevertheless, Yang et
al (31) reported that TSG-6
from bone marrow mesenchymal stem cells can protect against
intervertebral disc degeneration via the TLR2/NF-κB signaling
pathway. Moreover, TLR2 takes part in the inflammation response by
activating the STAT3 and MAPK pathways (32,33).
Additionally, Wu et al (34) showed that resveratrol blocks the
IL-6/JAK/STAT3 pathway to protect HNPCs from degeneration. Another
study has showed that the MAPK signaling proteins JNK, ERK and p38
are involved in the apoptosis and activation of HNPCs (35). In the current study, S1PR3
overexpression led to decreased TLR2 level and reduced levels of
STAT3 and MAPK pathways. When TLR2 was overexpressed, it was shown
that TLR2 overexpression reversed the effects of S1PR3
overexpression on LPS-induced NP cell inflammation and apoptosis,
along with ECM degradation.
There are several limitations in the present study.
Since the environment for cellular experiments is different from
that in living organisms, thus the result of this study might not
be suitable for in vivo experiments and clinical trials. The
animal experiments and clinical trials will be performed in the
further study. In addition, the current experiments only
demonstrated that S1PR3 regulated TLR2, thus mediating TLR2/STAT3
and TLR2/MAPK signaling, but the specific mechanism by which S1PR3
regulates TLR2 remains to be explored.
To conclude, the results of the present study
indicated that S1PR3 overexpression alleviated the inflammatory
response and ECM degradation induced by LPS in HNPCs via
suppression of the STAT3 and MAPK signaling pathways, which might
provide novel sights into the exploration of S1PR3 as a promising
candidate for intervertebral disc degeneration.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZT and ZL designed the study, drafted and revised
the manuscript. HG and WX analyzed the data and searched the
literature. ZT, ZL, HG and WX performed the experiments. All
authors read and approved the final manuscript. ZT and ZL confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Urits I, Burshtein A, Sharma M, Testa L,
Gold PA, Orhurhu V, Viswanath O, Jones MR, Sidransky MA, Spektor B
and Kaye AD: Low back pain, a comprehensive review:
Pathophysiology, diagnosis, and treatment. Curr Pain Headache Rep.
23(23)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Will JS, Bury DC and Miller JA: Mechanical
low back pain. Am Fam Physician. 98:421–428. 2018.PubMed/NCBI
|
|
3
|
Vlaeyen JWS, Maher CG, Wiech K, Van
Zundert J, Meloto CB, Diatchenko L, Battié MC, Goossens M, Koes B
and Linton SJ: Low back pain. Nat Rev Dis Primers.
4(52)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xin J, Wang Y, Zheng Z, Wang S, Na S and
Zhang S: Treatment of intervertebral disc degeneration. Orthop
Surg. 14:1271–1280. 2022.PubMed/NCBI View
Article : Google Scholar
|
|
5
|
Chen S, Fu P, Wu H and Pei M: Meniscus,
articular cartilage and nucleus pulposus: A comparative review of
cartilage-like tissues in anatomy, development and function. Cell
Tissue Res. 370:53–70. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Bhujel B, Shin HE, Choi DJ and Han I:
Mesenchymal stem cell-derived exosomes and intervertebral disc
regeneration: Review. Int J Mol Sci. 23(7306)2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Kirnaz S, Capadona C, Wong T, Goldberg JL,
Medary B, Sommer F, McGrath LB Jr and Härtl R: Fundamentals of
intervertebral disc degeneration. World Neurosurg. 157:264–273.
2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Risbud MV and Shapiro IM: Role of
cytokines in intervertebral disc degeneration: Pain and disc
content. Nat Rev Rheumatol. 10:44–56. 2014.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vergroesen PP, Kingma I, Emanuel KS,
Hoogendoorn RJ, Welting TJ, van Royen BJ, van Dieën JH and Smit TH:
Mechanics and biology in intervertebral disc degeneration: A
vicious circle. Osteoarthritis Cartilage. 23:1057–1070.
2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Krut Z, Pelled G, Gazit D and Gazit Z:
Stem cells and exosomes: New therapies for intervertebral disc
degeneration. Cells. 10(2241)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Cartier A and Hla T: Sphingosine
1-phosphate: Lipid signaling in pathology and therapy. Science.
366(eaar5551)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Meshcheryakova A, Mechtcheriakova D and
Pietschmann P: Sphingosine 1-phosphate signaling in bone
remodeling: Multifaceted roles and therapeutic potential. Expert
Opin Ther Targets. 21:725–737. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hu Y, Yang C, Shen G, Yang S, Cheng X,
Cheng F, Rao J and Wang X: Hyperglycemia-triggered
sphingosine-1-phosphate and sphingosine-1-phosphate receptor 3
signaling worsens liver ischemia/reperfusion injury by regulating
M1/M2 polarization. Liver Transpl. 25:1074–1090. 2019.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Weigel C, Hüttner SS, Ludwig K, Krieg N,
Hofmann S, Schröder NH, Robbe L, Kluge S, Nierhaus A, Winkler MS,
et al: S1P lyase inhibition protects against sepsis by promoting
disease tolerance via the S1P/S1PR3 axis. EBioMedicine.
58(102898)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hou J, Chen Q, Wu X, Zhao D, Reuveni H,
Licht T, Xu M, Hu H, Hoeft A, Ben-Sasson SA, et al: S1PR3 signaling
drives bacterial killing and is required for survival in bacterial
sepsis. Am J Respir Crit Care Med. 196:1559–1570. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yurube T, Takada T, Hirata H, Kakutani K,
Maeno K, Zhang Z, Yamamoto J, Doita M, Kurosaka M and Nishida K:
Modified house-keeping gene expression in a rat tail compression
loading-induced disc degeneration model. J Orthop Res.
29:1284–1290. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Francisco V, Pino J, González-Gay M, Lago
F, Karppinen J, Tervonen O, Mobasheri A and Gualillo O: A new
immunometabolic perspective of intervertebral disc degeneration.
Nat Rev Rheumatol. 18:47–60. 2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Li Z, Chen X, Xu D, Li S, Chan MTV and Wu
WKK: Circular RNAs in nucleus pulposus cell function and
intervertebral disc degeneration. Cell Prolif.
52(e12704)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
He R, Wang Z, Cui M, Liu S, Wu W, Chen M,
Wu Y, Qu Y, Lin H, Chen S, et al: HIF1A Alleviates
compression-induced apoptosis of nucleus pulposus derived stem
cells via upregulating autophagy. Autophagy. 17:3338–3360.
2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Shao Z, Wang B, Shi Y, Xie C, Huang C,
Chen B, Zhang H, Zeng G, Liang H, Wu Y, et al: Senolytic agent
Quercetin ameliorates intervertebral disc degeneration via the
Nrf2/NF-κB axis. Osteoarthritis Cartilage. 29:413–422.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li Z, Yu X, Shen J, Chan MT and Wu WK:
MicroRNA in intervertebral disc degeneration. Cell Prolif.
48:278–283. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Lin J, Du J, Wu X, Xu C, Liu J, Jiang L,
Cheng X, Ge G, Chen L, Pang Q, et al: SIRT3 mitigates
intervertebral disc degeneration by delaying oxidative
stress-induced senescence of nucleus pulposus cells. J Cell
Physiol. 236:6441–6456. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kunkel GT, Maceyka M, Milstien S and
Spiegel S: Targeting the sphingosine-1-phosphate axis in cancer,
inflammation and beyond. Nat Rev Drug Discov. 12:688–702.
2013.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Muppidi JR, Lu E and Cyster JG: The G
protein-coupled receptor P2RY8 and follicular dendritic cells
promote germinal center confinement of B cells, whereas S1PR3 can
contribute to their dissemination. J Exp Med. 212:2213–2222.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bajwa A, Huang L, Kurmaeva E, Gigliotti
JC, Ye H, Miller J, Rosin DL, Lobo PI and Okusa MD: Sphingosine
1-phosphate receptor 3-deficient dendritic cells modulate splenic
responses to ischemia-reperfusion injury. J Am Soc Nephrol.
27:1076–1090. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Nussbaum C, Bannenberg S, Keul P, Gräler
MH, Gonçalves-de-Albuquerque CF, Korhonen H, von Wnuck Lipinski K,
Heusch G, de Castro Faria Neto HC, Rohwedder I, et al:
Sphingosine-1-phosphate receptor 3 promotes leukocyte rolling by
mobilizing endothelial P-selectin. Nat Commun.
6(6416)2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Dong YF, Guo RB, Ji J, Cao LL, Zhang L,
Chen ZZ, Huang JY, Wu J, Lu J and Sun XL: S1PR3 is essential for
phosphorylated fingolimod to protect astrocytes against
oxygen-glucose deprivation-induced neuroinflammation via inhibiting
TLR2/4-NFκB signalling. J Cell Mol Med. 22:3159–3166.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang K, Li H and Li C: Expression and role
of Sphingosine 1-phosphate receptors in intervertebral disc
degeneration. J Back Musculoskelet Rehabil. 33:255–262.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lin Y, Jiao Y, Yuan Y, Zhou Z, Zheng Y,
Xiao J, Li C, Chen Z and Cao P: Propionibacterium acnes induces
intervertebral disc degeneration by promoting nucleus pulposus cell
apoptosis via the TLR2/JNK/mitochondrial-mediated pathway. Emerg
Microbes Infect. 7(1)2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang H, Tian W, Wang S, Liu X, Wang Z, Hou
L, Ge J, Zhang X, He Z and Wang X: TSG-6 secreted by bone marrow
mesenchymal stem cells attenuates intervertebral disc degeneration
by inhibiting the TLR2/NF-κB signaling pathway. Lab Invest.
98:755–772. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Jin Y, Nguyen TLL, Myung CS and Heo KS:
Ginsenoside Rh1 protects human endothelial cells against
lipopolysaccharide-induced inflammatory injury through inhibiting
TLR2/4-mediated STAT3, NF-κB, and ER stress signaling pathways.
Life Sci. 309(120973)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Xu Z, Hao X, Li M and Luo H: Rhodococcus
equi-derived extracellular vesicles promoting inflammatory response
in macrophage through TLR2-NF-κB/MAPK pathways. Int J Mol Sci.
23(9742)2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu C, Ge J, Yang M, Yan Q, Wang Y, Yu H,
Yang H and Zou J: Resveratrol protects human nucleus pulposus cells
from degeneration by blocking IL-6/JAK/STAT3 pathway. Eur J Med
Res. 26(81)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sun K, Zhu J, Yan C, Li F, Kong F, Sun J,
Sun X, Shi J and Wang Y: CGRP regulates nucleus pulposus cell
apoptosis and inflammation via the MAPK/NF-κB signaling pathways
during intervertebral disc degeneration. Oxid Med Cell Longev.
2021(2958584)2021.PubMed/NCBI View Article : Google Scholar
|