Introduction
Diabetes is one of the key factors posing a threat
to human health (1). The global
prevalence of diabetes is expected to rise notably, with the number
of patients projected to increase from 171 million in 2000 to 366
million globally in 2030(2),
emphasizing the need for innovative therapeutic strategies.
Diabetic nephropathy (DN), as the most common complication of
diabetes, leads to chronic kidney and end-stage renal disease
(3,4), presenting a challenge in diabetes
management. While the primary causes of DN, including renal
hemodynamic abnormality and metabolic disorders caused by high
blood sugar, are well-recognized (5,6),
there remains a lack of effective treatments targeting these
underlying mechanisms. The present study aims to address this gap
by exploring the potential of vitamin D3 as a novel therapeutic
agent in mitigating renal damage in DN, thereby offering a
translational perspective to understanding of DN treatment.
In diabetes, elevated blood glucose levels lead to
increased generation of reactive oxygen species (ROS) in cells,
which serves a crucial role in the development of DN (7,8). ROS
can activate the thioredoxin interacting protein (TXNIP/NLRP3
inflammatory pathway, leading to renal fibrosis (9,10).
Furthermore, oxidative stress responses aggravate cellular
apoptosis by promoting DNA damage within cells (11). Therefore, inhibiting oxidative
stress is an important target for treating DN.
1,25-dihydroxyvitamin D3 (1,25(OH)2D3) is
the active metabolite of vitamin D and possesses various biological
functions such as calcium regulation, pleiotropy, blood pressure
control, immune modulation, apoptosis inhibition and
anti-angiogenesis (12-15).
Additionally, in endothelial cells, vitamin D3 has
anti-inflammatory and antioxidant effects (16). Vitamin D3 can protect endothelial
cells by decreasing oxidative stress reactions and inhibiting the
TXNIP/NLRP3 inflammatory response pathway (9). However, it is unclear whether vitamin
D3 has therapeutic effects on renal tubular epithelial cells in
DN.
The present study aimed to investigate the effects
of vitamin D3 on oxidative stress reactions and the TXNIP/NLRP3
inflammatory response pathway in diabetic mice. Specifically, we
sought to determine whether vitamin D3 could alleviate apoptosis
and fibrosis in renal tubular epithelial cells, thus offering a
potential therapeutic approach for diabetic nephropathy (DN)'.
Materials and methods
Animals
Male C57BL/6J mice (age, 8 weeks; weight, 20-25 g;
number, 24) were obtained from the Animal Research Center of Hebei
Medical University (Shijiazhuang, Hebei, China). The animals were
housed in a specific-pathogen-free facility and maintained at a
controlled temperature of 22±2˚C and relative humidity of 50±10%.
They were subjected to a 12/12-h light/dark cycle, with ad libitum
access to food and water. The animal facility used in the study was
certified by the International Association for Assessment and
Accreditation of Laboratory Animal Care and all animal protocols
used in this study were approved by the Experimental Animal Welfare
Ethics Committee of Hebei Medical University (2022-021).
Mice were randomly divided into groups and
intraperitoneally injected with 60 mg/kg streptozotocin (STZ). Mice
were classified as diabetic if their blood glucose levels exceeded
16.7 mmol/l 48 h post-streptozotocin (STZ) administration. The
glucose measurements were carried out using a standard glucometer
(LifeScan) following a 12 h fasting period to minimize dietary
impact. Blood samples were obtained via non-invasive tail vein
sampling, and glucose levels were measured at the 48-h mark. Each
mouse's glucose level was measured twice to ensure accuracy, with
the average value being recorded. All procedures were conducted
under consistent conditions to ensure the reliability and
reproducibility of the experimental outcomes. Age-matched control
mice received an equivalent amount of sodium citrate instead of
STZ. The mice were randomly divided into four groups (n=6/group):
i) Normal control (NC), mice were injected with sodium citrate and
received intramuscular injection of DMSO at a concentration of 10%,
dissolved in PBS; ii) NC + vitamin D3 (NC + VD), mice received
sodium citrate and intramuscular injection of 233.3 U/kg body
weight/week calcitriol (Shanghai Titan Technology Co., Ltd.) in
DMSO at a concentration of 10%, dissolved in PBS. This treatment
commenced 4 weeks following diabetes induction and continued for 6
months; iii) type 1 diabetes group (T1D), STZ-induced diabetic mice
received intramuscular injection of DMSO at a concentration of 10%,
dissolved in PBS; iv) DM+VD, STZ-induced diabetic mice received
intramuscular injections of calcitriol dissolved in 10% DMSO in PBS
at a dose of 233.3 U/kg body weight/week, starting from 4 weeks
after diabetes induction for 6 months.
In consideration of the solvent properties required,
DMSO was used based on its well-established solubility properties
and widespread use as a solvent in similar experimental settings
(17,18). This concentration was chosen to
ensure the adequate solubility of the compounds under
investigation, while minimizing potential adverse effects
associated with higher concentrations. Preliminary experiments
supported the efficacy and safety of this concentration,
reinforcing its suitability (data not shown). Other studies
corroborate the safe administration of 10% DMSO, aligning with the
present findings and supporting the selection of this concentration
(17,18).
The 6-month analysis period was selected based on
previous literature (9). This
timeframe has been shown to be optimal for evaluating the
therapeutic impact of vitamin D3 on the progression of DN, allowing
comprehensive assessment of disease dynamics and treatment efficacy
(9).
Due to the clinical symptoms of polydipsia and
polyuria in diabetic mice, adequate feed and water was provided and
increase the frequency of bedding replacement. Health and behavior
of the animals was monitored 2-3 times/week.
No animals were found dead. After the model was
established, collect 24-h urine samples and blood specimens, and
then conduct biochemical indicator tests. At the end of the
experimental period, the mice were euthanized by inhalation
anesthesia with a high dose of isoflurane. For euthanasia,
anesthesia was induced at a low concentration (1.0-1.5%) of
isoflurane, followed by high concentration (5%) for 3 min, causing
the animal to lose consciousness. Cardiac arrest and cessation of
breathing were used for determining animal death.
Histology
Following 24 weeks of treatment, kidneys were
isolated and fixed at room temperature) in 4% paraformaldehyde for
24 h, followed by gradual dehydration. The kidney tissues were
embedded in paraffin and cut into 4-µm sections. Masson's trichrome
staining was performed using the MARC SOP method and the specimens
were imaged using an Olympus inverted microscope (Olympus
Corporation; cat. no. BX51).
Cell culture
HK-2 cells (American Type Culture Collection) were
cultured in DMEM-F12 (Gibco; Thermo Fisher Scientific, Inc.)
containing 5% fetal bovine serum. At specified time points, HK-2
cells were stimulated with normal glucose (5.6 mM), normal glucose
+ mannitol (24.4 mM), high glucose (30 mM glucose), high glucose +
50 nM vitamin D3) and high glucose + 10 nM NAC.
ELISA
Protein was extracted from kidney tissue and HK-2
cells. The levels of pro-inflammatory cytokines (IL-1β, IL-6,
TNF-α) were determined using ELISA kits (R&D Systems) according
to the manufacturer's instructions. Urine samples were centrifuged
(4˚C) at 377.3 g. for 10 min to remove particulates. The levels of
8-hydroxy-2'-deoxyguanosine (8-OHDG) in urine were measured using a
competitive ELISA kit (Nanjing Jiancheng Bioengineering
Institute).
Immunohistochemistry
Kidney specimens were fixed in 4% paraformaldehyde
and embedded in paraffin as aforementioned. Kidney sections (0.4
µm) were immunostained using the PV kit (ZS-GB BIO cat. no.
PV-6000) according to the manufacturer's instructions. Primary
antibodies against TXNIP (1/200;ab188865;Abcam), NLRP3
(1/500;68102-1-Ig;Proteintech.), Bax
(1/200;50599-2-Ig;Proteintech.), Bcl2 (1/200; ab32124;Abcam),
α-smooth muscle actin (18:200;ab7817; Abcam), and E-cadherin
(1/300;20874-1-AP;Proteintech) were used for staining (4˚C,12 h).
Positive staining was evaluated using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc.) for quantitative analysis Specimens were
imaged using an Olympus inverted light microscope (Olympus
Corporation; cat. no. BX51; light; 400X).
Protein extraction and western
blotting
Protein was extracted from renal cortex tissue and
HK-2 cells using lysis buffer (MilliporeSigma) according to
standard procedures. Nuclear and cytoplasmic proteins were
extracted from HK-2 cells using a commercial nuclear extraction kit
(Active Motif, Inc.). Proteins (50 µg/lane) were separated by
SDS-PAGE and transferred onto PVDF membranes. The membranes were
incubated overnight at 4˚C with primary antibodies against TXNIP
(MBL International Co.), NLRP3 (Santa Cruz Biotechnology, Inc.),
Bax (Cell Signaling Technology, Inc.), Bcl2 (Cell Signaling
Technology, Inc.), α-SMA (Abcam) and E-cadherin (Abcam). After
incubation with goat anti-rabbit or mouse IgG horseradish
peroxidase-conjugated secondary antibodies, the membranes were
scanned using the Odyssey Fc system (LI-COR Biosciences). Density
analysis was performed using ImageJ software (National Institutes
of Health).
Reverse transcription-quantitative
(RT-q)PCR
Total RNA and cDNA were prepared from kidney tissue
and HK-2 cells using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and RNA PCR kit (Takara Bio Inc.) according to
the manufacturer's instructions. The primers sequences are listed
in Table SI. The PCR
amplification conditions were as follows: Initial denaturation at
95˚C for 10 min, followed by 40 cycles at 95˚C for 15 sec and 60˚C
for 60 sec. Relative mRNA levels of IL-1β, IL-6, TNF-α, TXNIP,
NLRP3, Bax, Bcl2, α-SMA and E-cadherin were determined using the
SYBR Green PCR master mix (Applied Biosystems; cat. no. 4309155)
and reactions were performed on an Agilent MX3000P QPCR system
(Agilent Technologies, Inc.). The relative changes in gene
expression were calculated using the 2-ΔΔCq method and all
experiments were performed at least three times.
Statistical analysis
Data are presented as the mean ± SD. Continuous
variable data that exhibited normality and homogeneity of variance
were analyzed using one-way ANOVA followed by Tukey's HSD and
Games-Howell post hoc test. Otherwise, Mann-Whitney U test was
applied. Statistical analysis was conducted using SPSS Statistics
21.0 (IBM Corp.). P<0.05 was considered to indicate a
statistically significant difference.
Results
Vitamin D3 improves renal function
damage and inflammatory response in diabetic mice
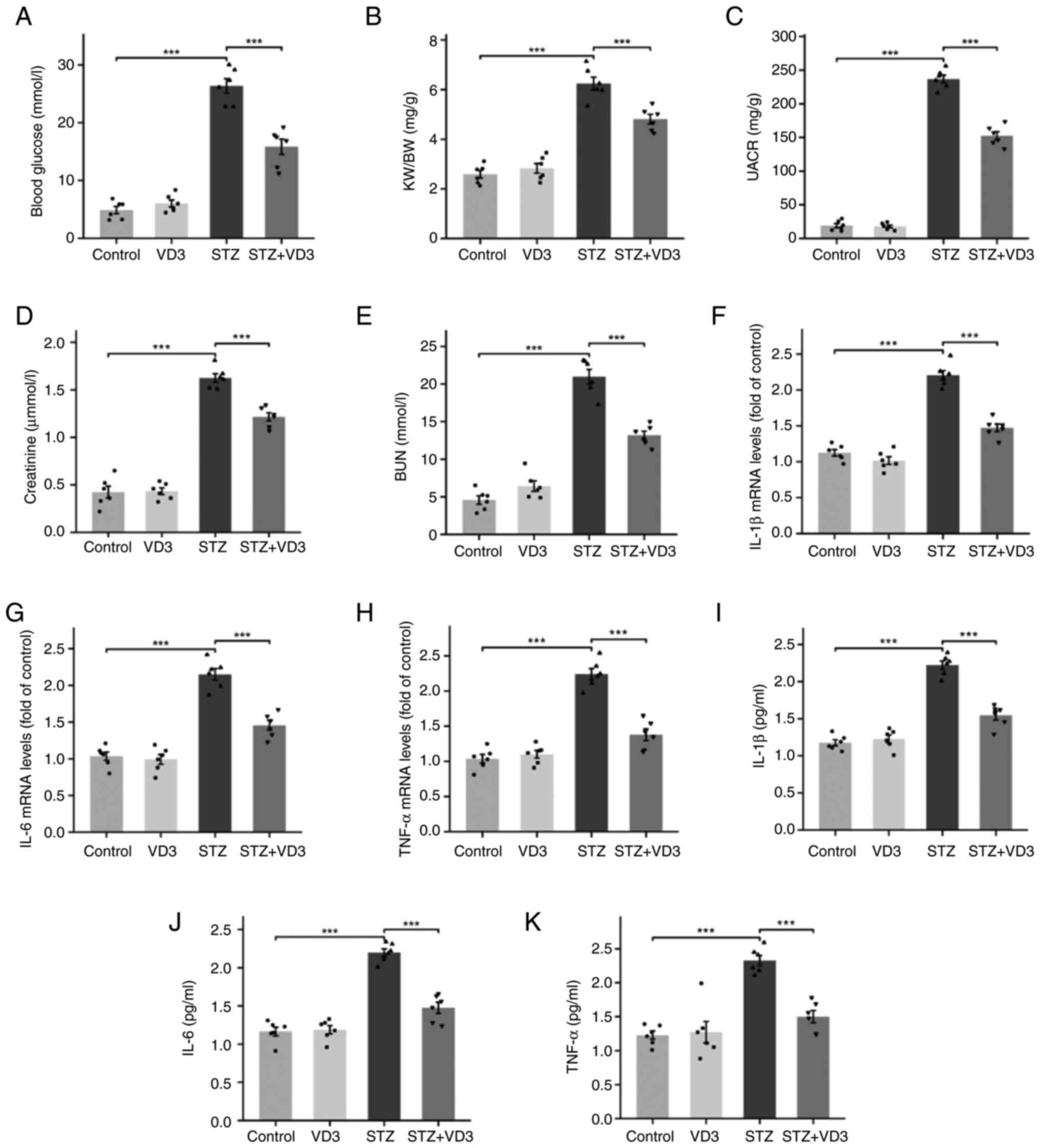
Blood glucose levels in diabetic mice were
significantly higher than in non-diabetic mice (Fig. 1A). Treatment with vitamin D3
significantly decreased the kidney-to-body weight ratio, urinary
albumin-to-creatinine ratio, serum creatinine and blood urea
nitrogen levels in diabetic mice (Fig.
1B-E). To evaluate the intensity of renal inflammation, RT-qPCR
and ELISA were used to detect expression levels of pro-inflammatory
factors such as IL-1β, IL-6 and TNF-α (Fig. 1F-K). Compared with the control, the
expression of pro-inflammatory factors was elevated in diabetic
mice but attenuated after treatment with vitamin D3. These findings
suggest that vitamin D3 improved renal function damage and
inflammatory response in diabetic mice.
Vitamin D3 inhibits tubulointerstitial
fibrosis and cell apoptosis in diabetic mice
Tubulointerstitial fibrosis and cell apoptosis are
key features in DN. To investigate whether vitamin D3 can improve
reverse fibrosis and cell apoptosis, Masson's trichrome staining
was performed on kidney sections (Fig.
2A). Compared with the control group, diabetic mice exhibited
severe tubulointerstitial fibrosis, which was restored to a similar
state as the control group following treatment with vitamin D3.
Immunohistochemistry was used to detect expression of E-cadherin,
α-SMA, Bcl2 and Bax (Fig. 2B). The
results indicated a significant increase in the expression of the
fibrosis marker α-SMA in diabetic mice, which decreased following
treatment with vitamin D3. E-cadherin demonstrated a significant
decrease in its expression in diabetic mice, with an increase
following vitamin D3 treatment. Similarly, the Bax/Bcl-2 ratio, an
indicator of apoptosis, showed a similar trend in diabetic mice.
These findings demonstrated that vitamin D3 not only alleviated
tubulointerstitial fibrosis in diabetic mice but also inhibited
apoptosis in renal tubular epithelial cells.
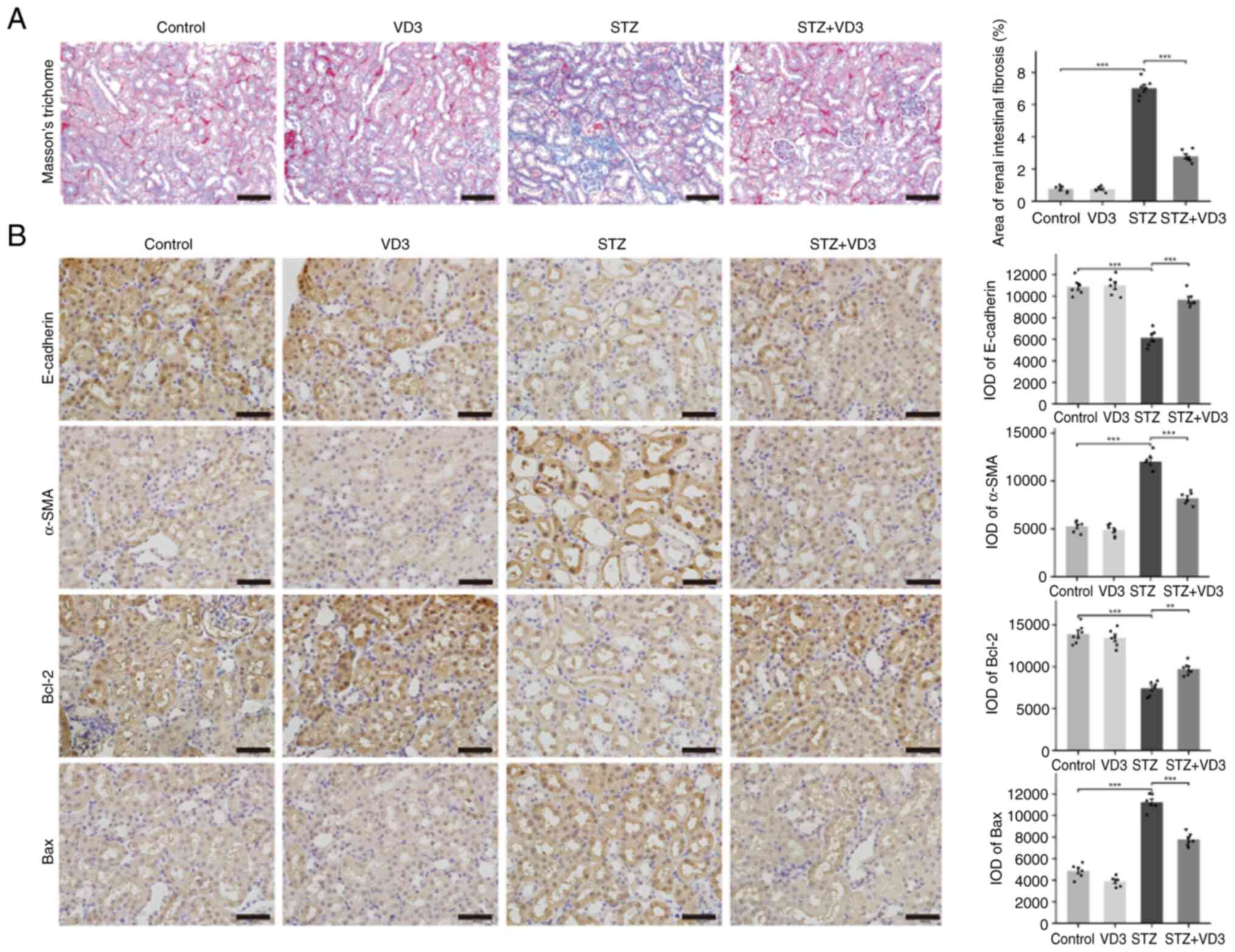 | Figure 2Vitamin D3 inhibits tubulointerstitial
fibrosis and cell apoptosis in diabetic mice. (A) Masson's
trichrome staining of animal tissue sections. (B) Expression of
E-cadherin, α-SMA, Bcl2, and Bax detected by immunohistochemistry.
Scale bar, 50 µm. Magnification, x400. n=6. **P<0.01,
***P<0.001. SMA, smooth Muscle Actin; VD3, vitamin
D3; STZ, streptozotocin; IOD, integrated Optical Density. |
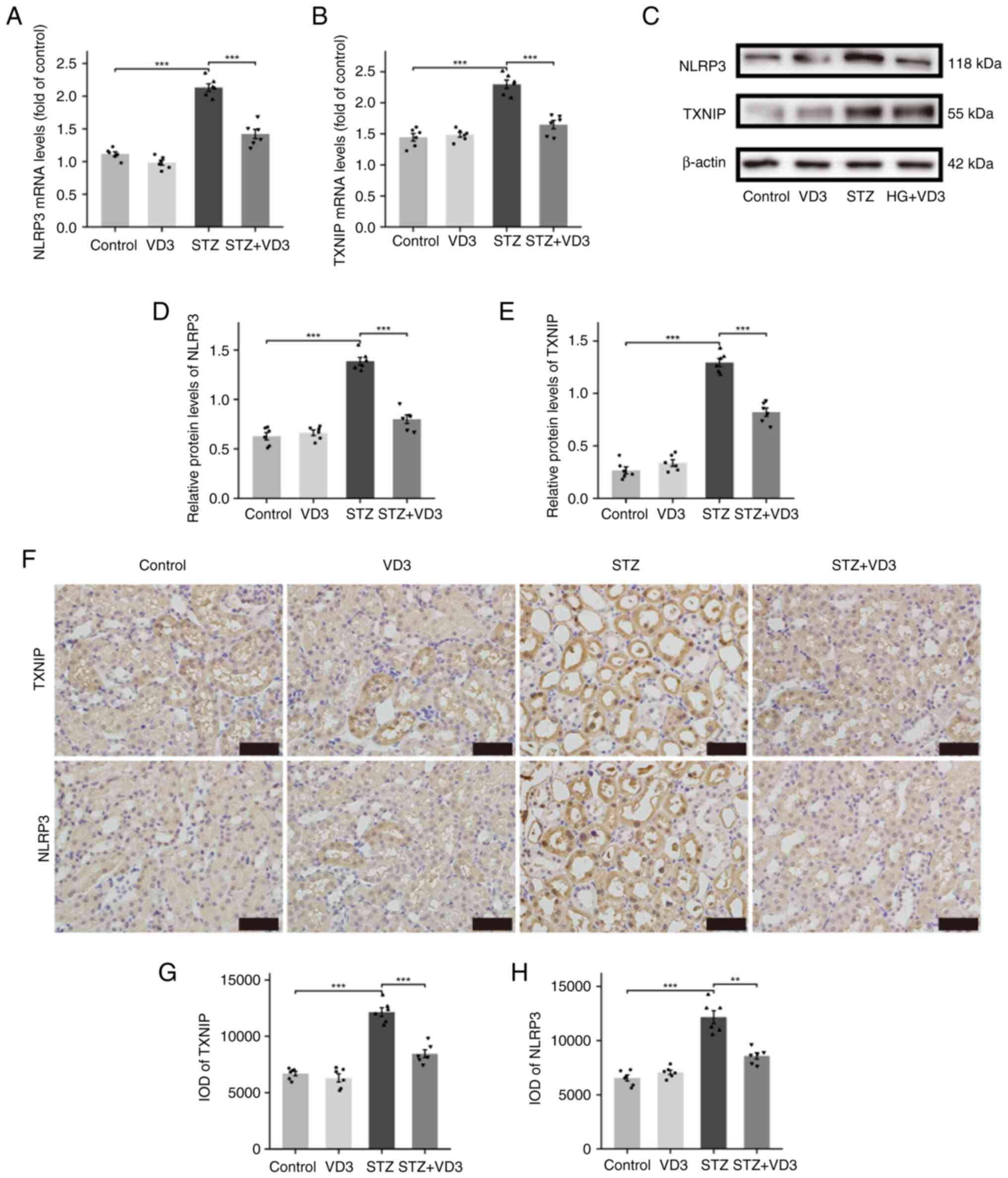
TXNIP/NLRP3 pathway mediates the
effects of vitamin D3 in renal tubules
TXNIP/NLRP3 pathway is known to mediate endogenous
inflammatory responses. Expression of TXNIP and NLRP3 significantly
decreased following treatment with vitamin D3 in diabetic mice
(Fig. 3A-F). Therefore, it was
hypothesized that in DN, vitamin D3 exerted its effects through the
TXNIP/NLRP3 inflammatory pathway.
Vitamin D3 alleviates fibrosis and apoptosis in HK-2
cells by inhibiting oxidative stress and the TXNIP/NLRP3
inflammatory pathway. 8-OHDG is a commonly used marker for
oxidative stress. ELISA was used to measure the levels of 8-OHDG in
mouse urine (Fig. 4A). The results
showed a significant increase in 8-OHDG levels in the urine of
diabetic mice, which was not observed in diabetic mice treated with
vitamin D3. HK-2 cells were treated with vitamin D3 under different
glucose concentrations before RT-qPCR experiments. Following 48 h
incubation in HG medium, gene expression of TXNIP and NLRP3 was
significantly increased in HK-2 cells. However, there was a
significant decrease in expression of the fibrosis marker
E-cadherin (Fig. 4C, G and I).
The expression of other fibrosis markers and apoptosis factors
generally increased. The addition of vitamin D3 to HG medium
notably prevented the upregulation of TXNIP and NLRP3 gene
expression and decreased overall fibrosis and apoptosis in the
cells (Fig. 4B-F). Immunoblotting
experiments with protein extract confirmed the trend observed in
the RT-qPCR results, particularly the decrease in E-cadherin
expression under HG conditions (Fig.
4G-L). Taken together, these findings suggested that vitamin D3
alleviates fibrosis and cell apoptosis in HK-2 cells by inhibiting
oxidative stress and the TXNIP/NLRP3 inflammatory pathway.
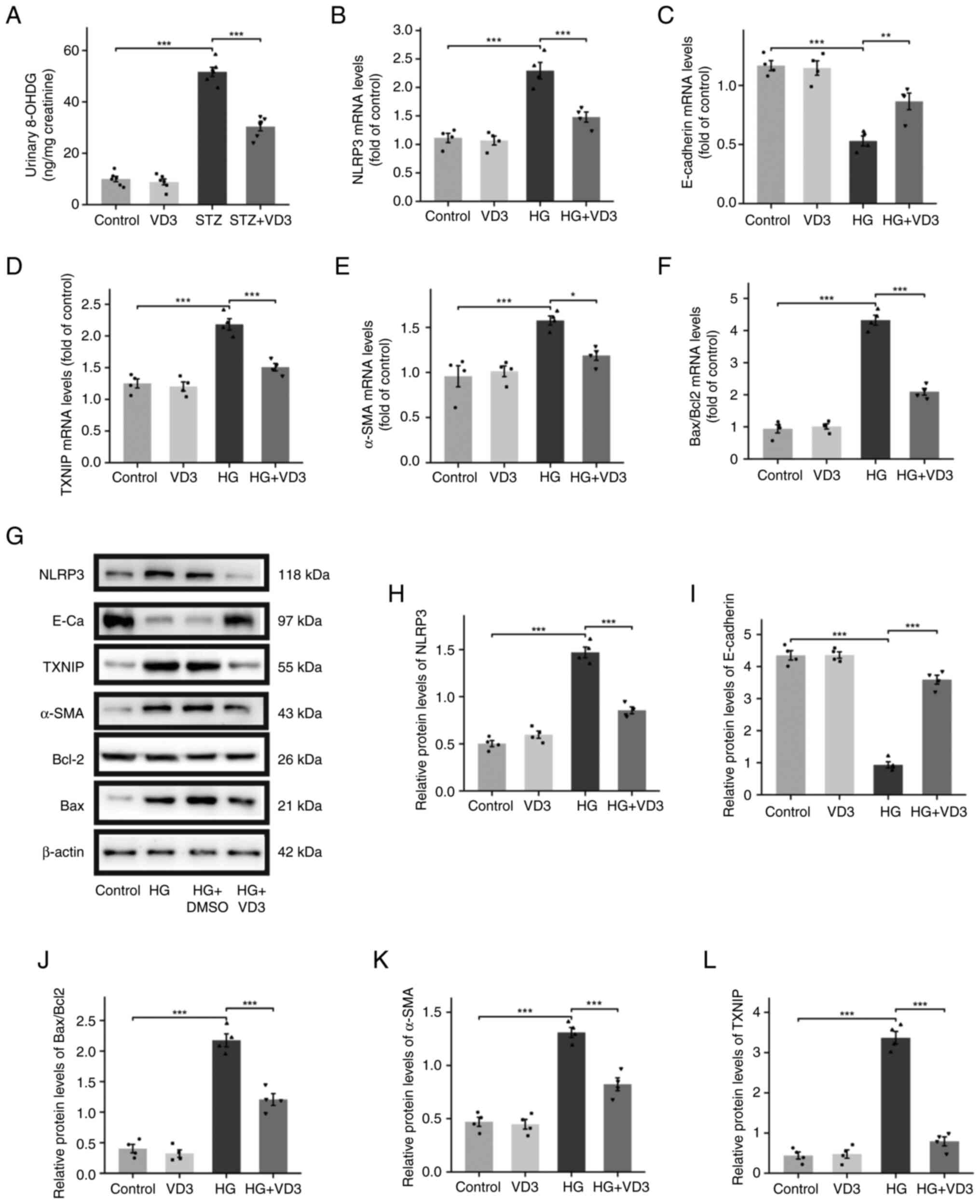 | Figure 4Vitamin D3 alleviates fibrosis and
apoptosis in HK-2 cells by inhibiting oxidative stress and the
TXNIP/NLRP3 inflammatory pathway. (A) Detection of 8-OHDG in mouse
urine by ELISA (n=6). Expression of (B) NLRP3, (C) E-cadherin, (D)
TXNIP, (E) α-SMA and (F) Bax/Bcl2 in cells detected by reverse
transcription-quantitative PCR. (G) Expression of (H) NLRP3, (I)
E-cadherin, (J) Bax/Bcl2, (K) α-SMA and (L) TXNIP in cells detected
by western blot. n=4. *P<0.05,
**P<0.01, ***P<0.001. TXNIP,
thioredoxin interacting protein; VD3, vitamin D3; 8-OHDG,
8-Hydroxy-2'-deoxyguanosine; α-SMA, α-smooth muscle actin; STZ,
streptozotocin; HG, high glucose. |
Vitamin D3 mediates the TXNIP/NLRP3
inflammatory pathway by inhibiting oxidative stress
To confirm that vitamin D3 inhibits the TXNIP/NLRP3
inflammatory pathway by suppressing oxidative stress, HK-2 cells
were cultured with the oxidative stress inhibitor NAC under
different glucose concentrations. NAC-mediated inhibition of
intracellular oxidative stress effectively suppressed the
TXNIP/NLRP3 inflammatory pathway at both mRNA and protein levels,
thereby alleviating fibrosis and apoptosis in HK-2 cells (Fig. 5A-K).
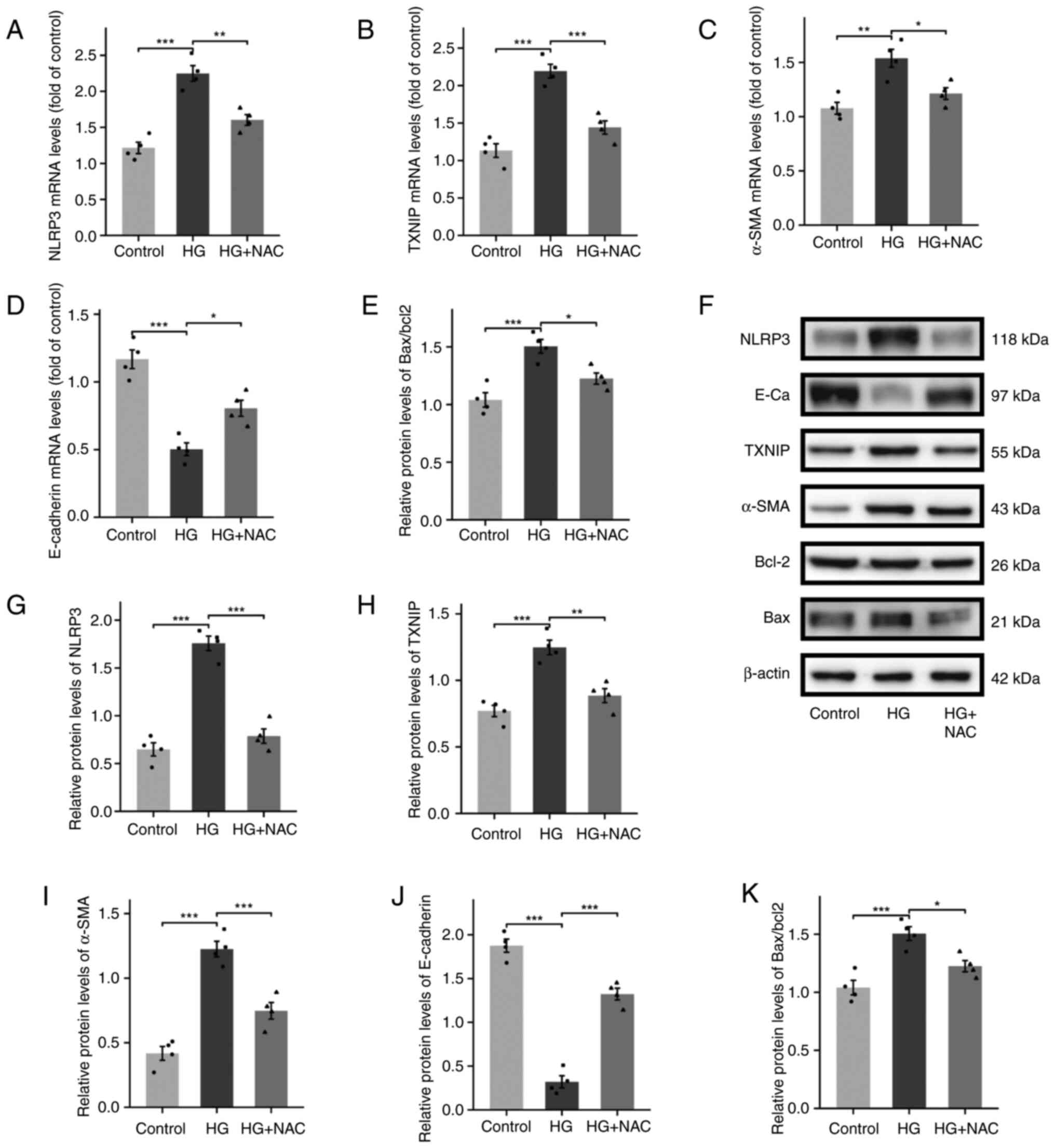 | Figure 5Vitamin D3 mediates the TXNIP/NLRP3
inflammatory pathway by inhibiting oxidative stress. Expression of
(A) NLRP3, (B) TXNIP, (C) α-SMA, (D) E-cadherin and (E) Bax/Bcl2 in
cells detected by reverse transcription-quantitative PCR and (F)
western blotting. Protein expression of (G) NLRP3, (H) TXNIP, (I)
α-SMA, (J) E-cadherin and (K) Bax/Bcl2. n=4. *P<0.05,
**P<0.01, ***P<0.001. TXNIP,
thioredoxin interacting protein; VD3, vitamin D3; 8-OHDG,
8-hydroxy-2'-deoxyguanosine; α-SMA, α-smooth muscle actin; STZ,
streptozotocin; HG, high glucose; NAC, N-acetyl-L-cysteine. |
Discussion
In recent years, there has been extensive discussion
regarding the functions of vitamin D3. In addition to its role in
calcium and bone homeostasis, vitamin D3 also serves a role in
anti-inflammatory and oxidative stress inhibition (19). A wealth of evidence suggests that
vitamin D3 deficiency can lead to various types of inflammatory
disease, such as transplant rejection and inflammatory bowel and
cardiovascular diseases (20).
Therefore, understanding the anti-inflammatory pathways of vitamin
D3 may lead to identification of potential targets for treating
these diseases.
The relationship between vitamin D3 and
complications of diabetes has become a research hotspot in recent
years (21-23).
Here, vitamin D3 had a protective effect in DN. Treatment with
vitamin D3 improved renal function and decreased inflammation in
diabetic mice. Additionally, it partially reversed fibrosis and
apoptosis in renal tubular epithelial cells. Activation of the
TXNIP/NLRP3 inflammatory pathway was primarily due to HG-induced
oxidative stress. Vitamin D3 was able to downregulate expression of
TXNIP by inhibiting oxidative stress, thereby blocking the
activation of NLRP3 and decreasing the inflammatory response,
protecting renal tissue and preventing fibrosis and apoptosis in
renal tubular epithelial cells. Additionally, the potential role of
vitamin D3 in modulating blood glucose levels suggests involvement
in several key molecular pathways. Specifically, vitamin D3 may
exert direct effects on pancreatic β cells, promoting insulin
secretion via modulation of calcium homeostasis and β cell gene
expression (24). Future research
will further elucidate these pathways, exploring their interactions
and potential for therapeutic targeting to manage hyperglycemia and
associated metabolic disorders.
Increased expression of TXNIP in diabetes can lead
to pathological damage such as glomerulosclerosis, interstitial
fibrosis, cell apoptosis and inflammation (25-28).
TXNIP, as an oxidative stress sensor, serves a critical role in the
regulation of NLRP3 inflammasome activation. Under normal
conditions, TXNIP is bound to thioredoxin (TRX), which inhibits its
function. However, under oxidative stress, TRX dissociates from
TXNIP, leading to activation of the NLRP3 inflammasome and the
production of inflammatory cytokines (29). By understanding the regulation of
NLRP3 inflammasome activation, specific inhibitors may be
rationally designed for the treatment and prevention of numerous
types of immune- or metabolic-based disease (30,31).
HG induces production of ROS, which in turn activates oxidative
stress responses (17,32,33).
Activation of oxidative stress responses can convert the function
of sensitive TXNIP from a thioredoxin inhibitor to an activator of
the NLRP3 inflammasome (34). In
the present study, there was an increase in the expression of
8-OHDG, a marker of oxidative stress, after HG stimulation of HK-2
cells. Therefore, it was hypothesized that vitamin D3 exerts its
protective effects on the kidney by inhibiting oxidative stress and
blocking the TXNIP/NLRP3 inflammatory pathway. To test this
hypothesis, NAC was added to HG-treated HK-2 cells. NAC decreased
expression of TXNIP and NLRP3, consistent with the effects of
vitamin D3. Thus, the present study confirmed that vitamin D3
blocks the TXNIP/NLRP3 pathway by inhibiting oxidative stress. The
present study investigated the mechanisms by which vitamin D3 may
alleviate DN by inhibiting oxidative stress and the TXNIP/NLRP3
inflammatory pathway in renal tubular epithelial cells, offering
new insights for potential therapeutic intervention.
The present study aimed to investigate the impact of
nuclear and cytoplasmic components on the outcomes. However, upon
analysis, no significant differences in protein expression between
the nuclear and cytoplasmic fractions extracted from HK-2 cells
were observed. Therefore, these observations were integrated into a
unified analysis to provide a coherent and comprehensive discussion
of the overall protein expression profile.
In conclusion, the present study demonstrated that
vitamin D3 has a protective effect on renal tubular epithelial
cells in diabetic mice. It alleviated interstitial fibrosis and
cell apoptosis in renal tubules. Additionally, this protective
effect is due to the ability of vitamin D3 to inhibit oxidative
stress in renal tubules, thereby blocking the TXNIP/NLRP3
pathway.
However, it is important to recognize the
limitations of the present study. The present findings are based on
a specific animal model and focused primarily on the TXNIP/NLRP3
inflammatory pathway, which may limit the generalizability of
results to other models or human subjects. Furthermore, the present
study did not explore the broader impact of vitamin D3 on systemic
glucose homeostasis. To address these limitations, future studies
should investigate the mechanistic pathways by which vitamin D3
influences glucose homeostasis, alongside its renal protective
effects.
The present study not only confirms that vitamin D3
can protect renal tubular epithelial cells in DN by inhibiting
oxidative stress and blocking the TXNIP/NLRP3 inflammatory pathway
but also highlights its potential as a novel therapeutic approach.
These findings may facilitate application of vitamin D3 in the
clinical management of DN, suggesting that it could be a valuable
addition to current treatment strategies. Future clinical trials
are warranted to explore the efficacy of vitamin D3 in improving
outcomes for patients with diabetic kidney disease, thereby
translating the present findings from the laboratory to the clinic
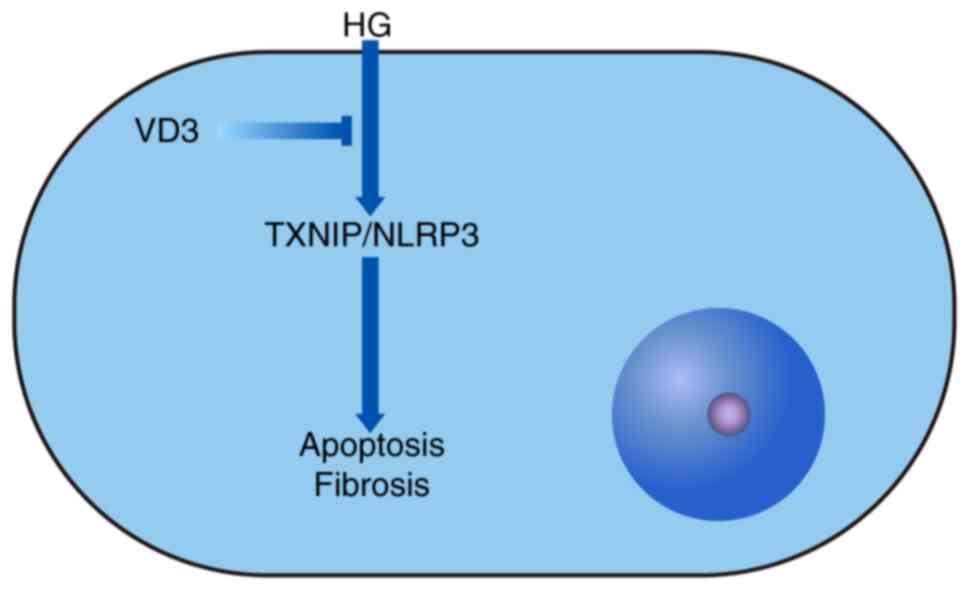
(Fig. 6).
Supplementary Material
Primer sequences.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by Hebei Province
Medical Science Research Key Project Plan (grant no. 20180200).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
GL performed experiments and wrote the manuscript.
SH and TL searched the literature and analyzed the data. NZ and TL
searched the literature and revised the manuscript. NZ designed the
experiments and revised the manuscript. All authors have read and
approved the final manuscript. NZ and GL confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
The present study was approved (approval no.
2016043) by the Ethics Committee of the Hebei General Hospital
(Shijiazhuang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Diabetes Control and Complications
Trial/Epidemiology of Diabetes Interventions and Complications
(DCCT/EDIC) Research Group. Nathan DM, Zinman B, Cleary PA,
Backlund JC, Genuth S, Miller R and Orchard TJ: Modern-day clinical
course of type 1 diabetes mellitus after 30 years' duration: The
diabetes control and complications trial/epidemiology of diabetes
interventions and complications and pittsburgh epidemiology of
diabetes complications experience (1983-2005). Arch Intern Med.
169:1307–1316. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes Care. 27:1047–1053.
2004.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Collins AJ, Foley RN, Chavers B,
Gilbertson D, Herzog C, Ishani A, Johansen K, Kasiske BL, Kutner N,
Liu JN, et al: US renal data system 2013 annual data report. Am J
Kidney Dis. 63 (1 Suppl)(A7)2014.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Paneni F, Beckman JA, Creager MA and
Cosentino F: Diabetes and vascular disease: pathophysiology,
clinical consequences, and medical therapy: Part I. Eur Heart J.
34:2436–2443. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Gall MA, Rossing P, Skøtt P, Damsbo P,
Vaag A, Bech K, Dejgaard A, Lauritzen M, Lauritzen E, Hougaard P,
et al: Prevalence of micro- and macroalbuminuria, arterial
hypertension, retinopathy and large vessel disease in European type
2 (non-insulin-dependent) diabetic patients. Diabetologia.
34:655–661. 1991.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Remuzzi G, Benigni A and Remuzzi A:
Mechanisms of progression and regression of renal lesions of
chronic nephropathies and diabetes. J Clin Invest. 116:288–296.
2006.PubMed/NCBI View
Article : Google Scholar
|
|
7
|
Baynes JW and Thorpe SR: Role of oxidative
stress in diabetic complications: A new perspective on an old
paradigm. Diabetes. 48:1–9. 1999.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ceriello A, Morocutti A, Mercuri F,
Quagliaro L, Moro M, Damante G and Viberti GC: Defective
intracellular antioxidant enzyme production in type 1 diabetic
patients with nephropathy. Diabetes. 49:2170–2177. 2000.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lu L, Lu Q, Chen W, Li JW, Li CX and Zheng
Z: Vitamin D3 protects against diabetic retinopathy by
inhibiting high-glucose-induced activation of the ROS/TXNIP/NLRP3
inflammasome pathway. J Diabetes Res. 2018(8193523)2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Simon AR, Rai U, Fanburg BL and Cochran
BH: Activation of the JAK-STAT pathway by reactive oxygen species.
Am J Physiol. 275:C1640–C1652. 1998.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kang BPS, Frencher S, Reddy V, Kessler A,
Malhotra A and Meggs LG: High glucose promotes mesangial cell
apoptosis by oxidant-dependent mechanism. Am J Physiol Renal
Physiol. 284:F455–F466. 2003.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhu XJ, Wu SH and Guo HC: Active vitamin D
and vitamin D receptor help prevent high glucose induced oxidative
stress of renal tubular cells via AKT/UCP2 signaling pathway.
Biomed Res Int. 2019(9013904)2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zehnder D, Bland R, Williams MC, McNinch
RW, Howie AJ, Stewart PM and Hewison M: Extrarenal expression of
25-hydroxyvitamin d(3)-1 alpha-hydroxylase. J Clin Endocrinol
Metab. 86:888–894. 2001.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Plum LA and DeLuca HF: Vitamin D, disease
and therapeutic opportunities. Nat Rev Drug Discov. 9:941–955.
2010.PubMed/NCBI View
Article : Google Scholar
|
|
15
|
Prietl B, Treiber G, Pieber TR and Amrein
K: Vitamin D and immune function. Nutrients. 5:2502–2521.
2013.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Chagas CE, Borges MC, Martini LA and
Rogero MM: Focus on vitamin D, inflammation and type 2 diabetes.
Nutrients. 4:52–67. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Wei H, Bu R, Yang QH, Jia J, Li T, Wang QP
and Chen YJ: Exendin-4 protects against hyperglycemia-induced
cardiomyocyte pyroptosis via the AMPK-TXNIP pathway. J Diabetes
Res. 2019(8905917)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yates AG, Weglinski CM, Ying Y, Dunstan
IK, Strekalova T and Anthony DC: Nafamostat reduces systemic
inflammation in TLR7-mediated virus-like illness. J
Neuroinflammation. 19(8)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hewison M: Vitamin D and the immune
system: New perspectives on an old theme. Rheum Dis Clin North Am.
38:125–139. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Guillot X, Semerano L,
Saidenberg-Kermanac'h N, Falgarone G and Boissier MC: Vitamin D and
inflammation. Joint Bone Spine. 77:552–557. 2010.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Aksoy H, Akçay F, Kurtul N, Baykal O and
Avci B: Serum 1,25 dihydroxy vitamin D (1,25(OH)2D3), 25 hydroxy
vitamin D (25(OH)D) and parathormone levels in diabetic
retinopathy. Clin Biochem. 33:47–51. 2000.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Kaur H, Donaghue KC, Chan AK,
Benitez-Aguirre P, Hing S, Lloyd M, Cusumano J, Pryke A and Craig
ME: Vitamin D deficiency is associated with retinopathy in children
and adolescents with type 1 diabetes. Diabetes Care. 34:1400–1402.
2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Prabhu RA and Saraf K: Vitamin D in
diabetic nephropathy. J Postgrad Med. 64:5–6. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cheng Q, Li YC, Boucher BJ and Leung PS: A
novel role for vitamin D: Modulation of expression and function of
the local renin-angiotensin system in mouse pancreatic islets.
Diabetologia. 54:2077–2081. 2011.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Shah A, Xia L, Masson EA, Gui C, Momen A,
Shikatani EA, Husain M, Quaggin S, John R and Fantus IG:
Thioredoxin-interacting protein deficiency protects against
diabetic nephropathy. J Am Soc Nephrol. 26:2963–2977.
2015.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Shi Y, Ren Y, Zhao L, Du C, Wang Y, Zhang
Y, Li Y, Zhao S and Duan H: Knockdown of thioredoxin interacting
protein attenuates high glucose-induced apoptosis and activation of
ASK1 in mouse mesangial cells. FEBS Lett. 585:1789–1795.
2011.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ha H, Hwang IA, Park JH and Lee HB: Role
of reactive oxygen species in the pathogenesis of diabetic
nephropathy. Diabetes Res Clin Pract. 82 (Suppl 1):S42–S45.
2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chen N, Song S, Yang Z, Wu M, Mu L, Zhou T
and Shi YH: ChREBP deficiency alleviates apoptosis by inhibiting
TXNIP/oxidative stress in diabetic nephropathy. J Diabetes
Complications. 35(108050)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Susztak K, Raff AC, Schiffer M and
Böttinger EP: Glucose-induced reactive oxygen species cause
apoptosis of podocytes and podocyte depletion at the onset of
diabetic nephropathy. Diabetes. 55:225–233. 2006.PubMed/NCBI
|
|
30
|
Ortega MA, De Leon-Oliva D, García-Montero
C, Fraile-Martinez O, Boaru DL, de Castro AV, Saez MA,
Lopez-Gonzalez L, Bujan J, Alvarez-Mon MA, et al: Reframing the
link between metabolism and NLRP3 inflammasome: Therapeutic
opportunities. Front Immunol. 14(1232629)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Fraile-Martinez O, García-Montero C,
Pekarek L, Saz JV, Álvarez-Mon MÁ, Barrena-Blázquez S,
García-Honduvilla N, Buján J, Asúnsolo Á, Coca S, et al: Decreased
survival in patients with pancreatic cancer may be associated with
an increase in histopathological expression of inflammasome marker
NLRP3. Histol Histopathol. 39:35–40. 2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zheng Z, Chen HB, Ke GJ, Fan Y, Zou HD,
Sun XD, Gu Q, Xu X and Ho PCP: Protective effect of perindopril on
diabetic retinopathy is associated with decreased vascular
endothelial growth factor-to-pigment epithelium-derived factor
ratio: Involvement of a mitochondria-reactive oxygen species
pathway. Diabetes. 58:954–964. 2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Zheng Z, Chen H, Wang H, Ke B, Zheng B, Li
Q, Li P, Su L, Gu Q and Xu X: Improvement of retinal vascular
injury in diabetic rats by statins is associated with the
inhibition of mitochondrial reactive oxygen species pathway
mediated by peroxisome proliferator-activated receptor gamma
coactivator 1alpha. Diabetes. 59:2315–2325. 2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Schroder K, Zhou RB and Tschopp J: The
NLRP3 inflammasome: A sensor for metabolic danger? Science.
327:296–300. 2010.PubMed/NCBI View Article : Google Scholar
|