Introduction
Hyperphenylalaninemia (HPA) occurs due to a
deficiency in phenylalanine (Phe) hydroxylase, thus preventing the
smooth conversion of Phe and resulting in its accumulation in the
body. The inability to metabolize Phe normally leads to the
accumulation of phenylketones, especially phenylacetone and
phenylethylketone. One of the primary clinical features of HPA is
damage to the nervous system, including delays in intellectual
developmental, cognitive impairments and motor disturbances. The
accumulation of phenylketones may also induce the dilation of
cerebral blood vessels, causing instability in the cerebral
vascular system (1). The
prevalence of HPA exhibits substantial global variation. The
average incidence of HPA is ~1:8,000 in Europe (2), 1:15,000 in the United States
(3) and 1:12,000 in mainland China
(4). If left undiagnosed and
untreated in a timely manner, HPA can lead to cognitive impairment,
neurodevelopmental disorders and even severe intellectual
disabilities. Thus, early and highly accurate screening and
diagnosis of HPA are paramount for the quality of life and
long-term prognosis of affected individuals (5). In clinical practice, HPA is
categorized into two distinct types: phenylalanine hydroxylase
deficiency (PAHD) and tetrahydrobiopterin deficiency (BH4D). PAHD
and BH4D play crucial roles in the Phe metabolic pathway. PAHD is
responsible for converting Phe to tyrosine, while BH4D is involved
in the regeneration of tetrahydrobiopterin, a cofactor essential
for PAHD activity. When both PAHD and BH4D are deficient, the
normal pathway of Phe metabolism is hindered, leading to the
accumulation of phenylketones in the body and toxic effects on the
organism (6).
Neonatal screening for the HPA has been implemented
in China since the early 1980s, leading to the accumulation of
substantial expertise spanning three decades. In 2014, Chinese
experts established a consensus regarding HPA diagnosis and
treatment (7). Moreover, in 2019,
there was a recommendation for a consensus on dietary interventions
and nutrition management for PAHD (8). The clinical diagnosis of HPA
typically relies on laboratory testing, with the most commonly used
methods being fluorometric assays and tandem mass spectrometry
(MS/MS). These two methods are widely employed in clinical
practice; however, their diagnostic accuracy and predictive value
have remained the subject of ongoing research and clinical scrutiny
(9). Fluorometric assays, which
are relatively simple, cost-effective and amenable to large-scale
application, are nonetheless associated with debates regarding its
specificity and accuracy (10). By
contrast, MS/MS offers higher resolution and sensitivity but
requires more complex instrumentation and specialized expertise
(11). Given the complexity and
heterogeneity of HPA, as well as the variations among different
subtypes of HPA, there is a pressing need to gain a deeper
understanding of the performance of these two commonly utilized
methods to improve guidance of clinical practice and neonatal
screening. To address this issue, the present study aimed to
conduct a comprehensive meta-analysis to assess the diagnostic
value of fluorometric assays and MS/MS for HPA and its
subtypes.
Materials and methods
Literature inclusion and exclusion
criteria
The inclusion criteria were as follows: The study
type was cross-sectional; the study reported the newborn screening
and genetic features of patients with HPA; and the language was
limited to English. The exclusion criteria were as follows:
Repeated publication; studies without full text, incomplete
information or inability to conduct data extraction; animal
experiments; and reviews and systematic reviews.
Search strategy
The present meta-analysis searched the PubMed,
Embase and Cochrane Library databases from inception to October
2023. The search strategies were as follows:
‘(((genetic[Title/Abstract]) OR (screening[Title/Abstract])) AND
((hyperphenylalaninemia[Title/Abstract]) OR (HPA[Title/Abstract])))
AND ((China[Title/Abstract]) OR (Chinese[Title/Abstract]))’ for
PubMed; ‘screening:ab,ti AND genetic:ab,ti AND
(hyperphenylalaninemia:ab,ti OR hpa:ab,ti) AND (china:ab,ti OR
chinese:ab,ti)’ for Embase; and ‘((genetic):ti,ab,kw OR
(screening):ti,ab,kw) AND ((hyperphenylalaninemia):ti,ab,kw OR
(HPA):ti,ab,kw) AND ((China):ti,ab,kw OR (Chinese):ti,ab,kw)’ for
the Cochrane Library.
Literature screening and data
extraction
Two researchers independently carried out the
literature search, screening and information extraction.
Disagreements were resolved by discussion or by consulting a third
person. The following data were extracted: Author, publication year
of the article, country, study design, sample size, age, sample
size of patients with suspected positive HPA, sample size of
patients with confirmed HPA and screening method.
Literature quality assessment
The quality of evidence for each study was assessed
by two independent researchers using the Methodological Index for
Nonrandomized Studies (MINORS) scale (12). There were a total of 12 items, each
with a score of 0-2 and a total score of 24. Studies were
classified as moderate quality (9-16)
or high quality (17-24).
The Meta-analysis was performed in accordance with the relevant
items in the Preferred Reporting Items for Systematic Reviews and
Meta Analysis statement.
Data synthesis and statistical
analysis
All the data were analyzed with STATA (15.1,
StataCorp LLC) (13). The
I2 and Q tests were used to evaluate heterogeneity. A
P-value ≥0.1 and an I2 value ≤50% indicate that there is
homogeneity between studies and in such cases, a fixed effects
model was used for pooled analysis; a P-value <0.1 or an
I2 value >50% indicated that there was heterogeneity
and in such cases, sensitivity analysis was conducted by
eliminating each included study individually and performing a
summary analysis of the remaining studies to determine the source
of heterogeneity. If the heterogeneity was still high, a random
effects model was used, or descriptive analysis was performed
instead of pooled analysis. A funnel plot and Egger's test were
used to assess publication bias. Age of newborns is presented as
mean ± SD.
Results
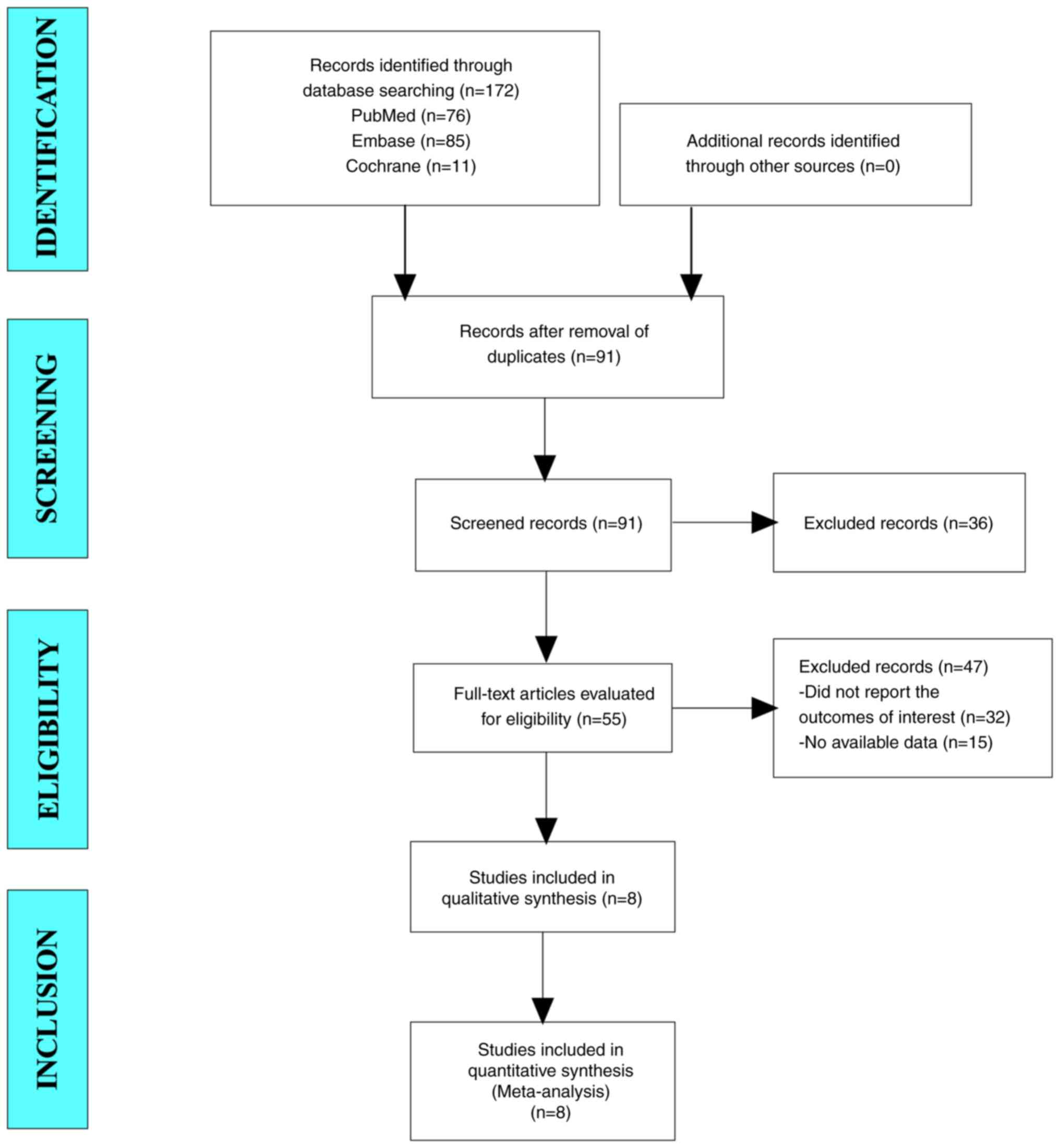
Results of the literature search
In the present meta-analysis, a total of 172 studies
were retrieved from databases, including PubMed, Embase and the
Cochrane Library. After eliminating duplicate studies, 91 studies
remained. After screening the titles and abstracts, 55 studies
remained. Ultimately, eight articles were included in the
meta-analysis (Fig. 1).
Baseline characteristics and quality
assessment of the included studies
A total of eight cross-sectional studies were
included in this meta-analysis. The total screening population
included 113,280,754 participants. All patients were newborns from
China. The screening methods used were fluorometric and MS/MS. In
the studies reporting sex distributions, a total of 2,429,813 boys
and 2,057,178 girls were included, indicating that the sex
distributions were comparable. The MINORS scores were all >16
points, indicating that the included studies were of moderate or
high quality (Table I).
 | Table IBaseline characteristics and quality
assessment of the included studies. |
Table I
Baseline characteristics and quality
assessment of the included studies.
| Author, year | Study design | Total screening
population | Patients with
suspected HPA | Patients with
confirmed HPA | Age (days) | Sex
(male/female) | Screening method | MINORS score | (Refs.) |
|---|
| Qiu et al,
2023 | Cross-sectional | 3,204,067 | 2,195 | 189 | Newborns |
1,714,014/1,490,053 | Fluorometric | 16 | (14) |
| Wang et al,
2021 | Cross-sectional | 1,243,957 | 1,082 | 181 | 20.0±7.0 | / | MS/MS | 18 | (15) |
| Wang et al,
2019 | Cross-sectional | 401,660 | / | 48 | 7.35±8.14 | 210,359/191,259 | MS/MS | 16 | (16) |
| Li et al,
2022 | Cross-sectional | 300,849 | / | 18 | Newborns | 178,265/122,584 | MS/MS | 17 | (17) |
| Yuan et al,
2021 | Cross-sectional | 107,078,115 | / | 10,553 | Newborns | / | MS/MS | 18 | (18) |
| Wang et al,
2018 | Cross-sectional | / | / | 1,020 | Newborns | / | MS/MS | 17 | (19) |
| Lin et al,
2022 |
Cross-sectional | 580,460 | 295 | 56 | Newborns |
327,175/253,285 | MS/MS | 17 | (20) |
| Wang et al,
2019 |
Cross-sectional | 418,831 | 25 | 19 | Newborns | / | Fluorometric | 16 | (21) |
| | | 52,815 | 9 | 9 | Newborns | / | MS/MS | 16 | |
Results of the meta-analysis.
Screening positive rate
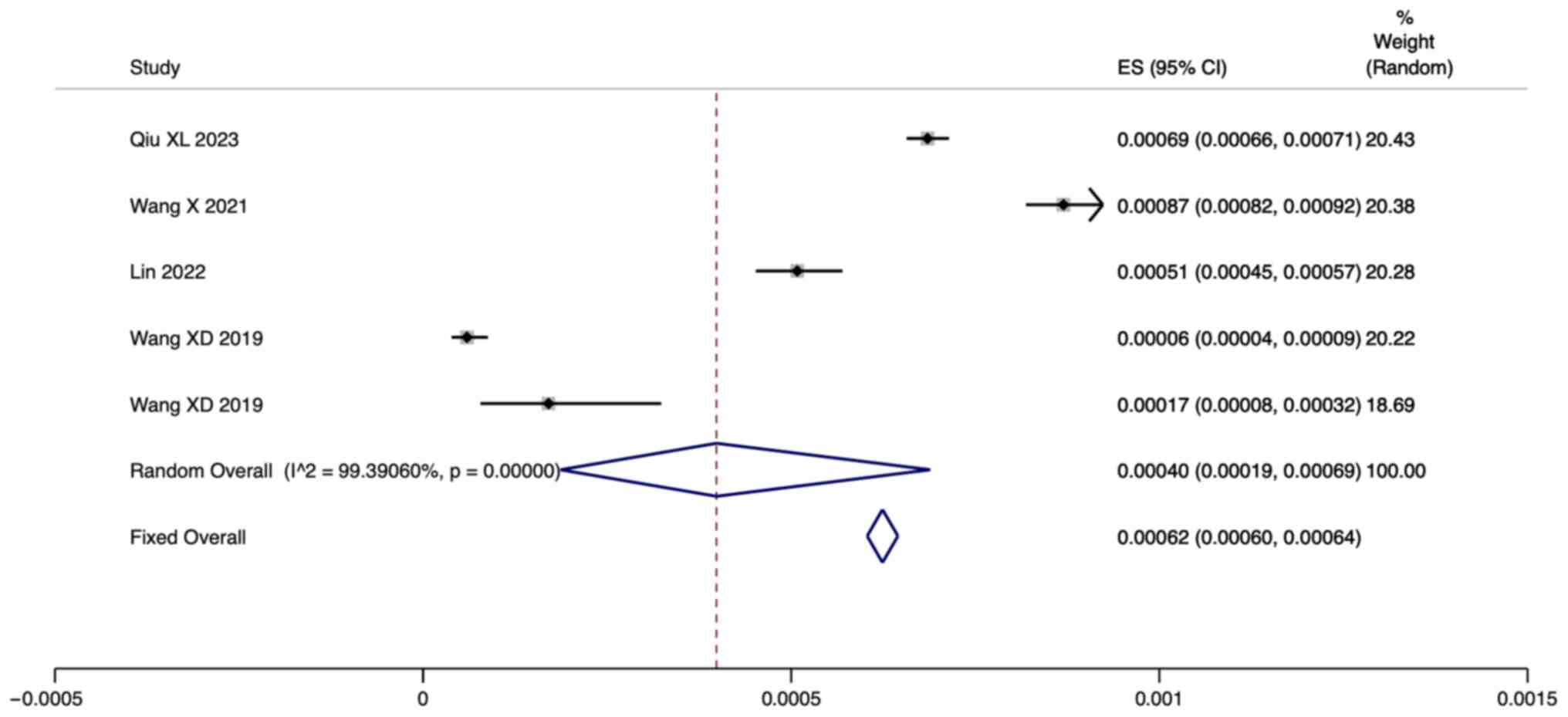
A total of five studies reported the positive rate
of newborn screening for HPA. Since there was significant
heterogeneity (I2=99.39%; P=0.000), a
meta-analysis was conducted through a random effects model. The
pooled results showed that the positive rate of neonatal HPA
screening using fluorometric assays and tandem mass spectrometry
was 0.04% [95% confidence interval (CI): 0.019-0.069] (Fig. 2).
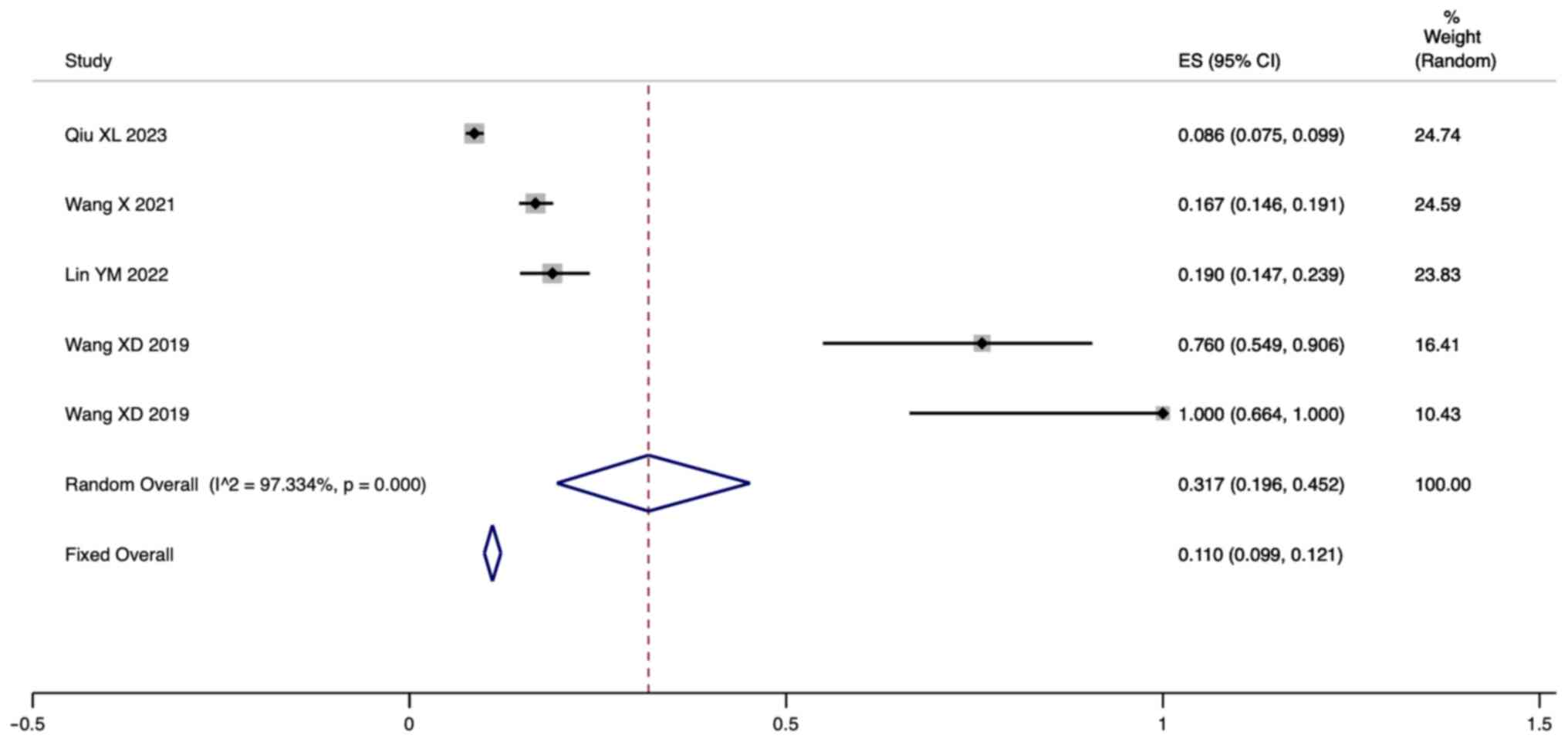
PPV. A total of five studies reported the PPV
of newborn screening for HPA. Since there was significant
heterogeneity (I2=97.33%; P=0.000), a
meta-analysis was conducted through a random effects model. The
pooled results showed that the PPV of neonatal HPA screening using
fluorometric assays and tandem mass spectrometry was 31.7% (95% CI:
19.6-45.2; Fig. 3).
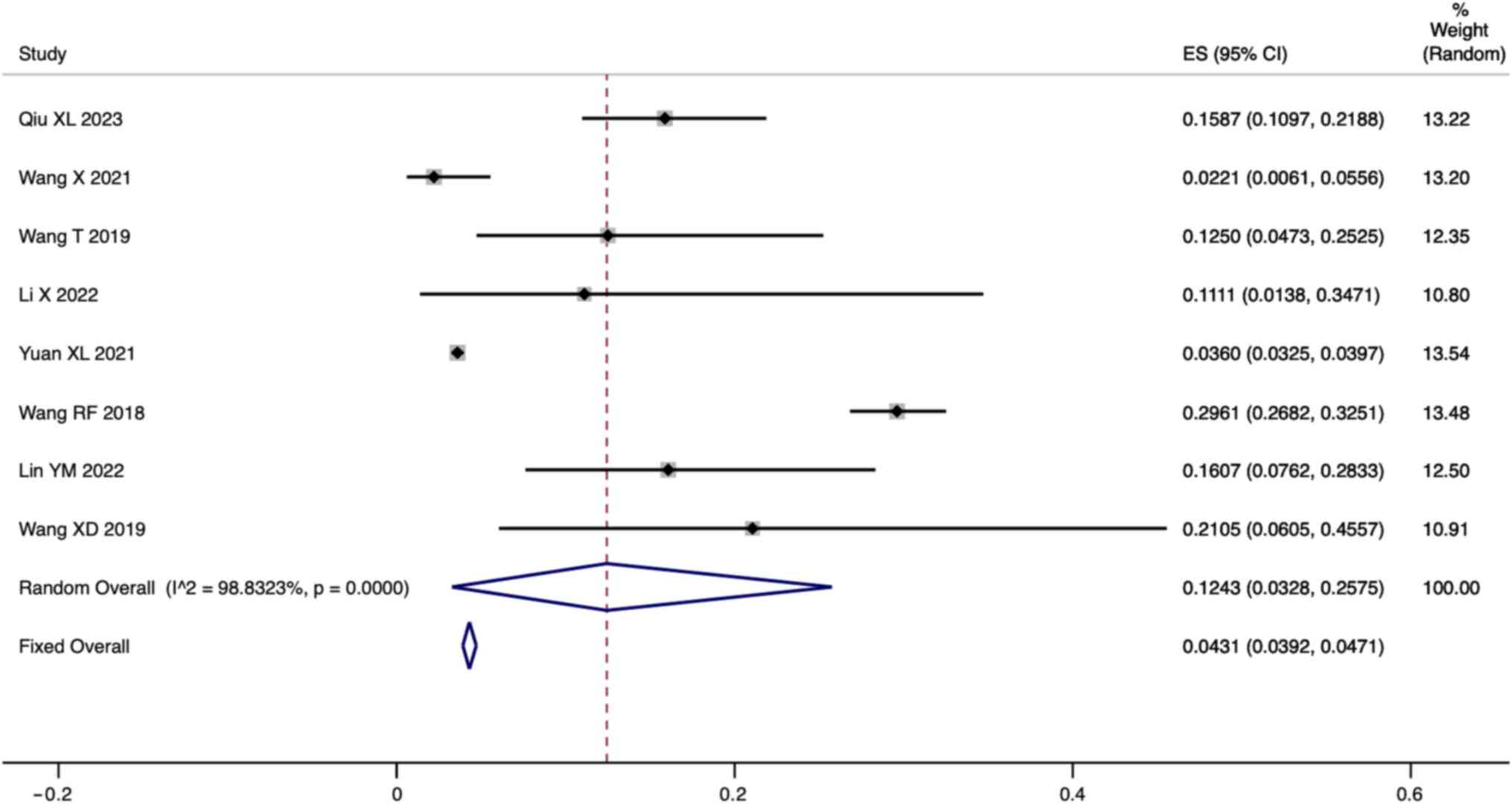
Incidence of HPA patients with BH4D. A total
of eight studies reported the incidence of BH4D in HPA patients.
Since there was significant heterogeneity
(I2=98.83%, P=0.000), a meta-analysis was
conducted through a random effects model. The pooled results showed
that the incidence of BH4D in HPA patients was 12.43% (95% CI:
3.28-25.75; Fig. 4).
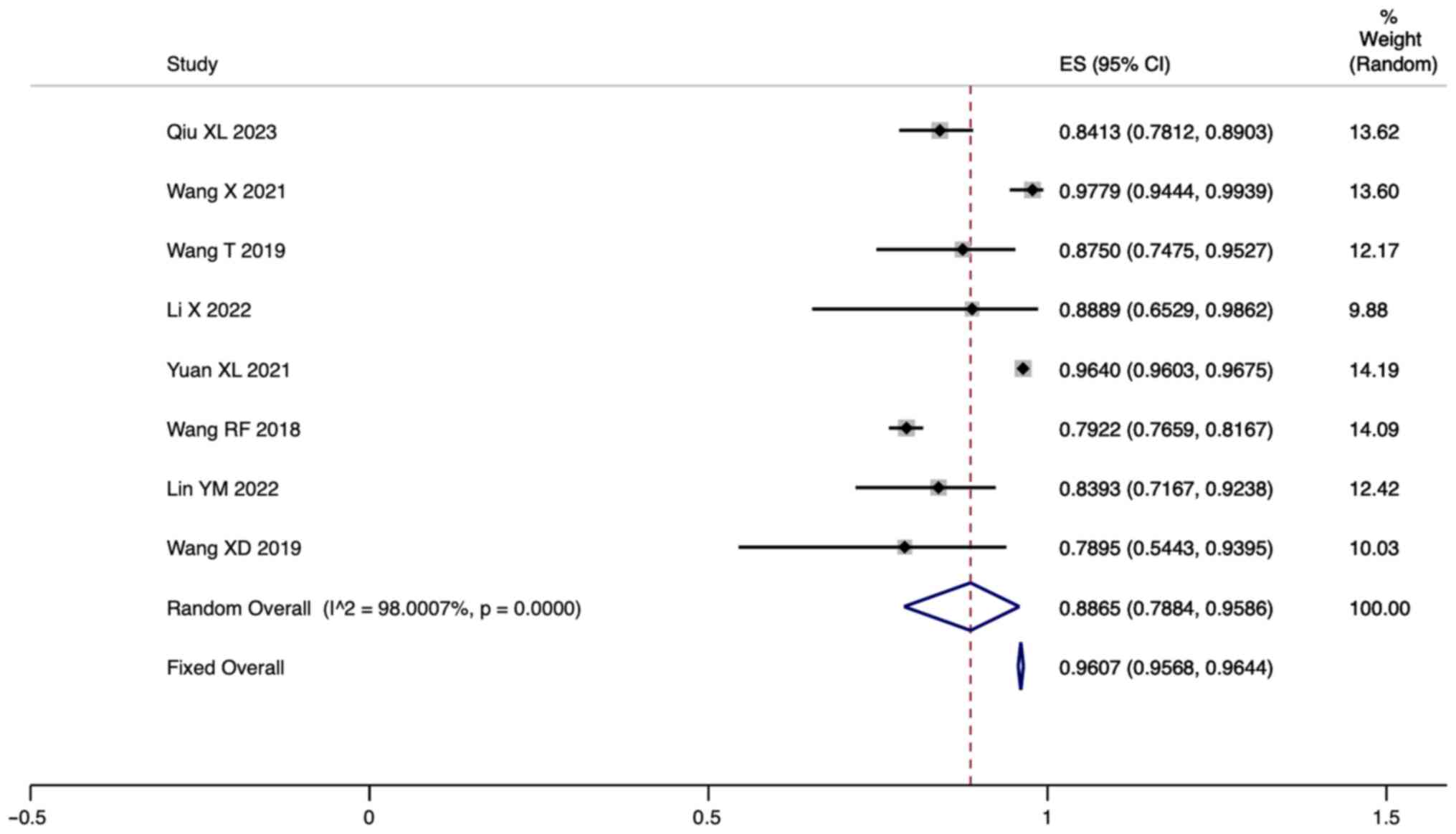
Incidence of PAHD in HPA patients. A total of
eight studies reported the incidence of PAHD in HPA patients. Since
there was significant heterogeneity (I2=98.00%,
P=0.000), a meta-analysis was conducted through a random effects
model. The pooled results showed that the incidence of PAHD in HPA
patients was 88.65% (95% CI: 78.84-95.86; Fig. 5).
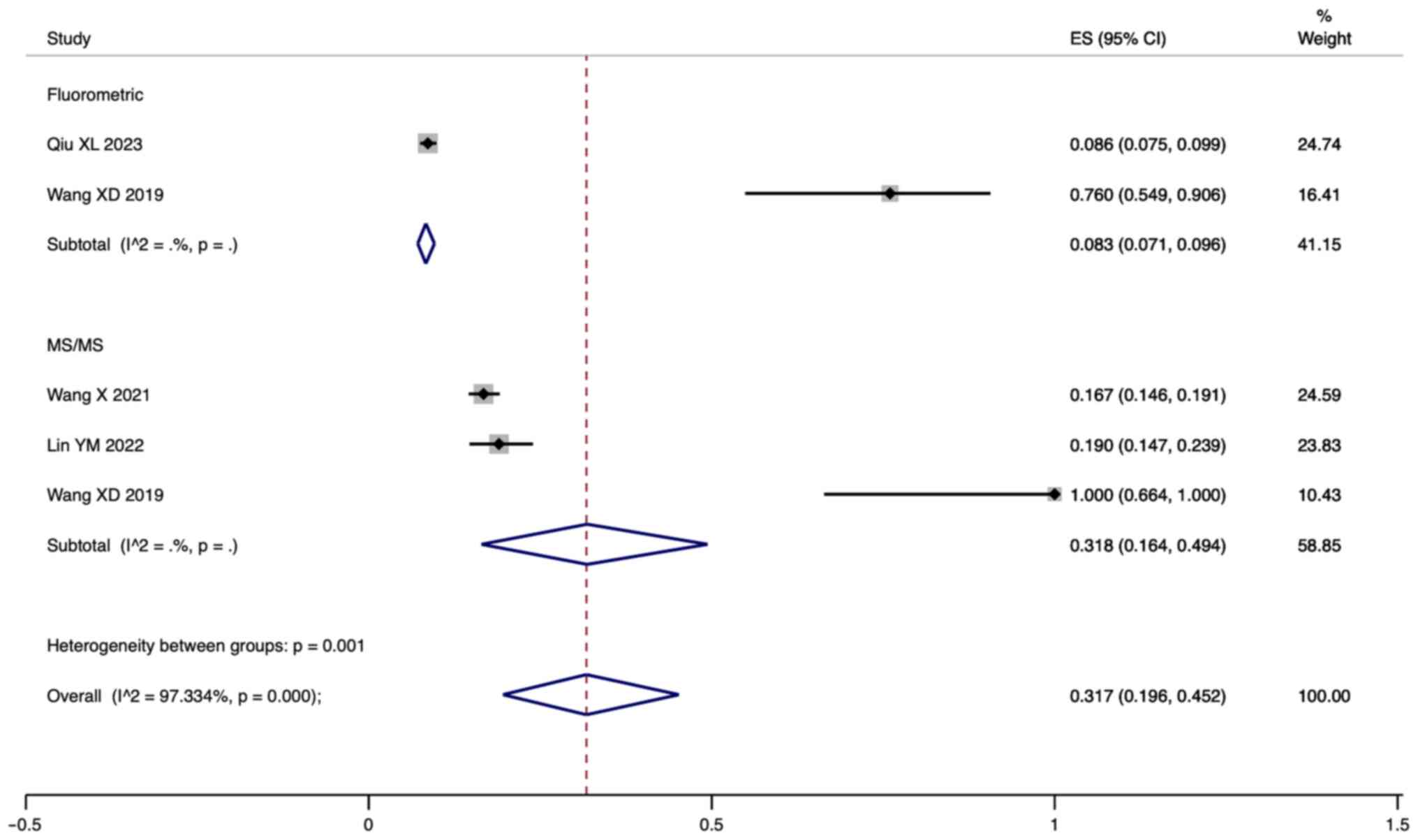
Subgroup analysis
The present study performed a subgroup analysis of
PPVs based on different screening methods. The pooled results
showed that the PPV of neonatal HPA screening using fluorometric
assays was 8.3% (95% CI: 7.1-9.6; Fig.
6), while the PPV of neonatal HPA screening using tandem mass
spectrometry was 31.8% (95% CI: 16.4-49.4) and the difference in
the percentage of positive HPA signals between the two screening
methods was statistically significant (P=0.001).
Sensitivity analysis
The present study revealed that none of the studies
had an outsized effect on the pooled results of the meta-analysis,
which suggested that the results of the meta-analysis were stable
and reliable.
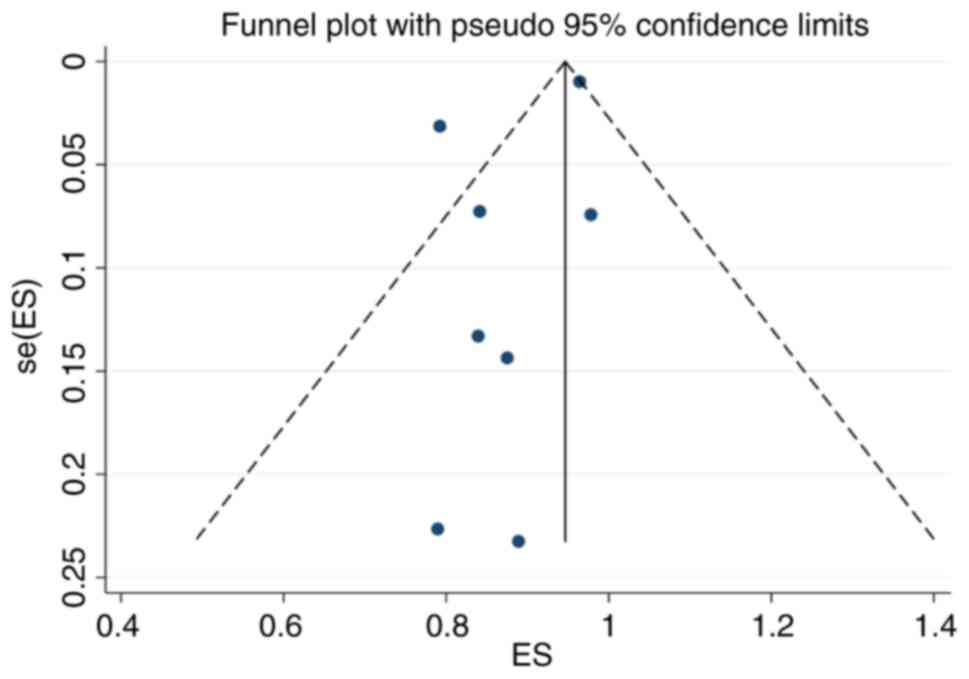
Publication bias
The funnel plot drawn in the present study is shown
in Fig. 7. The funnel plot was
basically symmetrical and the P-value of Egger's test was 0.204,
indicating that there was no obvious publication bias.
Discussion
Newborn screening for Phe metabolism defects is a
highly effective approach for the early identification and swift
management of HPA. This can significantly enhance survival and
overall quality of life for individuals affected by this condition.
The rate of HPA positivity varies by country, region and race. The
positive rate is estimated to be 1/43,449 in Brazil (22), 1/10,000 in Europe (23), 1/70,000 in Japan (24) and 1/19,000-1/13,500 in the United
States (25). In the present
study, the pooled results showed that the positivity rate of
neonatal HPA screening in China using fluorometric assays or MS/MS
was 0.04% (1/2,500). This shows that the positivity rate in China
is higher than that in other countries. A comparison with similar
international studies showed that the positivity rate of HPA in
China is relatively high, which may reflect the genetic
heterogeneity and geographic variation of the population. However,
there are some similarities between our findings and those of other
countries, such as the positive rate of 1/10,000 in Europe
(23). These comparisons provide
insight into the differences in HPA performance across populations,
providing a basis for more consistent screening standards
worldwide.
The present study further analyzed the PPVs of
fluorometric assays and MS/MS screening for HPA. The pooled results
showed that the PPV of neonatal HPA screening using fluorometric
assays and tandem mass spectrometry was 31.7% (95% CI: 19.6-45.2).
Subgroup analysis revealed that the PPV of neonatal HPA screening
using fluorometric assays was 8.3% (95% CI: 7.1-9.6), the PPV of
neonatal HPA screening using MS/MS was 31.8% (95% CI: 16.4-49.4)
and the difference in the percentage of positive HPA signals
between the two screening methods was statistically significant
(P=0.001). It is suggested that the PPV of MS/MS is greater and has
greater predictive value. Consistent with the findings of other
international studies, MS/MS has shown greater accuracy and
reliability in neonatal phenylketonuria screening. For example,
Perko et al (9) found that
the PPV for MS/MS in their sample was 5.36, compared with 3.21 for
fluorometric assays. This is consistent with the findings of the
present study, indicating the superior performance of MS/MS in HPA
screening. The implementation of MS/MS technology represents a
major breakthrough in neonatal HPA screening. This technology,
known for its high throughput, specificity and sensitivity, has
found extensive use in the identification of inherited metabolic
disorders. MS/MS technology outperforms fluorometric assays by
allowing for the concurrent assessment of Phe levels, Tyr levels
and the Phe/Tyr ratio. This capability substantially diminishes the
occurrence of false-positive results in HPA screening. Furthermore,
MS/MS technology has the capacity to identify >inherited
metabolic disorders (comprising amino acid, fatty acid and organic
acid disorders) within a single cost-effective test, thereby
meeting the requirements of newborn screening programs efficiently
(26). The screening of newborns
for HPA is highly important for future screening strategies and
public health policies. The results of the present study revealed
differences between fluorescence and MS/MS in HPA screening,
providing guidance for the development of more precise and
effective screening methods. Specifically, the present study
revealed that MS/MS has a greater PPV for the diagnosis of HPA,
highlighting its importance as an outstanding diagnostic tool in
clinical practice. Future screening strategies may tend to more
widely adopt MS/MS technology to enhance the accuracy and
reliability of screening. In terms of public health policy, the
results of the present study have significant implications for the
development of early intervention policies for the HPA. As HPA can
potentially lead to intellectual developmental delay and
neurological damage, timely early intervention is crucial for the
long-term prognosis of patients. Therefore, the management and
treatment policies for HPA-positive patients should place increased
emphasis on the importance of early intervention to minimize
neurological damage and cognitive impairments.
In addition, the pooled results suggested that the
number of HPA patients with PAHD was significantly greater than the
number of HPA patients with BH4D. BH4D, although less prevalent
than other forms of HPA, can lead to significant developmental
challenges if it is managed solely with a Phe-restricted diet. It
is vital to distinguish BH4D from other conditions, such as PAHD,
because individuals with BH4D require prompt treatment with BH4 and
neurotransmitter precursors to mitigate the risk of neurological
deterioration (27).
There were several limitations to the present study.
First, heterogeneity existed in the present study, which may be due
to differences in the basic characteristics of the population.
However, the insufficient number of studies made it impossible to
analyze the source of heterogeneity, which is a problem that needs
to be overcome in the future. Second, for the comparison of the
diagnostic value of fluorometric assays and MS/MS, only the PPV has
been reported and there is a lack of data on sensitivity and
specificity. Additionally, no individual study has specifically
explored the differences in diagnostic and prognostic value of the
fluorometric method and MS/MS for hyperphenylalaninemia patients
across different countries. Future efforts should focus on
conducting clinical research to comprehensively analyze this
aspect.
In summary, the PPV of neonatal HPA screening using
MS/MS was significantly greater than that of fluorometric assays,
underscoring MS/MS as a superior diagnostic tool in clinical
practice. The elevated PPV of MS/MS indicates its heightened
accuracy and reliability in diagnosing HPA and its subtypes,
namely, PAHD and BH4D.
This finding has significant practical implications
for real clinical practice. First, the high PPV of MS/MS in
neonatal HPA screening implies its greater accuracy and
reliability, positioning it as the preferred diagnostic modality.
In clinical practice, this signifies that health care professionals
can rely more confidently on MS/MS for early HPA diagnosis,
enabling timely intervention and management. Second, the
outstanding performance of MS/MS in diagnosing HPA and its subtypes
(PAHD and BH4D) underscores its importance in formulating
individualized treatment plans. The precision of identifying
specific subtypes aids medical teams in choosing targeted treatment
approaches, enhancing therapeutic outcomes. This has a positive
effect on improving the quality of life and long-term prognosis of
patients. Thus, the results of the present study not only provided
crucial guidance for clinicians in selecting HPA diagnostic methods
but also have a direct and practical impact on improving patient
management and treatment outcomes. This emphasized the direct
applicability of the research in actual medical practice, offering
a reliable basis for health care decision-making.
The results of the present meta-analysis provided
valuable insights into the predictive value of different screening
methods for HPA. Future research should further explore the
sensitivity and specificity of these methods to provide a more
comprehensive understanding of their diagnostic performance.
Additionally, exploring the factors contributing to the observed
heterogeneity in our analysis could enhance the precision of
screening outcomes.
The practical implications of the findings of the
present study underscore the importance of incorporating MS/MS
technology in routine newborn screening programs, particularly in
regions with a higher prevalence of HPA. This approach not only
enhances diagnostic accuracy but also allows for the concurrent
assessment of various metabolic disorders, making it a
cost-effective and efficient tool in clinical practice.
As the field of metabolic disorders evolves,
continuous research is essential to refine screening methods,
improve diagnostic precision and tailor treatment strategies based
on the distinct characteristics of PAHD and BH4D. The present study
laid the groundwork for future investigations and contributed to
ongoing advancements in the early diagnosis and management of
hyperphenylalaninemia.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by 2020 research topic
of Sichuan Provincial Health Commission (approval no. 20PJ270) and
2019 Sichuan Medical Research project (approval no. S19027).
Availability of data and materials
The present study has been officially registered on
the INPLASY website (https://inplasy.com/), with the registration number
INPLASY202430036 and DOI number 10.37766/inplasy2024.3.0036. The
data generated in the present study may be requested from the
corresponding author.
Authors' contributions
ZS, PX and KP wrote the manuscript and made
substantial contributions to study conception and design. JL, CH
and WX participated in the acquisition of data and the literature
revie. YH and LT participated in data extraction and literature
quality assessment. LT, QL and SL participated in data synthesis
and statistical analysis. ZS and CH confirm the authenticity of all
the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van Spronsen FJ, Blau N, Harding C,
Burlina A, Longo N and Bosch AM: Phenylketonuria. Nat Rev Dis
Primers. 7(36)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Loeber JG: Neonatal screening in Europe;
the situation in 2004. J Inherit Metab Dis. 30:430–438.
2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
National Institutes of Health Consensus
Development Panel: national institutes of health consensus
development conference statement. Phenylketonuria: Screening and
management, October 16-18, 2000. Pediatrics. 108:972–982.
2001.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Gu X, Wang Z, Ye J, Han L and Qiu W:
Newborn screening in China: Phenylketonuria, congenital
hypothyroidism and expanded screening. Ann Acad Med Singap. 37
(Suppl 12):S107–S104. 2008.PubMed/NCBI
|
|
5
|
Mitchell JJ, Trakadis YJ and Scriver CR:
Phenylalanine hydroxylase deficiency. Genet Med. 13:697–707.
2011.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Blau N, Hennermann JB, Langenbeck U and
Lichter-Konecki U: Diagnosis, classification, and genetics of
phenylketonuria and tetrahydrobiopterin (BH4) deficiencies. Mol
Genet Metab. 104 (Suppl 1):S2–S9. 2011.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yang Y and Ye Y: Subspecial Group of
Endocrine, Hereditary and Metabolic Diseases; Society of
Pediatrics, Chinese Medical Association; Newborn Screening
Committee of Professional Society of Birth Defect Prevention and
Control; Chinese Assocation of Preventive Medical. Consensus about
the diagnosis and treatment of hyperphenylalaninemia. Zhonghua Er
Ke Za Zhi. 52:420–425. 2014.PubMed/NCBI(In Chinese).
|
|
8
|
Subspecialty Group of Newborn Screening,
Society of Birth Defects Prevention and Control, Chinese Preventive
Medicine Association; Subspecialty Group of Clinical Nutrition, the
Society of Pediatrics, Chinese Medical Association; Subspecialty
Committee of Clinical Biochemistry and Genetics, the Society of
Medical Genetics, Chinese Medical Doctor Association; Subspecialty
Group of Clinical Genetics. Consensus statement on dietary
treatment and nutritional management for phenylalanine hydroxylase
deficiency. Zhonghua Er Ke Za Zhi. 57:405–409. 2019.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
9
|
Perko D, Groselj U, Cuk V, Iztok Remec Z,
Zerjav Tansek M, Drole Torkar A, Krhin B, Bicek A, Oblak A,
Battelino T and Repic Lampret B: Comparison of tandem mass
spectrometry and the fluorometric method-parallel phenylalanine
measurement on a large fresh sample series and implications for
newborn screening for phenylketonuria. Int J Mol Sci.
24(2487)2023.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Feng S, Mei J, Yang L, Luo P, Wang X, Wang
Y, Yao J, Cui L, Pan L, Wang Z and Xin L: Benzene derivatives from
ink lead to false positive results in neonatal
hyperphenylalaninemia screening with ninhydrin fluorometric method.
Int J Neonatal Screen. 6(14)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Groselj U, Murko S, Zerjav Tansek M, Kovac
J, Trampus Bakija A, Repic Lampret B and Battelino T: Comparison of
tandem mass spectrometry and amino acid analyzer for phenylalanine
and tyrosine monitoring--implications for clinical management of
patients with hyperphenylalaninemia. Clin Biochem. 48:14–18.
2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Slim K, Nini E, Forestier D, Kwiatkowski
F, Panis Y and Chipponi J: Methodological index for non-randomized
studies (minors): Development and validation of a new instrument.
ANZ J Surg. 73:712–716. 2003.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ma Y, Huo X, Kong S, Xu W, Zhao W and Zhu
M: A review about C-TIRADS, ACR-TIRADS, and K-TIRADS combined with
real-time tissue elastography to diagnose thyroid nodules. Discov
Med. 35:1–10. 2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Qiu X, Zhao P, Luo J, Li G, Deng L, Zeng
Y, Xu L and Zhou J: Biochemical and molecular features of
tetrahydrobiopterin deficiency in Fujian Province, southeastern
China. Front Genet. 14(1250568)2023.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Wang X, Wang Y, Ma D, Zhang Z, Li Y, Yang
P, Sun Y and Jiang T: Neonatal screening and genotype-phenotype
correlation of hyperphenylalaninemia in the Chinese population.
Orphanet J Rare Dis. 16(214)2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Wang T, Ma J, Zhang Q, Gao A, Wang Q, Li
H, Xiang J and Wang B: Expanded newborn screening for inborn errors
of metabolism by tandem mass spectrometry in Suzhou, China: Disease
spectrum, prevalence, genetic characteristics in a Chinese
population. Front Genet. 10(1052)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li X, He J, He L, Zeng Y, Huang X, Luo Y
and Li Y: Spectrum analysis of inherited metabolic disorders for
expanded newborn screening in a Central Chinese population. Front
Genet. 12(763222)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yuan X, Zhu J, Liu H, Xiang L, Yao Y, Li
Q, Deng K and Li X: Birth prevalence of tetrahydrobiopterin
deficiency in China: Data from the national newborn screening
program, 2013-2019. J Pediatr Endocrinol Metab. 34:835–841.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang R, Shen N, Ye J, Han L, Qiu W, Zhang
H, Liang L, Sun Y, Fan Y, Wang L, et al: Mutation spectrum of
hyperphenylalaninemia candidate genes and the genotype-phenotype
correlation in the Chinese population. Clin Chim Acta. 481:132–138.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lin Y, Lin W, Su R, Zheng Z, Fu Q and Wang
G: Newborn screening and genetic features of patients with
hyperphenylalaninemia in a southern Chinese population. Clin Chim
Acta. 535:13–18. 2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Wang X, He Y, Jiang Y, Feng X, Zhang G,
Xia Z and Zhou Y: Screening and mutation analysis of
hyperphenylalaninemia in newborns from Xiamen, China. Clin Chim
Acta. 498:161–166. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ramalho AR, Ramalho RJ, Oliveira CR,
Magalhães MM, Santos EG, Sarmento PM, Matos DO, Oliveira MC,
Oliveira AL and Aguiar-Oliveira MH: Evaluation of effectiveness and
outcome of PKU screening and management in the State of Sergipe,
Brazil. Arq Bras Endocrinol Metabol. 58:62–67. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Steinfeld R, Kohlschütter A, Ullrich K and
Lukacs Z: Efficiency of long-term tetrahydrobiopterin monotherapy
in phenylketonuria. J Inherit Metab Dis. 27:449–453.
2004.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Aoki K: Long term follow-up of patients
with inborn errors of metabolism detected by the newborn screening
program in Japan. Southeast Asian J Trop Med Public Health. 34
(Suppl 3):S19–S23. 2003.PubMed/NCBI
|
|
25
|
Kaye CI: Committee on Genetics. Accurso F,
La Franchi S, Lane PA, Hope N, Sonya P, G Bradley S and Michele
ALP: Newborn screening fact sheets. Pediatrics. 118:e934–e963.
2006.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lin Y, Zheng Q, Zheng T, Zheng Z, Lin W
and Fu Q: Expanded newborn screening for inherited metabolic
disorders and genetic characteristics in a southern Chinese
population. Clin Chim Acta. 494:106–111. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Opladen T, López-Laso E,
Cortès-Saladelafont E, Pearson TS, Sivri HS, Yildiz Y, Assmann B,
Kurian MA, Leuzzi V, Heales S, et al: Consensus guideline for the
diagnosis and treatment of tetrahydrobiopterin (BH4) deficiencies.
Orphanet J Rare Dis. 15(126)2020.PubMed/NCBI View Article : Google Scholar
|