Introduction
Parkinson's disease (PD) is a chronic, progressive
degenerative disease associated with dopaminergic neuron loss of
the substantia nigra, striatum and other brain structures, in which
the balance between dopamine and acetylcholine neurotransmitters is
disrupted. It is most common in middle-aged and older adults, and
is characterized by resting tremors, muscle rigidity, bradykinesia
and postural instability (1,2).
Myasthenia gravis (MG) is a B cell-mediated, acquired autoimmune
disease associated with antibodies that are directed mainly against
the acetylcholine receptor (AChR) in the postsynaptic membrane at
the neuromuscular junction. It occurs in patients of all ages, and
is associated with partial or whole skeletal muscle weakness and
fatigue (3). The coexistence of PD
and MG is an uncommon phenomenon since they differ in their
etiological and pathological features. Moreover, they share some
similar clinical symptoms, such as fatigue, ocular symptoms,
dysphagia, dysarthria and head drop (4), which may overlap and lead to
diagnostic ambiguity, making co-diagnosis of PD and MG more
challenging.
It is currently unclear whether the coexistence of
PD and MG is coincidental or etiologically related. The imbalance
between cholinergic and dopamine systems may link PD and MG.
Notably, trihexyphenidyl (THP), which can reduce acetylcholine
levels in the treatment of PD, has been reported to induce MG
symptoms (5,6), and pyridostigmine, which can increase
acetylcholine levels in the treatment of MG, can exacerbate PD
symptoms (6,7). However, most patients develop the
diseases without the being treated with such drugs (8-13),
suggesting other mechanisms are involved in the coexistence of the
two diseases. Multiple lines of evidence have indicated that immune
system dysfunction serves as a critical component in susceptibility
to and progression of PD, including shared molecular pathways (such
as NLRP3 inflammasome activation) and polygenic risk variants (such
as LRRK2 and PRKN genes) with autoimmune diseases, increased risk
of PD in patients with autoimmune diseases, impaired humoral and
cellular immunity, activated microglia in the brain, altered gut
microbiota and inflammatory markers in the feces, as well as a
lower risk after treatment with anti-inflammatory drugs and
immunosuppressants (14). MG is a
classic mainly AChR antibody-mediated disorder at the neuromuscular
junction, which is accompanied by an activated immune response with
the support of autoreactive B cells (15-17).
Therefore, some links, such as immune system dysfunction, might
result in the coexistence of the two diseases, even though they
initially appear quite different in terms of pathogenesis.
To the best of our knowledge, there are only a few
studies on MG comorbidity in patients with PD (4-13,18-28),
and fewer studies (9,13,18)
have summarized the clinical features of PD and MG coexistence. The
present study describes the case of a patient with concomitant PD
and MG, and a literature search was conducted to identify reports
on all patients diagnosed with concomitant PD and MG in an attempt
to analyze the clinical characteristics and explore the possible
mechanisms of such comorbidity. The present study aims to alert
clinicians to this potential overlap between PD and MG, with the
purpose of improving diagnostic accuracy and optimizing the
management of both diseases.
Case report
A 68-year-old man developed right-hand resting
tremor, bradykinesia, hypomimia, constipation and sleep disturbance
in January 2015, showing decreased function of dopamine
transporters in the bilateral caudate nuclei, and bilateral
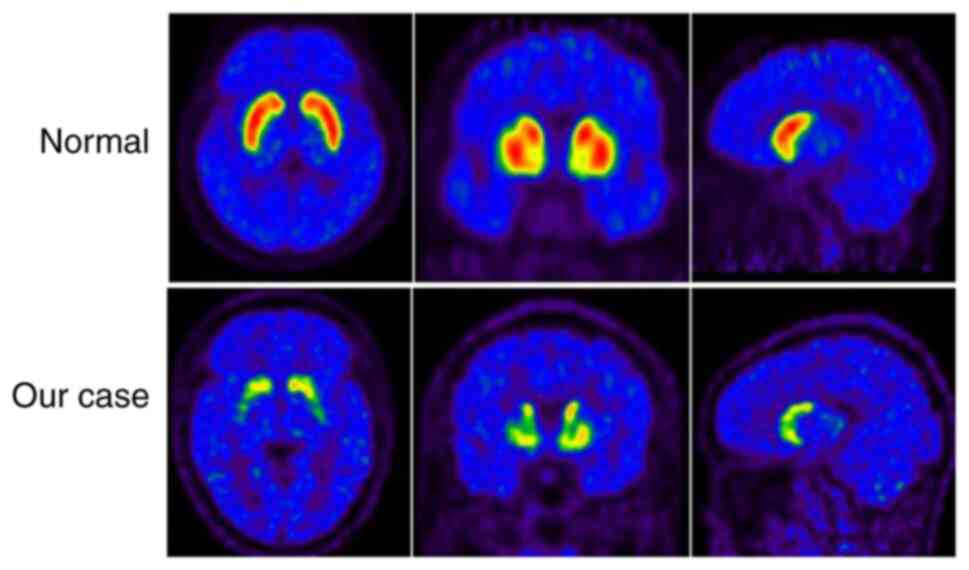
posterior and anterior putamen as revealed by 11C-CFT
PET/CT (Fig. 1). The patient was
therefore diagnosed with PD. The patient responded well to levodopa
(LD) (125 mg tid) and entacapone (100 mg tid). A total of 6 months
before admission, the patient gradually developed fluctuating
double eyelid drooping without diplopia or abnormality of ocular
movements, and ophthalmic examination showed no abnormality. The
patient experienced weakness in the neck muscles, with problems
raising their head soon afterward, and the symptoms of muscle
weakness gradually deteriorated. Furthermore, no improvement was
observed after adjusting the anti-PD drugs (LD, 125 mg tid;
pramipexole, 0.25 mg tid; entacapone, 200 mg tid). On admission to
Tongji Hospital (Wuhan, China) in January 2021, besides ptosis and
head drooping, the patient complained of symptoms of bulbar palsy
(such as choking and dysphagia) and mild dyspnea. Considering the
fluctuating and deteriorating muscle weakness, a neostigmine test
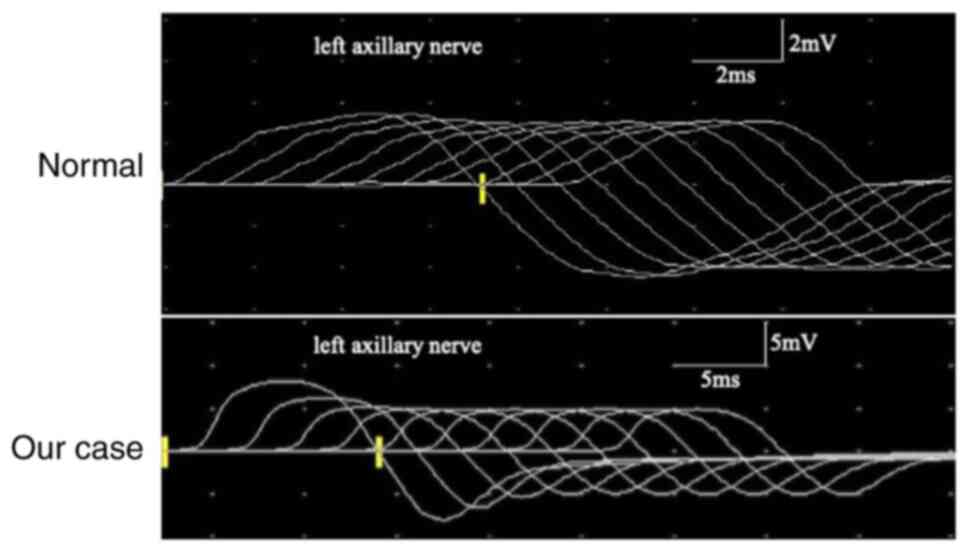
was performed, which was positive. Repetitive nerve stimulation
(RNS) revealed a progressive decrement in low-frequency RNS in both
the accessory and facial nerves (Fig.
2). Nerve conduction studies were normal. Thymic hyperplasia
and thymoma were not observed. There were no obvious abnormalities
in thyroid function or serum creatine levels. Furthermore, tumors,
autoimmune disorders (including rheumatoid arthritis, systemic
lupus erythematosus and Sjogren's syndrome) or systemic autoimmune
antibodies (such as antinuclear antibody) were not detected. The
serum anti-AChR antibody was 30.9 nmol/l (normal range <0.40
nmol/l, enzyme-linked immunosorbent assay) and the anti-muscle
specific tyrosine kinase (MuSK) antibody was negative. The patient
was finally diagnosed with MG based on fluctuating weakness,
positive neostigmine test results and the classical decrement in
low-frequency RNS, as well as the positive anti-AChR antibody.
Following treatment with intravenous immunoglobulin (IVIG, 0.4
g/kg/day for 5 days), cholinesterase inhibitors (pyridostigmine, 60
mg tid), steroids (prednisone, 20 mg qd) and immunosuppressant
therapy (tacrolimus, 3 mg qd), the symptoms of MG improved, as
evidenced by no drooping eyelids or drooping head, and an
improvement in swallowing.
After a 3-year follow-up, both MG and PD symptoms
remained stable. At this time point, the patient had discontinued
the steroid treatment, whereas a low dosage of tacrolimus (1 mg
daily) and pyridostigmine (60 mg, twice daily) was maintained to
prevent MG relapse. For PD treatment, LD (125 mg tid), pramipexole
(0.25 mg tid) and Stalevo (325 mg tid) were used as maintenance
medications.
Subsequently, a systematic literature review was
conducted by searching the PubMed (https://pubmed.ncbi.nlm.nih.gov/) and Embase
(http://www.embase.com). databases for reports on
all patients with concomitant PD and MG up to October 31, 2023.
Search terms included ‘Parkinson's disease’ or ‘Parkinson's
syndrome’ or ‘Parkinsonism’ AND ‘myasthenia gravis’ or
‘myasthenia’. The reference lists of the included articles were
also reviewed. All cases of concomitant PD and MG were summarized
and analyzed, then grouped and compared according to different
preexisting diseases.
Finally, a total of 47 cases of concomitant PD and
MG, including the current case, were analyzed. The patients were
categorized into three groups based on the sequential occurrence of
these two diseases. In the prePD-MG group, 30 patients were
initially diagnosed with PD, followed by a subsequent diagnosis of
MG (Table I). In the preMG-PD
group, MG was diagnosed in 12 patients, followed by a subsequent PD
diagnosis (Table II). The
remaining 5 cases failed to note the order of diagnosis occurrence
and were considered the coPD-MG group (Table III).
 | Table IDemography and clinical
characteristics of the prePD-MG group. |
Table I
Demography and clinical
characteristics of the prePD-MG group.
| First author,
year | Sex/Age, years | PD symptoms | PD treatment | Comorbidities | Interval | MG antibodies | Ocular
symptoms | Dysphagia | Dysarthria | Head drop | MG treatment | (Refs.) |
|---|
| Ueno, 1987 | M/55 | RT + Hm | THP, LD | DM | 5.5 years | AChR: P | Y | Y | N | Y | Pyd | (5) |
| Tasić, 1991 | M/74 | RT + R | THP,
amantadine | Tuberculous
lymphadenitis | 3 years | NR | Y | Y | Y | N | Pyd | (21) |
| Kao, 1993 | F/54 | R + RT + Hm +
BK | THP, Sinemet | NR | 7 years | AChR: P | Y | Y | Y | Y | Pyd, CS | (6) |
| Levin, 2003 | M/76 | RT + R | LD | NR | 5 years | AChR: P | N | N | N | Y | Pyd, AZA | (8) |
| Levin, 2003 | M/62 | R + RT | LD, pergolide,
selegiline, amantadine | NR | 4 years | AChR: P | Y | N | N | N | Pyd | (8) |
| Levin, 2003 | M/61 | R | LD | GD,
hyperthyroidism | NR | NR | Y | N | N | N | Pyd | (8) |
| Fasano, 2008 | F/53 | RT + R + BK | NR | NR | 5 years | AChR: Ne; MuSK:
Ne | N | N | N | Y | Pyd, CS, AZA,
PE | (19) |
| Unal-Cevik,
2009 | M/80 | RT + R + BK | NR | Hypothyroidism,
HPT | 4 years | AChR: Ne | N | N | N | Y | Pyd | (22) |
| Uludag, 2011 | M/66 | RT + R | LD,
benserazide | HPT, COPD,
hyperlipidemia | 9 years | AChR: P | N | N | N | Y | IVIG, Pyd | (10) |
| Lanfranconi,
2011 | M/70 | RT + BK + R +
Hm | LD | NR | 3 years | AChR:Ne; MuSK:
P | Y | Y | Y | N | CS | (11) |
| Zis, 2014 | M/64 | R | NR | HPT, RA | 5 years | AChR: P; Musk:
Ne | N | N | N | Y | Pyd, CS | (20) |
| Neuman, 2014 | F/68 | RT + R + BK | LD | NR | 7 years | AChR: P | N | N | N | Y | Pyd, AZA, IVIG | (12) |
| Sciacca, 2016 | F/57 | NR | NR | NR | 9 years | NR | Y | N | N | N | Pyd | (18) |
| Sciacca, 2016 | M/60 | NR | NR | NR | 3 years | NR | Y | N | N | Y | Pyd | (18) |
| Sciacca, 2016 | M/67 | NR | NR | NR | 3 years | AChR: P | Y | N | N | N | Pyd | (18) |
| Tung-Chen,
2016 | F/71 | BK + Hm + R +
RT | LD | Anemia | 19 years | AChR: Ne | Y | Y | Y | N | Pyd, CS, AZA,
IVIG | (13) |
| Aiba, 2016 | F/71 | R + BK | LD | NR | 5 years | AChR: P | N | N | N | Y | Pyd, CS | (23) |
| Hogg, 2017 | M/75 | BK + Hm + R | NR | Myopathy | 3-4 months | AChR: P | Y | Y | Y | Y | Pyd | (24) |
| Urban, 2018 | M/76 | NR | NR | NR | 6 years | AChR: P | N | Y | N | N | Pyd, CS, IVIG,
AZA | (25) |
| Urban, 2018 | M/90 | NR | NR | NR | 5 years | AChR: P | N | Y | N | N | Pyd, CS | (25) |
| Urban, 2018 | M/78 | NR | NR | NR | 5 years | AChR: P | N | Y | N | N | Pyd, CS. AZA | (25) |
| Urban, 2018 | M/74 | NR | NR | NR | 7 years | AChR: P | N | Y | Y | N | Pyd, IVIG, CS,
AZA | (25) |
| Marano, 2019 | M/65 | RT + BK + R | LD | NR | 8 years | AChR: P | Y | Y | N | Y | Pyd, CS, AZA | (26) |
| Odajiu, 2019 | M/49 | BK + R + Hm | Stalevo,
ropinirole rasagiline | Crohn's
disease | 11 years | AChR: P | Y | N | N | N | Pyd | (9) |
| Odajiu, 2019 | F/64 | BK + RBD + Hm +
R | Stalevo,
pramipexole, rasagiline | HPT | 4 years | AChR: Ne | Y | Y | Y | N | Pyd | (9) |
| Odajiu, 2019 | M/52 | RT + BK + R | Stalevo,
rasagiline, THP | DM, HPT | 3 years | AChR:Ne; MuSK:
Ne | Y | N | N | N | Pyd | (9) |
| Alshaikh, 2021 | M/61 | NR | NR | Vitamin B12
deficiency | 6 years | AChR: P | Y | Y | Y | N | NR | (4) |
| Alshaikh, 2021 | M/61 | NR | NR | Neuropathy, vitamin
B12 deficiency | 1 year | AChR: P | Y | Y | Y | Y | NR | (4) |
| Alshaikh, 2021 | M/82 | NR | NR | NR | NR | AChR: P | NR | Y | Y | N | NR | (4) |
| Current case | M/62 | RT + BK | LD, entacapone | None | 6 years | AChR: P; MuSK:
Ne | Y | Y | N | Y | Pyd, CS, IVIG,
tacrolimus (FK506) | |
 | Table IIDemography and clinical
characteristics of the preMG-PD group. |
Table II
Demography and clinical
characteristics of the preMG-PD group.
| First author,
year | Sex/Age, years | MG antibodies | Ocular
symptoms | Dysphagia | Dysarthria | Head drop | MG treatment | Comorbidities | Interval | PD symptoms | PD treatment | (Refs.) |
|---|
| Iwasaki, 1988 | F/62 | AChR: P | Y | N | N | N | Pyd | NR | 2 months | R + RT + BK | None | (7) |
| Levin, 2003 | F/68 | AChR: P | Y | N | N | N | Pyd, AZA | NR | 3 years | R +
restlessness | LD | (8) |
| Neuman, 2014 | M/72 | AChR: P | Y | N | N | N | Pyd | NR | 4 years | RT + R + BK | NO | (12) |
| Ozer, 2016 | M/67 | NR | N | N | Y | N | Pyd | Chronic subdural
hematoma | 16 months | BK | LD,
benserazide | (27) |
| Alshaikh, 2021 | M/70 | AChR: P | Y | N | N | Y | NR | Thyroid
disease | 8 years | NR | NR | (4) |
| Alshaikh, 2021 | M/66 | AChR: P | Y | Y | Y | N | NR | Neuropathy, thyroid
disease, vitamin B12 deficiency, childhood polio | 1 year | NR | NR | (4) |
| Alshaikh, 2021 | M/66 | AChR: P | Y | Y | Y | Y | NR | Neuropathy, thyroid
disease, vitamin B12 deficiency | 8 years | NR | NR | (4) |
| Alshaikh, 2021 | F/85 | AChR: P | Y | Y | Y | Y | NR | Neuropathy | 4 years | NR | NR | (4) |
| Alshaikh, 2021 | M/49 | SN | Y | Y | Y | N | NR | Thyroid
disease | 22 years | NR | NR | (4) |
| Alshaikh, 2021 | F/55 | NR | Y | Y | N | N | NR | Neuropathy | 19 years | NR | NR | (4) |
| Alshaikh, 2021 | M/59 | SN | N | Y | N | N | NR | Neuropathy, thyroid
disease, Lyme disease | 10 years | NR | NR | (4) |
| Alshaikh, 2021 | M/52 | NR | Y | N | N | N | NR | Thyroid disease,
vitamin B12 deficiency | 20 years | NR | NR | (4) |
 | Table IIIDemography and clinical
characteristics of the co MG-PD group. |
Table III
Demography and clinical
characteristics of the co MG-PD group.
| First author,
year | Sex | Age at diagnosis of
PD/MG, years | Comorbidities | MG antibodies | Ocular
symptoms | Dysphagia | Dysarthria | Head drop | (Refs.) |
|---|
| Albassam, 2021 | F | 72/72 | HPT, DM,
dyslipidemia | AChR: N; MuSK:
P | Y | Y | N | N | (28) |
| Alshaikh, 2021 | M | 78/- | Thyroid
disease | AChR: P | Y | N | N | N | (4) |
| Alshaikh, 2021 | M | 60/- | Neuropathy, vitamin
B12 deficiency, CVID | AChR: P | Y | Y | Y | N | (4) |
| Alshaikh, 2021 | M | -/- | Thyroid disease,
hemophilia A | AChR: P | Y | Y | Y | N | (4) |
| Alshaikh, 2021 | M | 84/- | Thyroid
disease | AChR: P | NR | NR | NR | NR | (4) |
The overall median age of patients at first
diagnosis was 66.59±9.91 years (range: 49-90 years), with 35
(74.47%) men and 12 (25.53%) women (Table IV). Notably, some of the cases
counted in the present study did not report detailed clinical
information; therefore, proportions were calculated as the
percentage of individuals reporting on that indicator. Various
comorbidities were observed, particularly autoimmune diseases, with
hypertension present in 6 patients (21.43%) of 28 patients, thyroid
disease in 12 patients (42.86%), neuropathy in 7 patients (25.00%),
and rheumatoid arthritis, Crohn's disease or myopathy in 1 patient
each (3.58%).
 | Table IVGeneral characteristics of PD
combined with MG. |
Table IV
General characteristics of PD
combined with MG.
| Characteristic | Concomitant PD and
MG (n=47) | PrePD-MG
(n=30) | PreMG-PD
(n=12) | CoPD-MG (n=5) |
P-valuea |
|---|
| Mean ± SD PD-onset
age, years | 68.76±9.44 | 66.60±9.81 | 72.58±6.72 | 73.50±10.25 | 0.061 |
| Mean ± SD MG-onset
age, years | 69.67±10.25 | 71.91±9.92 | 64.25±9.78 | - | 0.030b |
| Sex, M/F | 35/12 | 23/7 | 8/4 | 4/1 | 0.505 |
| History diseases,
% | | | | | |
|
Hypertension | 21.43 | 35.71 | 0 | 20.00 | 0.039b |
|
Diabetes
mellitus | 10.71 | 14.29 | 0 | 20.00 | 0.360 |
| Other immune
diseases, % | | | | | |
|
Thyroid
disease | 42.86 | 21.43 | 66.67 | 60.00 | 0.042b |
|
Neuropathy | 25.00 | 7.14 | 55.56 | 20.00 | 0.018b |
|
RA | 3.58 | 7.14 | 0 | 0 | 0.609 |
|
Crohn's
disease | 3.58 | 7.14 | 0 | 0 | 0.609 |
|
Myopathy | 3.58 | 7.14 | 0 | 0 | 0.609 |
| Median disease
interval, years | 5 | 5 | 5 | - | |
| PD symptoms, % | | | | | |
|
R | 88.00 | 90.00 | 75.00 | 100.00 | 0.437 |
|
RT | 64.00 | 70.00 | 50.00 | 0 | 0.407 |
|
BK | 64.00 | 65.00 | 50.00 | 100.00 | 0.486 |
|
Hm | 32.00 | 35.00 | 0 | 100.00 | 0.224 |
| PD treatment,
% | | | | | |
|
Levodopa | 85.71 | 93.75 | 50.00 | 100.00 | 0.088 |
|
THP | 19.05 | 25.00 | 0 | 0 | 0.376 |
|
DA | 14.29 | 18.75 | 0 | 0 | 0.491 |
|
MAO-B | 19.05 | 25.00 | 0 | 0 | 0.376 |
|
COMT | 19.05 | 25.00 | 0 | 0 | 0.376 |
|
Amantadine | 9.52 | 12.50 | 0 | 0 | 0.632 |
| MG symptoms, % | | | | | |
|
Ocular
symptoms | 69.57 | 62.07 | 83.33 | 100.00 | 0.138 |
|
Head
drop | 54.54 | 71.43 | 25.00 | 0 | 0.013b |
|
Dysphagia | 55.32 | 54.84 | 50.00 | 75.00 | 0.521 |
|
Dysarthria | 38.30 | 35.48 | 41.67 | 50.00 | 0.485 |
|
Limb
weakness | 36.36 | 32.14 | 50.00 | 100.00 | 0.427 |
| MG-antibodies,
% | | | | | |
|
AChR-Ab | 77.50 | 76.92 | 77.78 | 80.00 | 0.670 |
|
MuSK-Ab | 5.00 | 3.85 | 0 | 20.00 | 0.743 |
| MG-treatment,
% | | | | | |
|
Pyd | 96.88 | 96.30 | 100.00 | 100.00 | 0.871 |
|
CS | 40.63 | 44.44 | 0 | 100.00 | 0.123 |
|
IVIG | 12.8 | 18.52 | 0 | 100.00 | 0.475 |
|
AZA | 31.25 | 29.63 | 25.00 | 100.00 | 0.673 |
The initial symptoms of PD included rigidity
(88.00%), resting tremors (64.00%), bradykinesia (64.00%) and
hypomimia (32.00%), which could be present in any combination with
other symptoms. The anti-PD therapy was mainly LD (85.71%), THP
(19.05%), dopamine receptor agonists (14.29%), monoamine oxidase-B
inhibitors (19.05%), catechol-O-methyltransferase inhibitors
(19.05%), and amantadine (9.52%).
In terms of MG clinical signs, 32 patients (69.57%)
had ocular symptoms, including ptosis and diplopia, 26 (55.32%) had
dysphagia, 18 (38.30%) had dysarthria, 18 (54.54%) had head drop
and 12 (36.36%) had limb weakness. Among the 40 patients who
underwent antibody detection, 31 (77.50%) were positive for AChR
antibodies and 2 (5.00%) were positive for MuSK antibodies. None of
the patients had thymoma or thymic hyperplasia (data not shown).
Anti-MG therapy was mainly pyridostigmine (96.88%), and some
patients were treated with prednisone/methylprednisolone (40.63%),
azathioprine (31.25%) or IVIG (6 cases, 12.8%).
In the prePD-MG group, the average age at diagnosis
with MG was 71.91±9.92 years, which was older than that in the
preMG-PD group. GraphPad Prism (v8.0.2; Dotmatics) was applied for
the statistical analysis. P-values were calculated using
independent t-tests or Fisher's exact test. The interval between PD
and MG diagnosis ranged from 3-4 months to 20 years (median, 5
years). These patients were more prone to comorbidities, such as
hypertension. Most patients had typical PD symptoms and anti-PD
therapy was diverse. As for MG clinical signs, head drop was more
common in this group than in the preMG-PD group. However, due to
limited information, the present study could not determine which
stage of PD was prone to MG and which type of MG was more likely to
occur.
Patients in the preMG-PD group were more prone to
immune disease comorbidities, such as thyroid disease and
neuropathy, and there were higher proportion of women in this group
than in the prePD-MG group. The interval from MG to PD diagnosis
ranged from 2 months to 22 years (median, 5 years). Moreover, in
this group, although there was no statistical difference, the
proportion of ocular symptoms and limb weakness seemed higher,
whereas PD symptoms were relatively fewer than those in the
prePD-MG group and anti-PD therapy was relatively single (only LD)
The present study attempted to analyze which type of MG was more
prone to PD; however, due to limited information, conclusions could
not be drawn.
Although the number of CoPD-MG cases was relatively
low, their clinical characteristics was similar to that of the
prePD-MG group. Both the basic treatment of MG and PD were
effective in the three groups.
Discussion
The coexistence of neurodegenerative diseases and
autoimmune diseases is not uncommon. However, as a rare disease,
the incidence rate of MG worldwide was only 10-29 per million
person-years based on data from the past decade (29), which makes the coexistence of PD
and MG relatively rare. In clinical practice, the overlap of some
indicators, such as ocular symptoms, limb weakness and head drop,
leads to a misdiagnosis or delayed diagnosis; therefore, the actual
reported cases of PD and MG comorbidity may be less. The present
study described the case of a patient co-diagnosed as having PD and
MG with positive anti-AChR antibodies. After searching the
literature, 47 cases of PD and MG comorbidity, including the
current case, were identified. Similar to previous studies
(9,13,18),
in order to better present the cases, the clinical data of each
patient were listed in tables. In contrast to other studies, the
present study added newly reported cases, summarized their
demographic and clinical characteristics, and categorized and
compared them based on different preexisting diseases. Overall, the
average age of the three groups was relatively old. The interval
between the two diseases varied from 2 months to 22 years. In
addition to MG, autoimmune diseases, such as thyroid disease and
rheumatoid arthritis, were also observed in these patients. AChR
antibodies were the most common among those who underwent antibody
testing. Consistent with recent studies, the present study revealed
that most of the cases were male patients (30,31),
and most patients were diagnosed with PD before being diagnosed
with MG (30).
With varying initial diseases, each of the three
groups exhibited distinct characteristics. The mean age at
diagnosis of MG was older in the prePD-MG group than that in the
preMG-PD group. One possible explanation for this phenomenon is the
varying initial diseases, as PD tends to manifest in the older
population. Moreover, hypertension was most frequently observed in
this group, which may be due to the same reason as that
aforementioned. Another possibility is the increase in the
incidence of MG in older adults (32). The main clinical features of MG,
such as ocular symptoms and limb weakness, were easily masked by
bradykinesia and rigidity in the prePD-MG group, which challenged
the diagnosis of MG in such a group. Moreover, the sign of head
drop was more prominent in the prePD-MG group, which was consistent
with a previous finding that the proportion of head drop in PD was
higher than that in MG (33).
Since the head drop was less affected by bradykinesia and rigidity,
the comorbidity of MG should be considered once a fluctuation in
head drop has been observed in patients with PD. In the preMG-PD
group, it may be easier to make a certain diagnosis of both
diseases because the symptoms related to MG are more clearly
discernible in this pattern. Moreover, ophthalmoplegia and limb
weakness appeared to be more common, as they were not masked by PD
symptoms. Patients in this group were more prone to comorbid
autoimmune diseases, with female patients being more susceptible to
the preMG-PD pattern, which could be related to the fact that they
were more prone to autoimmune diseases (34).
The mechanisms underlying concomitant PD and MG
remain unknown. Whether it is a casual phenomenon or if there is a
causative relationship between both diseases requires further
clarification. Iatrogenic causes have been reported in some
comorbidity cases (5-7).
THP for treating PD has been reported to induce MG symptoms
(5,6), and pyridostigmine for treating MG can
exacerbate PD symptoms (6,7). Moreover, systematic immune
dysfunction has been revealed in PD and MG comorbidity, with
reports of thyroid disease, MG and PD (8), and rheumatoid arthritis, MG and PD
comorbidities (20). However, it
is currently unknown whether patients with PD and MG comorbidity
share similar genetic associations, such as LRRK2 gene mutations,
observed between PD and inflammatory bowel disease (35).
Some studies have detected autoantibodies to
α-synuclein (36,37) and autoreactive T cells that
recognize specific α-synuclein epitopes in patients with PD
(38), thus indicating that immune
reactions participate in the pathophysiology of PD. In addition,
studies have revealed that B cells contribute to the pathogenesis
of PD (39), including deposits of
immunoglobulin G (IgG) found on dopaminergic neurons (40), Lewy bodies coated with IgG
(41), and increased levels of
anti-α-synuclein antibodies in the cerebrospinal fluid and the
blood (36,37). Aberrant functioning of the immune
system, as aforementioned, has been proposed as a critical
component of susceptibility to and progression of PD (14). In addition, MG is a classic
antibody-mediated disorder dependent on autoreactive B cells that
require T-cell support (15,16),
accompanied by an activated immune response in the neuromuscular
junction from the early stage of the disease (17). Therefore, a common
pathophysiological mechanism of immune dysfunction may result in PD
and MG comorbidity.
Inflammatory mechanisms might also be involved in
the process of concomitant PD and MG. An increase in cytokines,
such as TNF-α, IL-1β and IL-6, in the peripheral blood has been
detected and proven to have a significant role in the progression
of PD (42). Adaptive immune
components in the peripheral blood of patients with PD have also
been observed, such as type 1 T-helper cells and
interleukin-17-producing T-helper cells, contributing to high blood
levels of interferon-γ and tumor necrosis factor (43). In a recent study, the
neutrophil-to-lymphocyte ratio (NLR), a marker of peripheral
inflammation, was used to detect peripheral inflammation in PD, and
the NLR ratio was revealed to be higher in the PD group than that
in the control group (44).
Microglia, local innate immune cells in the brain, become activated
in response to inflammation and are involved in dopaminergic neuron
damage via various mechanisms in PD (14). Furthermore, different
proinflammatory or inflammatory mediators have been reported to
contribute to the pathogenesis of MG (45). The initial activation of peripheral
immunocytes and cytokines in MG might infiltrate the brain
parenchyma once the blood-brain barrier is compromised, thus
leading to the subsequent activation of central inflammation, such
as antigen-antibody reaction associated with α-synuclein, or the
direct infiltration of the microglia, monocytes and dendritic cells
(14).
Gut-derived inflammation may also be associated with
the co-occurrence of PD and MG. The gut microbiota and its
metabolites have been shown to be involved in the regulation of
neuroinflammation, barrier function and neurotransmitter activity
in PD (46). The
microbiota-gut-brain axis, a form of bidirectional communication
between the enteric nervous system and the central nervous system,
may provide a pathway for the transmission of α-synuclein (46). Similarly, accumulating evidences
has endorsed the key role of gut microbiota in the pathogenesis of
MG (47,48), particularly the effects on T-helper
17/regulatory T cell balance, the imbalance of which can result in
the progression of MG.
Taken together, it is plausible to assume that
immune dysfunction and inflammatory mechanisms may contribute to
the comorbidity of MG and PD, with the initial peripheral immune
dysfunction or inflammation triggering neuroinflammation resulting
in activation of the degenerative process. However, it is
challenging to determine which mechanisms are involved in the
different groups assessed in the present study.
The present study had some limitations. First, due
to the small number of reported comorbidities and the lack of
detailed patient-specific information, the analysis based on the
existing data has a certain degree of bias, and it cannot be graded
based on the severity of patients. However, the data of most
patients were relatively detailed, and a small amount of unprovided
data did not affect most results. Larger scale studies are required
to examine the co-occurrence of PD and MG, which may improve
understanding of this complication. Second, all mechanisms of PD
and MG comorbidity were speculative based on current reported
studies, and more basic research is required to understand the
pathogenesis of the comorbidity. New immune-based therapeutic
options may focus on eliminating circulating autoantibodies or
inhibiting effector mechanisms by targeting B cells, B-cell growth
factors or other immunosuppressive treatments in such
comorbidities.
In conclusion, the coexistence of PD and MG is very
rare. The overlapping symptoms of these two diseases can be
challenging, especially when the initial disease is PD. Clinicians
should pay attention to this potential overlap in order to improve
the accuracy of PD and MG co-diagnosis, and to optimize the
management of these two diseases. Immune dysfunction and
inflammation may result in the coexistence of neurodegenerative and
neuroimmune diseases due to interactions between the brain
parenchyma and blood circulation. Further therapeutic interventions
aimed at immune-associated mechanisms could be of great use to
delay disease progression and pathological processes.
Acknowledgements
Not applicable.
Funding
Funding: This study was supported by grants from the Natural
Science Foundation of Hubei Province (grant no. 2020CFB744) and the
National Natural Science Foundation of China (grant no.
81901303).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZM and QN collected the data and confirm the
authenticity of all the raw data. ZX processed the statistical
data. ZM and ZL drafted and revised the manuscript. ZM and ZL
designed and guided the study. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Tongji Hospital, Tongji Medical College, Huazhong University of
Science and Technology (approval no. TJ-IRB20211273).
Patient consent for publication
The patient and healthy individuals providing normal
images provided written informed consent for publication.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Armstrong MJ and Okun MS: Diagnosis and
treatment of Parkinson disease: A review. JAMA. 323:548–560.
2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Jankovic J and Tan EK: Parkinson's
disease: Etiopathogenesis and treatment. J Neurol Neurosurg
Psychiatry. 91:795–808. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gilhus NE: Myasthenia Gravis. N Engl J
Med. 375:2570–2581. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Alshaikh JT and Mills K: Coincident
parkinsonism and myasthenia gravis: A case series. Parkinsonism
Relat Disord. 89:4–5. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Ueno S, Takahashi M, Kajiyama K, Okahisa
N, Hazama T, Yorifuji S and Tarui S: Parkinson's disease and
myasthenia gravis: Adverse effect of trihexyphenidyl on
neuromuscular transmission. Neurology. 37:832–833. 1987.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kao KP, Kwan SY, Lin KP and Chang YC:
Coexistence of Parkinson's disease and myasthenia gravis: A case
report. Clin Neurol Neurosurg. 95:137–139. 1993.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Iwasaki Y, Wakata N and Kinoshita M:
Parkinsonism induced by pyridostigmine. Acta Neurol Scand.
78(236)1988.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Levin N, Karussis D and Abramsky O:
Parkinson's disease associated with myasthenia gravis. A report of
4 cases. J Neurol. 250:766–767. 2003.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Odajiu I, Davidescu EI, Mitu C and Popescu
BO: Patients with Parkinson's disease and myasthenia Gravis-A
report of three new cases and review of the literature. Medicina
(Kaunas). 56(5)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Uludag IF, Korucuk M, Sener U and Zorlu Y:
Myasthenia gravis as a cause of head drop in Parkinson disease.
Neurologist. 17:144–146. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Lanfranconi S, Corti S, Baron P, Conti G,
Borellini L, Bresolin N and Bersano A: Anti-MuSK-Positive
myasthenia gravis in a patient with parkinsonism and cognitive
impairment. Neurol Res Int. 2011(859802)2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Neuman LA and Cheema FZ: Two cases of
Parkinson disease and concurrent myasthenia gravis, generalized and
ocular. Neurohospitalist. 4:117–118. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tung-Chen Y, Bataller L, Sevilla T and
López-Aldeguer J: Co-occurrence of myasthenia gravis with
Parkinson's disease: A not to be missed diagnosis. Geriatr Gerontol
Int. 16:528–530. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tan EK, Chao YX, West A, Chan LL, Poewe W
and Jankovic J: Parkinson disease and the immune
system-associations, mechanisms and therapeutics. Nat Rev Neurol.
16:303–318. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Gilhus NE and Verschuuren JJ: Myasthenia
gravis: Subgroup classification and therapeutic strategies. Lancet
Neurol. 14:1023–1036. 2015.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Yi JS, Guptill JT, Stathopoulos P, Nowak
RJ and O'Connor KC: B cells in the pathophysiology of myasthenia
gravis. Muscle Nerve. 57:172–184. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Malaspina A, Puentes F and Amor S: Disease
origin and progression in amyotrophic lateral sclerosis: An
immunology perspective. Int Immunol. 27:117–129. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sciacca G, Nicoletti A, Mostile G, Dibilio
V, Raciti L, Luca A, Reggio E and Zappia M: Is it just a
coincidence? Three new cases of Myasthenia Gravis associated with
Parkinson's disease. Parkinsonism Relat Disord. 28:166–168.
2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Fasano A, Evoli A, Piano C, Tonali PA and
Bentivoglio AR: Myasthenia gravis: An unrecognized cause of head
drop in Parkinson's disease. Parkinsonism Relat Disord. 14:164–165.
2008.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zis P, Argiriadou V, Temperikidis PP,
Zikou L, Tzartos SJ and Tavernarakis A: Parkinson's disease
associated with myasthenia gravis and rheumatoid arthritis. Neurol
Sci. 35:797–799. 2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Tasić Z, Stefanović P and Apostoloski S:
An unusual association of myasthenia gravis and Parkinsonism in a
female patient with tuberculous lymphadenitis. Srp Arh Celok Lek.
119:103–106. 1991.PubMed/NCBI(In Serbian).
|
|
22
|
Unal-Cevik I and Temucin CM: Head drop in
an elder Parkinson's disease after development of myasthenia
gravis. Mov Disord. 24:2025–2026. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Aiba Y, Iwakawa M, Sakakibara R, Tsuyusaki
Y, Tateno F, Kishi M, Tateno H and Ogata T: Myasthenia gravis
manifesting as head drop in an elderly adult with Parkinson's
disease. J Am Geriatr Soc. 64:e120–e122. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hogg EJ, Lewis RA, Bannykh S and Tagliati
M: Head drop in Parkinson's disease complicated by myasthenia
gravis and myopathy. J Neurol Sci. 376:216–218. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Urban PP and Stammel O: Myasthenia gravis
should be considered in cases of Parkinson's disease and
progressive dysphagia. Nervenarzt. 89:443–445. 2018.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
26
|
Marano M, Lanzone J, di Biase L, Pepe A,
Di Santo A and Di Lazzaro V: A rare cause of axial worsening in
Parkinson's disease: A case of myasthenic pseudo-parkinsonism. Clin
Neurol Neurosurg. 179:1–3. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Ozer AB, Catak T, Ozdemir M and Kilinc M:
Anesthesia management in the coexistence of myasthenia gravis and
parkinsonism. J Clin Anesth. 34:350–351. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Albassam MS, Thabet SA, Hmoud M and
Makkawi S: Anti-Muscle specific kinase (Anti-MuSK) positive
myasthenia gravis overlapping with Parkinson's disease: A
challenging diagnosis. Cureus. 13(e14839)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Punga AR, Maddison P, Heckmann JM, Guptill
JT and Evoli A: Epidemiology, diagnostics, and biomarkers of
autoimmune neuromuscular junction disorders. Lancet Neurol.
21:176–188. 2022.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Zhang Q, Xu E, Li HF, Chan P, Zhao Z and
Ma J: Parkinson's disease and comorbid myasthenia gravis: A case
report and literature review. Front Neurol.
14(1303434)2024.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Iori E, Mazzoli M, Ariatti A, Salviato T,
Rispoli V, Valzania F and Galassi G: Myasthenia Gravis crossing
Parkinson's disease: A 20 year study from single Italian center.
Int J Neurosci. 134:429–435. 2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Somnier FE: Increasing incidence of
late-onset anti-AChR antibody-seropositive myasthenia gravis.
Neurology. 65:928–930. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Drain JP, Virk SS, Jain N and Yu E:
Dropped head syndrome: A systematic review. Clin Spine Surg.
32:423–429. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Berrih-Aknin S, Panse RL and Dragin N:
AIRE: A missing link to explain female susceptibility to autoimmune
diseases. Ann N Y Acad Sci. 1412:21–32. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Peter I, Dubinsky M, Bressman S, Park A,
Lu C, Chen N and Wang A: Anti-Tumor necrosis factor therapy and
incidence of Parkinson disease among patients with inflammatory
bowel disease. JAMA Neurol. 75:939–946. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Akhtar RS, Licata JP, Luk KC, Shaw LM,
Trojanowski JQ and Lee VM: Measurements of auto-antibodies to
α-synuclein in the serum and cerebral spinal fluids of patients
with Parkinson's disease. J Neurochem. 145:489–503. 2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Bach JP and Falkenburger BH: What
autoantibodies tell us about the pathogenesis of Parkinson's
disease: An Editorial for ‘Measurements of auto-antibodies to
α-synuclein in the serum and cerebral spinal fluids of patients
with Parkinson's disease’ on page 489. J Neurochem. 145:433–435.
2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Sulzer D, Alcalay RN, Garretti F, Cote L,
Kanter E, Agin-Liebes J, Liong C, McMurtrey C, Hildebrand WH, Mao
X, et al: T cells from patients with Parkinson's disease recognize
α-synuclein peptides. Nature. 546:656–661. 2017.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sabatino JJ Jr, Pröbstel AK and Zamvil SS:
B cells in autoimmune and neurodegenerative central nervous system
diseases. Nat Rev Neurosci. 20:728–745. 2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Brochard V, Combadière B, Prigent A,
Laouar Y, Perrin A, Beray-Berthat V, Bonduelle O, Alvarez-Fischer
D, Callebert J, Launay JM, et al: Infiltration of CD4+ lymphocytes
into the brain contributes to neurodegeneration in a mouse model of
Parkinson disease. J Clin Invest. 119:182–192. 2009.PubMed/NCBI View
Article : Google Scholar
|
|
41
|
Orr CF, Rowe DB, Mizuno Y, Mori H and
Halliday GM: A possible role for humoral immunity in the
pathogenesis of Parkinson's disease. Brain. 128(Pt 11):2665–2674.
2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Collins LM, Toulouse A, Connor TJ and
Nolan YM: Contributions of central and systemic inflammation to the
pathophysiology of Parkinson's disease. Neuropharmacology.
62:2154–2168. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Kustrimovic N, Comi C, Magistrelli L,
Rasini E, Legnaro M, Bombelli R, Aleksic I, Blandini F, Minafra B,
Riboldazzi G, et al: Parkinson's disease patients have a complex
phenotypic and functional Th1 bias: Cross-sectional studies of CD4+
Th1/Th2/T17 and Treg in drug-naïve and drug-treated patients. J
Neuroinflammation. 15(205)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kara SP, Altunan B and Unal A:
Investigation of the peripheral inflammation (neutrophil-lymphocyte
ratio) in two neurodegenerative diseases of the central nervous
system. Neurol Sci. 43:1799–1807. 2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Huda R: Inflammation and autoimmune
myasthenia gravis. Front Immunol. 14(1110499)2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Wang Q, Luo Y, Ray Chaudhuri K, Reynolds
R, Tan EK and Pettersson S: The role of gut dysbiosis in
Parkinson's disease: Mechanistic insights and therapeutic options.
Brain. 144:2571–2593. 2021.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Kapoor B, Gulati M, Gupta R and Singla RK:
Microbiota dysbiosis and myasthenia gravis: Do all roads lead to
Rome? Autoimmun Rev. 22(103313)2023.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Chen P and Tang X: Gut Microbiota as
Regulators of Th17/Treg balance in patients with myasthenia gravis.
Front Immunol. 12(803101)2021.PubMed/NCBI View Article : Google Scholar
|