Introduction
Groove pancreatitis (GP) is a unique form of chronic
pancreatitis with an insidious onset, first reported by Becker in
1973(1). The lesions mainly
involve the groove area between the head of the pancreas, duodenum,
and lower part of the common bile duct, with a scar plate between
the areas (2). In 1982, Stolte
et al (3) introduced the
term ‘groove pancreatitis’ to describe this specific type of
chronic pancreatitis, and in 1991, Becker and Mischke (4) classified it into pure, segmental and
non-segmental types based on its effect on the pancreatic
parenchyma (5).
Despite these advancements in understanding GP, its
diagnosis remains challenging due to limited awareness among
clinicians and the consequent potential for misdiagnosis (6). This lack of recognition often leads
to a treatment dilemma for patients grappling with this condition.
The exact prevalence of GP is difficult to determine, but based on
several surgical series, it appears to range from 3-24% of patients
operated for chronic pancreatitis (6,7). The
exact etiology of GP has not been fully elucidated, but several
factors have been proposed as potential contributors, including
chronic alcohol consumption, smoking, anatomical abnormalities in
the pancreaticobiliary junction and previous pancreatic surgery
(6,8).
Patients with GP typically present with nonspecific
symptoms, and diagnostic imaging plays a crucial role in confirming
the presence of GP and differentiating it from other pancreatic
disorders (9,10). Treatment strategies aim to
alleviate symptoms, manage complications and improve the overall
quality of life for affected individuals. Conservative management
is often the initial approach, but in some cases, surgical
intervention may be necessary, such as pancreaticoduodenectomy or
local resection of the affected pancreatic segment (6,8,11).
Increased awareness and a multidisciplinary approach can help
improve the clinical outcomes for patients with this rare and
complex condition.
Case report
A 59-year-old male patient was admitted to the
Affiliated People's Hospital of Ningbo University (Zhejiang, China)
in May, 2019, due to a 2-week history of recurrent vomiting,
diarrhea and oliguria. The patient mainly presented with nausea and
vomiting after eating and had experienced diarrhea for a day, 7-8
times per day, but with small amounts each time. The patient had no
abdominal pain, blood in stool, or fever. The patient had taken
medicine to alleviate the diarrhea but continued to experience
repeated nausea and vomiting, leading to a weight loss of 8 pounds
over the 2-week period. The patient had a 20-year history of
smoking and a 30-year history of alcohol consumption. Additionally,
the patient had a history of hypertension and surgery for
hemorrhoids, but denied any other medical history.
The initial outpatient examination showed normal
white blood cell count, C-reactive protein level, platelet count
and hemoglobin level of 169 g/l, but abnormal renal function with
elevated urea nitrogen level of 28.12 mmol/l, creatinine level of
201 µmol/l and uric acid level of 794 µmol/l. The patient also had
low blood sodium level of 130 mmol/l and low blood calcium level of
2.67 mmol/l. The patient was admitted to the nephrology department
with a diagnosis of acute kidney injury, gastroenteritis,
hypertension and hyponatremia.
Following admission, laboratory tests revealed
carcinoembryonic antigen (CEA) level of 5.49 ng/ml, and ferritin
level of 645.1 ng/ml, with a normal carbohydrate antigen 19-9 (CA
19-9) level. The rest of the patient's liver function, troponin,
thyroid function, parathyroid hormone, coagulation function, HIV,
hepatitis B, hepatitis C, autoantibodies, immunoglobulin, blood and
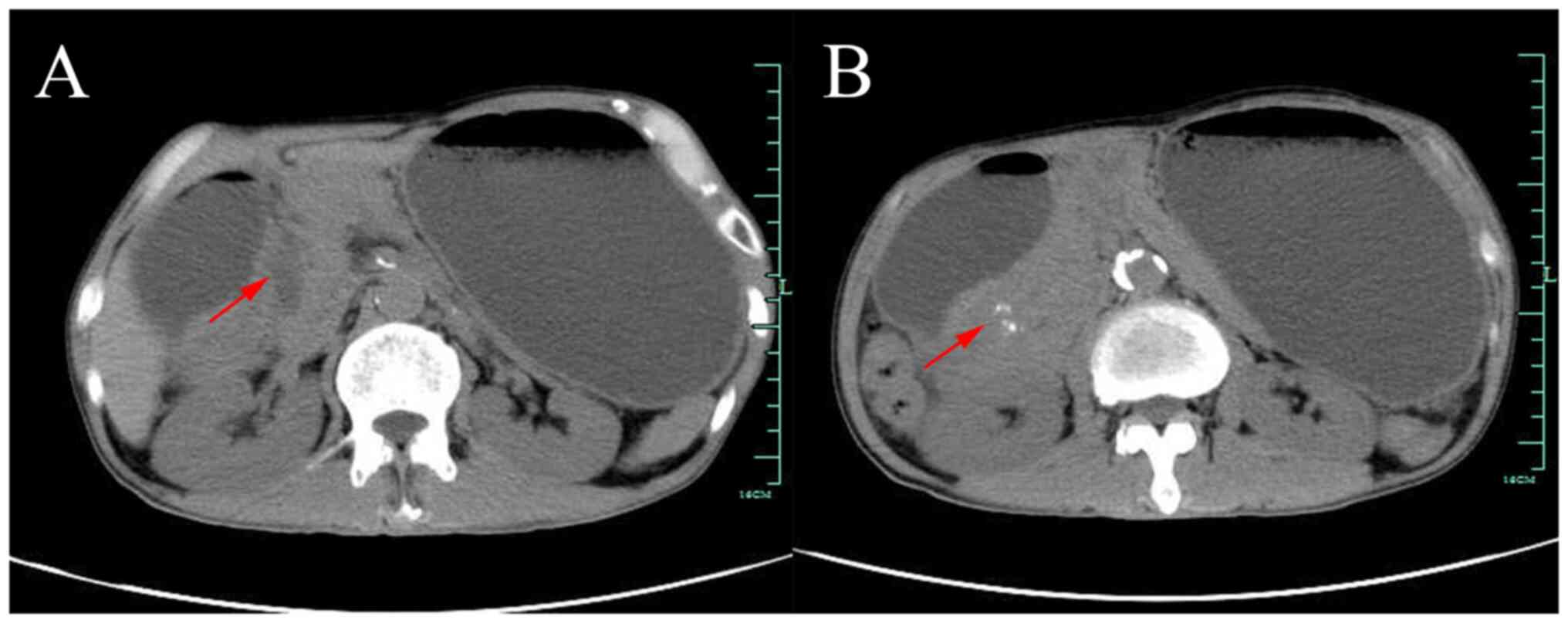
urine light chain were all normal. Abdominal computed tomography
(CT) suggested thickening of the sinusoidal wall with gastric
retention, patchy shadow of the pancreatic-gastric gap with
calcification, mild dilatation of the intra- and extrahepatic bile
ducts and dilated pancreatic ducts (Fig. 1). At this point, it became clear
that the patient was not simply suffering from gastroenteritis but
rather from gastric retention, so the patient was transferred to
the gastroenterology department. The patient was treated with
fasting, gastrointestinal decompression, acid suppression and
rehydration support. After treatment, the patient's urine output
increased, creatinine level returned to normal, blood calcium level
returned to normal and hyponatremia was more persistent at 127
mmol/l.
Initially, it was suspected that the patient's
gastric retention was due to obstruction caused by stenosis from
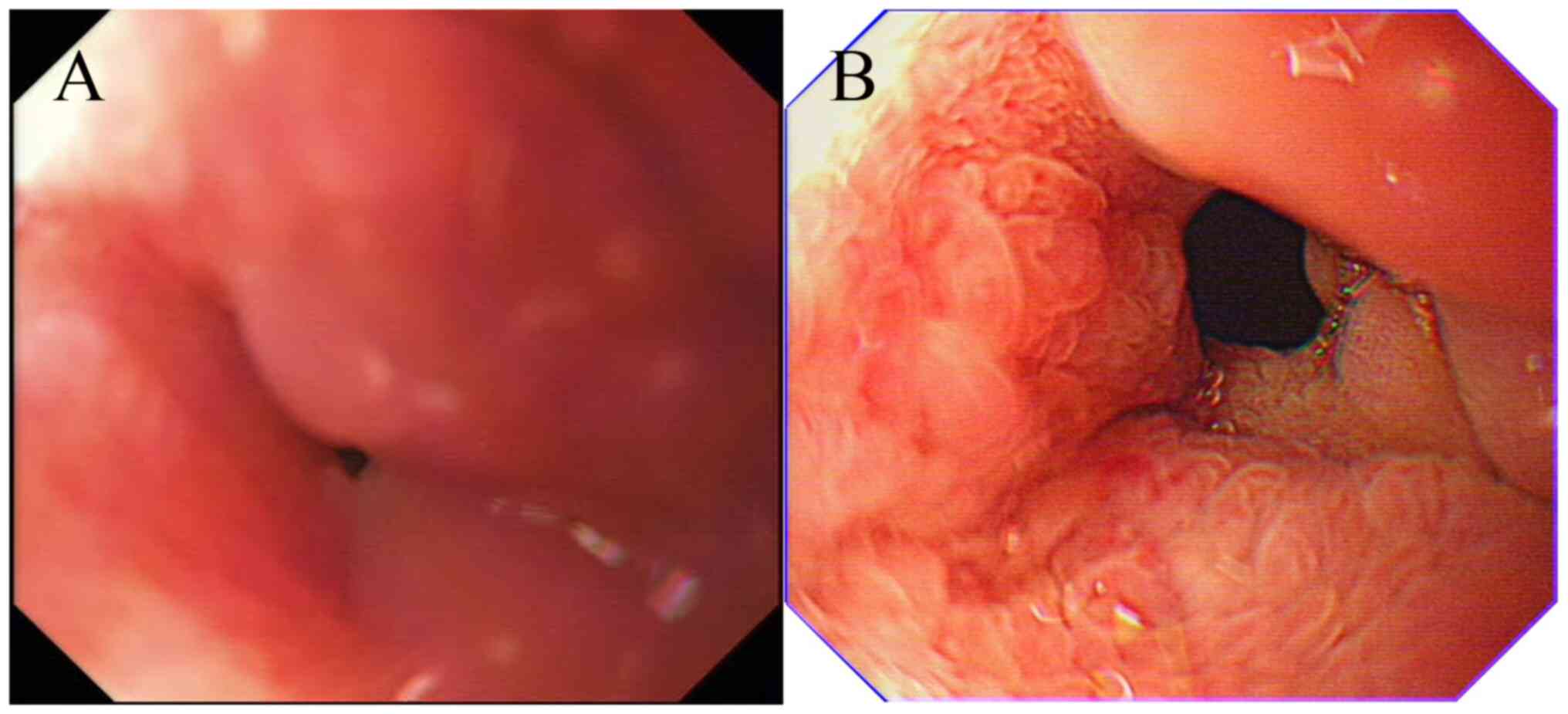
peptic ulcer scarring. However, gastroscopy revealed a significant
narrowing of the lumen in the descending duodenal bulb that was too
severe for ordinary gastroscopy to pass through. The mucosal
surface displayed coarse granular changes, but no obvious ulcer or
heterogeneous glands were seen (Fig.
2A). Endoscopic ultrasonography (EUS) indicated that the
mucosal layer was predominantly hyperechoic and fused with the
submucosal layer, with unclear demarcation from the muscular layer.
The duodenal wall was markedly thickened, and the structural level
was disturbed, with an indistinguishable echogenic line of the
plasma layer. A stenotic lesion in the descending duodenal bulb was
considered, with a high possibility of extravasation or
inflammation. Biopsy pathology results indicated chronic
inflammation.
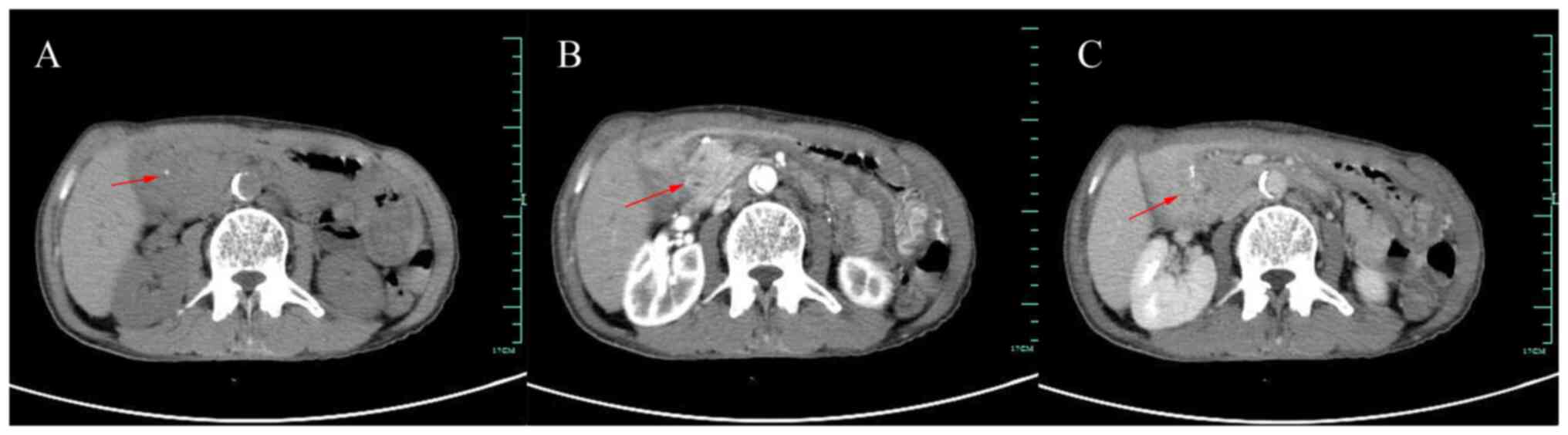
Magnetic resonance cholangiopancreatography of the
patient showed mild dilatation of the bile duct and pancreatic
duct, narrowing of the lower part of the common bile duct, no
obvious occupancy and no abnormal signal in the pancreatic
parenchyma. The contrast-enhanced CT of the abdomen showed patchy
soft tissue density shadow in the pancreatic-gastroduodenal space
with speckled calcification, poorly demarcated from the surrounding
organs and no abnormal enhancement was seen. Additionally, the
intra- and extra-hepatic bile ducts and pancreatic ducts were
dilated (Fig. 3). We accessed the
patient's endoscopic and imaging data at the time of
hospitalization for hemorrhoid surgery 3 years before. At that
time, the patient had no discomfort, CEA 8.04 ng/ml, ferritin 338.2
ng/ml and gastroscopy suggestive of duodenal erosion (recommended
in combination with CT examination; Fig. 2B). No abnormalities were reported
by the abdominal contrast-enhanced CT then but our review of the
images revealed the presence of a lesion in the pancreaticoduodenal
space at that time. For differential diagnosis, we refined
tuberculin purified protein derivative test, T-spot, and the
results were positive. Blood amylase and chest CT showed no
abnormalities. Although there was no manifestation of pulmonary
tuberculosis and the possibility of abdominal tuberculosis was
small, rare cases could not be excluded. In addition, it was
suggested to the patient to have a positron emission tomography
computed tomography examination to exclude tumor disease, however
the patient refused due to financial factors.
For further treatment to relieve the obstruction and
clarify the diagnosis, the patient was referred to surgery for
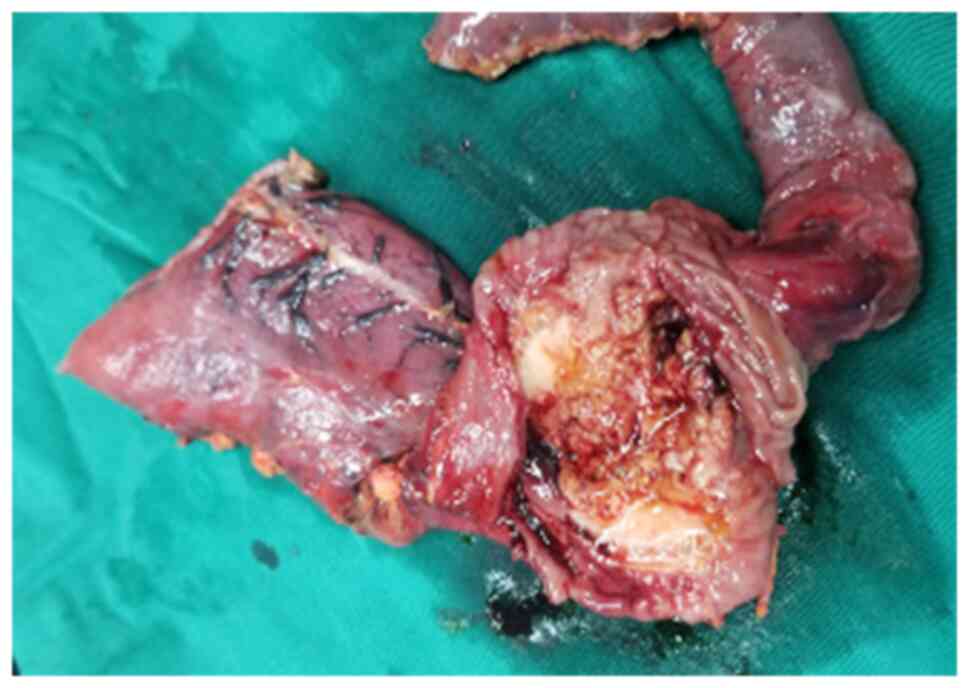
laparoscopic exploration. Intraoperatively, a duodenal and
pancreatic head mass measuring ~4x5 cm was seen (Fig. 4), and intraoperative cytopathology
suggested collagenous fibrous tissue and vascular tissue with a
small amount of inflammatory cell infiltration, and tuberculosis
was not considered, so pancreaticoduodenectomy was performed. The
excised lesion tissues were sent for postoperative pathological
examination. The specimen was fixed with 10% neutral formalin,
dehydrated at room temperature for 24 h, and paraffin embedded.
Next, 3- to 4-µm thick sections were prepared and stained with
hematoxylin-eosin at room temperature (18-25˚C) for ~5-10 min. The
stained sections were viewed at x400 magnification under a bright
field optical microscope. The surgical pathology suggested chronic
inflammation of the duodenal mucosa with nodular hyperplasia of
Brunner's glands, consistent with a so-called Brunner's adenoma
(4x3.5x0.5 cm; Fig. 5), ductal
hyperplasia with focal dilated cystic changes within the muscular
layer of part of the duodenum and within the pancreatic tissue.
Following surgery, the patient recovered well and
was discharged. The follow-up results showed that the patient had
no discomfort and the levels of CEA and ferritin had returned to
normal. The patient was followed up for three years and did not
experience any relapse or readmission during that period.
Discussion
Due to the rarity of the disease and a lack of
clinical recognition, no large clinical studies have been reported
and most of the literature consists of only case reports. As a
result, the true incidence of GP is unclear. The onset age is more
common in individuals aged 40-50, with the youngest reported case
being a 17-year-old patient (12).
A retrospective study involving 48 cases of GP reported by Ooka
et al (11) found a mean
age of 53 years and 79% of cases were male.
The pathogenesis of GP is still unclear, and there
are a number of different views. One of the main mechanisms
considered is obstruction of the small duodenal papilla (12) and a number of studies have found
congenital anomalies or absence of the Santorini duct with protein
plugs obstructing pancreatic fluid drainage, which may lead to GP
(13). Therefore, the appearance
of the Santorini duct on endoscopic retrograde
cholangiopancreatography is a critical factor for the diagnosis of
GP (14).
Numerous patients have a history of medium to
long-term alcohol consumption and smoking, with long-term alcohol
consumption leading to increased protein content and viscosity of
pancreatic fluid, which exacerbates the inflammatory response
(15). Additionally, patients with
GP often have duodenal Brunner's gland hyperplasia, but it is
uncertain whether gland hyperplasia is the cause or effect. Ectopic
pancreas may also play a role in the development of GP (12). The present case fits the
aforementioned description with the presence of a large adenoma of
the Brunner's glands.
Endoscopic biopsy or endoscopic ultrasound-guided
fine needle aspiration (EUS-FNA) reveals evidence of chronic
inflammation. Gross specimens show grayish scar tissue in the
pancreas grooves, thickening of the duodenal wall, cystic changes
and mild dilation of the peripancreatic and common bile ducts.
Microscopic examination shows fibrosis and cystic changes in the
duodenal wall (the most distinctive feature, with an incidence of
~49%), atypical hyperplasia of Brunner's glands, fibrosis and
scarring in the groove area, mild dilation of the peripancreatic
and pancreatic ducts with visible protein plugs in the lumen and
significant proliferation of duodenal submucosal myofibroblasts
(12). Gábos et al
(8) reported a case of GP with
duodenal stenosis and a mass in the head of the pancreas. The
authors used EUS-FNA to clarify that the mass was chronic
inflammation and avoided misdiagnosis as a tumor. This patient did
not present with biliary obstruction or gastric outlet obstruction
and was discharged with relief of symptoms after conservative
management. Otherwise, the patient might have had to be treated
surgically due to complete duodenal obstruction as in the present
case. This shows that EUS-FNA plays a crucial role in the diagnosis
of GP.
Clinical manifestations of GP typically include
acute and chronic epigastric pain, nausea, vomiting and weight loss
(8,16). Jaundice is rare and this can be
differentiated from pancreatic cancer (12). Tumor markers such as CEA and CA
19-9 levels are usually in the normal range or only mildly elevated
as in this case (17). Blood
leukocytes and C-reactive protein levels are usually normal, while
liver enzyme levels are often normal or slightly elevated. Serum
amylase and lipase levels are mostly normal or mildly elevated, but
not exceeding three times the normal range. A number of patients
exhibit symptoms of acute pancreatitis or are diagnosed with
chronic pancreatitis prior to the GP diagnosis. In the present
case, the patient did not experience significant symptoms in the
early stages until the development of severe duodenal obstruction,
leading to dehydration, electrolyte imbalance, and acute renal
insufficiency.
On CT, the classic presentation of GP is a
hypoenhancing slab-like hypodense soft tissue lesion in the middle
of the pancreas and duodenum (7).
Poor enhancement may be due to delayed circulation resulting from
fibrous tissue hyperplasia and secondary stenosis of the arteries
(18). Additionally, thickening of
the duodenal wall and narrowing of the lumen may be observed, with
cystic lesions often visible within the wall. In cases of segmental
GP, mild dilation of the bile duct and pancreatic duct may be
present and the lower portion of the common bile duct may be
narrowed without evidence of vascular invasion (10,19).
Unfortunately, early diagnosis of GP remains challenging through CT
imaging. In the present case, the abnormalities in the groove area
were overlooked by imaging specialists and clinicians three years
earlier. Inflammatory changes in the groove region, thickening of
the descending duodenal wall, and small cystic dilatations are
crucial signs that should be emphasized. Early detection via CT
provides prospective diagnostic value and can markedly guide
treatment (7). Conservative
management of patients with early-stage GP can often avoid
unnecessary surgical procedures (16). In clinical practice, there remain
instances wherein despite CT findings suggestive of inflammation
and negative results from puncture pathology, clinicians encounter
challenges in definitively excluding the prospect of malignant
tumors. Consequently, they may opt for surgical intervention
(10).
The main manifestation of GP is fibrosis in the
groove region, which generally appears as a low signal on magnetic
resonance imaging (MRI) (10,20).
Progressive enhancement is seen on contrast-enhanced MRI,
indicating that the lesion may be fibrous tissue. However, if the
inflammatory edema is more pronounced, the T2-weighted signal may
be high. Mild dilatation of the pancreatic duct and common bile
duct may occur when the lesion invades the pancreatic head. Smooth
stenosis in the lower part of the common bile duct may also be
present. Magnetic resonance cholangiopancreatography effectively
delineates abnormalities in the distal common bile duct and
downstream pancreatic duct, often characterized by constriction
near the ampulla. Assessment of the distance between the ampulla
and the duodenal lumen serves as an indicator for abnormalities
within the groove, commonly observed in GP. This widening typically
arises due to soft tissue deposition in the groove and thickening
of the duodenal wall (10,20,21).
In the early stages of GP, gastroscopy may not
reveal any significant abnormalities, or mild mucosal edema and
hyperplasia may be observed in the second segment of the duodenum.
However, as the disease progresses and fibrosis increases, severe
edema and thickening of the duodenal mucosa occur, leading to
significant luminal narrowing that may hinder endoscopic
examination. The comparative endoscopic performance of the present
case illustrates this evolution well. Under EUS, the majority of
hypoechoic masses are seen in the groove region, with severe
fibrosis appearing hyperechoic and occasionally displaying
indistinct borders (22).
Calcified foci are also visible. The lesion is poorly delineated
from the duodenum and pancreatic head, and cystic changes of
varying sizes may be present in the lesion or within the duodenal
wall. Fine needle aspiration under EUS can aid in the diagnosis
(23,24).
It can be challenging to distinguish GP from other
conditions such as duodenal tumors, bile duct cancer, periampullary
carcinoma and pancreatic cancer, particularly in the early stages
of the disease (9,25). In a case study by Kim et al
(12), a patient was initially
diagnosed with GP but was later found to have distal peribiliary
carcinoma on postoperative pathology, which can have similarities
in presentation to GP when the tumor contains a fibrous component.
However, GP typically does not invade blood vessels or the rest of
the pancreas, which can aid in the differential diagnosis (6,26).
Depending on the severity of the disease, various
treatment options are available. Conservative treatment may include
alcohol cessation, pain relief, growth inhibitor analogs and
nutritional support. Endoscopic therapies, such as cystgastrostomy,
pancreatic and biliary duct stenting, cystenterostomy, pancreatic
and/or biliary duct drainage and duodenal dilation, are also
available (27). A meta-analysis
including 1,404 patients revealed that endoscopic therapy achieved
success in 50% of cases (6).
Consequently, it is advisable to reserve this treatment for
patients experiencing recurrent pain unresponsive to conservative
measures and those presenting with moderate to marked morphological
alterations (6). Surgical
treatment options may include pancreaticoduodenectomy and
gastrojejunostomy, as well as choledochotomy with T-tube drainage
(11,28,29).
Pancreaticoduodenectomy has been shown to markedly reduce the use
of painkillers and completely eliminate clinical symptoms in
patients with late-stage complications such as obstruction and
severe pain, but there is a lack of relevant outcome studies
available to confirm its efficacy (6,12,30,31).
GP is a rare pancreatic disease with good overall
prognosis, but of varying severity. Conservative treatment can be
effective for early-stage patients, while endoscopic or surgical
intervention is often necessary for advanced complications
(11). Unfortunately, due to low
disease recognition, a number of patients are already diagnosed
with advanced complications. Although the prognosis of surgically
treated patients is mostly favorable, there is a lack of specific
clinical data. Moreover, there have been reported cases of
postoperative complications such as asphyxia and upper
gastrointestinal bleeding leading to patient mortality (12).
The diagnosis of GP is challenging due to the
complex anatomical findings around the paraduodenal groove of the
pancreatic head. Imaging and clinical presentations alone are often
insufficient, and pathological confirmation after
pancreaticoduodenectomy is frequently necessary. In cases where
malignancy cannot be excluded and symptoms persist despite
conservative treatment, surgical intervention may still be required
(6). It is imperative that
clinicians increase their awareness of this disease to avoid
unnecessary surgical procedures.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
XYF designed the present study, collected clinical
data and wrote the manuscript. CHS collected clinical data, managed
the patient and drafted the manuscript. XYF and DWL confirm the
authenticity of all the raw data. DWL made contributions to
conception and design of the work and wrote the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and accompanying
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Becker V: Proceedings: Fundamental
morphological aspects of acute and chronic pancreatitis (author's
transl). Langenbecks Arch Chir. 334:317–322. 1973.PubMed/NCBI View Article : Google Scholar : (In German).
|
|
2
|
Joshi SS, Dhok A, Mitra K and Onkar P:
Groove pancreatitis: A unique case of focal pancreatitis. J Clin
Imaging Sci. 12(54)2022.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Stolte M, Weiss W, Volkholz H and Rosch W:
A special form of segmental pancreatitis: ‘Groove pancreatitis’.
Hepatogastroenterology. 29:198–208. 1982.PubMed/NCBI
|
|
4
|
Becker V and Mischke U: Groove
pancreatitis. Int J Pancreatol. 10:173–182. 1991.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Manzelli A, Petrou A, Lazzaro A, Brennan
N, Soonawalla Z and Friend P: Groove pancreatitis. A mini-series
report and review of the literature. JOP. 12:230–233.
2011.PubMed/NCBI
|
|
6
|
Ukegjini K, Steffen T, Tarantino I, Jonas
JP, Rössler F, Petrowsky H, Gubler C, Müller PC and Oberkofler CE:
Systematic review on groove pancreatitis: Management of a rare
disease. BJS Open. 7(zrad094)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Patel BN, Brooke Jeffrey R, Olcott EW and
Zaheer A: Groove pancreatitis: A clinical and imaging overview.
Abdom Radiol (NY). 45:1439–1446. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gábos G, Nicolau C, Martin A and Mosteanu
O: Groove pancreatitis-tumor-like lesion of the pancreas.
Diagnostics (Basel). 13(866)2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Sanchez-Bueno F, Torres Salmeron G, de la
Pena Moral J, Ortiz Ruiz E, Fuster Quiñonero M, Gutiérrez Zárate
WV, Claver Valderas MA and Parrilla Paricio P: Groove pancreatitis
vs. pancreatic adenocarcinoma: A review of 8 cases. Cir Esp.
94:346–352. 2016.PubMed/NCBI View Article : Google Scholar : (In English,
Spanish).
|
|
10
|
Raman SP, Salaria SN, Hruban RH and
Fishman EK: Groove pancreatitis: Spectrum of imaging findings and
radiology-pathology correlation. AJR Am J Roentgenol. 201:W29–W39.
2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ooka K, Singh H, Warndorf MG, Saul M,
Althouse AD, Dasyam AK, Paragomi P, Phillips AE, Zureikat AH, Lee
KK, et al: Groove pancreatitis has a spectrum of severity and can
be managed conservatively. Pancreatology. 21:81–88. 2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kim JD, Han YS and Choi DL: Characteristic
clinical and pathologic features for preoperative diagnosed groove
pancreatitis. J Korean Surg Soc. 80:342–347. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Shudo R, Obara T, Tanno S, Fujii T,
Nishino N, Sagawa M, Ura H and Kohgo Y: Segmental groove
pancreatitis accompanied by protein plugs in Santorini's duct. J
Gastroenterol. 33:289–294. 1998.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Latham J, Sanjay P, Watt DG, Walsh SV and
Tait IS: Groove pancreatitis: A case series and review of the
literature. Scott Med J. 58:e28–e31. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Levenick JM, Gordon SR, Sutton JE,
Suriawinata A and Gardner TB: A comprehensive, case-based review of
groove pancreatitis. Pancreas. 38:e169–e175. 2009.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Triantopoulou C, Dervenis C, Giannakou N,
Papailiou J and Prassopoulos P: Groove pancreatitis: A diagnostic
challenge. Eur Radiol. 19:1736–1743. 2009.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Goransky J, Alvarez FA, Picco P, Spina JC,
Santibanes M and Mazza O: Groove pancreatitis vs groove pancreatic
adenocarcinoma. Report of two cases and review of the literature.
Acta Gastroenterol Latinoam. 43:248–253. 2013.PubMed/NCBI
|
|
18
|
Zaheer A, Haider M, Kawamoto S, Hruban RH
and Fishman EK: Dual-phase CT findings of groove pancreatitis. Eur
J Radiol. 83:1337–1343. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ray S, Ghatak S, Misra D, Dasgupta J,
Biswas J, Khamrui S, Bandyopadhyay D and Ghosh R: Groove
pancreatitis: Report of three cases with brief review of
literature. Indian J Surg. 79:344–348. 2017.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Castell-Monsalve FJ, Sousa-Martin JM and
Carranza-Carranza A: Groove pancreatitis: MRI and pathologic
findings. Abdom Imaging. 33:342–348. 2008.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Blasbalg R, Baroni RH, Costa DN and
Machado MC: MRI features of groove pancreatitis. AJR Am J
Roentgenol. 189:73–80. 2007.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lopez-Munoz P, Lorenzo-Zuniga V,
Alonso-Lazaro N, García-Campos M, Argüello L, Bustamante-Balén M
and Pons-Beltrán V: Endoscopic findings of paraduodenal or groove
pancreatitis. Endoscopy. 54:E735–E736. 2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Oria IC, Pizzala JE, Villaverde AM, Spina
JC, Pasqua AV, Lazarte JC, Mazza OM and Marcolongo MM: Endoscopic
ultrasound in the diagnosis of pancreatoduodenal groove pathology:
Report of three cases and brief review of the literature. Clin
Endosc. 52:196–200. 2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
She YM and Ge N: Diagnostic value of
endoscopic ultrasound in groove pancreatitis. Ann Med.
55(2295991)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Han C, Ling X, Sheng L, Yang M, Lin R and
Ding Z: Distal extrahepatic cholangiocarcinoma mimicking groove
pancreatitis: A case report and literature review. Front Oncol.
12(948799)2022.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Ito R, Shiba H, Okamoto T, Fujioka S,
Gocho T and Yanaga K: Groove pancreatitis with several cystic
lesions around the pancreatic head treated conservatively: Report
of a case. Case Rep Gastroenterol. 2:405–409. 2008.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Arvanitakis M, Rigaux J, Toussaint E,
Eisendrath P, Bali MA, Matos C, Demetter P, Loi P, Closset J,
Deviere J and Delhaye M: Endotherapy for paraduodenal pancreatitis:
A large retrospective case series. Endoscopy. 46:580–587.
2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Munthali Lovemore CE, Hsu JT, Chiu CT,
Chen HM and Chen MF: Groove pancreatitis: Case report and
literature review. Chang Gung Med J. 24:512–516. 2001.PubMed/NCBI
|
|
29
|
Aguilera F, Tsamalaidze L, Raimondo M,
Puri R, Asbun HJ and Stauffer JA: Pancreaticoduodenectomy and
Outcomes for Groove Pancreatitis. Dig Surg. 35:475–481.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Levenick JM, Sutton JE, Smith KD, Gordon
SR, Suriawinata A and Gardner TB: Pancreaticoduodenectomy for the
treatment of groove pancreatitis. Dig Dis Sci. 57:1954–1958.
2012.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ismail IB, Zenaidi H, Yahmadi A, Rebii S
and Zoghlami A: Surgical management of groove pancreatitis: A case
report. Pan Afr Med J. 36(99)2020.PubMed/NCBI View Article : Google Scholar
|