Introduction
Chitin, a cationic amino polysaccharide composed of
N-acetylglucosamine linked by β-1,4-glycosidic bonds, is a natural
macromolecule with a yield second only to cellulose in nature. It
is commonly found in the shells of crustaceans, insect epidermis
and fungal cell walls (1).
Complete or partial deacetylation of chitin can yield chitosan, a
cationic alkaline polysaccharide composed of D-glucosamine linked
by β-1,4-glycosidic bonds. Degradation of chitosan produces
chitosan oligosaccharides (COSs), characterized by low molecular
weight and high biological activity (2). Moreover, chitin degradation results
in the production of N-acetylated COS (NACOS), which exhibits good
solubility and multiple biological functions, including
antioxidant, anti-inflammatory, antitumor, antimicrobial and plant
elicitor activities, as well as immunomodulatory and prebiotic
effects (3). While chitin and its
derivative chitosan are known for their various functional
activities, their limited solubility hinders their use in food and
biomedical applications. However, hydrolyzed products of chitosan,
including COS (3-5)
and NACOS, exhibit increased water solubility due to their shorter
chain length, lower viscosity and potentially higher absorption
rates (6-8).
Numerous studies have shown that COS and its
derivatives possess biological activities, including
anti-inflammation, anti-tumor, anti-obesity, anti-Alzheimer's
disease, immunostimulation, tissue regeneration promotion, drug and
DNA delivery enhancement, anti-microbiome and anti-oxidation
effects (5,9-12).
Some studies also report that COS possesses robust neuroprotective
properties, such as β-amyloid and acetylcholinesterase inhibition,
anti-neuroinflammation and anti-apoptosis (13,14).
COS has demonstrated its potential to mitigate high-fat
diet-induced obesity and insulin resistance by modulating gut
microbiota dysfunction, mitigating low-grade inflammation and
preserving the integrity of the intestinal epithelial barrier
(15). Inflammatory factors serve
an important role in cardiovascular diseases such as
atherosclerosis, myocardial infarction and cardiac failure
(16). Several studies have
highlighted the anti-inflammatory effects of COS (17,18).
The mechanism underlying the anti-inflammatory effects of orally
administered COS may involve suppressing inflammatory processes,
including regulating the expression of NF-κB, Nrf2, TGF-β1/Smad,
inducible nitric oxide synthase and pro-inflammatory cytokines
(15). Notably, COS has been shown
to extend the survival of mice challenged with lipopolysaccharide
(19).
Oxidative stress and inflammation are linked, with
their interdependence consistently documented. Emerging evidence
implicates oxidative stress in various types of diseases, including
diabetes (20), atherosclerosis
(21) and cancer (22), as well as aging (23), prompting attention to the role of
antioxidants in biological systems. Extensive efforts have been
devoted to identifying suitable antioxidant compounds for
preventive and therapeutic purposes. Reactive oxygen species (ROS),
such as superoxide anion (O2-),
H2O2 and hydroxyl radicals (HO-),
are continually generated at a substantial rate as by-products of
aerobic metabolism and radiation exposure (24). These oxidants not only damage
cellular macromolecules, including DNA, protein and lipids, but
also contribute to NLRP3 inflammasome activation and mediate
inflammatory responses, establishing a cycle of localized
inflammation leading to oxidative damage (25). The persistent threat of
oxidant-induced damage to cells, tissue and organisms is
underscored by cellular defense mechanisms evolved to combat
reactive oxidants. Excessive ROS is readily converted to
H2O2, which easily diffuses across cell
membranes and generates highly reactive and toxic hydroxyl radicals
via the heme-catalyzed Fenton reaction, leading to rapid DNA damage
and strand breaks when superoxide anions react with nitric oxide
(NO) to form peroxynitrite, a reactive nitrogen species (23). In response to oxidative
stress-induced DNA damage, processes such as cell cycle arrest and
DNA damage repair are initiated as cellular defense mechanisms.
However, if DNA damage exceeds the cell repair capacity, it
ultimately leads to apoptotic cell death (26). Macrophages are key immune cells for
the host defense system to eliminate microbial pathogens. Studies
have verified that macrophages can produce ROS when infected by
some bacteria, making them a suitable model for antioxidant
research (27,28). The mouse macrophage cell line
RAW264.7 is the most commonly used mouse macrophage cell line in
medical research (29). Therefore,
the present study used H2O2-stimulated
RAW264.7 cells to establish a cellular oxidative damage model.
COS has shown potential in protecting against
oxidative stress-induced cell damage by modulating enzyme activity
and gene expression related to antioxidant defense mechanisms
(25,30-33),
thus exerting health benefits primarily through antioxidant
activity. Scholars have highlighted the impact of physicochemical
parameters such as molecular weight and degree of acetylation (DA)
on the bioactivities of COS (34,35).
However, the lack of well-defined and standardized criterion for
distinguishing the bioactivity of COS with varying degrees of
polymerization is compounded by the shared nomenclature of COS and
NACOS, leading to potential confusion. Therefore, the present study
aimed to investigate the impact of acetylation on COS biological
activity by comparing the protective effects of deacetylated COS
and its non-deacetylated form, NACOS, on
H2O2-induced apoptosis and oxidative stress
in RAW264.7 cells.
Materials and methods
Chemicals and reagents
Dulbecco's modified Eagle's medium (DMEM) medium was
purchased from Corning, Inc. Fetal bovine serum (FBS), penicillin
and streptomycin were from Gibco (Thermo Fisher Scientific, Inc.).
Annexin V-FITC kit and Cell Cycle Analysis kit were from Beyotime
Institute of Biotechnology. Colored standards and all the other
reagents for SDS-PAGE and immunoblotting were from Bio-Rad
Laboratories, Inc. Antibodies against β-actin, Bax, Bcl-2, PARP and
cleaved PARP, as well as horseradish peroxidase (HRP)-conjugated
goat anti-rabbit IgG and HRP-conjugated goat anti-mouse IgG were
obtained from Cell Signaling Technology, Inc. All chemicals used
were analytical grade unless otherwise stated.
Purification and quantitative analysis
of NACOS and COS
COS with a deacetylation degree of 88% and
polymerization degree of 2-6 was prepared through enzymatic
hydrolysis of chitosan, as described previously (36). NACOS was also prepared as described
previously (37). In brief, COS
was dissolved in 50 ml water with 3 ml methanol, 0.1 g
4-dimethylaminopyridine and 4.37 ml acetic anhydride. The mixture
was incubated at 60˚C for 4 h. Subsequently, 5X the volume of
acetone was added to obtain NACOS. DA and polymerization degree
were determined using liquid chromatography-mass spectrometry and
nuclear magnetic resonance analyses by Institute of Process
Engineering, Chinese Academy of Sciences. The mass spectrometry
detection conditions were: ESI source; positive ion scanning mode;
3 kV capillary voltage; 60 V cone hole voltage; 150˚C ion source
temperature; 500˚C desolvent gas temperature; 50 l/h flow rate of
conical hole gas; 800 l/h desolvent gas flow rate; 150-2,000 m/z
quality scanning range.
Cell culture
The mouse monocyte macrophage leukemia RAW264.7 cell
line was obtained from the Type Culture Collection of the Chinese
Academy of Sciences. RAW264.7 cells were cultured in DMEM
supplemented with 5% heat-inactivated FBS, 100 U/ml penicillin and
100 µg/ml streptomycin in a humidified atmosphere with 5%
CO2 at 37˚C and passaged twice/week. Cells at a
confluence of 70-80% were used for subsequent experiments.
Identification of cell viability using
MTT assay
Cell viability was determined by MTT colorimetric
assay. In brief, cells were treated with NACOS and COS (25, 50 or
100 µg/ml) for 12 h at 37˚C and cell viability was detected by MTT
assay. In addition, cells were pretreated with NACOS and COS (25,
50 or 100 µg/ml) for 12 h at 37˚C, followed by 300 µM
H2O2 for another 12 h at 37˚C. Treatment with
equal volume PBS was included as the vehicle control. A total of
100 µl 0.5 mg/ml MTT PBS solution was added to each well and the
samples were incubated for an additional 4 h at 37˚C. The
purple-blue MTT formazan precipitate was dissolved in 100 µl
dimethyl sulfoxide and the absorbance at 570 nm was measured using
a microplate reader.
Identification of cell cycle stage by
propidium iodide (PI) staining
Cell cycle analysis was performed by flow cytometry
using a fluorescence-activated cell sorting (FACS) MoFlo XDP
(Beckman Coulter) following PI staining. In brief, RAW264.7 cells
were exposed to 50 µg/ml NACOS and COS for 12 h and stimulated with
300 µM H2O2 for another 12 h at 37˚C. The
cells were harvested, adjusted to a concentration of
1x106 cells/ml by cell count and dilution, and fixed in
70% ethanol at 4˚C overnight. The fixed cells were washed twice
with cold PBS and incubated with RNase (8 µg/ml) and PI (10 µg/ml)
at 4˚C for 30 min. The fluorescent signal was detected through the
FL2 channel and the proportion of DNA in various phases was
analyzed using ModfitLT Version 3.0 (Verity Software House,
Inc.).
Identification of apoptosis by Annexin
V/PI staining
A total of 2x105 RAW264.7 cells in 2 ml
DMEM complete medium was seeded into 6-well plates and treated with
50 µg/ml NACOS or COS for 12 h and then stimulated with 300 µM
H2O2 for another 12 h at 37˚C. Apoptosis of
RAW264.7 cells was determined by flow cytometry analysis using a
FACS caliber (Becton-Dickinson) and Annexin V-fluorescein
isothiocyanate (FITC)/PI kit. Cell apoptosis was quantified using
FlowJo software (v.10.1.1; FlowJo LLC). Staining was performed
according to the manufacturer's instructions. Annexin V/PI
double-negative population represented viable cells, while Annexin
V-positive/PI-negative or Annexin V/PI double-positive population
represented cells undergoing early or late apoptosis,
respectively.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The total RNA was isolated from cells using
RNAisoPlus reagent (Takara Biotechnology Co., Ltd.). Reverse
transcription into cDNA was amplified according to the
manufacturer's instructions using the PrimeScript RT reagent kit
(Takara Biotechnology Co., Ltd.). An ABI 7500 Fast Real-Time PCR
System (Applied Biosystems) and the SYBR Premix Ex Tag (Takara
Biotechnology Co., Ltd.) was used for PCR. Following an initial
denaturation at 95˚C for 5 min, the PCR conditions were as follows:
35 cycles of denaturation at 95˚C for 30 sec, annealing at 55˚C for
30 sec and extension at 72˚C for 30 sec. The 2-ΔΔCq
method was used to calculate the mRNA expression levels in each
sample (38). Data were normalized
to the GAPDH content of the sample. The primer sequences were as
follows: L-1β forward, 5'-ATGATGGCTTATTACAGTGGCAA-3' and reverse
5'-GTCGGAGATTCGTAGCTGGA-3'; IL-6 forward,
5'-ACTCACCTCTTCAGAACGAATTG-3' and reverse,
5'-CCATCTTTGGAAGGTTCAGGTTG-3'; IL-8 forward,
5'-ACTGAGAGTGATTGAGAGTGGAC-3' and reverse,
5'-AACCCTCTGCACCCAGTTTTC-3'; TNF-α forward,
5'-CCTCTCTCTAATCAGCCCTCTG-3' and reverse,
5'-GAGGACCTGGGAGTAGATGAG-3'; MCP-1 forward,
5'-CAGCCAGATGCAATCAATGCC-3' and reverse,
5'-TGGAATCCTGAACCCACTTCT-3'; and GAPDH forward,
5'-CACATGGCCTCCAAGGAGTAA-3' and reverse,
5'-TGAGGGTCTCTCTCTTCCTCTTGT-3'.
Western blot analysis
Total protein extracts were obtained using RIPA
lysis buffer and concentrations were determined by the BCA assay
(both Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of
protein (50 µg) from each sample were separated using 10-15%
SDS-PAGE and transferred onto PVDF membranes. The membranes were
blocked with 5% non-fat milk for 1 h at room temperature and
incubated with specific primary antibodies (all 1:1,000) against
Bcl-2 (cat. no. 4223), Bax (cat. no. 5023), PARP, cleaved-PARP
(cat. no. 9542) and β-actin (cat. no. 4697) from Cell Signaling
Technology, Inc., at 4˚C overnight. After incubating with
horseradish peroxidase-conjugated secondary antibodies (1:250,000)
at room temperature for 2 h, the signals were visualized using
enhanced chemiluminescence. Protein bands were visualized using
enhanced chemiluminescence reagent (Thermo Fisher Scientific,
Inc.). Protein expression levels were semi-quantified using ImageJ
software (v2.1.4.7) with β-actin as the loading control.
Statistical analysis
All experiments were performed three times
independently. All data are presented as the mean ± SD. Data were
analyzed by one-way ANOVA followed by Tukey-Kramer's post hoc test.
P<0.05 was considered to indicate a statistically significant
difference. All statistical analysis was performed using the SPSS
package for Windows (version 17.0; SPSS, Inc.).
Results
Effects of NACOS and COS on viability
of H2O2-stimulated RAW264.7 cells
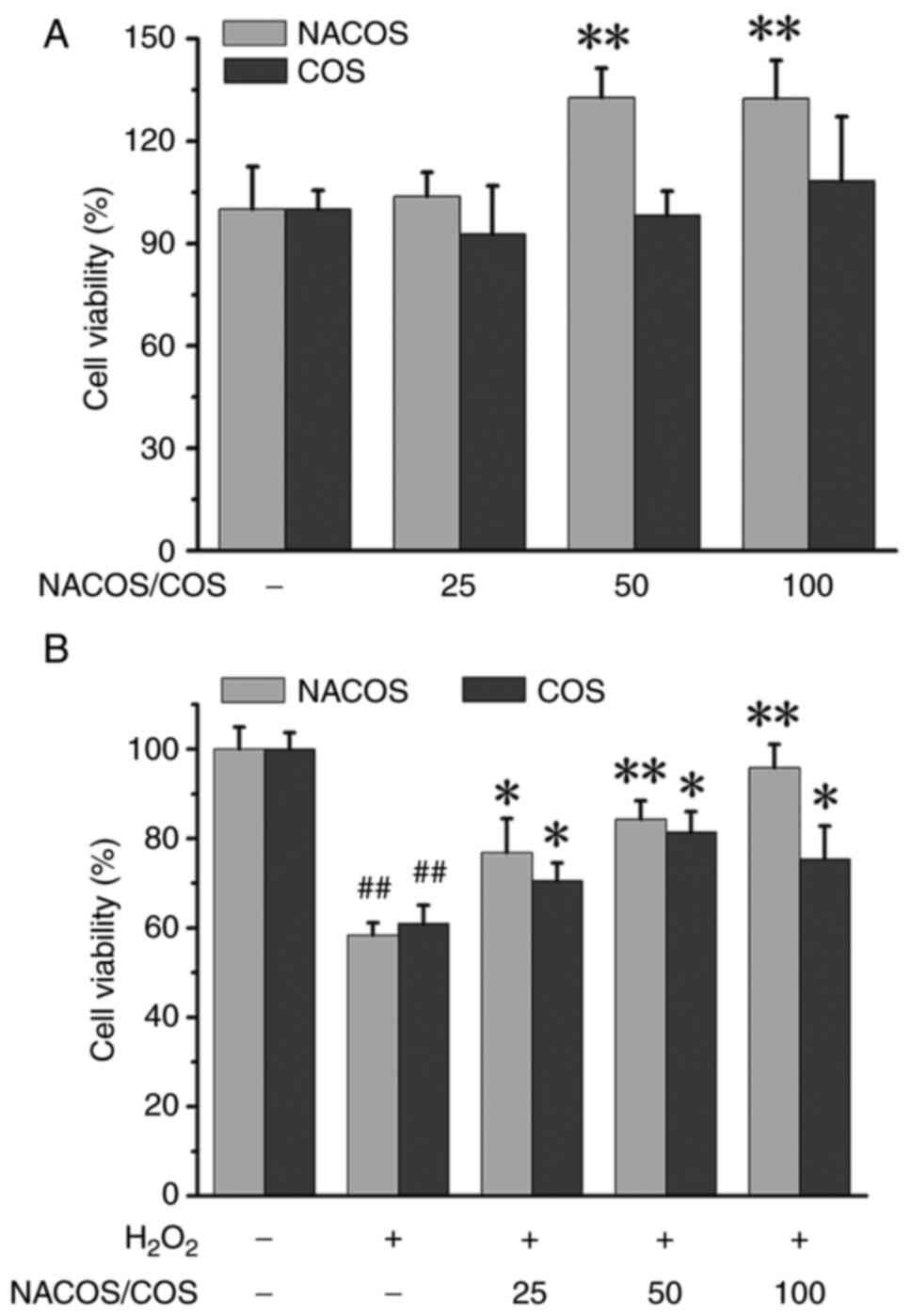
MTT assay was performed to assess the potential
cytotoxic effects of NACOS and COS treatments. The results of
liquid chromatography-mass spectrometry and nuclear magnetic
resonance analyses indicated that obtained COS had a deacetylation
degree of 88% and polymerization degree of 2-6, while NACOS had a
deacetylation degree of 97% and a polymerization degree of 3-10
(Fig. S1). COS (25, 50 or 100
µg/ml) for 24 h did not lead to a reduction in RAW264.7 cell
viability. By contrast, NACOS at 50 and 100 µg/ml remarkably
increased cell viability (Fig.
1A). Subsequently, the effects of NACOS and COS on the
viability of RAW264.7 cells was assessed following
H2O2-treatment. H2O2
led to a substantial decrease in cell viability, reaching
37.60±0.56% compared with untreated controls. However, NACOS and
COS treatment effectively attenuated the inhibitory effects of
H2O2 on cell viability. However, 100 µg/ml
COS had less effect than 50 µg/ml COS (Fig. 1B). NACOS and COS at 100 µg/ml
resulted in a significant increase compared with
H2O2 alone in terms of cell viability, with
values of 8.61±0.34 and 6.27±0.63%, respectively. This protective
effect of NACOS/COS may be associated with scavenging of oxygen
free radicals. Both NACOS and COS effectively scavenged oxygen free
radicals, albeit with varying scavenging abilities and impacts on
different types of oxygen free radicals, including ·OH,
·O2-, NO· and DPPH· (Table SI).
NACOS and COS protect against
H2O2-induced cellular apoptosis
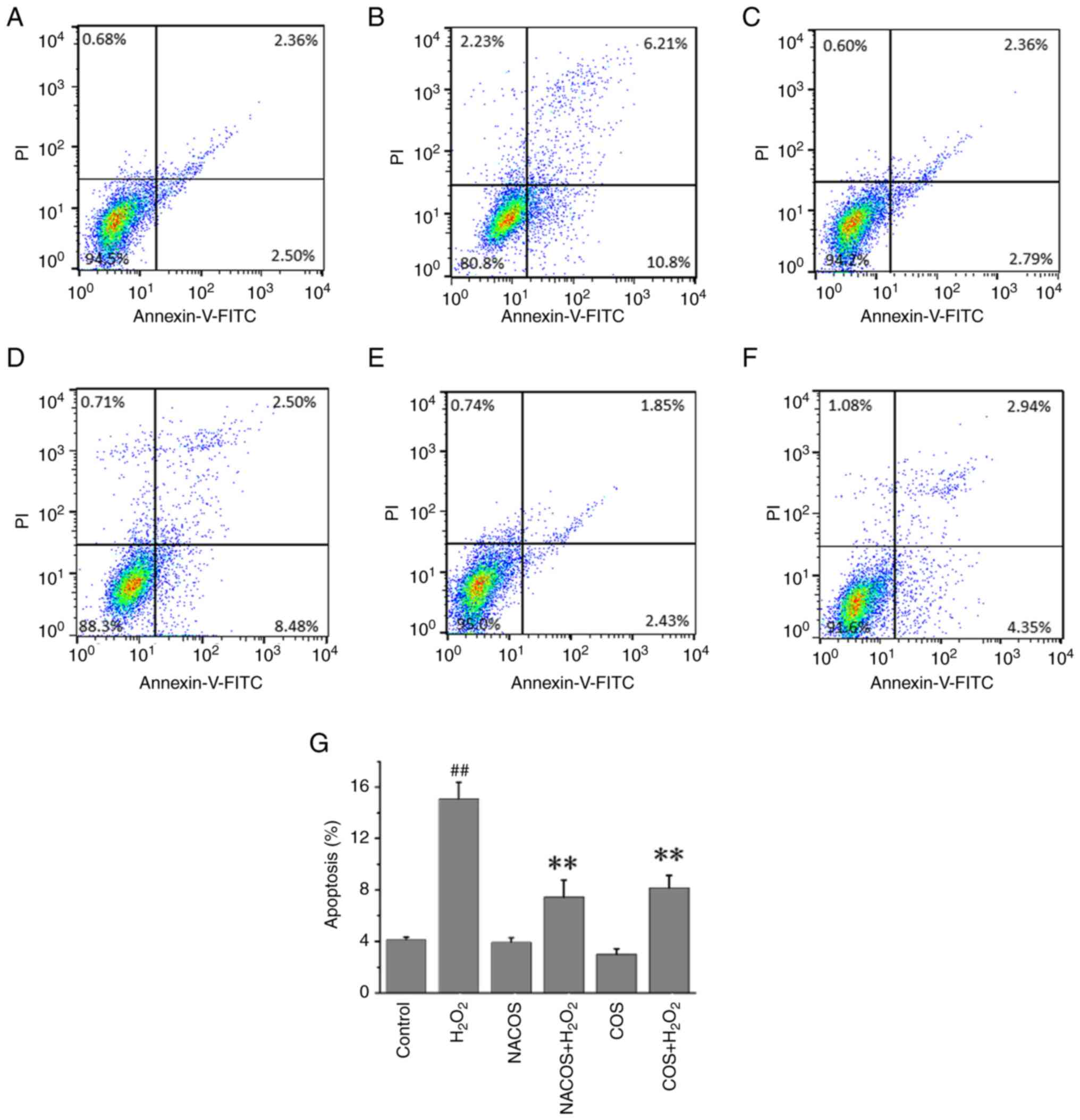
Flow cytometry showed that 15.74% of cells underwent
apoptosis following treatment with H2O2
alone, which was significantly higher than that of untreated
control cells. However, only 6.94 and 8.16% of cells that were
pretreated with COS or NACOS, respectively, (100 µg/ml) for 24 h
underwent apoptosis following H2O2 treatment,
showing a significant difference compared with
H2O2 alone (Fig.
2). NACOS and COS pretreatment protected against cellular
apoptosis induced by oxidative stress. In addition, NACOS and COS
decreased mRNA expression of IL-1β, IL-6, TNF-α and MCP-1 in
H2O2-stimulated RAW264.7 cells (Fig. S2).
Cell cycle changes following NACOS and
COS treatment
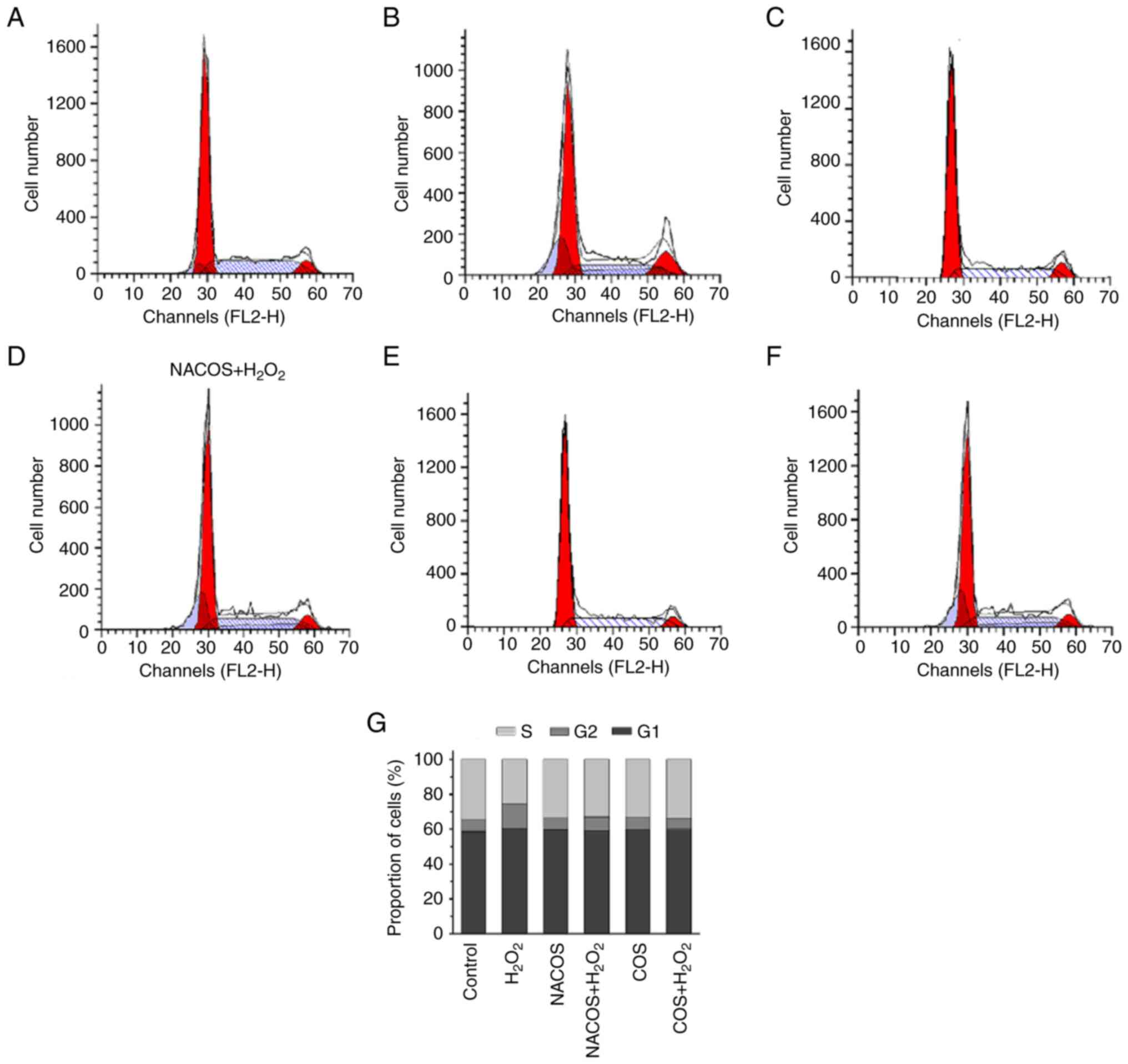
Effects of NACOS and COS treatment on
H2O2-induced G1/S phase arrest of RAW264.7
cells was assessed using FACS analysis. The results showed that COS
or NACOS alone had no significant effect on the proportion of cells
at different phases. The percentage of S phase cells was increased
by up to 10% following treatment with 100 µg/ml NACOS and COS
compared with H2O2 alone (Fig. 3), indicating that NACOS and COS
prevented H2O2-induced G1/S cell cycle
arrest.
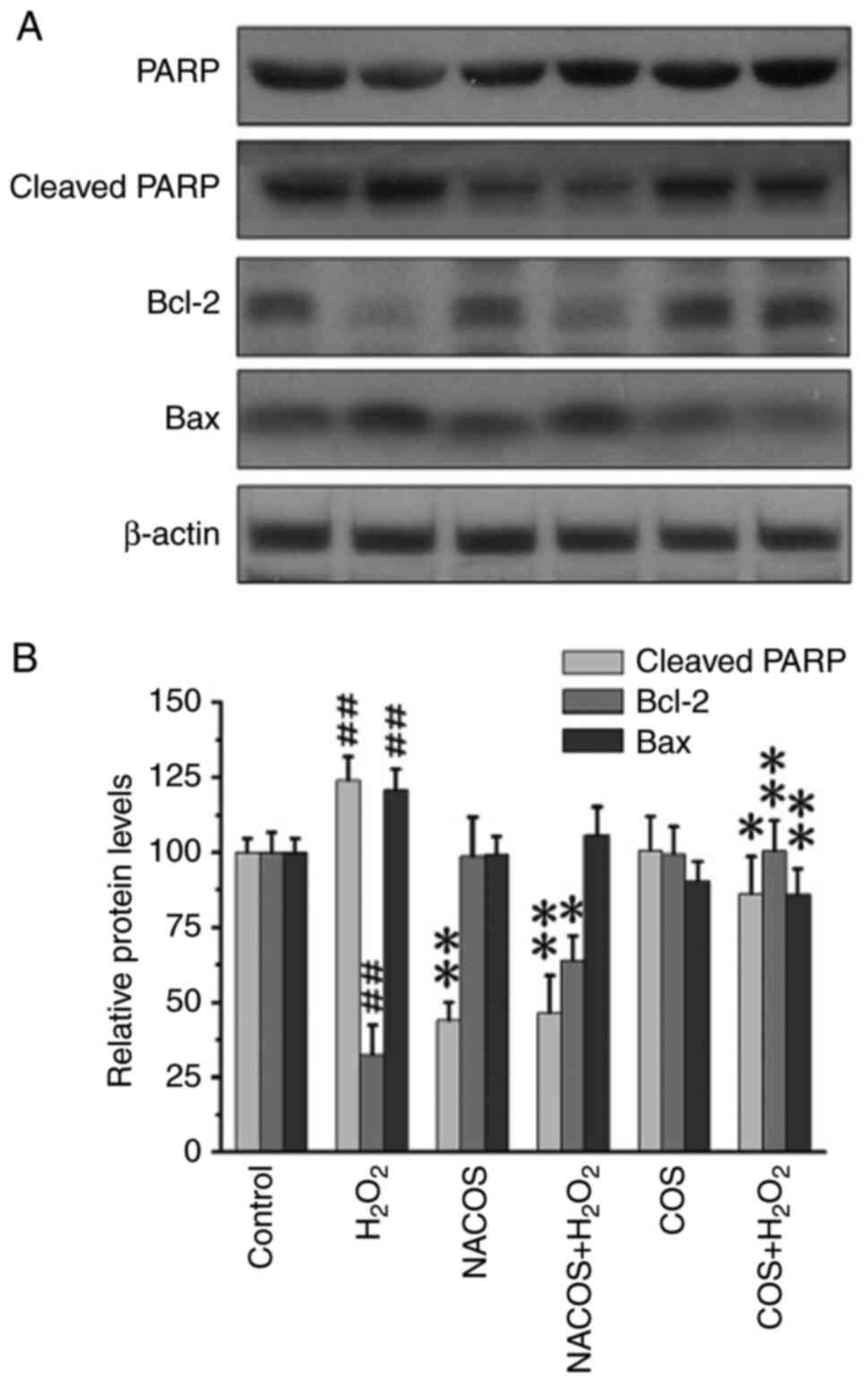
Effect of NACOS and COS on PARP
cleavage and Bcl-2 expression
PARP, a 116 kDa nuclear poly(ADP-ribose) polymerase
involved in DNA repair and cell proliferation, is be cleaved by
caspase-3, serving as an indicator of apoptotic cells (39). H2O2 is widely
recognized for inducing PARP cleavage (40). H2O2 treatment
increased the levels of cleaved PARP compared with untreated
control, confirming that H2O2 triggered
cellular apoptosis (Fig. 4).
However, pretreatment with COS and NACOS significantly inhibited
PARP cleavage, suggesting both COS and NACOS prevented
H2O2-induced apoptosis in RAW264.7 cells.
Moreover, the effect of NACOS in protecting against cellular
apoptosis was superior to that of COS. The rescue of cellular
apoptosis is typically associated with the upregulation of
anti-apoptotic molecules such as Bcl-2, which function in
antioxidant pathways to prevent apoptosis (41). Bax is also an apoptotic-induced
protein (42). COS and NACOS
upregulated Bcl-2 and downregulated Bax protein expression
following H2O2 treatment (Fig. 4). Moreover, NACOS showed a superior
protective effect compared with COS.
Discussion
The present study compared the antioxidant
activities of NACOS and COS against
H2O2-induced oxidative damage. NACOS and COS
effectively attenuated oxidative stress induced by
H2O2. Although COS did not significantly
affect cell viability, NACOS treatment significantly increased cell
viability. The improvement of cell viability by NACOS may be
associated to its antioxidant, antiapoptotic, cell proliferation
induction or cell cycle regulation effects, suggesting the need for
further investigation into the underlying mechanism. Furthermore,
both NACOS and COS significantly attenuated
H2O2-induced inhibition of cell viability in
a concentration-dependent manner. Cell cycle assay revealed that
NACOS and COS reversed H2O2-induced
inhibition of G1/S cell cycle arrest and significantly mitigated
H2O2-induced cell apoptosis. The protective
effects of NACOS and COS may be attributed to their ability to
scavenge oxygen free radicals, thereby preventing oxidative
damage.
Previous studies have suggested that COS exerts
potential free radical scavenging properties through both direct
and indirect mechanisms (43,44).
Here, both NACOS and COS effectively scavenged oxygen free
radicals, albeit with varying scavenging abilities and impacts on
different types of oxygen free radicals. Thus, both COS and NACOS
inhibited molecular damage by scavenging free radicals in
cells.
Furthermore, both NACOS and COS protected against
H2O2-induced apoptosis, as observed by flow
cytometry. Previous studies have also indicated that COS enhances
cell viability following H2O2 treatment and
decreases apoptosis rate (45,46).
In addition, COS increases extracellular matrix synthesis and
prevents its degradation (47).
Here, highly acetylated NACOS notably inhibited
H2O2-induced apoptosis. Furthermore, the
protective effect of NACOS was greater than that of COS. Previous
studies have indicated that degree of polymerization plays a
critical role in the neuroprotective effects of COS (47). β-amyloid (Aβ) aggregates containing
cross-β-sheet structures induce oxidative stress, neuroinflammation
and neuronal loss via multiple pathways. COS can directly bind to
Aβ42 in a polymerization-dependent manner, ameliorating
Aβ42-induced cytotoxicity (48).
Mitochondria serve as central integrators and
transducers of various pro-apoptotic signals, with their disruption
and subsequent release of pro-apoptotic proteins being a key aspect
of the apoptosis process (49,50).
The release of these factors from mitochondria is regulated by
Bcl-2 family proteins, which are key regulators for cell apoptosis
under both normal and oxidative stress conditions (41,51).
The present study showed that both NACOS and COS increased Bcl-2
expression and protected against H2O2-induced
apoptosis in RAW264.7 cells. Furthermore, activation of effector
caspases, such as caspase-3, leads to PARP cleavage, resulting in
apoptosis (52). Here, cleaved
PARP expression was ameliorated following NACOS and COS treatments
in RAW264.7 cells, with the effects of NACOS superior to those of
COS. These findings suggested that acetylation may be essential for
the protective effects of COS.
The present findings suggest that the anti-apoptotic
effect of COS and NACOS was associated with DA. Both COS and NACOS
were effective in inhibiting oxidative damage induced by
H2O2, with the antagonistic effect of NACOS
greater than that of COS. This implied that the degree of
deacetylation was positively associated with the protective effects
of COS. Acetylation has been reported to facilitate passive
diffusion of disaccharides across cell membranes, enabling them to
enter the Golgi (53). Thus,
acetylation modification may facilitate the entry of NACOS into
cells, allowing them to interfere with ROS-induced apoptosis;
however, the specific underlying mechanism requires further
investigation. DA may be a key factor influencing the ability of
COS to prevent oxidative damage-induced cell apoptosis. However,
functional gain or loss analysis experiments are needed. NACOS and
COS decreased mRNA expression of IL-1β, IL-6, TNF-α and MCP-1 in
H2O2 stimulated RAW264.7 cells and the
potential anti-inflammatory mechanisms of NACOS and COS may
associated with promoting protein O-GlcNA acylation, which
significantly inhibits the phosphorylation and nuclear
translocation of inflammatory transcription factors (54). However, the specific underlying
mechanism requires further investigation.
Supplementary Material
Mass spectrogram of COS and NACOS.
Liquid chromatography-mass spectrometry was used to determine the
acetylation degree and polymerization degree of COS and NACOS. A
represents N-Acetyl glucosamine and D represents glucosamine, while
the subsequent numbers represent the number of monosaccharides in
these predicted components. NACOS, N-acetylated chitosan
oligosaccharide; AU, arbitrary units.
Effect of NACOS and COS on mRNA levels
of inflammatory factors. Cells were pre-treated with NACOS or COS
(both 100 μg/ml) for 12 h and then exposed to
H2O2 (300 μM) for 12 h. mRNA
expression of IL-1β, IL-6, IL-8, TNF-α and MCP-1 was determined by
quantitative PCR analysis. N=3. *P<0.05 vs. control;
#P<0.05 vs. H2O2-alone. NACOS,
N-acetylated chitosan oligosaccharide; MCP-1, macrophage
chemoattractant protein-1.
Scavenging effects of NACOS and COS on
·OH, ·O2-, NO· and DPPH·.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Natural Science
Foundation of Fujian Province of China (grant no. 2021J05057).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
QL designed the study, analyzed data and wrote the
manuscript. QL, WS and YH performed the experiments. QL and WS
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Satitsri S and Muanprasat C: Chitin and
chitosan derivatives as biomaterial resources for biological and
biomedical applications. Molecules. 25(5961)2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Guan G, Azad MAK, Lin Y, Kim SW, Tian Y,
Liu G and Wang H: Biological effects and applications of chitosan
and chito-oligosaccharides. Front Physiol. 10(516)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shahbaz U: Chitin, characteristic,
sources, and biomedical application. Curr Pharm Biotechnol.
21:1433–1443. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wang W, Meng Q, Li Q, Liu J, Zhou M, Jin Z
and Zhao K: Chitosan derivatives and their application in
biomedicine. Int J Mol Sci. 21(487)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Naveed M, Phil L, Sohail M, Hasnat M, Baig
MMFA, Ihsan AU, Shumzaid M, Kakar MU, Mehmood Khan T, Akabar MD, et
al: Chitosan oligosaccharide (COS): An overview. Int J Biol
Macromol. 129:827–843. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fan Z, Wang L, Qin Y and Li P: Activity of
chitin/chitosan/chitosan oligosaccharide against plant pathogenic
nematodes and potential modes of application in agriculture: A
review. Carbohydr Polym. 306(120592)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zheng J, Cheng G, Li Q, Jiao S, Feng C,
Zhao X, Yin H, Du Y and Liu H: Chitin oligosaccharide modulates gut
microbiota and attenuates high-fat-diet-induced metabolic syndrome
in mice. Mar Drugs. 16(66)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yuan X, Zheng J, Jiao S, Cheng G, Feng C,
Du Y and Liu H: A review on the preparation of chitosan
oligosaccharides and application to human health, animal husbandry
and agricultural production. Carbohydr Polym. 220:60–70.
2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lan R, Chang Q, Wei L and Zhao Z: The
protect effects of chitosan oligosaccharides on intestinal
integrity by regulating oxidative status and inflammation under
oxidative stress. Mar Drugs. 19(57)2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Hao C, Wang W, Wang S, Zhang L and Guo Y:
An overview of the protective effects of chitosan and acetylated
chitosan oligosaccharides against neuronal disorders. Mar Drugs.
15(89)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zhai X, Yuan S, Yang X, Zou P, Shao Y, Abd
El-Aty AM, Hacımüftüoğlu A and Wang J: Growth-inhibition of S180
residual-tumor by combination of cyclophosphamide and chitosan
oligosaccharides in vivo. Life Sci. 202:21–27. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Shagdarova B, Konovalova M, Varlamov V and
Svirshchevskaya E: Anti-obesity effects of chitosan and its
derivatives. Polymers (Basel). 15(3967)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Hao C, Han M, Wang W, Yang C, Wang J, Guo
Y, Xu T, Zhang L and Li C: The neuroprotective effects of
peracetylated chitosan oligosaccharides against β-amyloid-induced
cognitive deficits in rats. Mar Life Sci Technol. 5:211–222.
2023.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Wang B, Wang L, Qu Y, Lu J and Xia W:
Chitosan oligosaccharides exert neuroprotective effects via
modulating the PI3K/Akt/Bcl-2 pathway in a Parkinsonian model. Food
Funct. 13:5838–5853. 2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
He N, Wang S, Lv Z, Zhao W and Li S: Low
molecular weight chitosan oligosaccharides (LMW-COSs) prevent
obesity-related metabolic abnormalities in association with the
modification of gut microbiota in high-fat diet (HFD)-fed mice.
Food Funct. 11:9947–9959. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Patoulias D, Stavropoulos K, Imprialos K,
Athyros V, Grassos H, Doumas M and Faselis C: Inflammatory markers
in cardiovascular disease; lessons learned and future perspectives.
Curr Vasc Pharmacol. 19:323–342. 2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yu Y, Luo T, Liu S, Song G, Han J, Wang Y,
Yao S, Feng L and Qin S: Chitosan oligosaccharides attenuate
atherosclerosis and decrease non-HDL in ApoE-/- mice. J Atheroscler
Thromb. 22:926–941. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yu Y, Wang S, Chen X, Gao Z, Dai K, Wang J
and Liu C: Sulfated oligosaccharide activates endothelial Notch for
inducing macrophage-associated arteriogenesis to treat ischemic
diseases. Proc Natl Acad Sci USA. 120(e2307480120)2023.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Guo C, Zhang Y, Ling T, Zhao C, Li Y, Geng
M, Gai S, Qi W, Luo X, Chen L, et al: Chitosan oligosaccharides
alleviate colitis by regulating intestinal microbiota and
PPARγ/SIRT1-Mediated NF-κB pathway. Mar Drugs.
20(96)2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Zhang P, Li T, Wu X, Nice EC, Huang C and
Zhang Y: Oxidative stress and diabetes: Antioxidative strategies.
Front Med. 14:583–600. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Batty M, Bennett MR and Yu E: The role of
oxidative stress in atherosclerosis. Cells. 11(3843)2022.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hayes JD, Dinkova-Kostova AT and Tew KD:
Oxidative stress in cancer. Cancer Cell. 38:167–197.
2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Jomova K, Raptova R, Alomar SY, Alwasel
SH, Nepovimova E, Kuca K and Valko M: Reactive oxygen species,
toxicity, oxidative stress, and antioxidants: Chronic diseases and
aging. Arch Toxicol. 97:2499–2574. 2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Sies H, Berndt C and Jones DP: Oxidative
stress. Annu Rev Biochem. 86:715–748. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Steven S, Frenis K, Oelze M, Kalinovic S,
Kuntic M, Bayo Jimenez MT, Vujacic-Mirski K, Helmstädter J,
Kröller-Schön S, Münzel T and Daiber A: Vascular inflammation and
oxidative stress: Major triggers for cardiovascular disease. Oxid
Med Cell Longev. 2019(7092151)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Senoner T and Dichtl W: Oxidative stress
in cardiovascular diseases: Still a therapeutic target? Nutrients.
11(2090)2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zhu H, Jia Z, Zhang L, Yamamoto M, Misra
HP, Trush MA and Li Y: Antioxidants and phase 2 enzymes in
macrophages: Regulation by Nrf2 signaling and protection against
oxidative and electrophilic stress. Exp Biol Med (Maywood).
233:463–474. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Grom AA and Mellins ED: Macrophage
activation syndrome: Advances towards understanding pathogenesis.
Curr Opin Rheumatol. 22:561–566. 2010.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li P, Hao Z, Wu J, Ma C, Xu Y, Li J, Lan
R, Zhu B, Ren P, Fan D and Sun S: Comparative proteomic analysis of
polarized human THP-1 and mouse RAW264.7 macrophages. Front
Immunol. 12(700009)2021.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mei QX, Hu JH, Huang ZH, Fan JJ, Huang CL,
Lu YY, Wang XP and Zeng Y: Pretreatment with chitosan
oligosaccharides attenuate experimental severe acute pancreatitis
via inhibiting oxidative stress and modulating intestinal
homeostasis. Acta Pharmacol Sin. 42:942–953. 2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Yang Z, Hong W, Zheng K, Feng J, Hu C, Tan
J, Zhong Z and Zheng Y: Chitosan oligosaccharides alleviate
H2O2-stimulated granulosa cell damage via
HIF-1α signaling pathway. Oxid Med Cell Longev.
2022(4247042)2022.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wu J, Xu Y, Geng Z, Zhou J, Xiong Q, Xu Z,
Li H and Han Y: Chitosan oligosaccharide alleviates renal fibrosis
through reducing oxidative stress damage and regulating
TGF-β1/Smads pathway. Sci Rep. 12(19160)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Wang Y and Xiong Y, Zhang A, Zhao N, Zhang
J, Zhao D, Yu Z, Xu N, Yin Y, Luan X and Xiong Y: Oligosaccharide
attenuates aging-related liver dysfunction by activating Nrf2
antioxidant signaling. Food Sci Nutr. 8:3872–3881. 2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Hao W, Li K, Ge X, Yang H, Xu C, Liu S, Yu
H, Li P and Xing R: The effect of N-acetylation on the
anti-inflammatory activity of chitooligosaccharides and its
potential for relieving endotoxemia. Int J Mol Sci.
23(8205)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Abd El-Hack ME, El-Saadony MT, Shafi ME,
Zabermawi NM, Arif M, Batiha GE, Khafaga AF, Abd El-Hakim YM and
Al-Sagheer AA: Antimicrobial and antioxidant properties of chitosan
and its derivatives and their applications: A review. Int J Biol
Macromol. 164:2726–2744. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zheng J, Yuan X, Cheng G, Jiao S, Feng C,
Zhao X, Yin H, Du Y and Liu H: Chitosan oligosaccharides improve
the disturbance in glucose metabolism and reverse the dysbiosis of
gut microbiota in diabetic mice. Carbohydr Polym. 190:77–86.
2018.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Xu Q, Liu M, Liu Q, Wang W, Du Y and Yin
H: The inhibition of LPS-induced inflammation in RAW264.7
macrophages via the PI3K/Akt pathway by highly N-acetylated
chitooligosaccharide. Carbohydr Polym. 174:1138–1143.
2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wang H, Ge W, Jiang W, Li D and Ju X:
SRPK1-siRNA suppresses K562 cell growth and induces apoptosis via
the PARP-caspase3 pathway. Mol Med Rep. 17:2070–2076.
2018.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Smith AJO, Ball SS, Bowater RP and
Wormstone IM: PARP-1 inhibition influences the oxidative stress
response of the human lens. Redox Biol. 8:354–362. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Singh R, Letai A and Sarosiek K:
Regulation of apoptosis in health and disease: The balancing act of
BCL-2 family proteins. Nat Rev Mol Cell Biol. 20:175–193.
2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Peña-Blanco A and García-Sáez AJ: Bax, Bak
and beyond-mitochondrial performance in apoptosis. FEBS J.
285:416–431. 2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Lan R, Chang Q, An L and Zhao Z: Dietary
supplementation with chitosan oligosaccharides alleviates oxidative
stress in rats challenged with hydrogen peroxide. Animals (Basel).
10(55)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Marmouzi I, Ezzat SM, Salama MM, Merghany
RM, Attar AM, El-Desoky AM and Mohamed SO: Recent updates in
pharmacological properties of chitooligosaccharides. Biomed Res
Int. 2019(4568039)2019.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Liu W, Liu Y, Li H and Rodgers GP:
Olfactomedin 4 contributes to hydrogen peroxide-induced NADPH
oxidase activation and apoptosis in mouse neutrophils. Am J Physiol
Cell Physiol. 315:C494–C501. 2018.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Zhang Y, Ahmad KA, Khan FU, Yan S, Ihsan
AU and Ding Q: Chitosan oligosaccharides prevent
doxorubicin-induced oxidative stress and cardiac apoptosis through
activating p38 and JNK MAPK mediated Nrf2/ARE pathway. Chem Biol
Interact. 305:54–65. 2019.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Jia P, Yu L, Tao C, Dai G, Zhang Z and Liu
S: Chitosan oligosaccharides protect nucleus pulposus cells from
hydrogen peroxide-induced apoptosis in a rat experimental model.
Biomed Pharmacother. 93:807–815. 2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Zhu L, Li R, Jiao S, Wei J, Yan Y, Wang
ZA, Li J and Du Y: Blood-brain barrier permeable chitosan
oligosaccharides interfere with β-amyloid aggregation and alleviate
β-amyloid protein mediated neurotoxicity and neuroinflammation in a
dose- and degree of polymerization-dependent manner. Mar Drugs.
18(488)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Abate M, Festa A, Falco M, Lombardi A,
Luce A, Grimaldi A, Zappavigna S, Sperlongano P, Irace C, Caraglia
M and Misso G: Mitochondria as playmakers of apoptosis, autophagy
and senescence. Semin Cell Dev Biol. 98:139–153. 2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Bock FJ and Tait SWG: Mitochondria as
multifaceted regulators of cell death. Nat Rev Mol Cell Biol.
21:85–100. 2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Zheng C, Liu T, Liu H and Wang J: Role of
BCL-2 family proteins in apoptosis and its regulation by nutrients.
Curr Protein Pept Sci. 21:799–806. 2020.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Henning RJ, Bourgeois M and Harbison RD:
Poly(ADP-ribose) polymerase (PARP) and PARP inhibitors: Mechanisms
of action and role in cardiovascular disorders. Cardiovasc Toxicol.
18:493–506. 2018.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Huang H, Ouyang Q, Mei K, Liu T, Sun Q,
Liu W and Liu R: Acetylation of SCFD1 regulates SNARE complex
formation and autophagosome-lysosome fusion. Autophagy. 19:189–203.
2023.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Dong X, Shu L, Zhang J, Yang X, Cheng X,
Zhao X, Qu W, Zhu Q, Shou Y, Peng G, et al: Ogt-mediated
O-GlcNAcylation inhibits astrocytes activation through modulating
NF-κB signaling pathway. J Neuroinflammation.
20(146)2023.PubMed/NCBI View Article : Google Scholar
|