Introduction
Obesity is characterized by the excessive
accumulation of body fat due to the imbalance between energy intake
and expenditure (1). Excessive
accumulation of body fat, leading to obesity, is attributed to an
increase in both the size and number of adipocytes differentiated
from preadipocytes (2). Obesity, a
precursor to various metabolic disorders such as type 2 diabetes,
cardiovascular diseases, non-alcoholic fatty liver disease and
cancer, is a global health concern owing its role in the etiology
of various conditions (3).
Furthermore, obesity leads to infectious diseases by weakening the
immune system, thereby increasing the risk of infection by foreign
pathogens and/or worsening the patient prognosis, ultimately
increasing the mortality rate (4,5).
Obese patients exhibited high infection and mortality rates during
the coronavirus disease-2019 (COVID-19) pandemic (6). Therefore, obesity treatment is
important for protection from such viral pandemics. To date,
numerous pharmaceuticals, such as orlistat, lorcaserin,
phentermine/topiramate and liraglutide, have been developed for
obesity treatment. However, these medications induce various side
effects, such as flatus with discharge, insomnia, dysgeusia, nausea
and headache, affecting their long-term use (7). Consequently, natural product-based
supplements without side effects have been extensively studied for
obesity treatment. Owing to increasing health consciousness,
natural substances are preferred for the development of
anti-obesity products (8).
Chrysosplenium flagelliferum (CF), belonging
to family Saxifragaceae, is a plant mainly found in the
Northern Hemisphere regions, such as Russia, China, Japan and South
Korea (9). CF is traditionally
used to treat inflammation (10).
Methanol extract of CF has been revealed to exert antioxidant and
antibacterial effects against Propionibacterium acnes
(10). However, the
pharmacological efficacy of CF and its potential therapeutic
effects remain largely unexplored. CF is listed as an approved food
ingredient by the Ministry of Food and Drug Safety of the Republic
of Korea, and no adverse effects associated with its use have been
reported to date. Therefore, the aim of the present study was to
assess the pharmacological efficacy of CF by evaluating its
anti-obesity and immunostimulatory effects and explore the
underlying mechanisms using a cell-based approach in vitro
to facilitate the development of new functional agents.
Materials and methods
Chemical reagents
Dexamethasone, 3-isobutyl-1-methylxanthine (IBMX),
insulin, Oil red O, PD98059 [extracellular signal-regulated kinase
(ERK)-1/2 inhibitor], SB203580 (p38 inhibitor), SP600125 [c-Jun
N-terminal kinase (JNK) inhibitor], C29 [Toll-like receptor (TLR)2
inhibitor], TAK-242 (TLR4 inhibitor) referred to as TAK hereafter
and BAY 11-7082 [BAY; nuclear factor (NF)-κB inhibitor] were
purchased from MilliporeSigma. Primary antibodies against the
peroxisome proliferator-activated receptor (PPAR)-γ (cat. no.
2435), CCAAT/enhancer-binding protein α (CEBPα; cat. no. 8178),
adipose triglyceride lipase (ATGL; cat. no. 2138),
hormone-sensitive lipase (HSL; cat. no. 4107), phosphorylated
(p)-HSL (cat. no. 4137), perilipin-1 (cat. no. 9349), AMP-activated
protein kinase, catalytic, α-1 (AMPKα also known as AMPK; cat. no.
5831), p-AMPKα (cat. no. 2535), and β-actin (cat. no. 5125), as
well as horseradish peroxidase-conjugated anti-rabbit (cat. no.
7074) and anti-mouse IgG (cat. no. 7076) secondary antibodies were
purchased from Cell Signaling Technology, Inc. The primary antibody
against PPARγ coactivator 1-α (PGC-1α; cat. no. sc-518025) was
purchased from Santa Cruz Biotechnology, Inc. Control small
interfering RNA (siRNA; cat. no. 6568) and β-catenin siRNA (cat.
no. sc-29210) were purchased from Cell Signaling Technology Inc.
and Santa Cruz Biotechnology, Inc., respectively.
Sample preparation
CF was obtained as a dehydrated powder from the
National Institute of Forest Science (Seoul, South Korea). To
prepare the extract, distilled water, with a volume 20-fold that of
CF, was added to 10 g of CF. This mixture underwent a 24-h
extraction process in a water bath at 60˚C. The resultant extract
was lyophilized, re-dissolved in distilled water, and subsequently
used in cellular assays.
Cell culture
RAW264.7 murine macrophages (cat. no. TIB-71) and
3T3-L1 mouse preadipocytes (cat. no. CL-173) were obtained from the
American Type Culture Collection. Culture of RAW264.7 cells was
conducted in a controlled CO2 incubator environment
(37˚C, 5% CO2) using Dulbecco's modified Eagle's
medium/nutrient mixture F-12 (DMEM/F-12) (cat. no. SH3023.01;
Cytiva), supplemented with 10% fetal bovine serum (FBS) (cat. no.
16000-044; Gibco; Thermo Fisher Scientific, Inc.) and a combination
of penicillin (100 U/ml) and streptomycin (100 µg/ml).
Additionally, 3T3-L1 cells were propagated under similar conditions
(37˚C, 5% CO2) in DMEM/F-12 medium supplemented with 10%
bovine calf serum (cat. no. 16170-078; Gibco; Thermo Fisher
Scientific, Inc.) and penicillin/streptomycin combination.
Measurement of the viability of
RAW264.7 and 3T3-L1 cells
Cell viability was assessed via
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay. RAW264.7 and 3T3-L1 cells (at >90% confluence in the
wells), following CF treatment (50-200 µg/ml), were incubated for
24 h and 8 days, respectively, in 96-well plates at 37˚C. Following
incubation, the cells were treated with the MTT reagent (1 mg/ml)
and incubated for 4 h at 37˚C. After the addition of dimethyl
sulfoxide, the cells were incubated for 10 min at room temperature.
Finally, absorbance of the resulting solution was measured at 570
nm using the UV/visible spectrophotometer (Xma-3000PC; Human
Corporation).
Differentiation of 3T3-L1 cells
A total of 2 days after reaching full confluence
[designated as day 0 (D0)] in a 6-well culture plate, 3T3-L1
preadipocytes were induced to differentiate. Briefly, the cells (at
100% confluence in the wells) were incubated in DMEM/F-12 enriched
with DMI cocktail (0.05 mM IBMX, 1 µM dexamethasone, and 10 µg/ml
insulin) until D2 at 37˚C. The cells were then cultured for 2 more
days in DMEM/F-12 supplemented with 10 µg/ml insulin until D4 at
37˚C. The culture continued from D6 to D8 at 37˚C, and the medium
was renewed every other day.
Transfection with siRNA
The 3T3-L1 cells (2x106 cells/well) were
plated in a 6-well plate and incubated overnight to achieve
adherence. Subsequently, the cells were transfected with the
control-siRNA (Cell Signaling Technology Inc.) and β-catenin-siRNA
(Santa Cruz Biotechnology, Inc.), each at a concentration of 100
nM. Transfection was performed for 48 h using the TransIT-TKO
transfection reagent (Mirus Bio), according to the manufacturer's
protocol. Control-siRNA was used as non-targeting siRNA for the
negative control. Immediately following the completion of siRNA
transfection, cell differentiation was initiated.
Oil red O staining of 3T3-L1
cells
Under differentiation, cells were treated with CF
for D0-D8, D0-D2, or D8-D10 and the concentration of CF used in the
experiments ranged from 50 to 200 µg/ml in the dose-dependent
studies, while it was set at 200 µg/ml in all other cases.
Following treatment, 3T3-L1 cells were fixed with 10% formalin at
an ambient temperature for 1 h. Following fixation, the cells were
rinsed thrice with distilled water and dehydrated using 60%
isopropanol for 5 min at room temperature. Fully dehydrated 3T3-L1
cells were subjected to Oil red O staining for 20 min at room
temperature for lipid droplet visualization. After staining, the
cells were rinsed twice with distilled water and examined under a
light microscope (Olympus Corporation) at a magnification of x400.
To quantify the accumulated lipid droplets, Oil red O was extracted
from the stained lipid droplets in completely dried cells using
100% isopropanol. Finally, absorbance was measured at 500 nm using
the microplate reader (Xma-3000PC).
Measurement of glycerol levels in
3T3-L1 cells
Upon reaching maximum confluency in a 6-well plate,
3T3-L1 cells (at 100% confluence in the wells) were cultured for an
additional 2 days. Post this period (D0), the cells were mixed with
DMI (0.05 mM IBMX, 1 µM dexamethasone and 10 µg/ml insulin) and
incubated for 2 days (D2). The 3T3-L1 cells were then exposed to
insulin (10 µg/ml) and cultured for another 2 days (D4). The cells
were further cultured for 4 days, with the medium refreshed every
other day (D6-D8). Following differentiation, the 3T3-L1 cells were
treated with CF (200 µg/ml) and were incubated for 2 days (D8-D10).
All the steps were performed at a constant temperature of 37˚C in a
5% CO2 environment. After 2 days of CF treatment (D10),
free glycerol content was evaluated using a Glycerol Cell-Based
Assay Kit (cat. no. 10011725; Cayman Chemical Company), according
to the manufacturer's instructions. Briefly, the cell culture
medium was mixed with the prepared free glycerol assay reagent at a
1:4 ratio and incubated at room temperature for 15 min.
Subsequently, absorbance was measured at 540 nm using the
microplate reader (Xma-3000PC).
Measurement of nitric oxide (NO)
levels in RAW264.7 cells
RAW264.7 cells (1x105 cells/well) were
propagated in a 96-well culture plate in DMEM/F-12 supplemented
with 10% FBS. The cells were exposed to CF for 24 h at 37˚C. In
addition, the cells were pretreated with C29 (100 µM, TLR2
inhibitor), TAK (5 µM, TLR4 inhibitor), PD (20 µM, ERK1/2
inhibior), SB (20 µM, p38 inhibitor), SP (20 µM, JNK inhibitor) or
BAY (10 µM, NF-κB inhibitor) at 37˚C for 2 h and then co-treated
with CF (200 µg/ml) at 37˚C for 24 h. To quantify NO levels, the
culture medium was mixed with the Griess reagent (cat. no.
G4410-10G; MilliporeSigma) and incubated for 15 min at ambient
temperature. Absorbance of the resulting mixture was measured at
540 nm using the UV/visible spectrophotometer (Xma-3000PC). The
concentration of CF used in the experiments ranged from 50 to 200
µg/ml in the dose-dependent studies, while it was set at 200 µg/ml
in all other cases.
Measurement of phagocytotic activity
in RAW264.7 cells
Phagocytic activity in RAW264.7 cells was assessed
using the neutral red uptake method. The cells (at over 90%
confluence in the wells) were cultured in DMEM/F-12 enriched with
10% FBS and treated with CF (50-200 µg/ml) at 37˚C for 24 h. In
addition, the cells were pretreated with C29 (100 µM, TLR2
inhibitor) or TAK (5 µM, TLR4 inhibitor) at 37˚C for 2 h and then
co-treated with CF (200 µg/ml) at 37˚C for 24 h. For phagocytic
assessment, the cells were stained with 0.01% neutral red at 37˚C
for 2 h and treated with a lysis solution (50:50 mixture of ethanol
and 1% acetic acid) to extract neutral red at room temperature.
Absorbance of the released dye was measured at 540 nm using the
UV/visible spectrophotometer (Xma-3000PC) to assess the extent of
phagocytic uptake.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
RAW264.7 cells (at >90% confluence in the wells)
were treated with CF for 24 h in the presence or absence of
chemical inhibitors. The concentration of CF used in the
experiments ranged from 50 to 200 µg/ml in the dose-dependent
studies, while it was set at 200 µg/ml in all other cases. Total
RNA was carefully extracted from RAW264.7 cells using the RNeasy
Mini Kit (Qiagen, Inc.). RNA (1 µg) was subsequently
reverse-transcribed into cDNA using the Verso cDNA Kit (Thermo
Fisher Scientific Inc.). RT-PCR analysis was performed targeting
inducible NO synthase (iNOS), interleukin (IL)-1β, IL-6, tumor
necrosis factor (TNF)-α, and glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) using the prepared cDNA and PCR Master Mix
Kit (Promega Corporation). All primers used for amplification are
listed in Table I. PCR cycles (30
in total) were executed in a PCR Thermal Cycler Dice (Takara Bio,
Inc.) as follows: Denaturation at 94˚C for 30 sec, annealing at
55˚C for 1 min, and extension at 72˚C for 1 min. The amplified
products were electrophoresed on a 1% agarose gel at 100 V for 15
min. The DNA bands on the agarose gel were visualized using Safe
Shine Green (10,000X) (cat. no. G6051; Biosesang). Band intensities
were quantitatively analyzed using the UN-SCAN-IT 5.1 software
(Silk Scientific, Inc). GAPDH was used as a loading control for
normalization in RT-PCR analysis.
 | Table ISequences of primers used in the
amplification of cDNA. |
Table I
Sequences of primers used in the
amplification of cDNA.
| Primers | Sequences |
|---|
| iNOS | Forward,
5'-TTGTGCATCGACCTAGGCTG GAA-3' |
| | Reverse,
5'-GACCTTTCGCATTAGCATGGA AGC-3' |
| IL-1β | Forward,
5'-GGCAGGCAGTATCACTCATT-3' |
| | Reverse,
5'-CCCAAGGCCACAGGTATTT-3' |
| IL-6 | Forward,
5'-GAGGATACCACTCCCAACAG ACC-3' |
| | Reverse,
5'-AAGTGCATCATCGTTGTTCAT ACA-3' |
| TNF-α | Forward,
5'-TGGAACTGGCAGAAGAGGCA-3' |
| | Reverse,
5'-TGCTCCTCCACTTGGTGGTT-3' |
| GAPDH | Forward,
5'-GGACTGTGGTCATGAGCCCTT CCA-3' |
| | Reverse,
5'-ACTCACGGCAAATTCAACGG CAC-3' |
Western blot analysis
Under differentiation, 3T3-L1 cells were treated
with CF for D0-D8, D0-D2, D0-D4 or D8-D10. The concentration of CF
used in the experiments ranged from 100 to 200 µg/ml in the
dose-dependent studies, while it was set at 200 µg/ml in all other
cases. RAW264.7 cells were treated with CF (200 µg/ml) for 1-6 h in
the absence of TAK (5 µM) or were treated with CF (200 µg/ml) for 3
h in the presence of TAK (5 µM) at 37˚C. After washing with
phosphate-buffered saline, proteins were extracted from RAW264.7
and 3T3-L1 cells using a radioimmunoprecipitation assay buffer
(cat. no. BP-115DG; Boston BioProducts, Inc.). The resulting
lysates were centrifuged at 4˚C and 25,200 x g for 30 min. Protein
concentrations were quantified using the bicinchoninic acid assay
kit (Thermo Fisher Scientific, Inc.). For electrophoresis, 30 µg of
protein from each sample was loaded per well, resolved via 10%
sodium dodecyl sulfate-polyacrylamide gel electrophoresis and
transferred to a nitrocellulose membrane (Thermo Fisher Scientific,
Inc.). Membranes were blocked with 5% skimmed milk for 1 h at room
temperature and incubated overnight with primary antibodies in a 5%
bovine serum albumin (BSA) solution (1:1,000 dilution) at 4˚C.
Subsequently, the membranes were incubated with 5% BSA solution
with secondary antibodies (1:1,000 dilution) for 1 h at room
temperature. An ECL Select™ Western Blotting Detection Reagent
(cat. no. RPN2232; Cytiva) was then added to visualize the
horseradish peroxidase activity. Protein bands were visualized
using the LI-COR C-DiGit Blot Scanner (LI-COR Biosciences) and
quantitatively analyzed using UN-SCAN-IT gel software version 5.1
(Silk Scientific, Inc.). Actin was used as a loading control for
normalization in western blot analysis.
Statistical analysis
All experiments were repeated at least three times.
Statistical analyses were conducted using GraphPad Prism v.5.0
(GraphPad; Dotmatics), and data are presented as the mean ±
standard deviation. All data were analyzed using one-way analysis
of variance, followed by Bonferroni's post-hoc test. P#x003C;0.05
was considered to indicate a statistically significant
difference.
Results
Effect of CF on lipid droplet
accumulation in 3T3-L1 cells
To investigate whether CF inhibits lipid droplet
accumulation in differentiated 3T3-L1 cells, the differentiation of
3T3-L1 cells was induced using DMI and insulin while administering
CF from D0 to D8. Subsequently, the extent of lipid formation and
triglyceride accumulation was analyzed (Fig. 1A). As illustrated in Fig. 1B and C, CF significantly reduced the
accumulation of lipid droplets and triglycerides in differentiated
3T3-L1 cells. To ascertain whether the inhibition of lipid droplets
and triglyceride accumulation mediated by CF is due to its
cytotoxic effects on 3T3-L1 cells, the impact of CF on cellular
viability was evaluated. CF exerted no detrimental effect on 3T3-L1
cell survival (Fig. 1D).
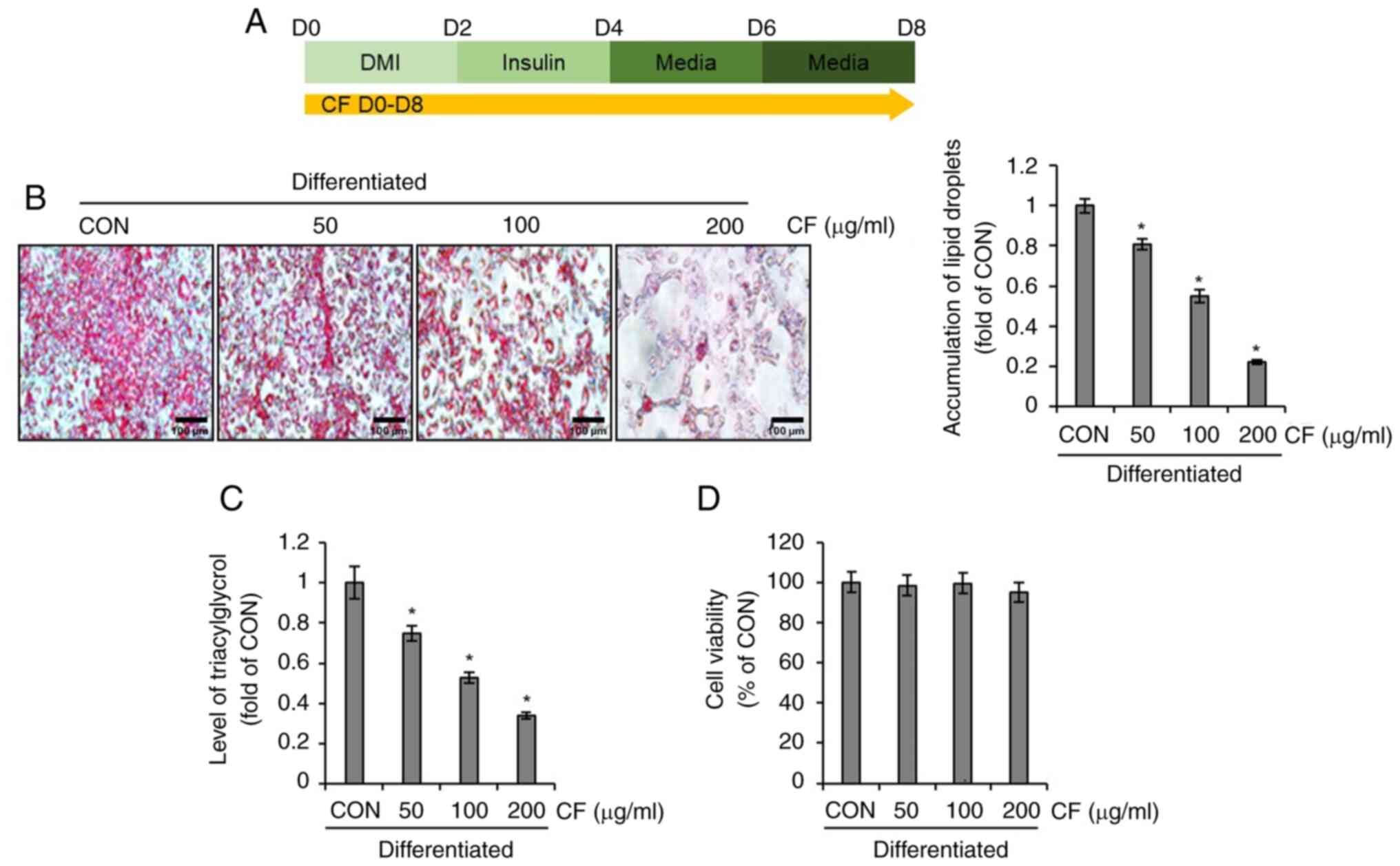 | Figure 1Effect of CF on the accumulation of
lipid droplets and triacylglycerol in 3T3-L1 cells. CF was
administered to 3T3-L1 cells undergoing differentiation induced by
DMI and insulin, from D0 to D8. Subsequently, the extent of lipid
accumulation, the content of triacylglycerol and cell viability
were measured. (A) Experimental design. (B) Oil Red O staining
(magnification, x400), (C) triacylglycerol content and (D) cell
viability in CF-treated 3T3-L1 cells. *P#x003C;0.05 vs.
CON. CF, Chrysosplenium flagelliferum; DMI, dexamethasone,
3-isobutyl-1-methylxanthine, insulin; D, day; CON, control. |
Effect of CF on the adipogenic
differentiation of 3T3-L1 cells
To evaluate whether CF inhibits adipogenic
differentiation in 3T3-L1 cells, differentiation was induced by the
use of DMI and insulin while administering CF from D0 to D8.
Subsequently, western blot analysis was conducted to determine the
expression levels of adipogenic differentiation-related molecules,
PPARγ and CEBPα. At 100 µg/ml, CF did not decrease the protein
levels of PPARγ and CEBPα. However, at 200 µg/ml, CF significantly
reduced the protein levels of PPARγ and CEBPα (Fig. 2A). Considering the necessity of
PPARγ and CEBPα for the differentiation of preadipocytes into
adipocytes, which is marked by the accumulation of lipid droplets
(11,12), it was investigated whether CF
reduces the protein levels of PPARγ and CEBPα during this critical
differentiation stage. First, the differentiation of preadipocytes
into adipocytes was induced using DMI while administering CF from
D0 to D2. Subsequently, western blot analysis was conducted to
assess the alterations in the protein levels of PPARγ and CEBPα
induced by CF. CF significantly reduced the protein levels of PPARγ
and CEBPα during the differentiation from preadipocytes to
adipocytes (Fig. 2B). To determine
whether the inhibition of preadipocyte differentiation to
adipocytes by CF results in decreased lipid droplet accumulation,
the extent of lipid accumulation was compared after treatment with
CF from D0 to D8 and D0 to D2. As revealed in Fig. 2C, a notable reduction in lipid
accumulation was observed in the 3T3-L1 cells treated with CF from
D0 to D2 or from D0 to D8. Furthermore, the reduction of the
accumulation in the D0 to D8 group was greater than that in the D0
to D2 group.
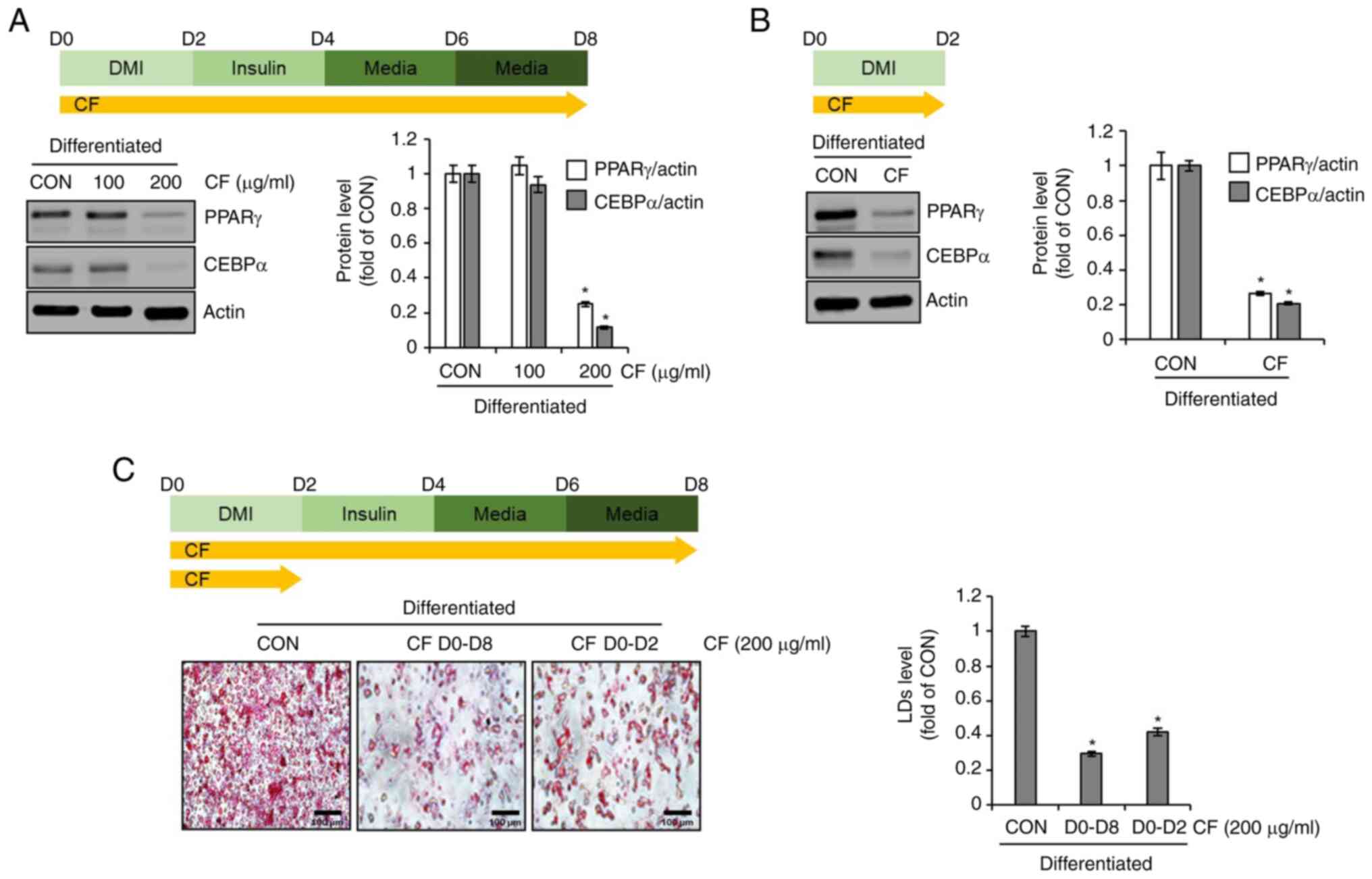 | Figure 2Effect of CF on the expression of
adipogenic markers in 3T3-L1 cells. (A) CF was administered to
3T3-L1 cells undergoing differentiation induced by DMI and insulin,
from D0 to D8. Experimental design and western blot analysis of
PPARγ and CEBPα in 3T3-L1 cells treated with CF (D0-D8). (B) CF
(200 µg/ml) was administered to 3T3-L1 cells undergoing
differentiation induced by DMI, from D0 to D2. Experimental design
and western blot analysis of PPARγ and CEBPα in 3T3-L1 cells
treated with CF (D0-D2). (C) CF was administered to 3T3-L1 cells
undergoing differentiation induced by DMI and insulin, from D0 to
D8 or from D0 to D2. Experimental design and Oil Red O staining
(magnification, x400) in 3T3-L1 cells treated with CF (D0-D8 or
D0-D2). *P#x003C;0.05 vs. CON. CF, Chrysosplenium
flagelliferum; D, day; DMI, dexamethasone,
3-isobutyl-1-methylxanthine, insulin; PPARγ, peroxisome
proliferator-activated receptor gamma; CEBPα,
CCAAT/enhancer-binding protein α; CON, control; LDs, lipid
droplets. |
Effect of β-catenin on CF-mediated
inhibition of adipogenic differentiation in 3T3-L1 cells
To investigate the impact of CF on the protein
levels of β-catenin in 3T3-L1 cells, CF was administered from D0 to
D2 and from D0 to D4. Subsequently, western blot analysis was used
to assess the alterations in the protein levels of β-catenin
induced by CF. As illustrated in Fig.
3A, CF increased the protein levels of β-catenin in a
time-dependent manner. To further ascertain the impact of increased
β-catenin protein levels induced by CF on the protein levels of
PPARγ and CEBPα, the changes in PPARγ and CEBPα protein levels were
analyzed in 3T3-L1 cells after β-catenin knockdown using the
β-catenin siRNA. Western blot analysis revealed that CF
significantly reduced the protein levels of PPARγ and CEBPα in
3T3-L1 cells without β-catenin knockdown (Fig. 3B). However, in 3T3-L1 cells with
β-catenin knockdown, CF significantly decreased the protein levels
of PPARγ and CEBPα, but to a more modest degree than the cells
without β-catenin knockdown (Fig.
3B). To further evaluate whether the inhibition of the
CF-mediated decrease in the protein levels of PPARγ and CEBPα due
to β-catenin knockdown affects the CF-mediated suppression of lipid
droplet accumulation, β-catenin-knockdown 3T3-L1 cells were treated
with CF from D0 to D2 and lipid droplet accumulation was analyzed
on D8. As revealed in Fig. 3C,
treatment of cells without β-catenin knockdown with CF resulted in
a notable decrease in lipid droplet accumulation. However, in cells
with β-catenin knockdown, the CF-mediated inhibitory effect on
lipid droplet accumulation was significantly diminished.
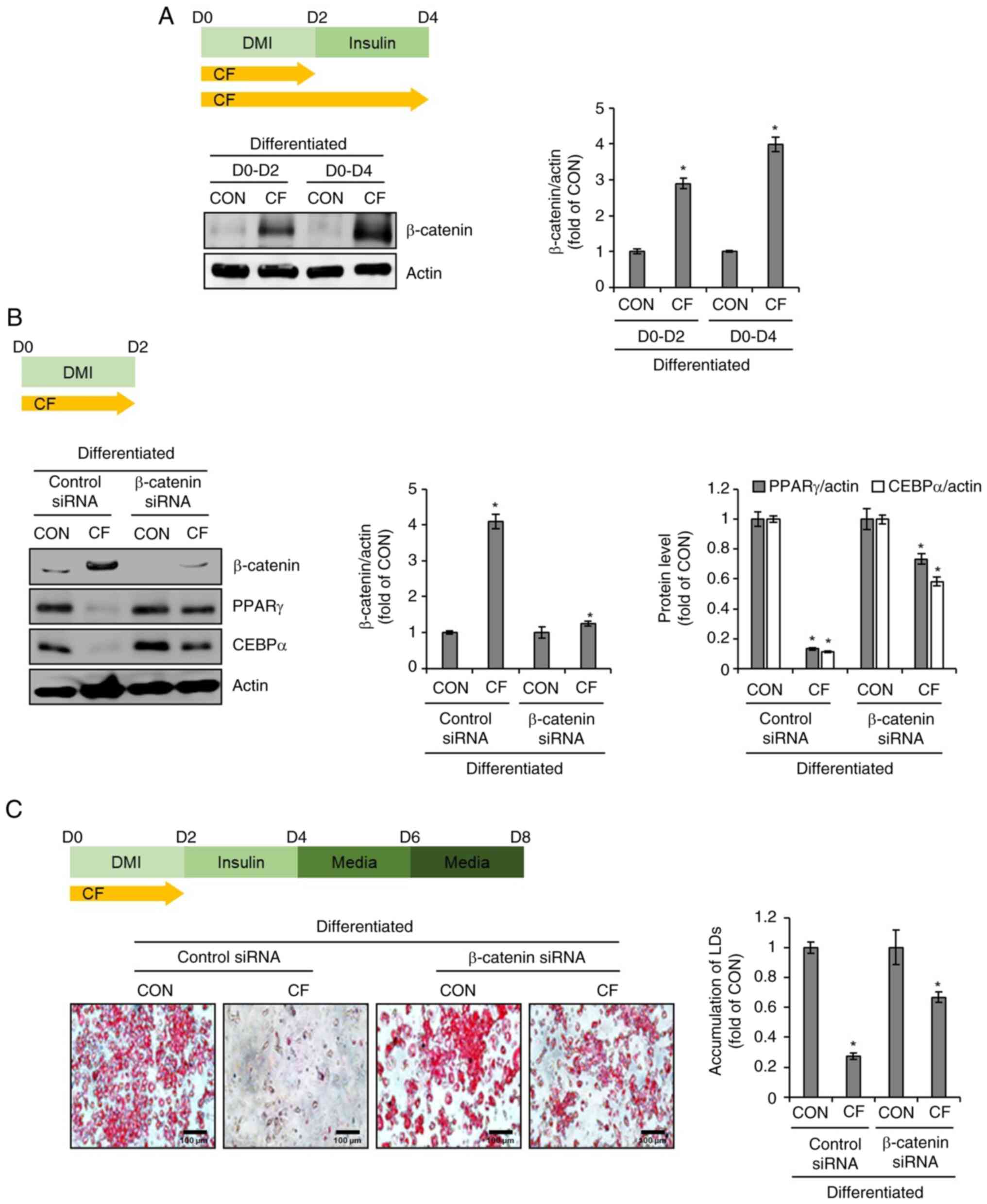 | Figure 3Effect of CF on β-catenin expression
in 3T3-L1 cells. (A) CF (200 µg/ml) was administered to 3T3-L1
cells undergoing differentiation induced by DMI and insulin, from
D0 to D2 or D0 to D4. Experimental design and western blot analysis
of β-catenin in 3T3-L1 cells treated with CF (D0-D2 or D0-D4). (B)
CF (200 µg/ml) was administered to β-catenin-knock downed 3T3-L1
cells undergoing differentiation induced by DMI and insulin, from
D0 to D2. Experimental design and western blot analysis of PPARγ
and CEBPα in β-catenin-knockdown 3T3-L1 cells treated with CF
(D0-D2). (C) CF (200 µg/ml) was administered to β-catenin-knockdown
3T3-L1 cells undergoing differentiation induced by DMI and insulin,
from D0 to D2. Experimental design and Oil Red O staining
(magnification, x400) in β-catenin-knockdown 3T3-L1 cells treated
with CF (D0-D2). *P#x003C;0.05 vs. CON. CF,
Chrysosplenium flagelliferum; D, day; DMI, dexamethasone,
3-isobutyl-1-methylxanthine, insulin; CON, control; siRNA, small
interfering RNA; PPARγ, peroxisome proliferator-activated receptor
gamma; CEBPα, CCAAT/enhancer-binding protein α; LDs, lipid
droplets. |
Effects of CF on lipolysis and
thermogenesis in 3T3-L1 cells
To explore the effects of CF on lipolysis and
thermogenesis in 3T3-L1 cells, the cells were treated with CF from
D0 to D8 during the differentiation process. Subsequently, western
blotting was conducted to assess the changes in protein levels of
lipolysis-related molecules, such as ATGL, p-HSL, HSL, perilipin-1
and thermogenesis-related molecules, such as p-AMPK, AMPK and
PGC-1α. As illustrated in Fig. 4A,
3T3-L1 cells treated with CF exhibited a concentration-dependent
increase in ATGL and the ratio of p-HSL to HSL, whereas perilipin-1
levels decreased. Additionally, CF treatment led to an increase in
the ratio of p-AMPK to AMPK and PGC-1α. Thus, to determine whether
the induction of lipolysis and thermogenesis mediated by CF
contributes to the reduction in accumulated lipid droplets, the
cells were treated with CF from D8 to D10, whereas lipid
accumulation was induced without CF treatment from D0 to D8.
Subsequently, the changes in lipid accumulation, glycerol release
and protein levels of molecules related to lipolysis and
thermogenesis were analyzed. As depicted in Fig. 4B, treatment of cells with complete
lipid accumulation with CF resulted in a decrease in accumulated
lipids and an increase in glycerol levels. Furthermore, CF
treatment led to an increase in the levels of lipolysis-related
molecules, such as ATGL and p-HSL, and a decrease in perilipin-1
(Fig. 4B). Additionally, CF
enhanced the levels of thermogenesis-related molecules, including
p-AMPK and PGC-1α (Fig. 4B).
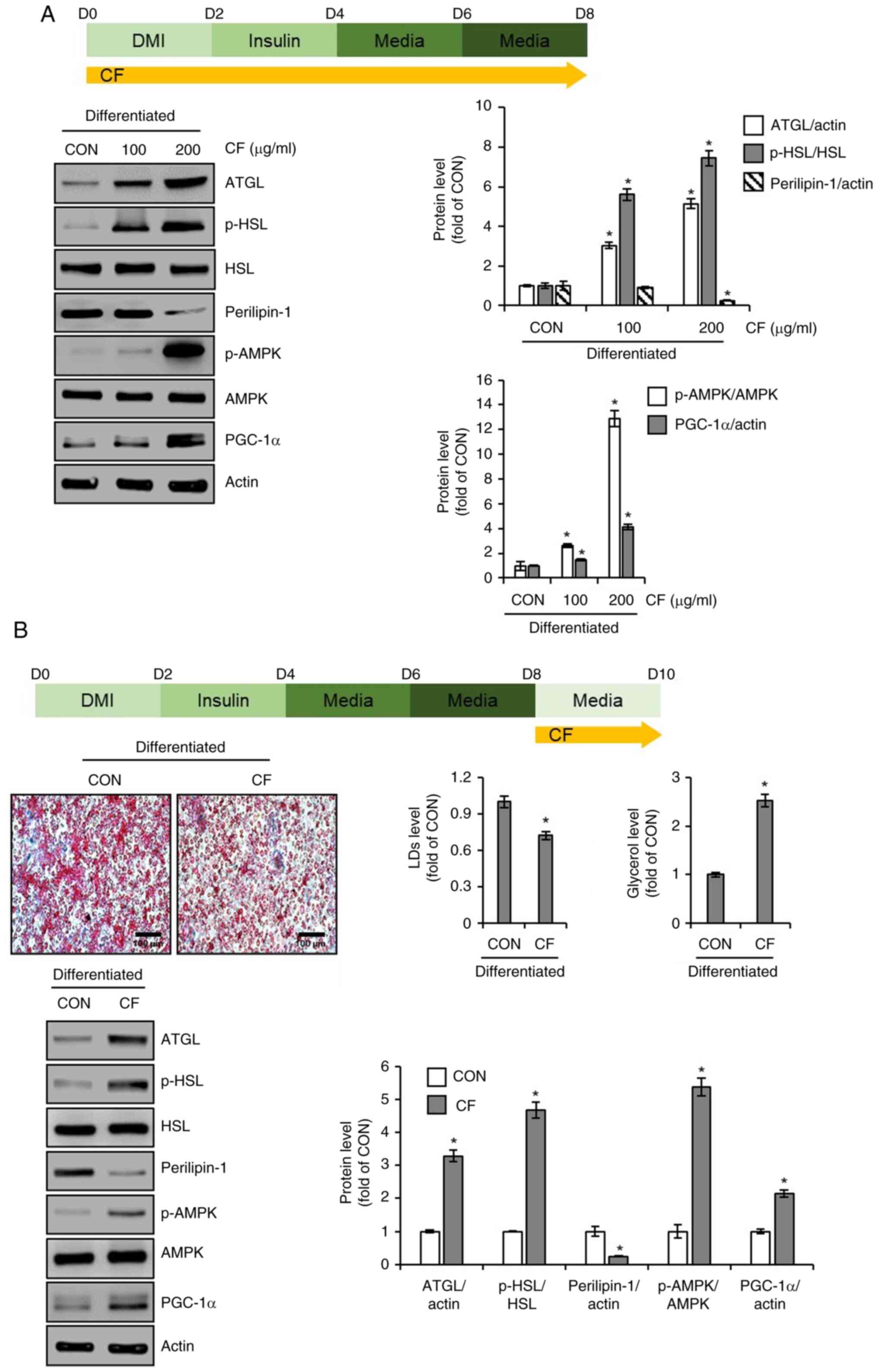 | Figure 4Effect of CF on the expression of
lipolytic and thermogenic markers in 3T3-L1 cells. (A) CF (200
µg/ml) was administered to 3T3-L1 cells undergoing differentiation
induced by DMI and insulin, from D0 to D8. Experimental design and
western blot analysis of ATGL, p-HSL, HSL, perilipin-1, p-AMPK,
AMPK, and PGC-1α in 3T3-L1 cells treated with CF (D0-D8). (B) CF
(200 µg/ml) was administered to differentiated 3T3-L1 by DMI and
insulin, from D8 to D10. Experimental design, Oil Red O staining
(magnification, x400), glycerol level, and western blot analysis of
ATGL, p-HSL, perilipin-1, p-AMPK, and PGC-1α in 3T3-L1 cells
treated with CF (D8-D10). *P#x003C;0.05 vs. CON. CF,
Chrysosplenium flagelliferum; DMI, dexamethasone,
3-isobutyl-1-methylxanthine, insulin; D, day; ATGL, adipose
triglyceride lipase; p-, phosphorylated; HSL, hormone-sensitive
lipase; AMPK, AMP-activated protein kinase; PGC-1α, peroxisome
proliferator-activated receptor-γ coactivator 1α; CON, control. |
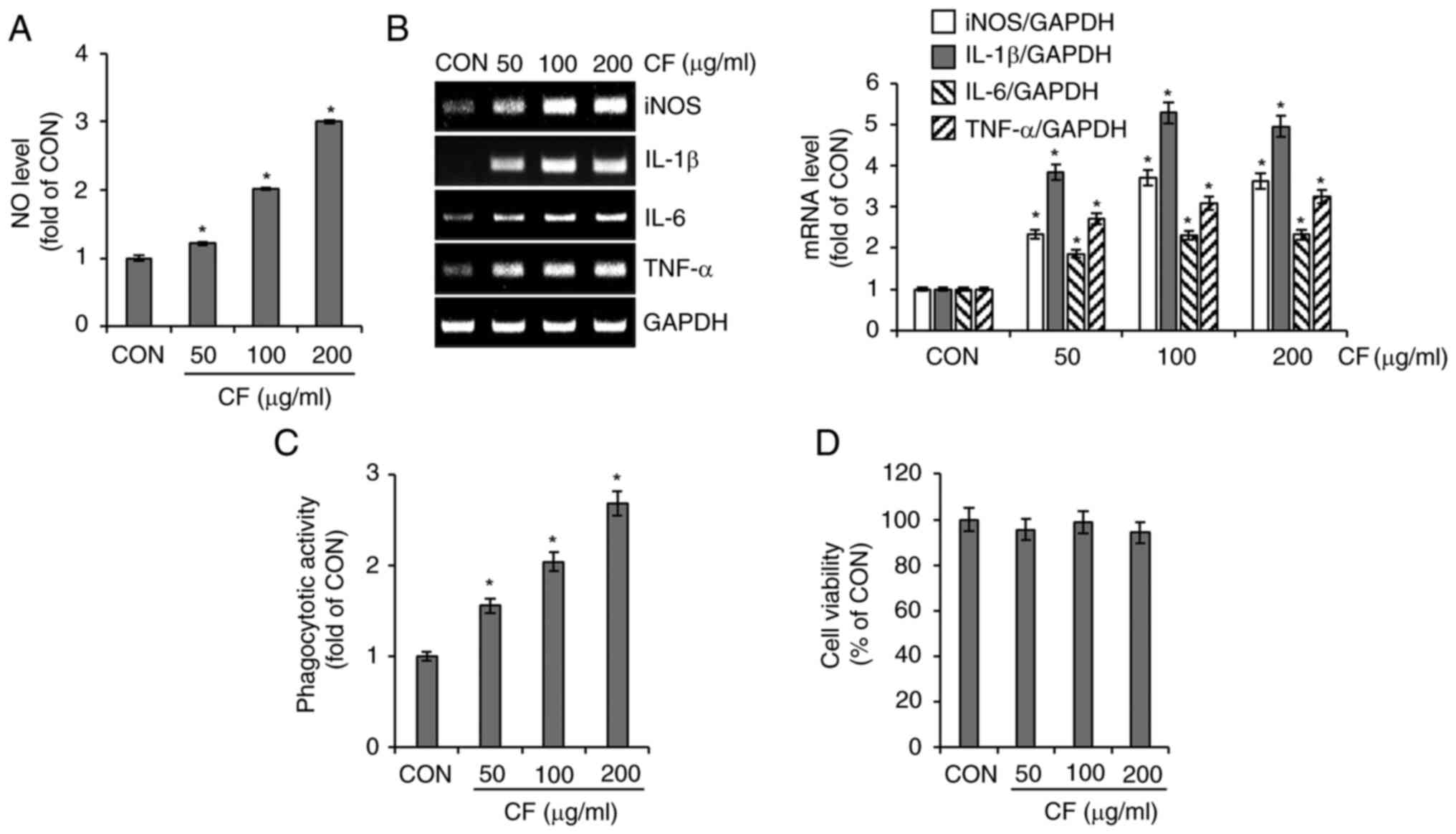
Effect of CF on macrophage activation
in RAW264.7 cells
To investigate the immunostimulatory activity of CF,
its effect on macrophage activation was analyzed in RAW264.7 cells.
As shown in Fig. 5A and B, cells treated with CF exhibited a
significant increase in the secretion of NO and the expression of
iNOS, IL-1β, IL-6 and TNF-α. Furthermore, CF significantly enhanced
the phagocytic activity of RAW264.7 cells (Fig. 5C). However, CF did not exhibit
cytotoxic effects on RAW264.7 cells (Fig. 5D).
Effects of TLR2 and TLR4 on
CF-mediated activation of RAW264.7 macrophages
To evaluate the impact of TLR2 and TLR4 on
CF-mediated activation of macrophages, RAW264.7 cells were treated
with CF, where TLR2 and TLR4 were inhibited using C29sp16 and TAK,
respectively. Subsequently, the changes in the levels of NO, the
expression of iNOS, IL-1β and TNF-α, as well as alterations in
phagocytic activity were analyzed. As illustrated in Fig. 6A, compared with untreated cells
(CON group), cells treated solely with CF (DM group) exhibited a
notable increase in NO production, whereas the inhibition of TLR2
by C29 only slightly suppressed CF-mediated NO production. However,
the inhibition of TLR4 by TAK resulted in an almost complete
absence of CF-mediated NO production. The expression of iNOS, IL-1β
and TNF-α mediated by CF was slightly decreased by the inhibition
of TLR2 but significantly reduced by the inhibition of TLR4
(Fig. 6B). Furthermore, it was
observed that the activation of phagocytic activity in RAW264.7
cells mediated by CF was more substantially reduced by the
inhibition of TLR4 than by the inhibition of TLR2 (Fig. 6C).
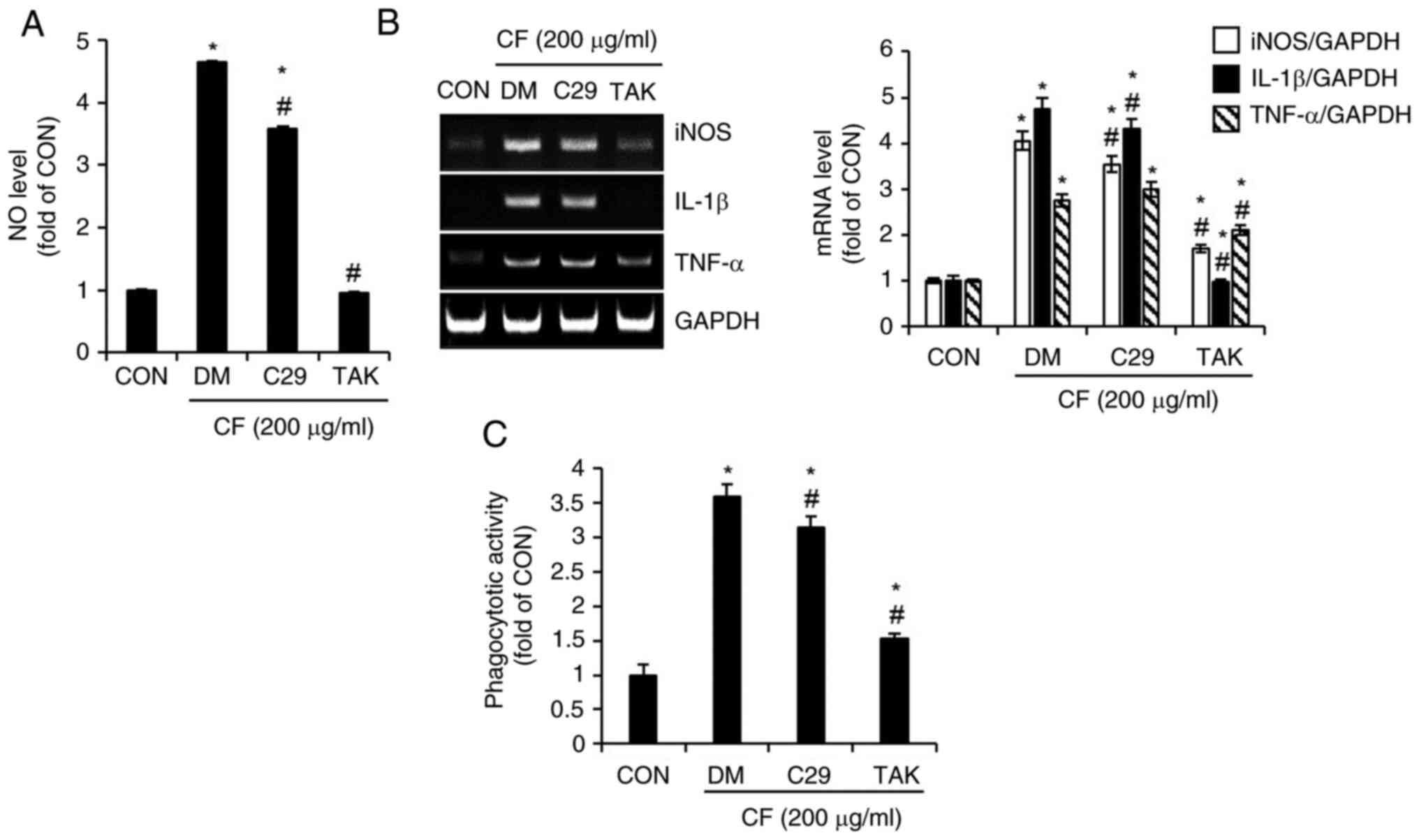 | Figure 6Effect of TLR2 and TLR4 on
CF-mediated activation of macrophages in RAW264.7 cells. RAW264.7
cells were pretreated with C29 (TLR2 inhibitor, 100 µM) or TAK
(TLR4 inhibitor, 5 µM) for 2 h and then co-treated with CF (200
µg/ml) for 24 h. (A) The level of NO, (B) reverse transcription PCR
analysis of iNOS, IL-1β and TNF-α, and (C) phagocytotic activity in
RAW264.7 cells treated with CF for 24 h in the presence of C29 or
TAK. *P#x003C;0.05 vs. CON. #P#x003C;0.05 vs.
DM. TLR, toll-like receptor; CF, Chrysosplenium
flagelliferum; TAK, TAK-242; NO, nitric oxide; iNOS, inducible
nitric oxide synthase; IL-1β, interleukin-1β; TNF-α, tumor necrosis
factor-α; CON, control; DM, DMSO group. |
Effects of mitogen-activated protein
kinase (MAPK) and NF-κB signaling pathways on CF-mediated
activation of RAW264.7 macrophages
To investigate the effects of MAPK and NF-κB
signaling pathways on CF-mediated activation of macrophages,
RAW264.7 cells were treated with CF and with the inhibitors of each
signaling pathway. Subsequently, the changes in NO production and
the expression of iNOS, IL-1β and TNF-α were examined. As
demonstrated in Fig. 7A, the
inhibition of ERK1/2 by PD had no effect on CF-mediated NO
production. However, the inhibition of p38 by SB and the inhibition
of JNK by SP both suppressed CF-mediated NO production, with the
suppression due to JNK inhibition being particularly pronounced.
Therefore, the impact of JNK inhibition by SP on the expression of
iNOS, IL-1β and TNF-α mediated by CF was analyzed. The results
revealed that the inhibition of JNK significantly decreased the
CF-mediated expression of iNOS, IL-1β and TNF-α (Fig. 7B). Furthermore, it was confirmed
that the inhibition of NF-κB by BAY also resulted in a significant
decrease in the CF-mediated production of NO and the expression of
iNOS, IL-1β and TNF-α (Fig. 7C).
Thus, it was then investigated whether CF activated the JNK and
NF-κB signaling pathways. The results indicated that CF increases
the phosphorylation of JNK and p65, which are the active forms of
JNK and NF-κB signaling, respectively (Fig. 7D). As the activation of macrophages
mediated by CF primarily occurs via TLR4 stimulation, it was
analyzed whether the activation of JNK and NF-κB by CF is
influenced by TLR4. Inhibition of TLR4 by TAK significantly
suppressed the CF-mediated phosphorylation of JNK and p65 (Fig. 7E).
 | Figure 7Effect of MAPK and NF-κB signaling
pathways on CF-mediated activation of macrophages in RAW264.7
cells. (A) RAW264.7 cells were pretreated with PD (ERK1/2
inhibitor, 20 µM), SB (p38 inhibitor, 20 µM) or SP (JNK inhibitor,
20 µM) and then co-treated with CF (200 µg/ml) for 24 h. The level
of NO and (B) RT-PCR analysis of iNOS, IL-1β and TNF-α in RAW264.7
cells treated with CF in the presence of PD, SB or SP. (C) RAW264.7
cells were pretreated with BAY (NF-κB inhibitor, 10 µM), and then
co-treated with CF (200 µg/ml) for 24 h. The level of NO and RT-PCR
analysis of iNOS, IL-1β, and TNF-α in RAW264.7 cells treated with
CF for 24 h in the presence of BAY. (D) RAW264.7 cells were treated
with CF (200 µM) for the indicated time-points. Western blot
analysis of p-JNK, JNK, p-p65 and p65 in RAW264.7 cells treated
with CF. (E) RAW264.7 cells were pretreated with TAK (TLR4
inhibitor, 5 µM) for 2 h and the co-treated with CF (200 µM) for 3
h. Western blot analysis of p-JNK, JNK, p-p65, and p65 in RAW264.7
cells treated with CF in the presence of TAK.
*P#x003C;0.05 vs. CON. #P#x003C;0.05 vs. DM.
CF, Chrysosplenium flagelliferum; PD, PD98059; SB, SB203580;
SP, SP600125; NO, nitric oxide; iNOS, inducible nitric oxide
synthase; IL-1β, interleukin-1β; TNF-α, tumor necrosis factor-α;
BAY, BAY11-7082; p-, phosphorylated; JNK, c-Jun N-terminal kinase;
TAK, TAK-242; CON, control; DM, DMSO group. |
Discussion
In the human body, excess energy is stored in
adipocytes in the form of lipids via a process that is a hallmark
of obesity (11). Obesity is
characterized by the enlargement of existing adipocytes and
increase in the number of new adipocytes due to preadipocyte
differentiation (11). To
investigate the potential anti-obesity effects of CF, the changes
in lipid accumulation within adipocytes after CF treatment were
examined in the present study. A significant reduction in lipid
accumulation in the adipocytes treated with CF was observed.
However, CF did not exert any adverse effects on adipocyte
viability. These findings suggested the anti-obesity activity of CF
and highlighted its potential as a safe and effective agent to
combat obesity. Lack of a detrimental effect on adipocyte viability
further underscored its suitability as a therapeutic agent for
obesity management. Adipogenesis, differentiation of preadipocytes
into mature adipocytes, plays a key role in increasing the number
of lipid-storing adipocytes (11).
Consequently, adipogenesis inhibition has been used as a molecular
target for the discovery of new anti-obesity agents, underlining
its significance in obesity research and treatment (11). In the present study, to evaluate
the inhibitory effect of CF on adipogenesis, preadipocytes
undergoing differentiation into adipocytes were treated with CF.
Post-treatment, western blot analysis was employed to assess the
expression changes of PPARγ and C/EBPα, key regulators instrumental
in the activation of adipogenesis (12). PPARγ has been demonstrated to be
essential for both adipogenesis and the maintenance of adipocyte
phenotype, as evidenced through in vivo and ex vivo
studies (13-16).
Similarly, C/EBPα has been proven to be crucial for the
differentiation and maintenance of white adipose tissue, also
validated through in vivo and ex vivo experiments
(17-20).
These existing reports collectively indicate that the suppression
of PPARγ and C/EBPα expression can effectively inhibit
adipogenesis. In the present study, it was revealed that CF
inhibits the expression of PPARγ and C/EBPα, which in turn leads to
the suppression of lipid accumulation within adipocytes. These
results demonstrate that the anti-obesity activity of CF may
primarily operate through the suppression of adipogenesis, mediated
by the inhibition of PPARγ and C/EBPα expression. Activation of the
Wnt signaling pathway inhibits the expression of PPARγ and C/EBPα,
thereby maintaining preadipocytes in an undifferentiated state and
suppressing adipogenesis. Conversely, inhibition of Wnt signaling
induces adipogenic differentiation (21). During adipogenic differentiation,
the degradation of β-catenin leads to an increase in the expression
of PPARγ and C/EBPα. However, an elevation in β-catenin levels,
even under conditions favoring adipogenic differentiation, results
in decreased expression of PPARγ and C/EBPα, thereby inhibiting
adipogenesis (22). Therefore, to
determine whether the CF-induced decrease in PPARγ and C/EBPα is
associated with Wnt signaling, adipocytes undergoing adipogenic
differentiation were treated with CF. Subsequently, western blot
analysis was conducted to assess the changes in the levels of
β-catenin protein. The results revealed that CF treatment led to an
increase in the levels of β-catenin protein. Furthermore, in
adipocytes where β-catenin was knocked down by siRNA, the
CF-mediated decrease in PPARγ and C/EBPα was notably attenuated.
This observation also led to the discovery that such β-catenin
knockdown by siRNA consequently reduced the CF-mediated inhibition
of lipid accumulation. These results indicated that CF inhibits
adipogenesis through the activation of Wnt signaling, leading to
the suppression of PPARγ and C/EBPα expression. However, a
limitation of this study is the inability to ascertain whether the
CF-mediated increase in β-catenin protein levels is due to the
inhibition of proteasomal degradation or a result of
transcriptional activation. Consequently, further mechanistic
studies are required to elucidate the specific pathway involved in
the CF-mediated elevation of β-catenin protein levels. It has been
reported that the most ideal mechanism for anti-obesity treatments
encompasses the augmentation of lipolysis and the enhancement of
energy expenditure (23).
Lipolysis is a catabolic reaction in which triacylglycerol stored
in adipocytes is hydrolyzed to glycerol and fatty acids (24). During lipolysis, triacylglycerol is
progressively hydrolyzed to diacylglycerol, monoacylglycerol, and
glycerol by a series of lipolytic enzymes (25). ATGL catalyzes the hydrolysis of
triacylglycerol to diacylglycerol, which is subsequently hydrolyzed
to monoacylglycerol by HSL (26).
As ATGL and HSL account for >90% of the triacylglycerol
hydrolysis (26), they are
considered pivotal indicators of lipolysis (27-29).
Furthermore, perilipin-1, which encircles lipid droplets within
adipocytes, has been revealed to play a role in lipolysis (30,31).
Perilipin-1 was shown to inhibit lipolysis while concurrently
promoting lipid synthesis and lipid droplet formation (32). Furthermore, perilipin-1 was
demonstrated to lead to increased lipolysis, resulting in a
reduction in the size of lipid droplets within adipocytes (33). In the present study, CF increased
the protein levels of ATGL and p-HSL in adipocytes, while
concurrently reducing the protein levels of perilipin-1. Upon
treatment of lipid-laden adipocytes with CF, a reduction in the
number of lipid droplets and an increase in glycerol content were
observed. These findings indicated that CF can induce lipolysis,
suggesting that its lipolytic action may contribute to the
inhibition of lipid accumulation. Lipolysis in white adipose tissue
is essential for thermogenesis (34). Furthermore, it has been reported
that the browning of white adipose tissue can facilitate
thermogenesis, thereby promoting energy expenditure (35). AMPK, a critical metabolic sensor
and regulator of energy balance, induces browning of white adipose
tissue (36). PGC-1α, known as a
master transcriptional coactivator in mitochondrial biogenesis, is
widely used as a key indicator for the differentiation of white
adipose tissue into brown adipocytes (37). In the present study, it was
confirmed that CF increases the protein levels of p-AMPK and PGC-1α
in adipocytes. Although the current study did not analyze the
impact of CF on the protein levels of uncoupling protein-1 and PR
domain containing 16, closely associated with the browning of white
adipocytes, which presents a limitation, the observed increase in
the protein levels of p-AMPK and PGC-1α by CF can be considered
evidence suggesting that CF may induce the browning of white
adipocytes.
Macrophages maintain homeostasis by defending
against foreign pathogens, processing internal waste and
facilitating tissue repair (38).
When foreign pathogens invade the human body, activated
macrophages, as a key component of the innate immune system,
phagocytize these invaders and secrete pro-inflammatory mediators,
such as NO, iNOS, IL-1β, IL-6 and TNF-α (38). Furthermore, macrophages possess the
ability to present antigens to adaptive immune cells, such as T and
B cells, and pro-inflammatory mediators secreted by macrophages
contribute to the activation of these T and B cells (39). In conclusion, these studies may
serve as evidence suggesting that the activation of macrophages can
play a positive role in both innate and adaptive immune responses.
In the present study, it was confirmed that CF enhances the
production of pro-inflammatory mediators, such as NO, iNOS, IL-1β,
IL-6 and TNF-α, in macrophages and activates their phagocytic
activity. These findings provided evidence that CF induces
macrophage activation. Although this study did not elucidate the
relationship between CF-mediated activation of macrophages and
adaptive immune cells, such as T and B cells, which is a
limitation, existing research that macrophage activation can induce
T and B cell activation (39)
suggests that CF may contribute to the activation of these cells
through its role in macrophage activation. The inflammatory
response of macrophages to eliminate foreign pathogens initiates
upon the recognition of these pathogens by macrophages (38). Macrophages recognize
pathogen-associated molecular patterns of foreign pathogens via
pattern recognition receptors (PRRs) (38). Among the various PRRs in
macrophages, TLRs play a pivotal role in the recognition of foreign
pathogens (38). TLR2 and TLR4
play crucial roles in the elimination of foreign pathogens by
inducing the production of proinflammatory mediators and activating
phagocytic activity in macrophages (40). In the present study, it was
observed that inhibition of TLR4 in cells resulted in a significant
decrease in CF-mediated pro-inflammatory mediator production and
phagocytic activation compared with cells with inhibition of TLR2.
Although the present study did not fully analyze the relationship
between CF-mediated macrophage activation and all PRRs present in
macrophages, which is a limitation, the findings suggested that
TLR4 plays a central role in the activation of macrophages mediated
by CF. TLR4-mediated macrophage activation involves the signaling
pathways of NF-κB and MAPK (41).
In the current study, it was observed that the inhibition of JNK, a
component of the MAPK signaling pathway, and NF-κB notably reduced
the production of CF-mediated pro-inflammatory mediators, and the
inhibition of TLR4 suppressed CF-mediated activation of JNK and
NF-κB. These findings may serve as evidence that CF-mediated
macrophage activation can be attributed to the activation of the
TLR4-dependent JNK and NF-κB signaling pathways.
In the present study, it was found that CF inhibited
adipogenesis and induced lipolysis and thermogenesis, thereby
suppressing excessive lipid accumulation in adipocytes.
Additionally, CF stimulated macrophage activation via
TLR4-dependent activation of the JNK and NF-κB signaling pathways.
These findings indicated that CF exerts anti-obesity and
immunostimulatory effects. Combined with the previously reported
antioxidant and antimicrobial effects, the anti-obesity and
immunostimulatory effects demonstrated in the present study expand
the known pharmacological activity spectrum of CF. However, this
study primarily relied on in vitro cellular assays and
lacked in vivo experiments using animal models, representing
a major limitation. Therefore, future in vivo studies using
animal models are necessary to verify these findings and facilitate
the development of new functional materials for the anti-obesity
and immunostimulatory applications of CF.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the National Institute
of Forest Science, Korea (grant no. FP0802-2023-01-2023), the
R&D Program for Forest Science Technology (grant no.
RS-2024-00405196) provided by the Korea Forest Service (Korea
Forestry Promotion Institute, Seoul, Korea).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JWC, GHP, HJC, JWL, HYK and MYC performed cell-based
experiments and analyzed the data. JWC and GHP wrote the
manuscript. JBJ designed the experiments and wrote and edited the
manuscript. JWC, GHP, HJC, JWL, HYK, MYC and JBJ confirm the
authenticity of all the raw data. All the authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jin H, Han H, Song G, Oh HJ and Lee BY:
Anti-obesity effects of GABA in C57BL/6J mice with high-fat
diet-induced obesity and 3T3-L1 adipocytes. Int J Mol Sci.
25(995)2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kang SA and Yu HS: Anti-obesity effects by
parasitic nematode (Trichinella spiralis) total lysates.
Front Cell Infect Microbiol. 13(1285584)2024.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Shi Q, Wang Y, Hao Q, Vandvik PO, Guyatt
G, Li J, Chen Z, Xu S, Shen Y, Ge L, et al: Pharmacotherapy for
adults with overweight and obesity: A systematic review and network
meta-analysis of randomised controlled trials. Lancet. 399:259–269.
2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Huttunen R and Syrjänen J: Obesity and the
outcome of infection. Lancet Infect Dis. 10:442–443.
2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Muscogiuri G, Pugliese G, Laudisio D,
Castellucci B, Barrea L, Savastano S and Colao A: The impact of
obesity on immune response to infection: Plausible mechanisms and
outcomes. Obes Rev. 22(e13216)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nour TY and Altintaş KH: Effect of the
COVID-19 pandemic on obesity and its risk factors: A systematic
review. BMC Public Health. 23(1018)2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tak YJ and Lee SY: Anti-obesity drugs:
Long-term efficacy and safety: An updated review. World J Mens
Health. 39:208–221. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sun NN, Wu TY and Chau CF: Natural dietary
and herbal products in anti-obesity treatment. Molecules.
21(1351)2016.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yan WJ, Yang TG, Liao R, Wu ZH, Qin R and
Liu H: Complete chloroplast genome sequence of Chrysosplenium
macrophyllum and Chrysosplenium flagelliferum
(Saxifragaceae). Mitochondrial DNA B Resour. 5:2040–2041.
2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Choi HA, Ahn SO, Lim HD and Kim GJ: Growth
suppression of a gingivitis and skin pathogen Cutibacterium
(Propionibacterium) acnes by medicinal plant extracts.
Antibiotics (Basel). 10(1092)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jakab J, Miškić B, Mikšić S, Juranić B,
Ćosić V, Schwarz D and Včev A: Adipogenesis as a potential
anti-obesity target: A review of pharmacological treatment and
natural products. Diabetes Metab Syndr Obes. 14:67–83.
2021.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Madsen MS, Siersbaek R, Boergesen M,
Nielsen R and Mandrup S: Peroxisome proliferator-activated receptor
γ and C/EBPα synergistically activate key metabolic adipocyte genes
by assisted loading. Mol Cell Biol. 34:939–954. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Barak Y, Nelson M, Ong E, Jones Y,
Ruiz-Lozano P, Chien K, Koder A and Evans R: PPAR gamma is required
for placental, cardiac, and adipose tissue development. Mol Cell.
4:585–595. 1999.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rosen E, Sarraf P, Troy A, Bradwin G,
Moore K, Milstone D, Spiegelman B and Mortensen R: PPAR gamma is
required for the differentiation of adipose tissue in vivo and in
vitro. Mol Cell. 4:611–617. 1999.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Duan SZ, Ivashchenko CY, Whitesall SE,
D'Alecy LG, Duquaine DC, Brosius FC III, Gonzalez F, Vinson C,
Pierre MA, Milstone DS and Mortensen RM: Hypotension,
lipodystrophy, and insulin resistance in generalized
PPARgamma-deficient mice rescued from embryonic lethality. J Clin
Invest. 117:812–822. 2007.PubMed/NCBI View
Article : Google Scholar
|
|
16
|
Imai T, Takakuwa R, Marchand S, Dentz E,
Bornert JM, Messaddeq N, Wendling O, Mark M, Desvergne B, Wahli W,
et al: Peroxisome proliferator-activated receptor gamma is required
in mature white and brown adipocytes for their survival in the
mouse. Proc Natl Acad Sci USA. 101:4543–4547. 2004.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Linhart HG, Ishimura-Oka K, DeMayo F, Kibe
T, Repka D, Poindexter B, Bick RJ and Darlington GJ: C/EBPalpha is
required for differentiation of white, but not brown, adipose
tissue. Proc Natl Acad Sci USA. 98:12532–12537. 2001.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Wang N, Finegold M, Bradley A, Ou C,
Abdelsayed S, Wilde M, Taylor L, Wilson D and Darlington G:
Impaired energy homeostasis in C/EBP alpha knockout mice. Science.
269:1108–1112. 1995.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Yang J, Croniger C, Lekstrom-Himes J,
Zhang P, Fenyus M, Tenen D, Darlington G and Hanson R: Metabolic
response of mice to a postnatal ablation of CCAAT/enhancer-binding
protein alpha. J Biol Chem. 280:38689–38699. 2005.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Rosen E, Hsu CH, Wang X, Sakai S, Freeman
M, Gonzalez F and Spiegelman B: C/EBPalpha induces adipogenesis
through PPARgamma: A unified pathway. Genes Dev. 16:22–26.
2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Rosen ED and MacDougald OA: Adipocyte
differentiation from the inside out. Nat Rev Mol Cell Biol.
7:885–896. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Chang E and Kim CY: Natural products and
obesity: A focus on the regulation of mitotic clonal expansion
during adipogenesis. Molecules. 24(1157)2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Yang XD, Ge XC, Jiang SY and Yang YY:
Potential lipolytic regulators derived from natural products as
effective approaches to treat obesity. Front Endocrinol (Lausanne).
13(1000739)2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Saponaro C, Gaggini M, Carli F and
Gastaldelli A: The subtle balance between lipolysis and
lipogenesis: A critical point in metabolic homeostasis. Nutrients.
7:9453–9474. 2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Song Z, Xiaoli AM and Yang F: Regulation
and metabolic significance of de novo lipogenesis in adipose
tissues. Nutrients. 10(1383)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Brejchova K, Radner FPW, Balas L,
Paluchova V, Cajka T, Chodounska H, Kudova E, Schratter M,
Schreiber R, Durand T, et al: Distinct roles of adipose
triglyceride lipase and hormone-sensitive lipase in the catabolism
of triacylglycerol estolides. Proc Natl Acad Sci USA.
118(e2020999118)2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Mottillo EP and Granneman JG:
Intracellular fatty acids suppress β-adrenergic induction of
PKA-targeted gene expression in white adipocytes. Am J Physiol
Endocrinol Metab. 301:E122–E131. 2011.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Chakrabarti P, English T, Karki S, Qiang
L, Tao R, Kim J, Luo Z, Farmer SR and Kandror KV: SIRT1 controls
lipolysis in adipocytes via FOXO1-mediated expression of ATGL. J
Lipid Res. 52:1693–1701. 2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Barbato DL, Aquilano K, Baldelli S,
Cannata SM, Bernardini S, Rotilio G and Ciriolo MR: Proline
oxidase-adipose triglyceride lipase pathway restrains adipose cell
death and tissue inflammation. Cell Death Differ. 21:113–123.
2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Granneman JG, Moore HP, Krishnamoorthy R
and Rathod M: Perilipin controls lipolysis by regulating the
interactions of AB-hydrolase containing 5 (Abhd5) and adipose
triglyceride lipase (Atgl). J Biol Chem. 284:34538–34544.
2009.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Contreras GA, Strieder-Barboza C and
Raphael W: Adipose tissue lipolysis and remodeling during the
transition period of dairy cows. J Anim Sci Biotechnol.
8(41)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Straub BK, Stoeffel P, Heid H, Zimbelmann
R and Schirmacher P: Differential pattern of lipid
droplet-associated proteins and de novo perilipin expression in
hepatocyte teratogenesis. Hepatology. 47:1936–1946. 2008.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Temprano A, Sembongi H, Han GS, Sebastián
D, Capellades J, Moreno C, Guardiola J, Wabitsch M, Richart C,
Yanes O, et al: Redundant roles of the phosphatidate phosphatase
family in triacylglycerol synthesis in human adipocytes.
Diabetologia. 59:1985–1994. 2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Shin H, Shi H, Xue B and Yu L: What
activates thermogenesis when lipid droplet lipolysis is absent in
brown adipocytes? Adipocyte. 7:143–147. 2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Nguyen VTT, Vu VV and Pham PV: Brown
adipocyte and browning thermogenesis: Metabolic crosstalk beyond
mitochondrial limits and physiological impacts. Adipocytes.
12(2237164)2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Van der Vaart JI, Boon MR and Houtkooper
RH: The role of AMPK signaling in brown adipose tissue activation.
Cells. 10(1122)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Gulyaeva O, Dempersmier J and Sul HS:
Genetic and epigenetic control of adipose development. Biochim
Biophys Acta Mol Cell Biol Lipids. 1864:3–12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hirayama D, Iida T and Nakase H: The
phagocytic function of macrophage-enforcing innate immunity and
tissue homeostasis. Int J Mol Sci. 19(92)2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Navegantes KC, de Souza Gomes R, Pereira
PAT, Czaikoski PG, Azevedo CHM and Monteiro MC: Immune modulation
of some autoimmune diseases: The critical role of macrophages and
neutrophils in the innate and adaptive immunity. J Transl Med.
15(36)2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Lagos LS, Luu TV, De Haan B, Faas M and De
Vos P: TLR2 and TLR4 activity in monocytes and macrophages after
exposure to amoxicillin, ciprofloxacin, doxycycline and
erythromycin. J Antimicrob Chemother. 77:2972–2983. 2022.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Wu J, Mo J, Xiang W, Shi X, Guo L, Li Y,
Bao Y and Zheng L: Immunoregulatory effects of Tetrastigma
hemsleyanum polysaccharide via TLR4-mediated NF-κB and MAPK
signaling pathways in Raw264.7 macrophages. Biomed Pharmacother.
161(114471)2023.PubMed/NCBI View Article : Google Scholar
|