Introduction
Cutaneous cancer is among one of the most common
types of cancer as ~1.5 million individuals worldwide are diagnosed
with cutaneous cancer annually, accounting for nearly 10% of all
new cancer cases (1). Cutaneous
squamous cell carcinoma (cSCC) is a common non-melanoma skin cancer
that accounts for ~20% of all skin cancers in the United States and
UV light is the main causative factor (2,3). The
ozone layer absorbs some of the UVB and all of the UVC, so the UV
that reaches the Earth's surface is mainly longwave UVA (320-400
nm) and shortwave UVB (280-320 nm) (4). Prolonged exposure to UV radiation
generates reactive oxygen species (ROS), which can directly damage
DNA, leading to the activation of proto-oncogenes and the
inhibition of anti-oncogenes (5).
ROS and its modified intermediates can also oxidize proteins,
lipids, nucleic acids and carbohydrates, thereby exacerbating
metabolic diseases such as obesity, diabetes and dyslipidemia
(6,7). In addition, ROS can also activate
inflammatory pathways, release inflammatory regulatory factors and
further damage DNA (8,9). Genomic instability occurs when DNA
damage is not promptly repaired but rather accumulates. If this
damage persists, it can eventually lead to the development of
cancer (10-12).
Nuclear factor-E2-related factor 2 (Nrf2) is an
essential regulator of certain transcription factors involved in
oxidative stress. When healthy cells are damaged, a functional
defect in the Nrf2 pathway can increase the tumorigenic potential
of early tissue damage (13).
However, a previous study reported that Nrf2 is continuously
abnormally activated during the progression of certain types of
carcinoma, including esophageal SCC, cSCC and non-small cell lung
cancer (14). He et al
(15) reported that Nrf2 is
involved in a number of metabolic processes in cancer cells,
including the pentose phosphate pathway, the regulation of
glycolysis and fatty acid metabolism. Aberrant activation of Nrf2
may be associated with the accumulation of p62/sequestosome-1, a
multidomain protein that competes with Nrf2 for binding to
Kelch-like ECH associated protein 1 (Keap1), which can lead to
aberrant Nrf2 activation (16).
Sirtuin1 (SIRT1) is a deacetylase that serves an
instrumental role in the inflammatory response. The elimination of
SIRT1 in the liver, pancreas and brain results in increased
inflammatory responses and ROS accumulation (17). Upregulation of SIRT1 can repair
UV-induced DNA injury and photoaging in human immortalized
keratinocytes and mouse embryonic fibroblasts (18,19).
The physiological functions of SIRT1 are primarily regulated
through its deacetylation of co-activators, histones and
transcription factors, such as E2F transcription factor 1, c-Myc,
FOXO1 and NF-κB (20). SIRT1
inhibits the function of NF-кB by deacetylation of the RelA/p65
subunit, which results in cells entering the TNF-α-mediated
apoptotic process (21).
Chrysanthemum indicum Linnén (C. indicum) is a
medicinal and food herb, which is readily available in East Asia
(22). Previous studies have
reported that C. indicum may have antihypertensive,
antioxidant, antiseptic and anticancer properties, as well as
inhibiting lipogenesis (22-25).
Supercritical carbon dioxide fluid extraction has been successfully
employed for the extraction process of flowers and buds of C.
indicum because it can ensure a high extraction rate and the
structural integrity of its volatile compounds, such as
monoterpenes, sesquiterpenes and alkenes (26). A previous study reported that the
supercritical carbon dioxide fluid extraction of C. indicum
(CISCFE) may show potential for the treatment of liver
and brain damage induced by D-galactose in an aging mouse model
(22). Moreover, CISCFE
combined with bleomycin was reported to improve the anticancer
ability of tumor-transplanted mice and reduce the toxicity of
bleomycin (27). Furthermore,
CISCFE inhibited UV-induced photoaging in a mouse model
by reducing inflammation and enhancing antioxidant capacity
(28). However, the effect of
CISCFE in UV-induced skin carcinogenesis is currently
unclear.
The present study aimed to investigate whether local
application of CISCFE could alleviate UV-induced skin
cancer in a mouse model through macroscopic, histological and
immunohistochemical evaluations, and determine whether
CISCFE could regulate oxidative stress and inflammation
related pathways.
Materials and methods
Preparation of CISCFE
The flowers and buds of C. indicum were
purchased from Qingping Chinese herbal medicine market of
Guangzhou, Guangdong, China, and were identified by Professor
Zi-Ren Su of Guangzhou University of Chinese medicine (Guangdong,
China). The extraction and identification procedures of
CISCFE were based on previously published literature
(27). Briefly, the flowers and
buds of C. indicum were loaded into the extraction vessel of
the 532 Supercritical Fluid Extraction Equipment (Applied
Separations). CISCFE was obtained using an extraction
time of 4 h, a pressure of 25 MPa, a temperature of 45˚C and a flow
rate of 20 l/h. High-performance liquid chromatography-pulsed
amperometric detection (HPLC-PAD) and gas chromatography-mass
spectrometry (GC-MS) were used to analyze the chemical composition
of CISCFE (Table SI
and Fig. S1). HPLC analysis was
performed on a Shimadzu LC40 HPLC system. The separation was
performed on a ACE Excel 5 Super C18 column (4.6x250 mm, 5 µm; cat.
no. EXL-1211-2546U; Advanced Chromatography Technologies) with a
flow rate of 1.0 ml/min, column temperature at 30˚C, and injection
volume of 10 µl. The mobile phase consisting of acetonitrile
(solvent A) and 0.1% aqueous formic acid (solvent B) was used to
elute the targets with the gradient mode (0-5 min: 5% A→25% A; 5-15
min: 25% A→25% A; 15-25 min: 25% A→45% A; 25-35 min: 45% A→55% A;
35-40 min: 55% A→70% A). Luteolin-7-glucoside (cat. no. B20887),
luteolin (cat. no. B20888), linarin (cat. no. B20860) and
chlorogenic acid (cat. no. B20782) standards were purchased from
Shanghai Yuanye Biotechnology Co., Ltd. The content of these
compounds was quantitatively analyzed with peak areas under the
standard curves at 334 nm. GC-MS analysis was performed on an
Agilent 6890-5975 GC-MS system (Agilent Technologies, Inc.). The
oven temperature was initially set at 60˚C, then ramped up to 100˚C
at a gradient of 10˚C/min (held for 1 min), then to 110˚C at a rate
of 1˚C/min (held for 1 min); then to 150˚C at a rate of 3˚C/min
(held for 1 min) and finally to 260˚C at a rate of 10˚C/min (held
for 5 min). Split injection (0.5 µl) was conducted with a split
ratio of 60:1 and helium was used as carrier gas of 1.0 ml/min flow
rate. The spectrometer was set to electron impact (EI) mode with an
ionization energy of 70 eV, a scanning range of 40-400 amu, and a
scanning rate of 0.34 sec/scan. The temperatures of the inlet and
ionization source were 230 and 250˚C, respectively. Ultimately,
CISCFE at a concentration of 0.48
mg/cm2/mouse (low concentration CISCFE) or
1.6 mg/cm2/mouse (high concentration CISCFE)
in 10% Tween 80 were used for subsequent animal experiments.
Animal model
A total of 75 specific-pathogen free male Kunming
mice (22-24 g, age 8 weeks) were obtained from the Animal
Experiment Center, Guangzhou University of Chinese Medicine (Animal
Quality Certificate No. 44005800007154). The mice were housed in an
environment conforming to the prescribed humidity (50±5%) and
temperature (23±2˚C), with food and water ad libitum and
were maintained under a 12 h light/dark cycle. The laboratory
animal license number was SCXK (Yue) 2018-0085 and the ethics
certification number was 20190304024. Under the supervision of
authorized researchers, all experiments in the present study were
approved by the Animal Care and Use Committee of Guangzhou
University of Chinese Medicine (approval no. 20190304024;
Guangzhou, China) based on the Guidelines for the ethical review of
laboratory animal welfare People's Republic of China National
Standard GB/T 35892-2018(29).
According to additional markers that may constitute humane
endpoints in tumor research, the experimental endpoint for tumor
size took into account the fact that UV exposure on the back of
mice causes damage to skin tumors. The experiment was halted when
the volume of any of the skin lesions >1,000 mm3, the
maximum diameter >10 mm or when the diameter ≤10 mm but
interfered with animal feeding or hindered animal movement.
According to the humane endpoint guidance of the present animal
experiments, two mice were euthanized, one from the
CISCFE-L group and one from NAA group (30).
Topical CISCFE treatments
and UV exposure
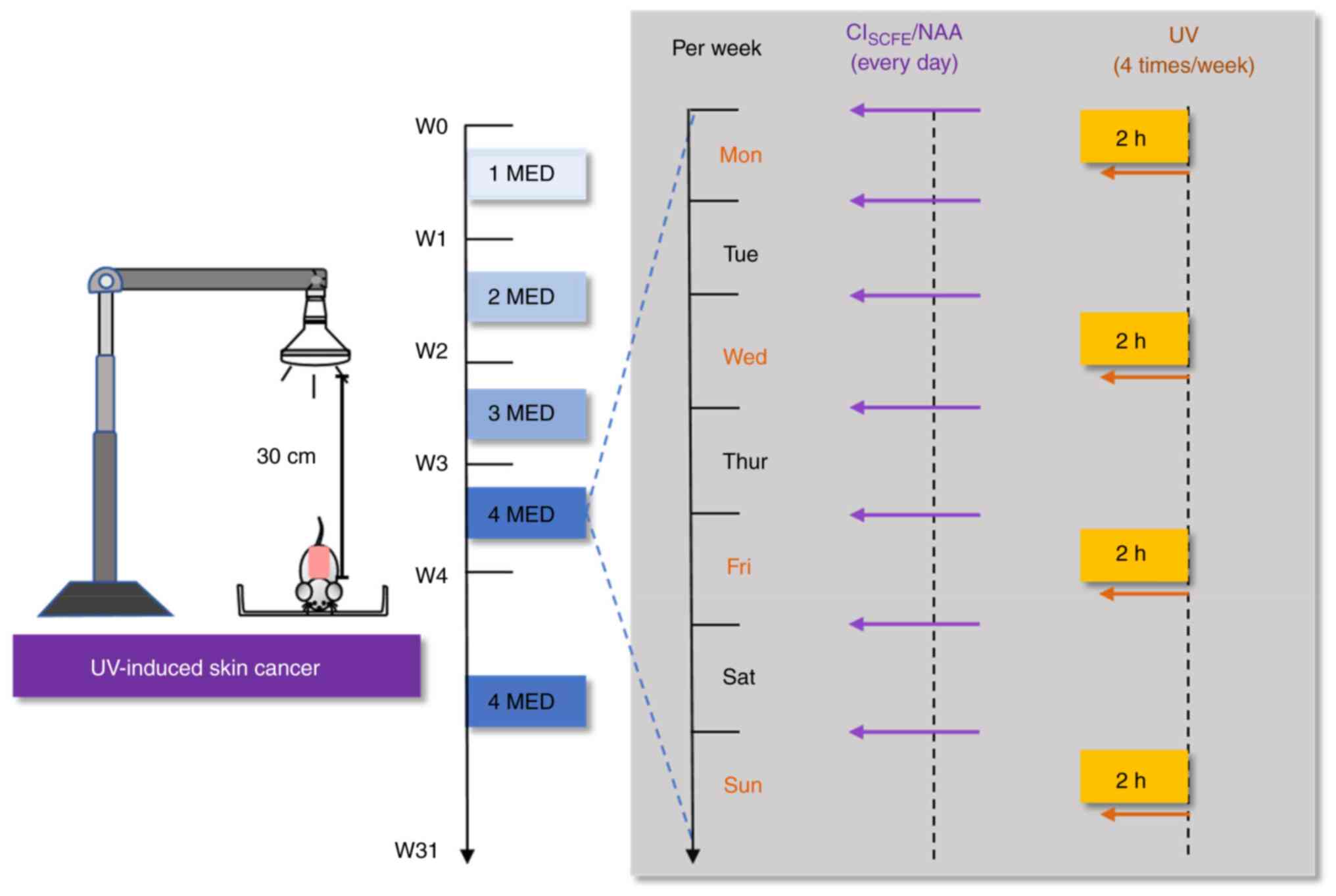
The skin area on the back (2.5x3.0 cm2)
of mice was depilated with a shaver (FS607; FLYCO) (Fig. 1). In our previous study, the
concentrations of 0.48 mg/cm2/mouse and 1.6
mg/cm2/mouse of CISCFE reduced skin damage
caused by UV exposure (28).
Therefore, the mice were randomly allocated to five treatment
groups: Sham (no medication or UV radiation), model (only UV
radiation without drug treatment), low concentration
CISCFE (0.48 mg/cm2/mouse), high
concentration CISCFE (1.6 mg/cm2/mouse) and
positive control nicotinamide (NAA; 0.65 mg/cm2/mouse;
Sigma-Aldrich; Merck KGaA) groups. CISCFE and NAA were
applied daily to the shaved area of the skin on the back of the
mouse.
Ultra-Vitalux light bulbs were used as a source of
UV light (UVA:UVB=93:7; Osram). Mice were positioned 30 cm away
from the UV lamp at 23˚C and were irradiated on Mondays,
Wednesdays, Fridays and Sundays for 31 weeks. According to our
previous study (31), the minimum
erythema dose (MED) for mice was 100 mJ/cm2. UV
intensity was 1 MED for the first week and was increased by 1
MED/week until 400 mJ/cm2 in the 4th week, which was
maintained until the end of the experiment. The experiment
continued for 31 weeks, with daily observations of mice health and
behavior. At 31 weeks, isoflurane (induction, 5%; maintenance, 2%)
was used for anesthesia and cervical dislocation was performed on
the mice after photographic documentation of the dorsal skin
status. Finally, skin tissue from the irradiated area was extracted
for subsequent experiments.
Histological analysis
The dorsal skin was fixed in 10% formalin at 26˚C
for 48 h. Tissue dehydration was then performed with the following
procedure: Soaking in 10% formalin at 26˚C for 1 h, soaked in 75%
ethanol at 26˚C for 1.5 h, soaked in 85% ethanol at 26˚C for 1.5 h,
soaking in 95% ethanol at 26˚C for 1.5 h, soaking in anhydrous
ethanol at 26˚C for 1 h three times, soaking in TO Bio-permeable
agent (cat. no. AYA0150; Shanghai Acmec Biochemical Technology Co.,
Ltd.) at 26˚C for 1.5 h twice and soaking in paraffin wax at 60˚C
for 2 h. Subsequently, the tissue was embedded in melted paraffin
at 65˚C and sectioned (4 µm). Paraffin sections were stained using
Gomori aldehyde fuchsin at 26˚C for 10 min (GAF; cat. no. G1593;
Beijing Solarbio Science & Technology Co., Ltd.), hematoxylin
and eosin at 26˚C for 20 min (H&E; cat. no. 0619A19; Beijing
Leagene Biotechnology Co., Ltd.) and Sirius red staining at 26˚C
for 1 h (cat. no. DC0041-100; Beijing Leagene Biotechnology Co.
Ltd.). The histological changes in the skin were examined using a
light microscope (BX53; Olympus Corporation).
Immunohistochemical analysis
The procedure for fixation, embedding and sectioning
of skin tissue is the same as in the Histological analysis
section. Skin sections (5 µm) were subsequently deparaffinized with
the following procedure: Placed in xylene at 26˚C for 10 min three
times, in anhydrous ethanol at 26˚C for 5 min and three times, in
95% ethanol at 26˚C for 3 min, in 85% ethanol at 26˚C for 3 min and
in 75% ethanol at 26˚C for 3 min. The skin sections were heated in
EDTA for 15 min at 95˚C and then the sections were soaked in 3%
hydrogen peroxide at 26˚C for 20 min. Tissue sections were then
sealed with 10% goat serum (cat. no. SL038; Beijing Solarbio
Science & Technology Co., Ltd.) for 30 min at 37˚C.
Subsequently, sections were incubated with Ki-67 (1:300; cat. no.
ab15580; Abcam) and CD11b (1:4,000; cat. no. ab133357; Abcam)
antibodies diluted in PBS at 4˚C overnight. Samples were incubated
with anti-rabbit IgG H&L (HRP; 1:1,000; cat. no. ab6721; Abcam)
at 37˚C for 1 h, followed by incubation with 3,3-diaminobenzidine
at 26˚C for 1 min (cat. no. ZLI-9017; ZSGB-BIO) and counterstaining
with hematoxylin at 26˚C for 3 min. Sections were imaged using a
light microscope (Olympus Co.; BX53). Samples were semi-quantified
using ImageJ software (version 1.53e; National Institutes of
Health).
ROS accumulation assay
At week 31, the UV-irradiated dorsal skin of mice
was removed and analyzed using a ROS assay. The mouse skin was
encapsulated in optimal cutting temperature encapsulant (cat. no.
4583; Sakura Finetek USA, Inc.) and frozen sections (-80˚C;
thickness, 8 µm) were incubated with DCFH-DA (cat. no. BB18081;
Bestbio) at 37˚C for 30 min. The samples were measured at a
wavelength of 525 nm using a fluorescence microscope (BX53; Olympus
Corporation). The average fluorescence intensity of ROS was
analyzed using ImageJ software (version 1.53e; National Institutes
of Health).
Catalase (CAT) and superoxide
dismutase (SOD) assays
Skin tissue was homogenized by adding 9 times the
volume of saline (g/ml) and the supernatant was collected after
centrifugation at 3,000 x g for 10 min at 4˚C. The protein
concentration of the supernatant was measured using a BCA kit (cat.
no. P0012; Beyotime Institute of Biotechnology). CAT and SOD levels
were measured according to the manufacturer instructions of the CAT
(cat. no. A007-1; Nanjing Jiancheng Bioengineering Institute) and
SOD (cat. no. A001-3; Nanjing Jiancheng Bioengineering Institute)
assay kits.
Measurement of
8-hydroxy-2'-deoxyguanosine (8-OHdG), IL-6 and TNF-α
Skin tissues were ground in PBS with a homogenizer
(KZ-III-FP; Wuhan Servicebio Technology Co., Ltd.) at 60 Hz for 6
min at 4˚C and subsequently centrifuged at 4˚C and 3,000 x g for 20
min. The supernatant was collected and ELISA kits were used
accordingly to the manufacturer's instructions to measure the
levels of TNF-α (cat. no. 430904; BioLegend, Inc.), IL-6 (cat. no.
431304; BioLegend, Inc.) and 8-OHdG (cat. no. MM-0221M1; Jiangsu
Meimian Industrial Co., Ltd.).
Western blotting
Skin samples were homogenized in RIPA lysis solution
(cat. no. P0013B; Beyotime Institute of Biotechnology). Samples
were centrifuged at 4˚C and 14,000 x g for 10 min and the protein
content was determined using a BCA kit (cat. no. P0010; Beyotime
Institute of Biotechnology). The proteins (40 µg/lane) were
electrophoresed using a 10% SDS-polyacrylamide gel and transferred
to PVDF membranes. PVDF membranes were blocked with 5% skimmed milk
for 1 h at 26˚C and incubated overnight at 4˚C with heme oxygenase
1 (HO-1; 1:1,000; cat. no. ab13248; Abcam), CD11b (1:1,000; cat.
no. ab133357; Abcam), VEGF (1:1,000; cat. no. SC7269; Santa Cruz
Biotechnology, Inc.), c-Myc (1:1,000; cat. no. SC40; Santa Cruz
Biotechnology, Inc.), p65 (1:1,000; cat. no. 8242S; Cell Signaling
Technology, Inc.), PTEN (1:1,000; cat. no. ET1606-43; HUABIO),
phosphorylated (p)-p62 (1:1,000; cat. no. ab211324; Abcam),
NAD-dependent protein deacetylase sirtuin-1 (SIRT1; 1:1,000; cat.
no. ab110304; Abcam), p-p65 (1:1,000; cat. no. ab76302; Abcam),
p-IκBα (1:1,000; cat. no. ab133462; Abcam), acetyl-p65 (1:250; cat.
no. ab19870; Abcam, IκBα (1:1,000; cat. no. ab32518; Abcam), Nrf2
(1:1,000; cat. no. ab137550; Abcam), NAD(P)H dehydrogenase
[quinone] 1 (NQO1; 1:50,000; cat. no. ab80588; Abcam), GAPDH
(1:1,000; cat. no. 5174; Cell Signaling Technology, Inc.), lamin B1
(1:2,000; cat. no. AF5161; Affinity Biosciences) and Keap1
(1:2,500; cat. no. ab139729; Abcam) antibodies. Subsequently,
membranes were incubated with anti-mouse IgG H&L (1:5,000; cat.
no. LK2003; Tianjin Sungene Biotech Co., Ltd.) or anti-rabbit IgG
H&L (1:5,000; cat. no. ab6721; Abcam) antibodies for 1 h at
26˚C. Blots were visualized using ECL reagents (cat. no. FD8000;
Hangzhou Fude Biotechnology Co., Ltd.) and the blot densities were
quantified using ImageJ software (version 1.53e; National
Institutes of Health). GAPDH or Lamin B1 were used as loading
controls (32).
Statistical analysis
Data were expressed as mean ± standard deviation.
Data were analyzed using a one-way ANOVA followed by Tukey's post
hoc test. P<0.05 was considered to indicate a statistically
significant difference. Data were visualized and analyzed using
GraphPad software (version 8.3.0; Dotmatics).
Results
Chemical Composition Analysis of
CISCFE.
GC-MS analysis and HPLC analysis were used to detect
the chemical constituents of CISCFE. As shown in
Table SI, CISCFE
mainly contains d-Camphor, Caryophyllene oxide, Endo-Borneol,
α-Curcumene, Cis-verbenol, β-Caryophyllene, Eucalyptol,
Thymol as detected by GC-MS. In addition, HPLC analysis detected
four compounds in CISCFE (Fig. S1), which were Chlorogenic acid,
Luteolin-7-glucoside, Linarin and Luteolin.
CISCFE alleviated cutaneous
injury induced by UV
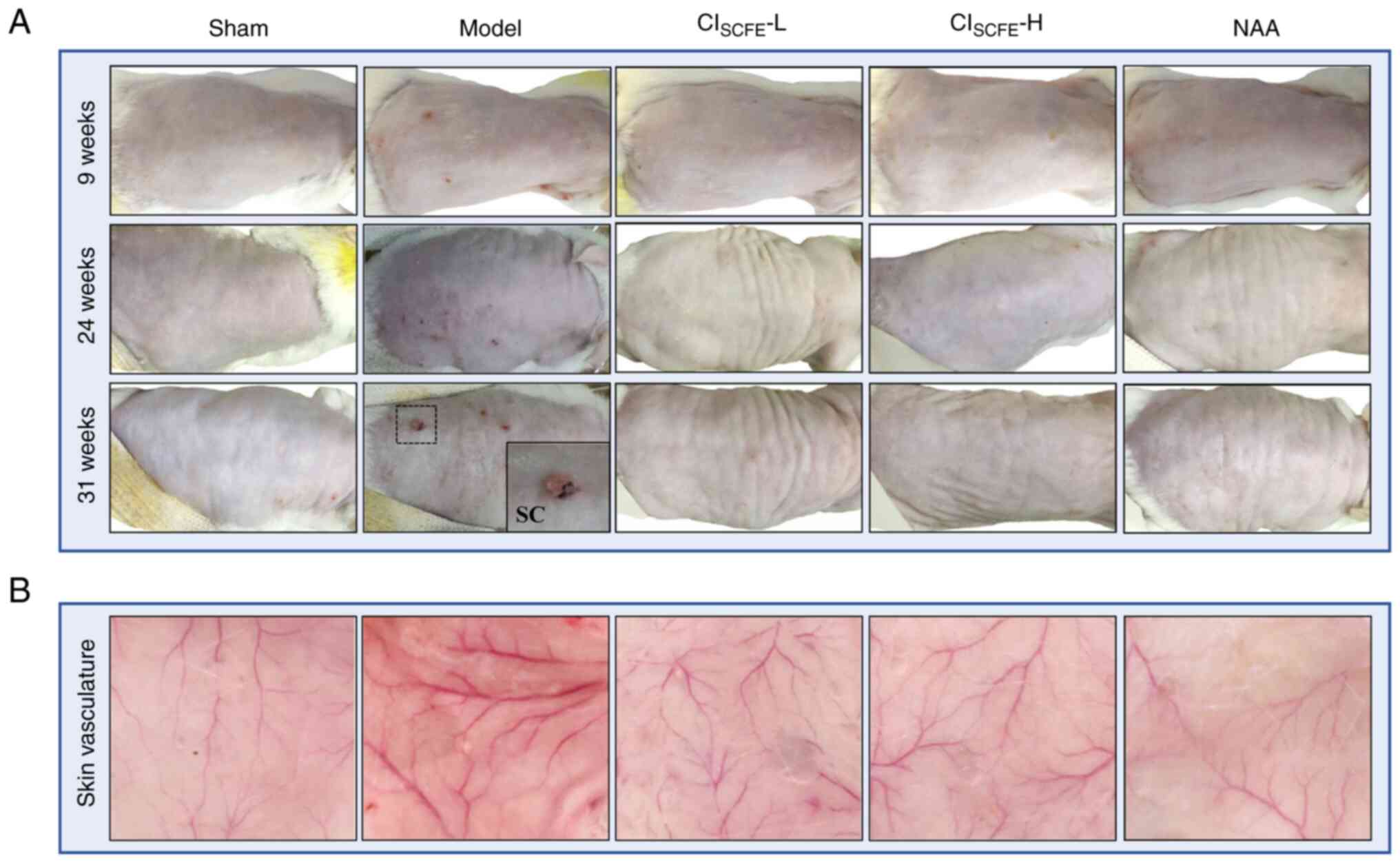
Over the course of the present study, it was
demonstrated that the skin of the model group showed shallow
wrinkles, erythema and a leathery appearance after 9 weeks of UV
exposure compared with the sham group (Fig. 2A). The skin in the low dose and
high dose CISCFE groups and the NAA group exhibited no
erythema and showed few wrinkles compared with the model group.
After 24 weeks of UV irradiation, papular lesions and broken crusts
were observed on the skin of model mice. At week 31, ulcerative
papules, adhesive scales, recurrent local bleeding and crusting on
the skin was observed on model mice. However, after pretreatment of
mice with low or high dose CISCFE and NAA, there were no
papules and deep wrinkles only appeared on the skin at 31 weeks of
UV irradiation. Moreover, the dermal vessels in the model group
were expanded and proliferated compared with the sham group after
31 weeks of UV irradiation. After pretreatment with
CISCFE, at both doses tested, vasodilation and
hyperplasia in the dermis caused by UV were markedly reduced
(Fig. 2B).
CISCFE reduced histological
damage caused by UV exposure
To observe the histopathologic changes of mouse skin
after UV irradiation, GAF, Sirius red and H&E staining were
used (Fig. 3A). H&E staining
showed that the skin of model mice exhibited abnormal proliferation
of the epidermis, keratinocytes extending into the dermis and
extensive infiltration of inflammatory cells at week 31. Sirius red
and GAF staining of the mouse skin showed that UV irradiation
damaged elastic and collagen fibers and reduced their density.
Nevertheless, compared with the model group, low and high dose
CISCFE and NAA treatment significantly reduced
inflammatory cell infiltration and the deformation and degradation
of elastin fibers and collagen fibers and markedly reduced
UV-induced abnormal epidermal hyperplasia (Fig. 3B; P<0.01).
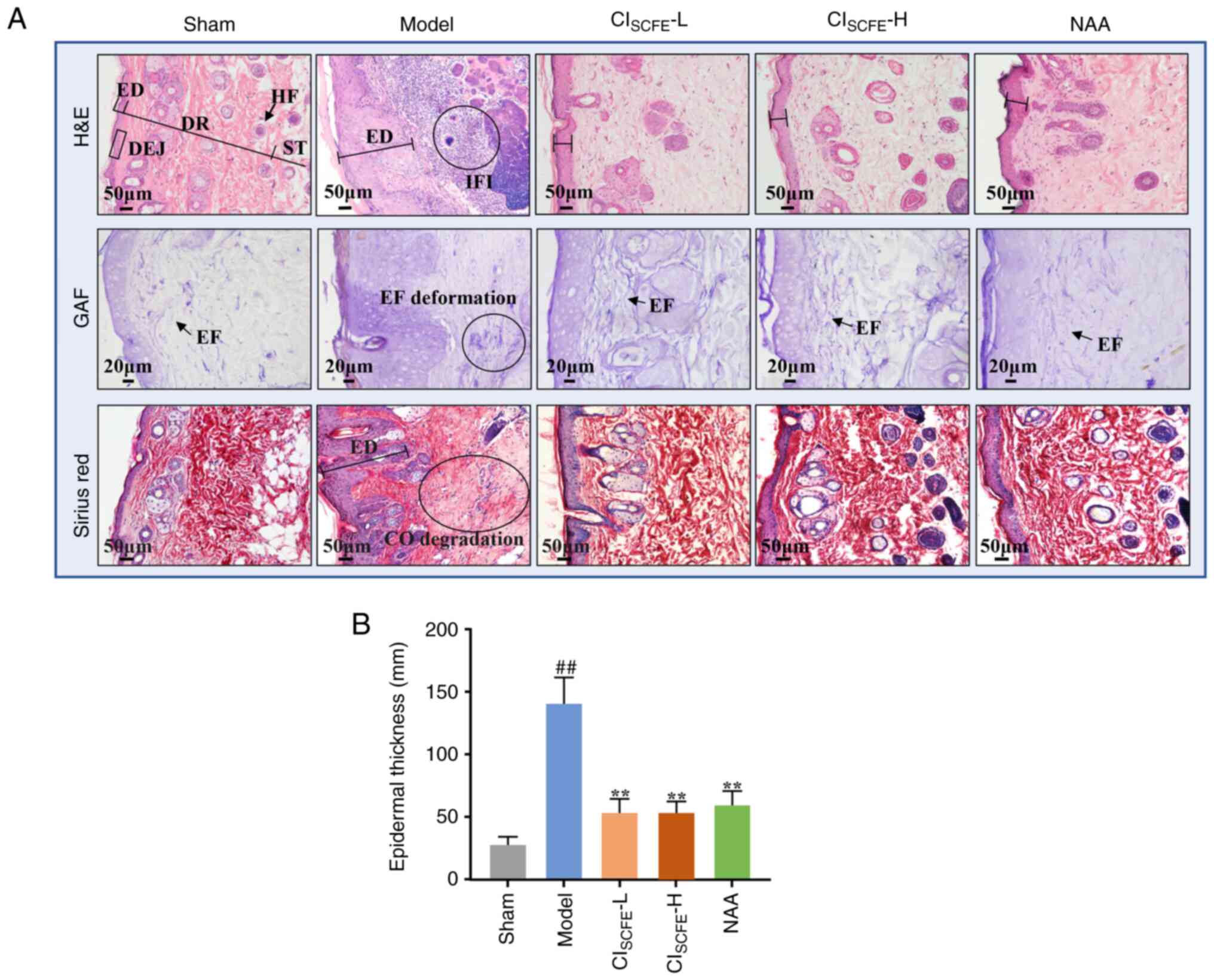 | Figure 3CISCFE reduces
histological damage caused by UV exposure. (A) H&E, (scale bar,
50 µm; magnification, x200), Gomori aldehyde fuchsin (GAF) staining
(scale bar, 20 µm; magnification, x400) and Sirius red staining
(scale bar, 50 µm; magnification, x200) of mouse skin after 31
weeks of UV irradiation. (B) Epidermal thickness of UV-irradiated
mouse skin. Data were expressed as mean ± standard deviation (n=5).
##P<0.01 vs. sham group;
**P<0.01 vs. model group. H&E, hematoxylin and
eosin; GAF, Gomori aldehyde fuchsin; ED, epidermis; DR, dermis;
DEJ, dermo-epidermal junction; ST, subcutaneous tissue; HF, hair
follicle; EF, elastic fiber; CO, collagen; IFI, inflammatory
infiltration; CISCFE, supercritical carbon dioxide fluid
extraction of Chrysanthemum indicum Linnén; NAA,
nicotinamide; L, low dose; H, high dose. |
CISCFE inhibited the
development of UV-induced skin cancer
To examine the effects of CISCFE on
proliferation, angiogenesis and the expression levels of
cancer-related proteins, immunohistochemical assays and western
blotting were used to detect Ki-67, VEGF, c-Myc and PTEN expression
levels. After 31 weeks of UV irradiation, the epidermis of the
model mice exhibited increased Ki-67 expression compared with the
sham group (Fig. 4A and B; P<0.001). The epidermal layer in the
CISCFE and NAA groups demonstrated a significant
reduction in Ki-67 expression compared with the model group
(P<0.001). VEGF and c-Myc protein expression levels were
significantly increased and protein expression levels of PTEN were
significantly decreased in the model mice compared with the sham
group (Fig. 4C-F;
P<0.001). After pretreatment with CISCFE or
NAA, VEGF and c-Myc protein expression levels were significantly
reduced while PTEN protein expression levels were significantly
increased compared with the model group (P<0.05).
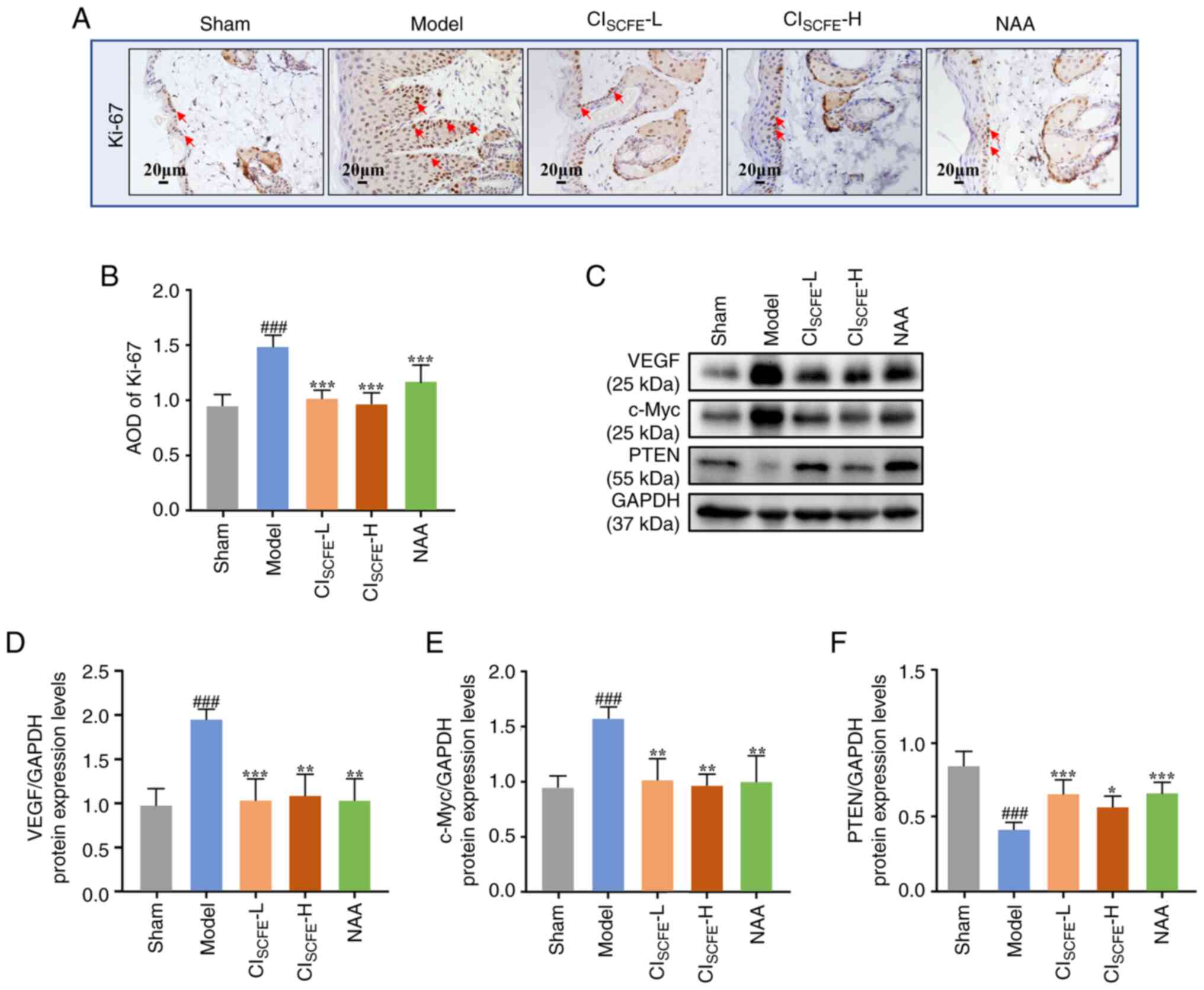 | Figure 4CISCFE suppresses
UV-induced skin carcinogenesis. (A) Immunohistochemical analysis of
Ki-67 expression in mice skin at the 31 weeks was conducted on 5 µm
sections. Red arrows, expression of Ki-67 in the epidermis; scale
bar, 20 µm; magnification, x400. (B) Semi-quantitative analysis of
Ki-67 staining. (C) VEGF, c-Myc and PTEN protein expression levels
in mouse skin specimens at the 31st week using western blot.
Relative changes in protein expression levels intensities of (D)
VEGF, (E) c-Myc and (F) PTEN were quantified by densitometric
analysis. Data were presented as mean ± standard deviation (n=6).
###P<0.001 vs. sham group; *P<0.05,
**P<0.01, ***P<0.001 vs. model group.
CISCFE, supercritical carbon dioxide fluid extraction of
Chrysanthemum indicum Linnén; NAA, nicotinamide; L, low
dose; H, high dose; AOD, average optical density. |
CISCFE suppressed
UV-induced oxidative stress and inflammation of skin
To investigate whether the inhibition of skin cancer
progression by CISCFE is related to its antioxidant
effects, the levels of ROS, SOD, CAT and 8-OHdG were assayed. An
increase in ROS accumulation was observed in the model group
compared with the sham group (Fig.
5A and B). However, compared
with the model group, CISCFE and NAA treatment
significantly reduced UV-induced ROS overexpression (P<0.05).
The activity levels of SOD and CAT were significantly decreased and
8-OHdG was significantly increased in the skin of the model mice at
31 weeks compared with the sham group (Fig. 5C-E; P<0.05). In mice treated
with CISCFE and NAA, the levels of SOD and CAT activity
were significantly increased (P<0.05), while 8-OHdG
levels were significantly decreased in mice treated with low dose
CISCFE compared with those in the model group
(P<0.01).
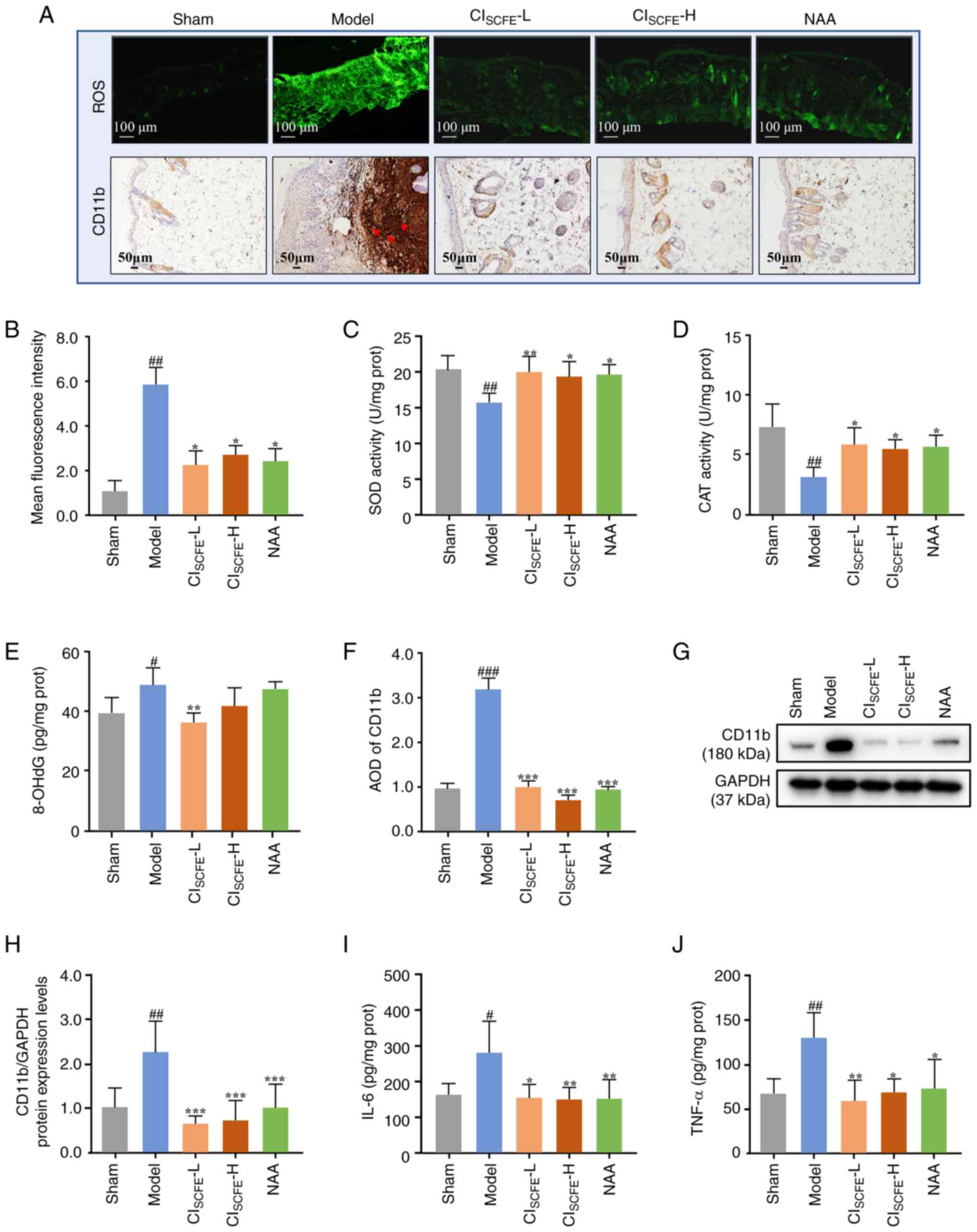 | Figure 5CISCFE suppresses
UV-induced oxidative stress and inflammation in mice. (A) ROS
accumulation in mouse skin was assessed by DCFH-DA at week 31.
Scale bar, 100 µm; magnification, x100. Green fluorescence
represents the intensity of the generated ROS. Immunohistochemical
analysis of CD11b expression in mouse skin at week 31 was conducted
on 5 µm skin sections (Red arrows, expression of CD11b in dermis;
scale bar, 50 µm; magnification, x200). Semi-quantitative analysis
of (B) ROS Data were expressed as mean ± SD (n=6). Activity of
antioxidant enzymes (C) SOD and (D) CAT activity levels in mouse
skin at the 31 weeks were detected using assay kits. Data were
presented as mean ± SD (n=8). (E) Levels of 8-OHdG in skin
specimens were measured by ELISA kit at 31 weeks. Data were
presented as mean ± SD (n=4). Semi-quantitative analysis of (F)
CD11b. Data were expressed as mean ± SD (n=6). (G) Analysis of
CD11b protein expression levels in mouse skin specimens at 31 weeks
using western blotting. (H) Protein expression levels of CD11b were
quantified by densitometric analysis. Data were presented as mean ±
SD (n=6). Expression levels of (I) IL-6 and (J) TNF-α in skin
specimens were measured using ELISA kits at 31 weeks.
#P<0.05, ##P<0.01,
###P<0.001 vs. sham group; *P<0.05,
**P<0.01, ***P<0.001 vs. model group.
CISCFE, supercritical carbon dioxide fluid extraction of
Chrysanthemum indicum Linnén; NAA, nicotinamide; L, low
dose; H, high dose; AOD, average optical density; ROS, reactive
oxygen species; prot, protein, CAT, catalase, SOD, superoxide
dismutase; SD, standard deviation; 8-OHdG,
8-hydroxy-2'-deoxyguanosine. |
To explore the level of inflammation in irradiated
mouse skin, the expression levels of CD11b, IL-6 and TNF-α were
assayed. After 31 weeks of UV irradiation, an increase in CD11b
expression was observed in the dermis of model mice compared with
the sham group (Fig. 5A and
F; P<0.001). The protein
expression level of CD11b in the skin of mice treated with
CISCFE and NAA was significantly reduced compared with
the model group (Fig. 5G and
H; P<0.01). The expression
levels of IL-6 and TNF-α in the model group were significantly
higher compared with the sham group (Fig. 5I and J; P<0.05). Compared with the model
group, topical application of CISCFE and NAA
significantly reduced the expression levels of IL-6 and TNF-α
(P<0.05).
CISCFE prevented UV-induced
tumorigenesis by inhibiting Nrf2 and NF-κB pathways
High expression of Nrf2 in tumor cells promotes
tumor development (15).
Furthermore, NFκB is an important inflammatory and oncogenic
transcription factor, and activation of SIRT1 inhibits the NF-κB
pathway and suppresses the inflammatory response (21). To explore the potential mechanism
of action of CISCFE against skin cancer in mice, the
p62/Keap1-Nrf2 and SIRT1/NF-κB pathway-related proteins were
examined. The results showed that the protein expression levels of
p62, p-p62, Nrf2, HO-1 and NQO1 were significantly increased, while
that of Keap1 significantly decreased in the model group compared
with the sham group (Fig. 6;
P<0.05). Furthermore, CISCFE treatment significantly
decreased the expression levels of p62, p-p62, Nrf2, HO-1 and NQO1
and significantly increased that of Keap1 compared with the model
group (P<0.05). However, the p-p62/p62 ratio was not
significantly altered in any treatment condition.
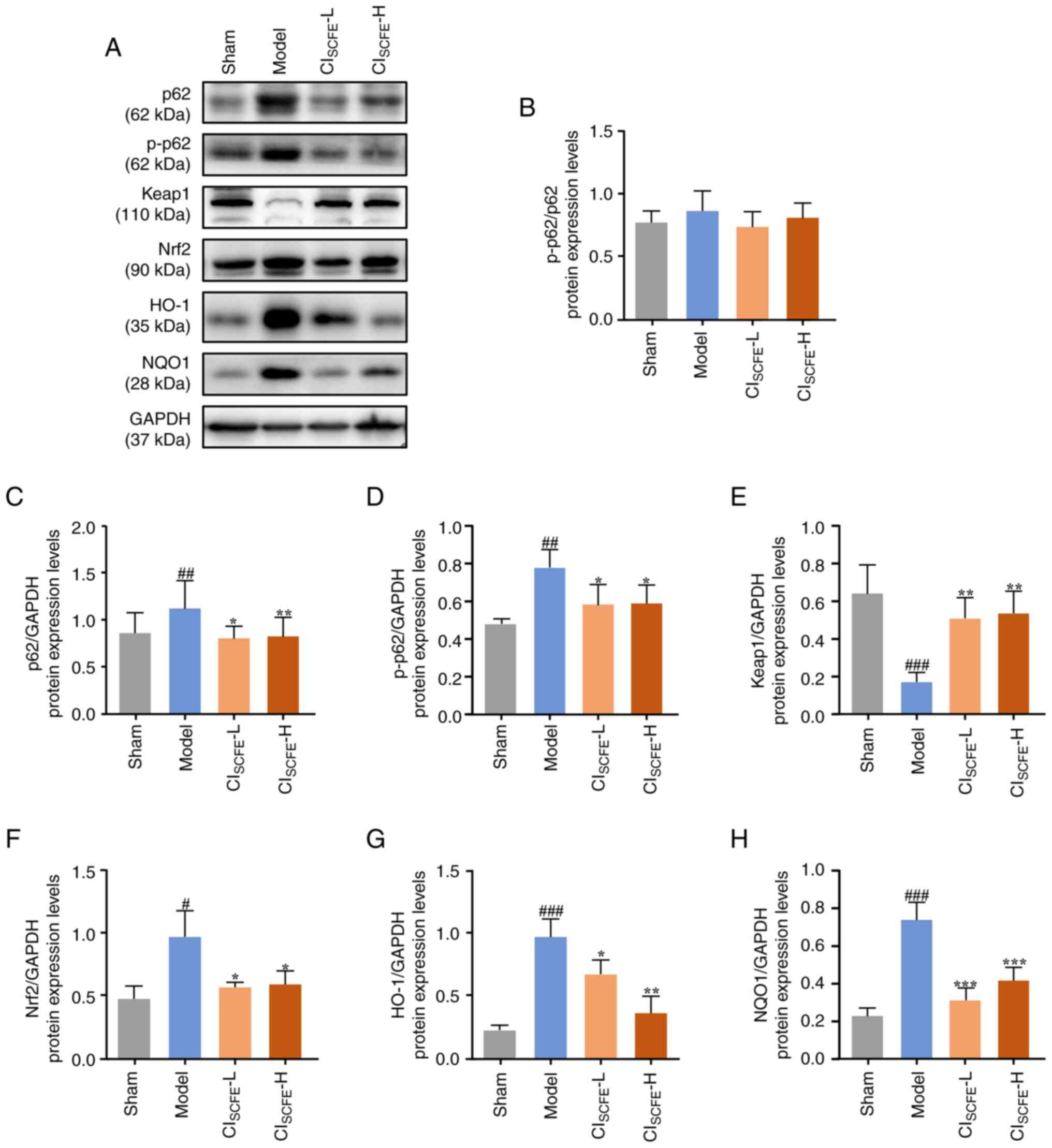 | Figure 6CISCFE inhibits the
UV-induced p62/Keap1/Nrf2 pathway in UV-irradiated mice. (A)
Protein expression levels of p62, p-p62, Keap1, Nrf2, HO-1 and NQO1
were analyzed in skin samples at week 31 using western blotting.
(B) Quantification of p-p62/p62 in mouse skin. Relative changes in
protein expression levels of (C) p62, (D) p-p62, (E) Keap1, (F)
Nrf2, (G) HO-1 and (H) NQO1 were quantified by densitometric
analysis. Data were presented as mean ± standard deviation (n=4).
#P<0.05, ##P<0.01,
###P<0.001 vs. sham group; *P<0.05,
**P<0.01, ***P<0.001 vs. model group.
CISCFE, supercritical carbon dioxide fluid extraction of
Chrysanthemum indicum Linnén; NAA, nicotinamide; L, low
dose; H, high dose; p, phosphorylated; Keap1, Kelch-like ECH
associated protein 1; Nrf2, nuclear factor-E2-related factor 2;
HO-1, heme oxygenase 1; NQO1, NAD(P)H dehydrogenase [quinone]
1. |
Furthermore, there was a significant increase in the
protein expression levels of p65, p-p65, acetyl-p65 and p-IκBα in
the skin of the model group compared with the sham group (Fig. 7; P<0.05). However, the
aforementioned protein expression levels were significantly lower
in the CISCFE group compared with the model group
(P<0.05). Additionally, the expression levels of IκBα and SIRT1
were significantly lower in the model group after 31 weeks of UV
irradiation compared with the sham group (P<0.01). Topical
pretreatment of mice with CISCFE significantly increased
the protein expression level of IκBα compared with the model group
(P<0.05). High dose treatment of mice with CISCFE
significantly increased protein expression levels of SIRT1 compared
with the model group (P<0.05). Furthermore, p-IκBα/IκBα and
p-p65/p65 expression ratios were significantly increased in the
model group compared with the sham group (P<0.01). Additionally,
p-IκBα/IκBα and p-p65/p65 expression ratios in the skin of
CISCFE mice were significantly decreased compared with
the model group (P<0.01).
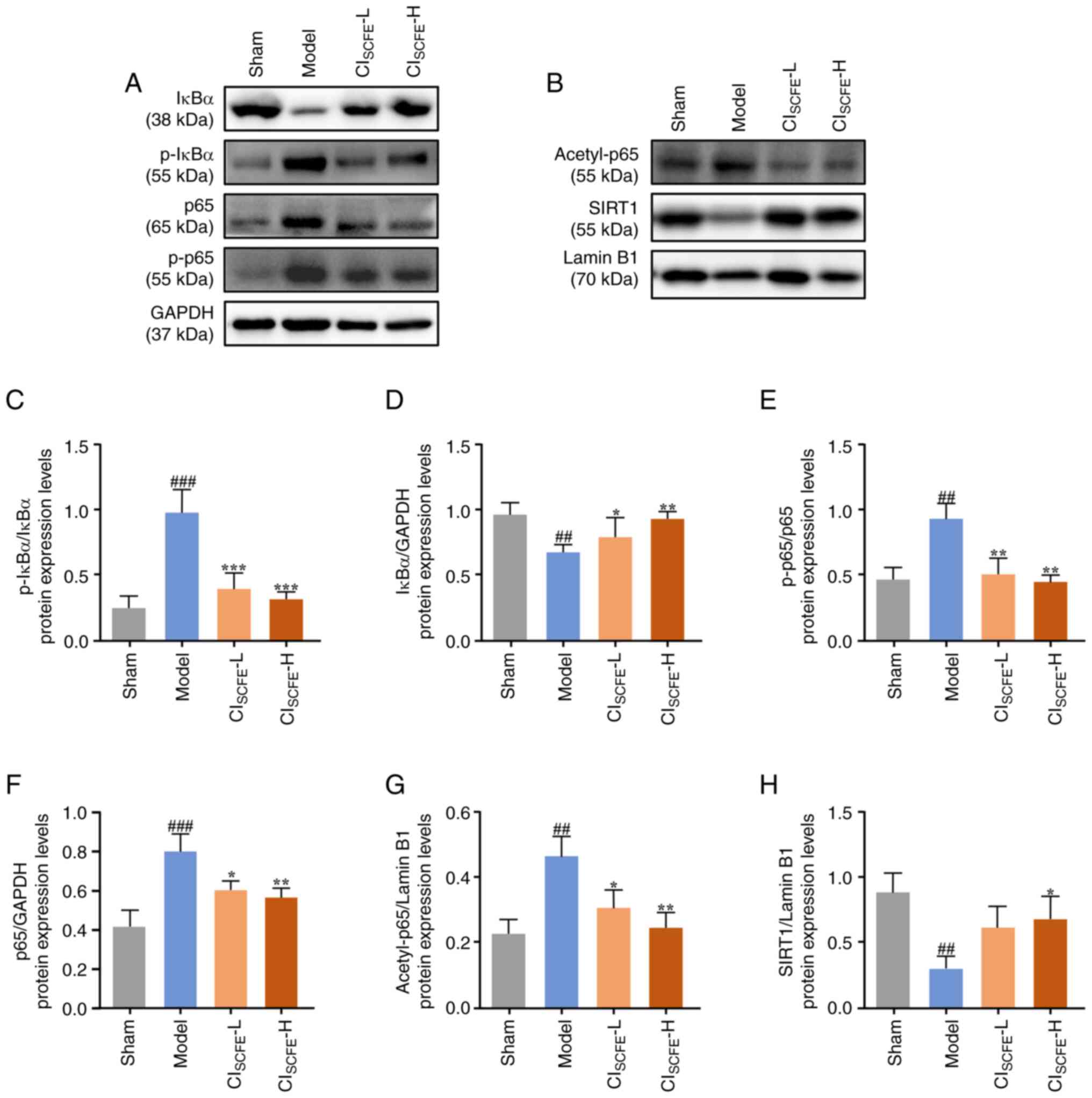 | Figure 7CISCFE affects the
SIRT1/NF-κB pathway in UV-irradiated mice. Protein expression
levels of (A) IκBα, p-IκBα, p65, p-p65 and (B) acetyl-p65 and SIRT1
were analyzed in mouse skin samples at 31 weeks using western
blotting. (C) Quantification of the p-IκBα/IκBα expression level in
mouse skin. (D) Protein expression levels of IκBα quantified by
densitometric analysis. (E) Quantification of the p-p65/p65
expression level ratio in mouse skin. Protein expression levels of
(F) p65, (G) acetyl-p65 and (H) SIRT1 quantified by densitometric
analysis. Data were presented as mean ± standard deviation (n=4).
##P<0.01, ###P<0.001 vs. sham group;
*P<0.05, **P<0.01,
***P<0.001 vs. model group. CISCFE,
supercritical carbon dioxide fluid extraction of Chrysanthemum
indicum Linnén; NAA, nicotinamide; L, low dose; H, high dose;
p, phosphorylated; SIRT1, NAD-dependent protein deacetylase
sirtuin-1. |
Discussion
Prolonged exposure to UV radiation can lead to
erythema, photoaging, photo immunosuppression and even skin cancer
(3). cSCC is a group of skin
cancers caused by the malignant growth of epithelial cells, which
accounts for 20-50% of skin cancers in the United States (3,33).
Although cSCC can be successfully treated surgically, its incidence
is still increasing (1).
Therefore, it is important to find a drug with low toxicity for
prevention and treatment of this condition. It is believed that
traditional Chinese herbs have been used for thousands of years to
prevent and treat a variety of ailments (34). C. indicum is one of these
herbs and is used both as a food source and also for the potential
prevention and treatment of skin-related diseases (24,34).
In the present study, a mouse model of UV-induced skin cancer was
used to investigate the potential chemoprevention effect and
mechanism of action of C. indicum on skin cancer. A number
of previous studies have reported the effect of NAA in the field of
dermatology and its actions in preventing photoaging and skin
cancers in humans (35-37).
Moreover, studies have reported that NAA prevented UV radiation
from reducing ATP levels and inhibiting glycolysis, thus preventing
the UV radiation-induced energy crisis in cells (36,37).
Therefore, NAA was used as a positive drug control in the present
study to evaluate the anti-skin cancer effect of
CISCFE.
The clinical manifestations of cSCC may present as
small spots and nodules in the early stages of disease, followed by
necrosis, ulceration or mycosis, which can present as flat ulcers
with raised edges and are accompanied by scaling (38). In the present study, the mice
demonstrated clinical manifestations similar to those of cSCC after
31 weeks of UV irradiation. In addition, the diagnosis of skin
cancer needs to be combined with histopathological analysis
(39). The pathological results of
the present study showed abnormal proliferation of the epidermis
into the deep dermis in the model mouse group, in addition to
increased inflammatory cell infiltration, which is consistent with
previously published literature (40,41).
The results of the present study suggested that CISCFE
treatment may effectively inhibit the development of UV-induced
skin cancer in mice.
The anticancer effects of CISCFE in the
present study may be closely related to the biological activities
of its chemical components. The chemical compositions of
CISCFE analyzed using GC-MS (Table SI) showed that the main components
were d-camphor, β-caryophyllene and thymol, which exhibit
anti-inflammatory and antioxidant capabilities (42-44).
In addition, caryophyllene oxide and thymol were reported to have
antitumor activities (45,46). Eucalyptol may inhibit skin
carcinogenesis in vivo and in vitro by reducing the
migration and invasion of cancer cells (43). HPLC analysis was used to quantify
four constituents in the present study, which were chlorogenic
acid, linarin, luteolin-7-glucoside and luteolin. Chlorogenic acid
and linarin inhibit the NF-κB signaling pathway and thereby inhibit
cancer cell growth and proliferation (47,48).
A previous study reported that lignan-7-glucoside inhibited the
migration and invasion of oral cancer cells by regulating matrix
metalloproteinase-2 expression and the extracellular
signal-regulated kinase pathway (49). Luteolin has also been reported to
act as an anti-inflammatory and anticancer agent (50). It could therefore be suggested that
the active compounds in CISCFE, particularly the active
components with potential anti-inflammatory and anticancer effects,
may be key to the prevention and treatment of skin cancer using
CISCFE. Therefore, it was investigated whether
CISCFE inhibited UV-induced skin cancer in mice by
exerting antioxidant and anti-inflammatory effects.
The activation of oncogenes and suppression of
anti-oncogenes are important for determining carcinogenesis
(51). c-Myc is a recognized
oncogenic gene and its variants are observed in >70% of human
cancers (52). Pelengaris et
al (53) reported that
sustained activation of c-Myc can induce abnormal skin hyperplasia,
related keratinization insufficiency and angiogenesis. The
hyperplastic epidermis was found to be accompanied by the
expression of Ki-67, which serves as an attractive prognostic,
predictive and potential therapeutic target for malignancies
(54). Tumors are often associated
with vascular dilation and proliferation, which provides adequate
oxygen and nutrients to the cancer cells for proliferation
(55). VEGF is crucial in skin
angiogenesis and its abnormal activation within tumors causes the
blood vessels in and around the tumor to grow exponentially
(56). In the present study, the
expression levels of c-Myc, VEGF and Ki-67 were increased following
UV irradiation, which led to vasodilation in the dermis and
hyperplasia in the epidermis. PTEN, a common mutant tumor
suppressor gene, has exhibited inactivation or partial loss of
function in numerous types of cancer (57). It has been reported that long-term
UV irradiation causes genetic alterations in PTEN and reduces its
expression level (58). In the
present study, it was demonstrated that CISCFE reduced
the expression levels of c-Myc, VEGF and Ki-67 and restored that of
PTEN, thereby potentially alleviating skin cancer progression.
Long-term UV exposure can cause oxidative imbalance
and lead to the accumulation of ROS, which induces a series of
signal transduction events contributing to inflammatory immune
imbalance, DNA injury and even cancer (59). Excessive accumulation of ROS can be
detected through measuring levels of 8-OHdG, a biomarker of
oxidative DNA damage (60).
Moreover, 8-OHdG has been reported to be elevated in a number of
types of cancer, such as colorectal, gastric and melanoma skin
cancers (61,62). SOD and CAT serve a crucial role in
the cellular antioxidant system, reducing the oxidative induction
of proto-oncogenes and structural DNA damage by oxidative
carcinogens (63). In the present
study, CISCFE reduced the excessive production of ROS
and oxidative DNA damage induced by UV and restored the activities
of antioxidants including SOD and CAT. Moreover, the inflammatory
factors induced by UV can also contribute to the progression of
skin cancer and CD11b may be expressed in various types of
inflammatory cells, which can be used to determine inflammatory
injury (64). Previous studies
have reported that photo-carcinogenesis is associated with
UV-induced infiltration of CD11b cell populations and
CD11b-mediated oxidative damage (65). Reduction of UV-induced infiltration
of CD11b+ cells can prevent UV-induced skin aging and skin cancer
(66). In the present study,
increased CD11b expression was demonstrated in mouse skin tumors,
and IL-6 and TNF-α protein expression levels were increased.
However, CISCFE reduced the UV-induced infiltration of
inflammatory cells, the protein expression levels of IL-6 and TNF-α
and the number of CD11b cells. Therefore, CISCFE may
potentially prevent skin cancer by inhibiting inflammation and
oxidative stress induced by UV.
Nrf2 activation is beneficial to the survival of
precancerous or cancer cells because oncogene mutations provide
these cells with a higher proliferative capacity and viability by
upregulating Nrf2 expression (67). Kim et al (68) reported that the mutation and
sustained activation of Nrf2 affected the differentiation of
squamous epithelial cells and was ubiquitous in cSCC. Moreover, p62
has been reported to be involved in the activation of Nrf2 in
cancer cells. P62 competes with Nrf2 for the Keap1 binding site,
especially when p62 is phosphorylated at serine 349 (Ser-349)
(69). Keap1 is subsequently
degraded and Nrf2 is released, leading to its persistent activation
and subsequent transfer to the nucleus to exert its effect
(16,70). In the present study, prolonged UV
irradiation increased the expression level of Nrf2, p62
phosphorylation at Serine 349 (Ser-349) and increased the
expression levels of downstream proteins NQO1 and HO-1. However,
CISCFE treatment diminished the UV-induced expression of
p-p62 and reduced the continuous expressions of Nrf2 and downstream
NQO1 and HO-1, thereby potentially inhibiting the development of
skin cancer.
The expression of NF-κB is triggered in tumor cells
and cells that constitute the tumor microenvironment, promoting the
production of cytokines and ultimately activating genes involved in
abnormal growth and malignant tumor expression (71). Nrf2 and activated antioxidant
enzymes, such as HO-1, inhibit the NF-κB pathway, thereby reducing
inflammatory damage (72).
However, CISCFE in the present UV-induced mouse skin
cancer model did not inhibit NF-κB expression via the Nrf2/HO-1
pathway, but instead potentially inhibited NF-κB expression via
SIRT1. A number of previous studies have reported that NF-κB is
present in the cytoplasm as a p50/RelA (p65) dimer or RelB/p52
dimer. SIRT1 can deacetylate lysine 310 of p65 to inhibit the
transcription of inflammation-related genes (73-76).
The present study indicated that UV irradiation decreased the
protein expression levels of SIRT1, which is in accordance with the
previous study by Ming et al (76) in which the level of SIRT1 in
patients with UV-related skin cancer was reduced. Moreover, the
present study found that UV radiation increased the acetylation and
phosphorylation of p65 to activate NF-κB. CISCFE
increased SIRT1 protein expression levels and inhibited the
activation of the NF-κB pathway, thereby potentially reducing the
occurrence of skin inflammation, and even cancer.
In summary, CISCFE exhibited potent
anti-inflammatory and anti-skin cancer activity (Fig. 8). CISCFE inhibited
UV-induced epidermal abnormal proliferation and dermal fiber
damage, reducing epidermal cell carcinogenesis. CISCFE
also suppressed oxidative stress and the inflammatory response in
mouse skin. CISCFE enhanced the protein expression level
of SIRT1, which suppressed the abnormal activation of the
pro-inflammatory factor NF-κB. Moreover, CISCFE reduced
the protein expression level of p62, which reduced the abnormal
activation of Nrf2. Therefore, the potential effect of
CISCFE on skin cancer may be related to its
anti-inflammatory and antioxidant chemical compositions. These
findings suggested that CISCFE may potentially be a
future prospective drug for the prevention and therapy of
UV-induced skin cancer. However, the present study evaluated the
anti-UV-induced skin cancer effects of CISCFE in mice
and was not evaluated in patients with skin cancer. In future
studies, the therapeutic effects of CISCFE should be
studied in patients with skin cancer. In addition, the present
study evaluated the expression levels of CD11b, which is commonly
used as a biomarker for NK cells, monocytes, dendritic cells and
neutrophils (77-80).
B cells and T cells are also important classes of immune cells that
serve an important role in skin inflammation and skin cancer
progression (81,82). In future studies, the relationship
between T cells and B cells and the progression of skin cancer
should be examined.
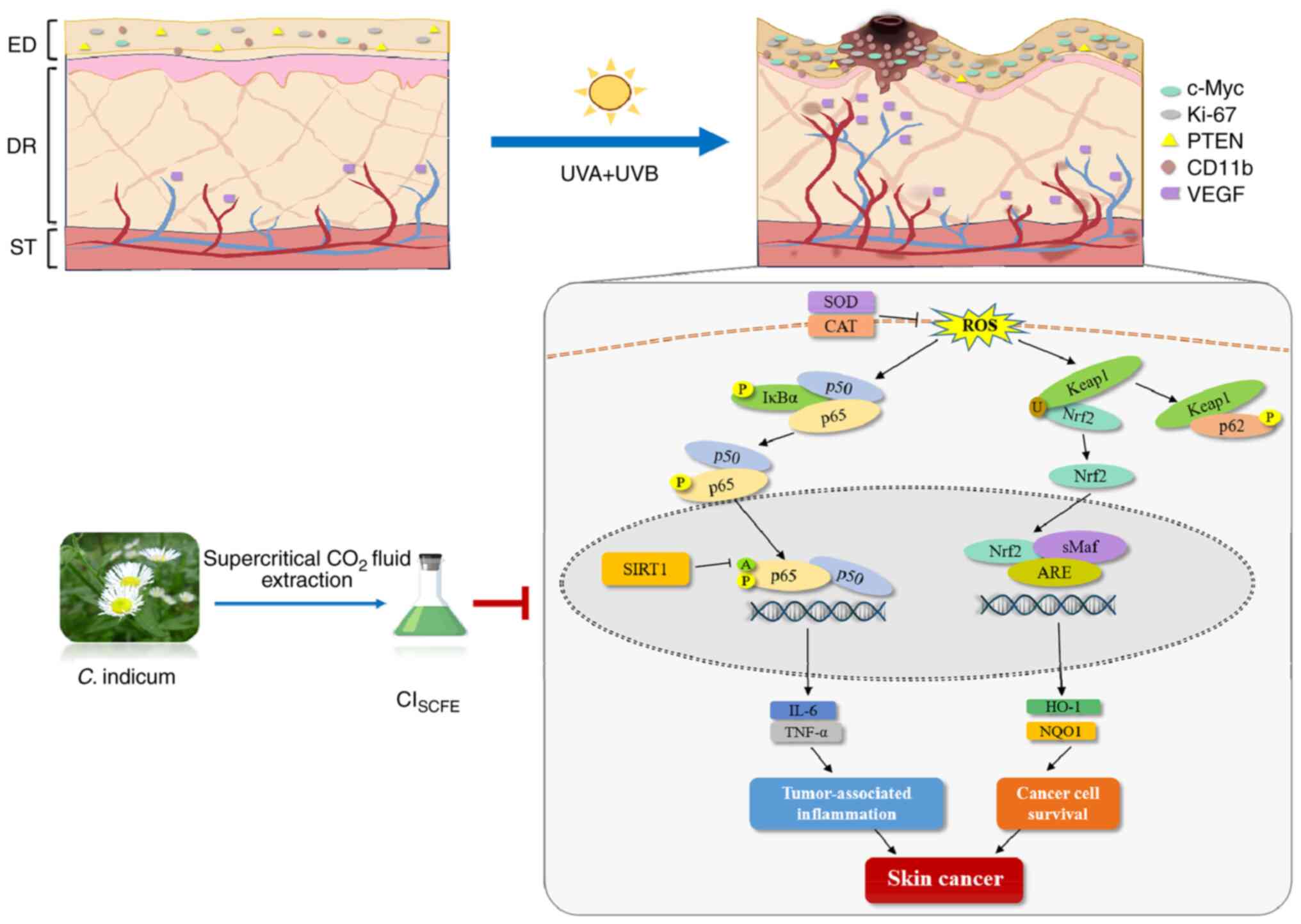 | Figure 8Summary schema of the protective
mechanism of CISCFE against UV-induced skin
carcinogenesis. CISCFE, supercritical carbon dioxide
fluid extraction of C. indicum; SOD, superoxide dismutase;
CAT, catalase; ROS, reactive oxygen species; Keap1, Kelch-like ECH
associated protein 1; Nrf2, nuclear factor 2 erythroid 2-related
factor 2; SIRT1, NAD-dependent protein deacetylase sirtuin-1; HO-1,
heme oxygenase 1; NQO1, NAD(P)H dehydrogenase [quinone] 1; P,
phosphorylated; A, acetylated; U, ubiquitinated; C. indicum,
Chrysanthemum indicum Linnén; sMaf, small musculoaponeurotic
fibrosarcoma; ARE, antioxidant response elements; ED, epidermis;
DR, dermis; ST, subcutaneous tissue. |
Supplementary Material
High-performance liquid chromatograph
of the 75% methyl alcohol layers of the supercritical carbon
dioxide fluid extraction of Chrysanthemum indicum Linnén.
mAU, m absorbance unit.
Chemical compositions of supercritical
carbon dioxide extract from Chrysanthemum indicum Linnén by
gas chromatography-mass spectrometry analysis (˃2%) and HPLC
analysis.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by The Science and
Technology Planning Project of Guangzhou (grant no.
202102021263).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YXZ and BQL developed and designed the study
concept. QHL, QYZ, HJC, HEH, YQH, YCL, BL, YQW and SLD conducted
the experiments and collected data. QHL, QYZ and HJC interpreted
the results and drafted the manuscript. BQL, XHD and YXZ analyzed
data and confirm the authenticity of all the raw data. All authors
have read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
guidelines of the Animal Care and Use Committee of Guangzhou
University of Chinese Medicine (approval no. 20190304024;
Guangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249.
2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Burton KA, Ashack KA and Khachemoune A:
Cutaneous squamous cell carcinoma: A review of high-risk and
metastatic disease. Am J Clin Dermatol. 17:491–508. 2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Rogers HW, Weinstock MA, Feldman SR and
Coldiron BM: Incidence estimate of nonmelanoma skin cancer
(keratinocyte carcinomas) in the U.S. population, 2012. JAMA
Dermatol. 151:1081–1086. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bachelor MA and Bowden GT: UVA-mediated
activation of signaling pathways involved in skin tumor promotion
and progression. Semin Cancer Biol. 14:131–138. 2004.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Winge MCG, Kellman LN, Guo K, Tang JY,
Swetter SM, Aasi SZ, Sarin KY, Chang ALS and Khavari PA: Advances
in cutaneous squamous cell carcinoma. Nat Rev Cancer. 23:430–449.
2023.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Forrester SJ, Kikuchi DS, Hernandes MS, Xu
Q and Griendling KK: Reactive oxygen species in metabolic and
inflammatory signaling. Circ Res. 122:877–902. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Singh A, Kukreti R, Saso L and Kukreti S:
Mechanistic insight into oxidative stress-triggered signaling
pathways and type 2 diabetes. Molecules. 27(950)2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Kim YE and Kim J: ROS-scavenging
therapeutic hydrogels for modulation of the inflammatory response.
ACS Appl Mater Interfaces: Dec 28, 2021 (Epub ahead of print).
|
|
9
|
Aggarwal V, Tuli HS, Varol A, Thakral F,
Yerer MB, Sak K, Varol M, Jain A, Khan MA and Sethi G: Role of
reactive oxygen species in cancer progression: molecular mechanisms
and recent advancements. Biomolecules. 9(735)2019.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kawanishi S, Ohnishi S, Ma N, Hiraku Y and
Murata M: Crosstalk between DNA damage and inflammation in the
multiple steps of carcinogenesis. Int J Mol Sci.
18(1808)2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Jomova K, Raptova R, Alomar SY, Alwasel
SH, Nepovimova E, Kuca K and Valko M: Reactive oxygen species,
toxicity, oxidative stress, and antioxidants: Chronic diseases and
aging. Arch Toxicol. 97:2499–2574. 2023.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhao Y, Ye X, Xiong Z, Ihsan A, Ares I,
Martínez M, Lopez-Torres B, Martínez-Larrañaga MR, Anadón A, Wang X
and Martínez MA: Cancer metabolism: the role of ROS in DNA damage
and induction of apoptosis in cancer cells. Metabolites.
13(796)2023.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Jaramillo MC and Zhang DD: The emerging
role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev.
27:2179–2191. 2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Kitamura H and Motohashi H: NRF2 addiction
in cancer cells. Cancer Sci. 109:900–911. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
He F, Antonucci L and Karin M: NRF2 as a
regulator of cell metabolism and inflammation in cancer.
Carcinogenesis. 41:405–416. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Ichimura Y, Waguri S, Sou YS, Kageyama S,
Hasegawa J, Ishimura R, Saito T, Yang Y, Kouno T, Fukutomi T, et
al: Phosphorylation of p62 activates the Keap1-Nrf2 pathway during
selective autophagy. Mol Cell. 51:618–631. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Singh V and Ubaid S: Role of silent
information regulator 1 (SIRT1) in regulating oxidative stress and
inflammation. Inflammation. 43:1589–1598. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jiang SB, Lu YS, Liu T, Li LM, Wang HX, Wu
Y, Gao XH and Chen HD: UVA influenced the
SIRT1-miR-27a-5p-SMAD2-MMP1/COL1/BCL2 axis in human skin primary
fibroblasts. J Cell Mol Med. 24:10027–10041. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wu P, Zhang B, Han X, Sun Y, Sun Z, Li L,
Zhou X, Jin Q, Fu P, Xu W and Qian H: HucMSC exosome-delivered
14-3-3ζ alleviates ultraviolet radiation-induced photodamage via
SIRT1 pathway modulation. Aging. 13:11542–11563. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Valente S, Mellini P, Spallotta F, Carafa
V, Nebbioso A, Polletta L, Carnevale I, Saladini S, Trisciuoglio D,
Gabellini C, et al: 1,4-Dihydropyridines active on the SIRT1/AMPK
pathway ameliorate skin repair and mitochondrial function and
exhibit inhibition of proliferation in cancer cells. J Med Chem.
59:1471–1491. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Puppala ER, Yalamarthi SS, Aochenlar SL,
Prasad N, Syamprasad NP, Singh M, Nanjappan SK, Ravichandiran V,
Tripathi DM, Gangasani JK and Naidu VGM: Mesua assamica
(King&Prain) kosterm. Bark ethanolic extract attenuates chronic
restraint stress aggravated DSS-induced ulcerative colitis in mice
via inhibition of NF-κB/STAT3 and activation of HO-1/Nrf2/SIRT1
signaling pathways. J Ethnopharmacol. 301(115765)2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zhang X, Wu JZ, Lin ZX, Yuan QJ, Li YC,
Liang JL, Zhan JY, Xie YL, Su ZR and Liu YH: Ameliorative effect of
supercritical fluid extract of Chrysanthemum indicum Linnén
against D-galactose induced brain and liver injury in senescent
mice via suppression of oxidative stress, inflammation and
apoptosis. J Ethnopharmacol. 234:44–56. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Shao Y, Sun Y, Li D and Chen Y:
Chrysanthemum indicum L.: A comprehensive review of its
botany, phytochemistry and pharmacology. Am J Chin Med. 48:871–897.
2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kim WJ, Yu HS, Bae WY, Ko KY, Chang KH,
Lee NK and Paik HD: Chrysanthemum indicum suppresses
adipogenesis by inhibiting mitotic clonal expansion in 3T3-L1
preadipocytes. J Food Biochem. 45(e13896)2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang X, Liu Y, Zhong C, Hu J, Xu S, Zhang
P and He L: Total flavonoids of Chrysanthemum indicum L
inhibit acute pancreatitis through suppressing apoptosis and
inflammation. BMC Complement Med Ther. 23(23)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Wu XL, Li CW, Chen HM, Su ZQ, Zhao XN,
Chen JN, Lai XP, Zhang XJ and Su ZR: Anti-inflammatory effect of
supercritical-carbon dioxide fluid extract from flowers and buds of
Chrysanthemum indicum Linnen. Evid Based Complement Alternat
Med. 2013(413237)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Yang HM, Sun CY, Liang JL, Xu LQ, Zhang
ZB, Luo DD, Chen HB, Huang YZ, Wang Q, Lee DY, et al:
Supercritical-carbon dioxide fluid extract from Chrysanthemum
indicum enhances anti-tumor effect and reduces toxicity of
bleomycin in tumor-bearing mice. Int J Mol Sci.
18(465)2017.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang X, Xie YL, Yu XT, Su ZQ, Yuan J, Li
YC, Su ZR, Zhan JY and Lai XP: Protective effect of super-critical
carbon dioxide fluid extract from flowers and buds of
Chrysanthemum indicum Linnén against ultraviolet-induced
photo-aging in mice. Rejuvenation Res. 18:437–448. 2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
MacArthur Clark JA and Sun D: Guidelines
for the ethical review of laboratory animal welfare people's
republic of china national standard GB/T 35892-2018 [Issued 6
February 2018 Effective from 1 September 2018]. Animal Model Exp
Med. 3:103–113. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Pillon A, Gomes B, Vandenberghe I, Cartron
V, Cèbe P, Blanchet JC, Sibaud V, Guilbaud N, Audoly L, Lamant L
and Kruczynski A: Actinic keratosis modelling in mice: A
translational study. PLoS One. 12(e0179991)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhong QY, Lin B, Chen YT, Huang YP, Feng
WP, Wu Y, Long GH, Zou YN, Liu Y, Lin BQ, et al: Gender differences
in UV-induced skin inflammation, skin carcinogenesis and systemic
damage. Environ Toxicol Pharmacol. 81(103512)2021.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang Y, Zhao Z, Jiao W, Yin Z, Zhao W, Bo
H, Bi Z, Dong B, Chen B and Wang Z: PRAF2 is an oncogene acting to
promote the proliferation and invasion of breast cancer cells. Exp
Ther Med. 24(738)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Que SKT, Zwald FO and Schmults CD:
Cutaneous squamous cell carcinoma: Incidence, risk factors,
diagnosis, and staging. J Am Acad Dermatol. 78:237–247.
2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Sun S, Jiang P, Su W, Xiang Y, Li J, Zeng
L and Yang S: Wild chrysanthemum extract prevents UVB
radiation-induced acute cell death and photoaging. Cytotechnology.
68:229–240. 2016.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Allen NC, Martin AJ, Snaidr VA, Eggins R,
Chong AH, Fernandéz-Peñas P, Gin D, Sidhu S, Paddon VL, Banney LA,
et al: Nicotinamide for skin-cancer chemoprevention in transplant
recipients. N Engl J Med. 388:804–812. 2023.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Snaidr VA, Damian DL and Halliday GM:
Nicotinamide for photoprotection and skin cancer chemoprevention: A
review of efficacy and safety. Exp Dermatol. 28 (Suppl 1):S15–S22.
2019.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Damian DL: Nicotinamide for skin cancer
chemoprevention. Australas J Dermatol. 58:174–180. 2017.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Stratigos A, Garbe C, Lebbe C, Malvehy J,
del Marmol V, Pehamberger H, Peris K, Becker JC, Zalaudek I, Saiag
P, et al: Diagnosis and treatment of invasive squamous cell
carcinoma of the skin: European consensus-based interdisciplinary
guideline. Eur J Cancer. 51:1989–2007. 2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Stratigos AJ, Garbe C, Dessinioti C, Lebbe
C, Bataille V, Bastholt L, Dreno B, Fargnoli MC, Forsea AM, Frenard
C, et al: European interdisciplinary guideline on invasive squamous
cell carcinoma of the skin: Part 1. Epidemiology, diagnostics and
prevention. Eur J Cancer. 128:60–82. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Dorrell DN and Strowd LC: Skin cancer
detection technology. Dermatol Clin. 37:527–536. 2019.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Firnhaber JM: Basal cell and cutaneous
squamous cell carcinomas: Diagnosis and treatment. Am Fam
Physician. 102:339–346. 2020.PubMed/NCBI
|
|
42
|
Wang X, Liu Y, Niu Y, Wang N and Gu W: The
chemical composition and functional properties of essential oils
from four species of Schisandra growing wild in the qinling
mountains, China. Molecules. 23(1645)2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Rahaman A, Chaudhuri A, Sarkar A,
Chakraborty S, Bhattacharjee S and Mandal DP: Eucalyptol targets
PI3K/Akt/mTOR pathway to inhibit skin cancer metastasis.
Carcinogenesis. 43:571–583. 2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Rawat A, Rawat M, Prakash OM, Kumar R,
Punetha H and Rawat DS: Comparative study on eucalyptol and camphor
rich essential oils from rhizomes of Hedychium spicatum Sm. and
their pharmacological, antioxidant and antifungal activities. An
Acad Bras Cienc. 94(e20210932)2022.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Li Y, Wen JM, Du CJ, Hu SM, Chen JX, Zhang
SG, Zhang N, Gao F, Li SJ, Mao XW, et al: Thymol inhibits bladder
cancer cell proliferation via inducing cell cycle arrest and
apoptosis. Biochem Biophys Res Commun. 491:530–536. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Xiu Z, Zhu Y, Han J, Li Y, Yang X, Yang G,
Song G, Li S, Li Y, Cheng C, et al: Caryophyllene oxide induces
ferritinophagy by regulating the NCOA4/FTH1/LC3 pathway in
hepatocellular carcinoma. Front Pharmacol.
13(930958)2022.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Zhen ZG, Ren SH, Ji HM, Ma JH, Ding XM,
Feng FQ, Chen SL, Zou P, Ren JR and Jia L: Linarin suppresses
glioma through inhibition of NF-κB/p65 and up-regulating p53
expression in vitro and in vivo. Biomed Pharmacother. 95:363–374.
2017.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Wang L, Du H and Chen P: Chlorogenic acid
inhibits the proliferation of human lung cancer A549 cell lines by
targeting annexin A2 in vitro and in vivo. Biomed Pharmacother.
131(110673)2020.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Velmurugan BK, Lin JT, Mahalakshmi B,
Chuang YC, Lin CC, Lo YS, Hsieh MJ and Chen MK:
Luteolin-7-O-glucoside inhibits oral cancer cell migration and
invasion by regulating matrix metalloproteinase-2 expression and
extracellular signal-regulated kinase pathway. Biomolecules.
10(502)2020.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Singh S, Gupta P, Meena A and Luqman S:
Acacetin, a flavone with diverse therapeutic potential in cancer,
inflammation, infections and other metabolic disorders. Food Chem
Toxicol. 145(111708)2020.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Weinstein IB and Joe A: Oncogene
addiction. Cancer Res. 68:3077–3080. 2008.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Wu H, Yang TY, Li Y, Ye WL, Liu F, He XS,
Wang JR, Gan WJ, Li XM, Zhang S, et al: Tumor necrosis factor
receptor-associated factor 6 promotes hepatocarcinogenesis by
interacting with histone deacetylase 3 to enhance c-Myc gene
expression and protein stability. Hepatology. 71:148–163.
2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Pelengaris S, Littlewood T, Khan M, Elia G
and Evan G: Reversible activation of c-Myc in skin: Induction of a
complex neoplastic phenotype by a single oncogenic lesion. Mol
Cell. 3:565–577. 1999.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Menon SS, Guruvayoorappan C, Sakthivel KM
and Rasmi RR: Ki-67 protein as a tumour proliferation marker. Clin
Chim Acta. 491:39–45. 2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Viallard C and Larrivée B: Tumor
angiogenesis and vascular normalization: Alternative therapeutic
targets. Angiogenesis. 20:409–426. 2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Carmeliet P: VEGF as a key mediator of
angiogenesis in cancer. Oncology. 69 (Suppl 3):S4–S10.
2005.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Álvarez-Garcia V, Tawil Y, Wise HM and
Leslie NR: Mechanisms of PTEN loss in cancer: It's all about
diversity. Semin Cancer Biol. 59:66–79. 2019.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Wang Y, Digiovanna JJ, Stern JB, Hornyak
TJ, Raffeld M, Khan SG, Oh KS, Hollander MC, Dennis PA and Kraemer
KH: Evidence of ultraviolet type mutations in xeroderma pigmentosum
melanomas. Proc Natl Acad Sci USA. 106:6279–6284. 2009.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Garg C, Sharma H and Garg M: Skin
photo-protection with phytochemicals against photo-oxidative
stress, photo-carcinogenesis, signal transduction pathways and
extracellular matrix remodeling-An overview. Ageing Res Rev.
62(101127)2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Al-Sadek T and Yusuf N: Ultraviolet
radiation biological and medical implications. Curr Issues Mol
Biol. 46:1924–1942. 2024.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Jelic MD, Mandic AD, Maricic SM and
Srdjenovic BU: Oxidative stress and its role in cancer. J Cancer
Res Ther. 17:22–28. 2021.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Murtas D, Piras F, Minerba L, Ugalde J,
Floris C, Maxia C, Demurtas P, Perra MT and Sirigu P: Nuclear
8-hydroxy-2'-deoxyguanosine as survival biomarker in patients with
cutaneous melanoma. Oncol Rep. 23:329–335. 2010.PubMed/NCBI
|
|
63
|
Cerutti P, Ghosh R, Oya Y and Amstad P:
The role of the cellular antioxidant defense in oxidant
carcinogenesis. Environ Health Perspect. 102 (Suppl 10):S123–S129.
1994.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Sluyter R and Halliday GM: Infiltration by
inflammatory cells required for solar-simulated ultraviolet
radiation enhancement of skin tumor growth. Cancer Immunol
Immunother. 50:151–156. 2001.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Mittal A, Elmets CA and Katiyar SK: CD11b+
cells are the major source of oxidative stress in UV
radiation-irradiated skin: possible role in photoaging and
photocarcinogenesis. Photochem Photobiol. 77:259–264.
2003.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Katiyar SK, Meleth S and Sharma SD:
Silymarin, a flavonoid from milk thistle (Silybum marianum L.),
inhibits UV-induced oxidative stress through targeting infiltrating
CD11b+ cells in mouse skin. Photochem Photobiol. 84:266–271.
2008.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Su H, Yang F, Fu R, Li X, French R, Mose
E, Pu X, Trinh B, Kumar A, Liu J, et al: Cancer cells escape
autophagy inhibition via NRF2-induced macropinocytosis. Cancer
Cell. 39:678–693.e11. 2021.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Kim YR, Oh JE, Kim MS, Kang MR, Park SW,
Han JY, Eom HS, Yoo NJ and Lee SH: Oncogenic NRF2 mutations in
squamous cell carcinomas of oesophagus and skin. J Pathol.
220:446–451. 2010.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Jiang T, Harder B, Rojo de la Vega M, Wong
PK, Chapman E and Zhang DD: p62 links autophagy and Nrf2 signaling.
Free Radic Biol Med. 88:199–204. 2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Lee DH, Park JS, Lee YS, Han J, Lee DK,
Kwon SW, Han DH, Lee YH and Bae SH: SQSTM1/p62 activates
NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and
protects mouse liver from lipotoxicity. Autophagy. 16:1949–1973.
2020.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Inoue J, Gohda J, Akiyama T and Semba K:
NF-kappaB activation in development and progression of cancer.
Cancer Sci. 98:268–274. 2007.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Bellezza I, Giambanco I, Minelli A and
Donato R: Nrf2-Keap1 signaling in oxidative and reductive stress.
Biochim Biophys Acta Mol Cell Res. 1865:721–733. 2018.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Li CX, Gao JG, Wan XY, Chen Y, Xu CF, Feng
ZM, Zeng H, Lin YM, Ma H, Xu P, et al: Allyl isothiocyanate
ameliorates lipid accumulation and inflammation in nonalcoholic
fatty liver disease via the Sirt1/AMPK and NF-κB signaling
pathways. World J Gastroenterol. 25:5120–5133. 2019.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Chen M, Chen Z, Huang D, Sun C, Xie J,
Chen T, Zhao X, Huang Y, Li D, Wu B and Wu D: Myricetin inhibits
TNF-α-induced inflammation in A549 cells via the SIRT1/NF-κB
pathway. Pulm Pharmacol Ther. 65(102000)2020.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Sun HJ, Xiong SP, Cao X, Cao L, Zhu MY, Wu
ZY and Bian JS: Polysulfide-mediated sulfhydration of SIRT1
prevents diabetic nephropathy by suppressing phosphorylation and
acetylation of p65 NF-κB and STAT3. Redox Biol.
38(101813)2021.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Ming M, Shea CR, Guo X, Li X, Soltani K,
Han W and He YY: Regulation of global genome nucleotide excision
repair by SIRT1 through xeroderma pigmentosum C. Proc Natl Acad Sci
USA. 107:22623–22628. 2010.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Scott NR, Swanson RV, Al-Hammadi N,
Domingo-Gonzalez R, Rangel-Moreno J, Kriel BA, Bucsan AN, Das S,
Ahmed M, Mehra S, et al: S100A8/A9 regulates CD11b expression and
neutrophil recruitment during chronic tuberculosis. J Clin Invest.
130:3098–3112. 2020.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Crinier A, Narni-Mancinelli E, Ugolini S
and Vivier E: SnapShot: Natural killer cells. Cell.
180:1280–1280.e1. 2020.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Subhi Y, Krogh Nielsen M, Molbech CR,
Krüger Falk M, Singh A, Hviid TVF, Nissen MH and Sørensen TL:
Association of CD11b+ monocytes and anti-vascular endothelial
growth factor injections in treatment of neovascular age-related
macular degeneration and polypoidal choroidal vasculopathy. JAMA
Ophthalmol. 137:515–522. 2019.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Rombouts M, Ammi R, Van Brussel I, Roth L,
De Winter BY, Vercauteren SR, Hendriks JM, Lauwers P, Van Schil PE,
De Meyer GR, et al: Linking CD11b (+) dendritic cells and natural
killer T cells to plaque inflammation in atherosclerosis. Mediators
Inflamm. 2016(6467375)2016.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Sluyter R and Halliday GM: Enhanced tumor
growth in UV-irradiated skin is associated with an influx of
inflammatory cells into the epidermis. Carcinogenesis.
21:1801–1807. 2000.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Scibiorek M, Mthembu N, Mangali S, Ngomti
A, Ikwegbue P, Brombacher F and Hadebe S: IL-4Rα signalling in B
cells and T cells play differential roles in acute and chronic
atopic dermatitis. Sci Rep. 13(144)2023.PubMed/NCBI View Article : Google Scholar
|