Introduction
Lower-extremity diseases are common, often
presenting a health-threatening problem that may lead to knee
amputation. The estimated global frequency in adults, primarily
those over 40 years old, was 9.7% (95% CI, 7.1-12.4), with women
having a greater prevalence (10.2%) than men (8.8%) (1-3).
Despite the therapeutic advances that have been made in this field,
limb salvage still carries a high risk of functional restrictions,
and is associated with pain for patients, which may eventually
force a late amputation. Increased hospital admissions, higher
costs, longer hospital stays, anxiety and depression have all been
linked to late amputation (4-7).
Below-knee amputation (BKA) is a rare form of surgery among
patients who are subjected to total knee arthroplasty. For BKA, no
set rules, indications or contraindications exist; decisions are
made on a case-by-case basis according to expert opinions. Every
patient is assessed for limb-sparing surgery before any amputations
are performed, as in the case of other types of amputation.
High-grade sarcoma of the lower limbs that is widespread and
infiltrative provides typical grounds for BKA (8). The main benefit of BKA is that the
knee joint is preserved, at least in cases where it remains
functional and has <15% flexion contracture. In contrast to
above-knee amputations, BKA enables improved prospects of recovery
and ambulation (9). If the
contralateral knee develops symptomatic osteoarthritis after BKA,
the prosthesis can be adjusted to unload the affected compartment
in addition to standard conservative therapy (10). Transmetatarsal amputation (TMA) may
appear to be the best option among below-knee patients, since it is
both quick and technically easy to perform, preserves lower
extremity length, causes low levels of blood loss, improves energy
conservation and is attractive to patients from a cosmetic
standpoint. One drawback of TMA, however, is that it may
conceivably result in a requirement for further revision surgeries,
including more severe amputations, when compared with BKAs
(11).
Especially with regard to studies analyzing the
mortality and morbidity of patients after surgery, those that
evaluate the ratios of blood values are currently increasing in
importance. Ratios, such as the neutrophile-to-lymphocyte ratio
(NLR) and the platelet-to-lymphocyte ratio, are used in an
increasing number of studies, and results on their predictive
values are being published. The general common goal of these
studies is to identify the ratios between blood values for specific
diseases that provide information about the mortality and morbidity
of patients (12-14).
Although the number of treatment options and the
opportunities for selecting BKA have increased, BKA remains an
important health problem in terms of mortality (1-7).
Conditions for selecting BKA include standardized prosthetic care
after trauma (4), the possibility
for more effective treatment if the cause of amputation is a
chronic disease, such as diabetic foot (5), and more dangerous situations, such as
vasopressor-dependent sepsis (6).
The mortality rate following BKA is 13-40% in the first year,
35-65% in the third year and 39-80% in the fifth year after
amputation (2). Better knowledge
of the indicators of mortality in the clinic, and especially the
predictive values of blood parameters that are easily and routinely
checked, can guide clinical practice and studies to predict and
prevent mortality. Therefore, determining indicators of mortality
according to the blood parameters may contribute to reducing
mortality in patients with BKA.
Although studies have been performed on the ratios
of post-surgical hemogram and blood values, at present, a small
number of studies have evaluated the predictive value of these
ratios on mortality and survival following BKA surgery (1-7).
Therefore, the aim of the present retrospective study was to
investigate the predictive value of blood parameters on the
mortality rates of patients with BKA. The main research objective
was to obtain information on clinical decision-making and treatment
processes through making mortality predictions based on blood
parameters in patients with BKA.
Patients and methods
Patients
This single-center, cross-sectional, retrospective
study included a total of 178 patients with BKA who were
hospitalized and operated on in the Orthopedics and Traumatology
Clinic in Kütahya Health Sciences University Hospital (Kütahya,
Turkey) between December 2016 and December 2022. In G*power 3.1.9.2
(G*Power Team), power analysis was performed for 0.50 effect size,
95% confidence inverval and 0.05 significance level. T critical was
found to be 1.6802300 and minimum patient number was found to be 45
patient files. The patients were divided into two groups, namely,
the exitus group (n=136; 76.4%) and the survivors group (n=42;
23.6%). Patients in the exitus group were divided further into
three subgroups: i) Those who experienced mortality in <1 month
(n=55; 40.4%); ii) those who experienced mortality between 1-12
months (n=48; 35.3%); and iii) those who experienced mortality in
>12 months after surgery (n=33; 24.3%). The present study was
approved by the Non-Invasive Clinical Research Ethics Committee of
Kütahya Health Sciences University (approval no.
E-41997688-050.99-74729).
Data on demographic characteristics, the number of
surgeries performed, postoperative mortality status and blood
parameters were obtained from the patients' files and the hospital
automation system. All data must have been present for inclusion in
the study. The blood parameters and ratios of patients were
evaluated at both the univariate and multivariate levels.
Since the study was retrospective, different risk
factors for mortality, epicrisis information and information about
which indication was followed in which center were not clear. For
this reason, indication-linked mortalities that were directly
reported as criteria in the study were excluded as BKA surgery
criteria. Patients with diabetic foot-related BKA were excluded.
The study was conducted in a public hospital where doctors may
change, patients often change addresses, epicrises may not be
complete, and certain patients may change institutions to benefit
from private health services. This makes it difficult to follow up
the patients in terms of risk factors for mortality.
Statistical analysis
Nominal data are presented as n (%), whereas
measurement data are presented as the mean ± SD. χ2 or
Fisher's exact test was used to analyze differences between
categorical variables. Missing values were excluded. The
Kolmogorov-Smirnov test was used to assess the normality of
measurement data. According to the normality test results,
non-parametric tests were used for statistical analysis, as the
measurement parameters did not conform to the standard normal
distribution. Therefore, Mann-Whitney U test was used to compare
two groups, and Kruskal-Wallis followed by Dunn's post hoc test was
used for pairwise comparisons between multiple groups. Binary
logistic regression and the generalized linear model (ordinal
logit) were used for linearization deviations of nonparametric data
(15,16), and the parameters with significant
differences between the mortality groups/subgroups were included in
these analyses. Receiver operating characteristic (ROC) curve
analysis was used, and in order to understand mortality rates, the
cut-off values used were selected to achieve the highest
sensitivity in the ROC analysis (17). Kaplan-Meier curve analysis was used
for survival analysis over time. As this was a retrospective study
that depended on the research design, Cox regression analysis was
not performed. SPSS version 25.0 (IBM Corp.) was used. P<0.05
was considered to indicate a statistically significant
difference.
Results
Sex and age distributions of
patients
The mean age of the survivors was 68.64±9.58 years
(range, 52-90 years), whereas that of the patients in the exitus
group was 74.91±0.01 years (range, 52-95 years). Of the survivor
and exitus groups of patients, 31.0 and 40.4% were female,
respectively. The baseline characteristics and results of the
analysis between the survivors and exitus groups of patients are
shown in Table I.
 | Table IBaseline characteristics and results
of the analysis between the survivors and exitus groups. |
Table I
Baseline characteristics and results
of the analysis between the survivors and exitus groups.
| | Group | |
|---|
| | Survivors (n=42) | Exitus (n=136) | |
|---|
| Parameter | Mean ± SD | Range | Mean ± SD | Range | P-value |
|---|
| Age, years | 68.64±9.58 | 52-90 | 74.911±0.01 | 52-95 |
<0.001a |
| Sex | | | | | |
|
Female | 13 (31.0) | | 55 (40.4) | | 0.178b |
|
Male | 29 (69.0) | | 81 (59.6) | | |
| RDW initial, n | 15.49±2.15 | 12.4-22.4 | 15.97±2.36 | 11.5-24.8 | 0.201a |
| Neutrophils, n
(x109/l) | 9.24±5.64 | 2.30-30.55 | 11.50±6.13 | 1.10-36.18 | 0.014a |
| Lymphocytes, n
(x109/l) | 1.71±0.74 | 0.75-3.50 | 1.35±0.61 | 0.21-3.19 | 0.008a |
| RDW discharge | 15.45±1.85 | 12.2-20.8 | 16.06±2.26 | 12.3-26.4 | 0.150a |
| RDW difference | 0.05±1.31 | -3-7 | -0.09±1.03 | -4-4 | 0.768a |
| Monocytes, n
(x109/l) | 0.73±0.26 | 0.31-1.57 | 0.59±0.31 | 0.01-1.40 | 0.004a |
| Eosinophill, n
(x109/l) | 0.22±0.22 | 0.01-1.30 | 0.16±0.37 | 0.01-3.70 | 0.000a |
| PLT, g/l | 355.36±151.65 | 157-756 | 249.38±147.42 | 16-1259 | 0.000a |
| MPV, g/l | 8.73±1.21 | 6.1-11.3 | 9.40±1.43 | 5.4-13.2 | 0.005a |
| Albumin, g/l | 2.74±0.43 | 2-4 | 2.45±0.46 | 2-4 |
<0.001a |
| Hemoglobin,
g/l | 13.05±14.15 | 8-102 | 10.45±1.64 | 7-16 | 0.073a |
| CRP, mg/dl | 77.48±55.24 | 3-262 | 143.83±97.87 | 1-502 |
<0.001a |
| NLR | 6.81±6.13 | 1.63-33.67 | 11.28±10.22 | 1.00-61.24 | 0.001a |
| ELR | 0.16±0.16 | 0-1 | 0.15±0.37 | 0-4 | 0.011a |
| MLR | 0.52±0.32 | 0-1 | 0.56±0.51 | 0-4 | 0.696a |
| PLR | 245.58±143.10 | 56-627 | 230.39±186.18 | 15-1114 | 0.227a |
| EMR | 0.32±0.32 | 0-2 | 1.42±10.40 | 0-116 | 0.040a |
| Hemoglobin/RDW | 0.81±0.61 | 0-5 | 0.67±0.14 | 0-1 | 0.037a |
| CRP/albumin | 30.10±24.16 | 1-105 | 62.78±46.76 | 0-245 | 0.000a |
| MPV/lymphocyte | 6.08±2.66 | 3-12 | 8.76±5.48 | 3-42 | 0.001a |
| HT | 2 (4.3) | | 2 (1.5) | | 0.272b |
| DM | 1 (2.2) | | 2 (1.5) | | 0.592b |
| CVE | 2 (4.3) | | 1 (0.8) | | 0.163b |
Clinical parameters and difference
analysis results
The mean values of lymphocytes, monocytes,
eosinophil-to-lymphocyte ratio (ELR), platelets (PLT), albumin,
eosinophils and the hemoglobin/red-blood-cell distribution width
(RDW) were found to be significantly higher in the survivors group
compared with those in the exitus groups (P<0.05). By contrast,
the mean values of patient age, neutrophils, mean platelet volume
(MPV), C-reactive protein (CRP), NLR, eosinophil-to-monocyte ratio
(EMR), CRP/albumin and MPV/lymphocyte ratios were significantly
higher in the exitus group (P<0.05) (Table I). In the present study, the
distribution of hypertension, cardiovascular events, diabetes
mellitus and other chronic comorbidities (such as asthma and artery
diseases) was found to be <5% in both groups, and the
distributions of both groups were similar in terms of these
comorbidities (Table I).
Logistic regression analysis
The binary logistic regression analysis results are
shown in Table II. Since the
single parameters among the significant parameters in Table I were also included in the ratios,
the ratios were used in the regression analysis. For example, while
the difference in neutrophils, lymphocytes and NLR was significant
between the survivors and exitus groups, only NLR was used in the
regression analysis, as it takes into account both neutrophils and
lymphocytes, and therefore the proportional value was taken to
avoid cointegration. Age, NLR, hemoglobin/RDW, and CRP/albumin and
MPV/lymphocyte ratios were significant at univariate level. The
results obtained showed that the exact age (B=0.061; P=0.01), ELR
(B=-2.861; P=0.03), and the CRP/albumin (B=0.027; P<0.01) and
MPV/lymphocyte (B=0.310; P<0.01) ratios had a significant effect
on mortality at the multivariate level. Moreover, regression
coefficients showed that the effects of the exact age, CRP/albumin
and MPV/lymphocyte ratios were positive, whereas that of the ELR
were negative (Table II).
 | Table IIBinary logistic regression analysis
results. |
Table II
Binary logistic regression analysis
results.
| | Univariate
analysis | Multivariate
analysis | 95% CI for
exponential (B) |
|---|
| Parameter | OR | SE | P-value | OR | SE | Wald | P-value | Exponential
(B) | Lower | Upper |
|---|
| Age | 0.065 | 0.019 | 0.001 | 0.061 | 0.022 | 7.652 | 0.006 | 1.062 | 1.018 | 1.109 |
| NLR | 0.087 | 0034 | 0.009 | -0.067 | 0.047 | 2.058 | 0.151 | 0.935 | 0.853 | 1.025 |
| ELR | -0.039 | 0.515 | 0.940 | -2.861 | 1.331 | 4.623 | 0.032 | 0.057 | 0.004 | 0.776 |
| EMR | 0.059 | 0.132 | 0.654 | 0.474 | 0.721 | 0.431 | 0.511 | 1.606 | 0.391 | 6.606 |
| Hemoglobin/RDW | -2.481 | 1.219 | 0.042 | -1.336 | 1.178 | 1.287 | 0.257 | 0.263 | 0.026 | 2.646 |
| CRP/albumin | 0.029 | 0.007 | <0.001 | 0.027 | 0.008 | 10.911 | 0.001 | 1.027 | 1.011 | 1.044 |
| MPV/lymphocyte | 0.207 | 0.464 | 0.002 | 0.310 | 0.104 | 8.944 | 0.003 | 1.363 | 1.113 | 1.670 |
| Regression
constant | | | | -4.788 | 1.864 | 6.600 | 0.010 | 0.008 | | |
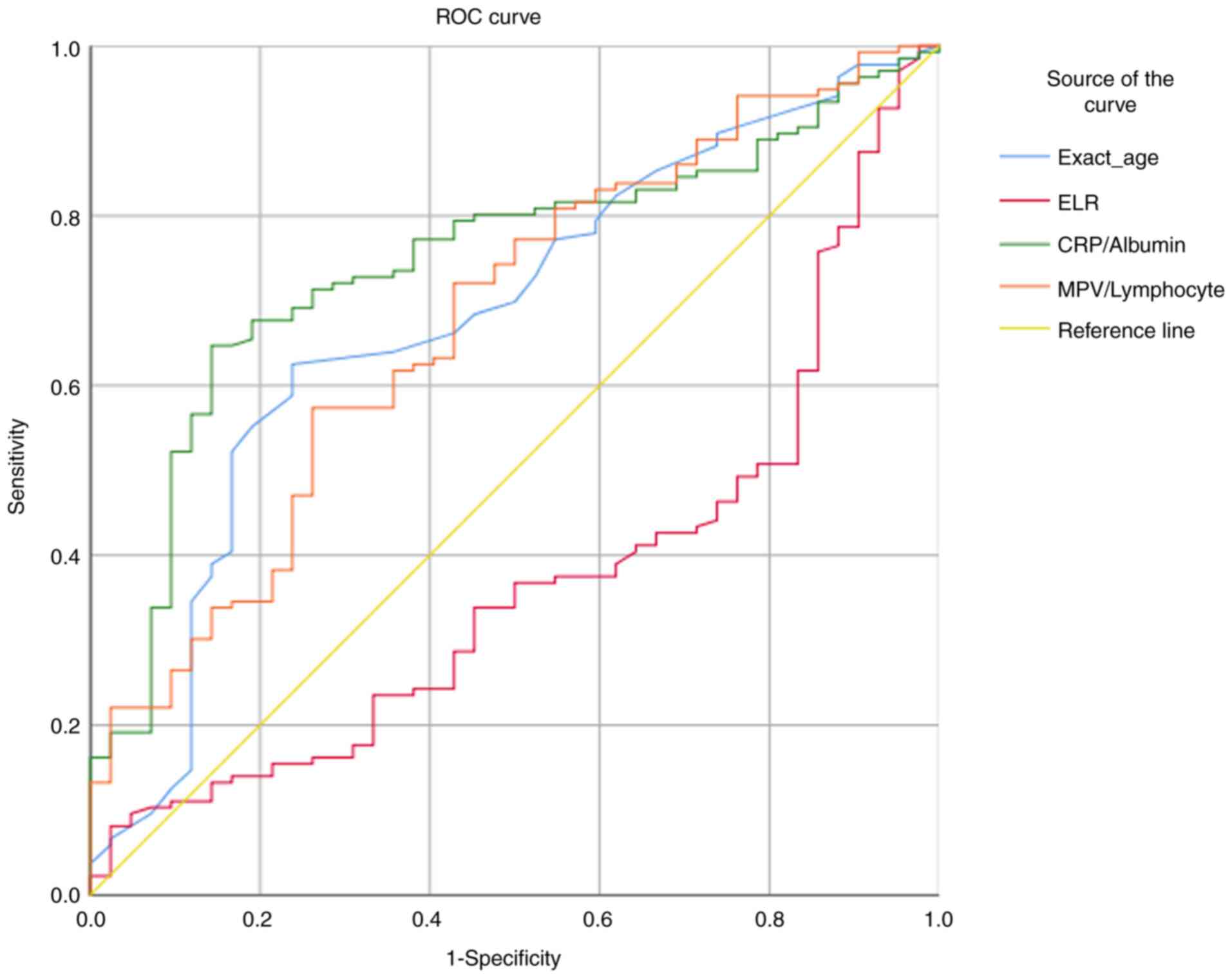
The ROC analysis results showed that the predictive
value of age on mortality [area under the curve (AUC)=0.681;
P=0.01], ELR (AUC=0.630; P=0.01), CRP/albumin (AUC=0.746; P=0.01)
and MPV/lymphocyte (AUC=0.676; P<0.01) ratios were statistically
significant. Although all factors were found to have significant
predictive value, the CRP/albumin ratio had the highest AUC value
and a predictive value of ~74.6%. For the 27.51 CRP/albumin cut-off
value, the sensitivity was found to be 80.1%, whereas the
specificity was 54.8%. By contrast, for the 36.93 CRP/albumin
cut-off value, the sensitivity was 71.3%, and the specificity was
73.8% (Fig. 1).
The baseline characteristics and results of the
analysis among the time-to-death subgroups are shown in Table III. The parameter female-to-male
sex ratio was significantly different between groups. The
parameters RDW discharge, MPV and CRP/albumin ratio were
significantly higher in the <1 month mortality subgroup
(P<0.05) compared with those in the 1-12-month mortality and
>12 month mortality subgroups. By contrast, age was found to be
significantly higher in the 1-12-month mortality subgroup
(P<0.05) compared with that in the <1 month mortality and
>12 month mortality subgroups. Finally, albumin, hemoglobin and
the hemoglobin/RDW ratio were significantly higher in the
>12-month mortality subgroup (P<0.05) compared with those in
the <1 month mortality and 1-12-month mortality subgroups
(Table III).
 | Table IIIBaseline characteristics and results
of the analysis between the time of death subgroups. |
Table III
Baseline characteristics and results
of the analysis between the time of death subgroups.
| | Subgroup | |
|---|
| Parameter | <1 month
(n=55) | 1-12 months
(n=48) | >12 months
(n=33) | P-value | Post hoc analysis
results |
|---|
| Age, years | 75.02±10.14 | 77.77±9.42 | 70.58±9.29 | 0.006a | II-III (0.003) |
| Sex | | | | | |
|
Female | 32 (58.2) | 18 (37.5) | 5 (15.2) |
<0.001b | |
|
Male | 23 (41.8) | 30 (62.5) | 28 (84.8) | | |
| RDW initial, n | 16.51±2.47 | 15.70±2.35 | 15.48±2.09 | 0.068a | |
| Neutrophils, n
(x109/l) | 13.05±6.75 | 10.08±4.31 | 10.96±6.84 | 0.061a | |
| Lymphocytes, n
(x109/l) | 1.37±0.61 | 1.33±0.66 | 1.36±0.55 | 0.853a | |
| RDW discharge | 16.65±2.38 | 15.70±2.06 | 15.62±2.19 | 0.026a | I-II (0.009) |
| RDW difference | -0.14±1.26 | 0.00±0.82 | -0.14±0.90 | 0.984a | |
| Monocytes, n
(x109/l) | 0.60±0.37 | 0.57±0.26 | 0.60±0.28 | 0.727a | |
| Eosinophils, n
(x109/l) | 0.17±0.28 | 0.12±0.13 | 0.20±0.63 | 0.964a | |
| PLT, g/l | 233.45±177.61 | 274.90±133.86 | 238.79±103.00 | 0.125a | |
| MPV, g/l | 9.87±1.50 | 9.05±1.30 | 9.14±1.32 | 0.014a | I-II (0.011) |
| Albumin, g/l | 2.30±0.37 | 2.40±0.43 | 2.76±0.52 |
<0.001a | I-III (0.000);
II-III (0.005) |
| Hemoglobin,
g/l | 10.01±1.71 | 10.49±1.26 | 11.15±1.81 | 0.005a | I-III (0.015) |
| CRP | 159.05±97.72 | 136.51±88.07 | 129.11±110.46 | 0.181a | |
| NLR | 12.19±10.56 | 10.37±9.60 | 11.09±10.69 | 0.451a | |
| ELR | 0.15±0.29 | 0.11±0.13 | 0.21±0.65 | 0.888a | |
| MLR | 0.55±0.47 | 0.53±0.39 | 0.62±0.72 | 0.940a | |
| PLR | 211.06±188.85 | 255.00±182.49 | 226.82±188.62 | 0.256a | |
| EMR | 2.47±15.62 | 0.27±0.34 | 1.32±6.41 | 0.947a | |
| Hemoglobin/RDW | 0.62±0.14 | 0.68±0.12 | 0.73±0.15 | 0.001a | I-III (0.003) |
| CRP/albumin | 70.69±44.50 | 60.80±43.75 | 52.47±53.36 | 0.046a | I-III (0.028) |
| MPV/lymphocyte | 8.58±3.86 | 8.89±5.71 | 8.85±7.30 | 0.564a | |
A generalized linear model (ordinal logit) was used
to associate the time to death with the factors that showed
significant differences among subgroups, and the results of this
analysis are presented in Table
IV. MPV and hemoglobin/RDW were significant at univariate
level. The results showed that MPV (B=-0.37; P=0.005) and the
hemoglobin/RDW ratio (B=5.20; P<0.001) had a significant effect
on the time to death (Table
IV).
 | Table IVGeneralized linear model (ordinal
logit) for the time of death. |
Table IV
Generalized linear model (ordinal
logit) for the time of death.
| | Multivariate
analysis |
|---|
| | Univariate
analysis | | 95% CI | Hypothesis
test |
|---|
| Parameter | B | SE | P-value | B | SE | Lower | Upper | Wald
χ2 | df | P-value |
|---|
| [Mortality, <1
month]a | | | | -1.920 | 1.520 | -4.910 | 1.060 | 1.590 | 1 | 0.207 |
| [Mortality, 1-12
months]a | | | | 0.080 | 1.520 | -2.890 | 3.060 | 0.010 | 1 | 0.956 |
| MPV | -0.322 | 0.118 | 0.006 | -0.370 | 0.130 | -0.623 | -0.110 | 7.960 | 1 | 0.005 |
| Hemoglobin/RDW | 4.604 | 1.229 | <0.001 | 5.200 | 1.320 | 2.61 | 7.780 | 15.480 | 1 | <0.001 |
| CRP/albumin | -0.007 | 0.004 | 0.066 | -0.010 | 0.010 | -0.015 | 0.010 | 2.120 | 1 | 0.145 |
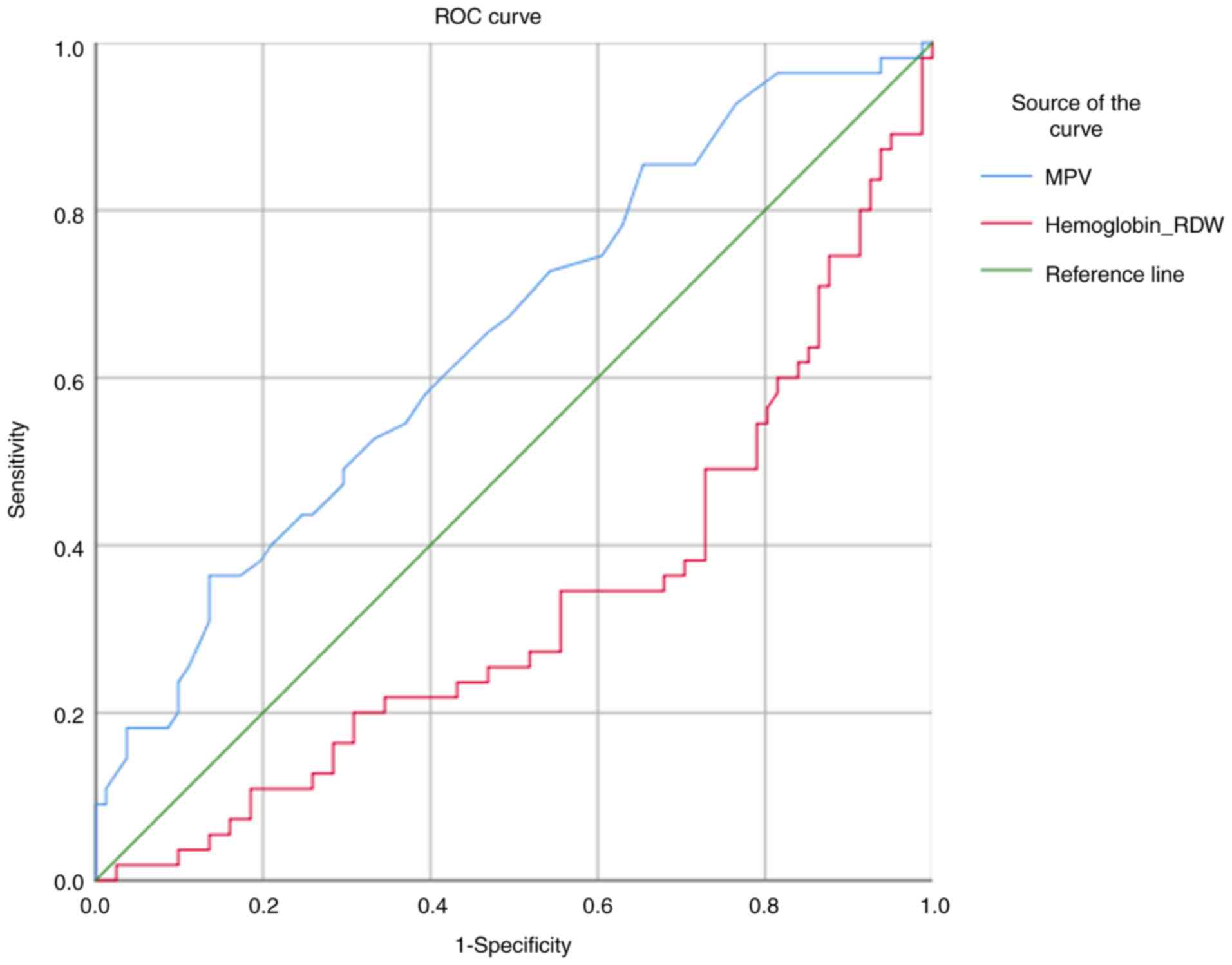
MPV (AUC=0.648; P=0.01) and the hemoglobin/RDW ratio
(AUC=0.673; P=0.01) were found to have predictive value in terms of
the time to death. The predictive value for MPV was 64.8%, whereas
that for the hemoglobin/RDW ratio was 67.3%. For the 0.54 cut-off
value for hemoglobin/RDW, the sensitivity was 74.5%, and the
specificity was 11.1%. In addition, for the 0.84 cut-off value for
hemoglobin/RDW, the sensitivity was found to be 10.9%, and the
specificity was 81.5% (Fig.
2).
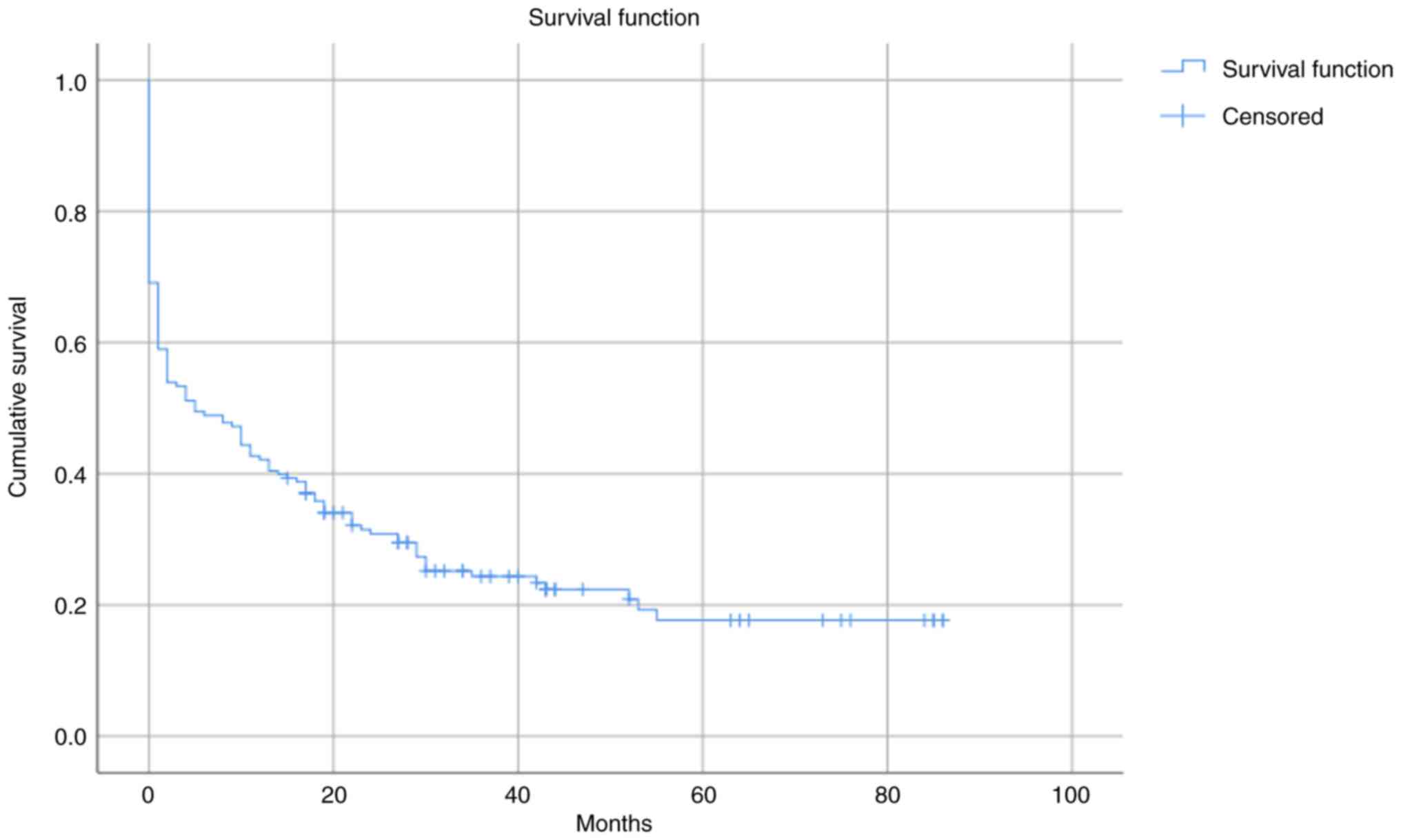
Finally, the results obtained from the Kaplan-Meier
analysis showed that the mean survival time for patients with BKA
was 23.616 months, with a 95% confidence interval of 18.620-28.612
months (Fig. 3).
Discussion
Predicting mortality in patients with BKA will be
able to guide future studies examining the underlying causes of
mortality in these patients, and also enable further research in
assessing patients who have a high probability of mortality. In
addition, the ability to make effective predictions based on
routinely measured blood parameters should contribute to improving
both clinical practice and the treatment process. Motivated by
these aims, the predictive value of blood parameters on mortality
in patients with BKA was investigated in the present study. During
the time period evaluated in the current study, 136 out of 178
patients died, whereas 42 patients survived.
Although BKA surgery enables superior recovery and
ambulation (9), BKA that is
performed early following trauma may increase the risk of
post-amputation revision surgery (4). In spite of the possibility of
revision surgery and the uncertainty in deciding to perform BKA
surgery as part of a general procedure, BKA does have certain
advantages for specific BKA patients compared with other types of
lower-extremity surgery (18-20).
However, the risk of mortality following BKA and the predictive
factors associated with mortality after BKA need to be
well-understood in order to both increase the success of the method
and save patient lives.
Mortality following a lower-extremity surgical
procedure and amputation presents a major problem that must be
evaluated with prognostic and risk factors, such as age and sex
(21-26).
Norvell and Czerniecki (27)
reported that age, white blood cell count, alcohol usage,
revascularization, kidney failure, diabetes and the ankle-brachial
index are risk factors associated with mortality following
amputation. In another study, Mustapha et al (28) reported that revascularization and
amputation techniques are associated with BKA risk factors and
mortality following limb ischemia. Ordaz et al (11) reported that TMA provides higher
ambulation rates compared with BKA with low BKA risk factors.
Finally, Bui et al (4)
reported that the stage at which BKA is performed (early vs. late)
may be a risk factor for mortality.
In the present study, the mean values of
lymphocytes, monocytes, eosinophils, PLT, albumin, ELR and the
hemoglobin/RDW ratio were significantly higher in the survivors
group compared with in the exitus group, whereas the mean values
for the exact age, number of neutrophils, MPV, CRP, NLR and EMR
were significantly higher in the exitus group. It could be argued
that the general blood parameters would be expected to be more
healthy and in accepted ranges, and the patients' age would be
expected to be higher in the mortality group. Therefore, the
health-associated parameters would be likely to be worse in
patients who lose their life. Blood ratios, such as the NLR and
EMR, may be of predictive value for assessing mortality in patients
with BKA. The results obtained in the present study were consistent
with the findings of Ordaz et al (11), Norvell and Czerniecki (27), and Mustapha et al (28) in showing that blood parameters may
help to predict mortality in patients with BKA. In our previous
study, systemic immune inflammation index and prognostic
nutritional index were associated with mortality in patients with
BKA and diabetic foot. In the present study, patients with diabetic
foot-related BKA were excluded (29). The agreement between the results
obtained in the present study and those reported in previous
studies may contribute to clinical practice, especially in terms of
assessing mortality risk and subsequently including this risk in
the treatment process. The development of metabolic disorders in
more elderly patients and patients following amputation may also
have an impact on these findings, as the the lifestyles would be
similar.
The multivariate regression analysis results showed
that age (B=0.061), ELR (B=-2.861), and the CRP/albumin (B=0.027)
and MPV/lymphocyte (B=0.310) ratios had a significant effect on
mortality at the multivariate level. According to the regression
coefficient, the most effective parameter was ELR (OR, -2.861),
followed by exact age (OR, 0.061) and CRP/albumin (OR, 0.027). The
effect of exact age may be associated with the presence of chronic
diseases and comorbidities in older patients. However, the effects
of ELR and CRP/albumin are also important factors that should be
evaluated further.
According to the results of the time-to-death
subgroup analysis, the female sex ratio differed among groups and
the parameters RDW discharge, MPV and CRP/albumin were
significantly higher in the <1-month mortality group compared
with the >12 months groups. Age was found to be significantly
higher in the 1-12-month mortality group, whereas albumin,
hemoglobin and the hemoglobin/RDW ratio were significantly higher
in the >12-month mortality group compared with the <1 month
group. The multivariate analysis results revealed that MPV
(B=-0.37) and the hemoglobin/RDW ratio (B=5.20) had a significant
effect on the time to death. For the <1-month mortality group,
the predictive value for MPV was 64.8%, and that for hemoglobin/RDW
was 67.3% for prediction of <1 month mortality. Moreover, the
predictive values for the exact age (AUC=0.681), ELR (AUC=0.630),
and the CRP/albumin (AUC=0.746) and MPV/lymphocyte (AUC=0.676)
ratios were statistically significant in terms of mortality. Taken
together, these results showed that ELR, CRP/albumin and
MPV/lymphocyte may be used for the prediction of mortality in
patients with BKA. Age may not be used for the prediction of
mortality due to cointegration. However, the survival predictive
parameter, hemoglobin/RDW, was not a predictive parameter for
mortality. This could be used to predict time-to-death for <1
month mortality. In this case, a patient having a high mortality
risk due to CRP/albumin may be further evaluated for survival by
analyzing the hemoglobin/RDW ratio.
The present study has a certain number of
limitations. Although a good sample size was reached in accordance
with the power analysis results of the current study, the number of
patients was still small, and BKA-associated mortality has only
recently started to be investigated. Furthermore, the fact that the
present study was single-center was another important limitation.
It would be beneficial to expand the study in the future with
larger samples and multicenter studies. Another limitation of the
present study is that it was retrospective. Patients having risk
factors for mortality, such as post-traumatic, tumor-related,
vessel-related or infections, will generally select private
hospitals or clinics for treatment. When the indication becomes
life-threatening or when there is organ amputation, financial
resources of public hospitals are strained and services are sought
from private health institutions. For the aforementioned reasons,
the lack of data on the association of different indications with
survival is a potential limitation of the current study. This
limitation points to the need for data integration in public
hospitals, and between the healthcare system and patient tracking
systems in private hospitals. Due to the limited prevalence and
incidence of BKA, prospective studies would require a long time to
be completed, during which both the populations and the
technologies are likely to change. Therefore, the present study was
designed as a retrospective study. As a result, analyses were made
only of the parameters available in the patient files. In the
future, further parameters, such as other comorbidities, genetic
predisposition or social factors, should also be included.
Furthermore, although the present study has provided important
results based on the analyzed group of patients and will be useful
in terms of guiding clinical practice, further research is required
to generalize these results. The aforementioned limitations prevent
the present findings from being generalized; however, they still
provide a basis for further research.
The most important contribution of the present study
to the field is that, although previous studies have analyzed
patients with different types of amputation, such as TMA,
ipsilateral re-amputation and critical limb ischemia, there have
been limited studies investigating BKA-associated mortality in
patients with BKA with no indication (11,27-29).
Furthermore, the results of the present study have suggested that
the predictive value of different parameters on mortality in
patients with BKA is high. In this regard, the present study may be
considered a pioneering study in the field and offer a pragmatic
approach for clinical applications. Another important contribution
that has been made by the present study is that it has examined
more than one blood value ratio, thereby allowing comparisons to be
made between them as predictive values. Therefore, the results of
the present study suggest that using more than one ratio in the
same model proportional to their coefficients will provide the most
effective results.
In clinical practice, previous studies have drawn
attention to patients with a high probability of mortality
following BKA (1-7).
If an improved understanding of routinely monitored blood
parameters is achieved, it may be possible to monitor individual
patients more closely according to their risk of mortality,
including performing further tests during follow-up and monitoring
other conditions that may cause mortality.
In conclusion, assessing ELR, CRP/albumin and
MPV/lymphocyte factors in patients with BKA may provide a better
understanding of postoperative mortality. In this context, however,
there is a need for more extensive studies to be performed, and
other demographic and comorbidity values should be included in
these further analyses. It is hoped that improved clinical outcomes
will be achieved against postoperative mortality in the future.
Acknowledgements
The authors would like to thank Dr Kadir Yilmaz
(Istanbul Commerce University, Istanbul, Turkey) for their valuable
help with statistics.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
Data collection was performed by TCD. Statistical
analysis was performed by TCD and MK. AOÜ, SY, SA and FK conducted
the literature search, writing of the article and confirm the
authenticity of all the raw data. TCD, AOÜ, SY, SA, MK and FK
analyzed the results and contributed to the final manuscript. The
original draft was written by TCD, AOÜ, SY, SA and FK. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Non-Invasive
Clinical Research Ethics Committee of Kütahya Health Sciences
University (approval no. E-41997688-050.99-74729; Kütahya, Turkey).
According to the research design and ethics approval, patient
consent was not required for this retrospective study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hsueh MC, Lin CY, Lai TF, Yu YC, Chang SH,
Bae JY and Liao Y: Is achieving 7,000 steps/day cross-sectionally
and prospectively associated with older adults' lower-extremity
performance? BMC Geriatr. 21(359)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Singh N, Armstrong DG and Lipsky BA:
Preventing foot ulcers in patients with diabetes. JAMA.
293:217–228. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Muehlbauer T, Gollhofer A and Granacher U:
Associations between measures of balance and lower-extremity muscle
strength/power in healthy individuals across the lifespan: A
systematic review and meta-analysis. Sports Med. 45:1671–1692.
2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bui G, Buckwalter J, Wilken J, Davison J,
Palmer J, Shurr D, Davidson N, Gracia-Fleury I and Willey M:
Comparison of early versus late below knee amputation after trauma
with standardized prosthetic care. Iowa Orthop J. 42:89–96.
2022.PubMed/NCBI
|
|
5
|
Liao X, Li SH, El Akkawi MM, Fu XB, Liu HW
and Huang YS: Surgical amputation for patients with diabetic foot
ulcers: A Chinese expert panel consensus treatment guide. Front
Surg. 9(1003339)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Reitz KM, Kennedy J, Rieser C, Hlavin C,
Gershengorn HB, Neal MD, Bensen N, Linstrum K, Prescott HC,
Rosengart MR, et al: The epidemiology of extremity threat and
amputation after vasopressor-dependent sepsis. Ann Am Thorac Soc.
19:625–632. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Jensen PS, Petersen J, Kirketerp-Møller K,
Poulsen I and Andersen O: Progression of disease preceding lower
extremity amputation in Denmark: A longitudinal registry study of
diagnoses, use of medication and healthcare services 14 years prior
to amputation. BMJ Open. 7(e016030)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Sugarbaker P, Bickels J and Malawer M:
Above-knee amputation. In: Musculoskeletal Cancer Surgery.
Springer, Dordrecht, pp351-362, 2004.
|
|
9
|
Randon C, Deroose J and Vermassen F: How
to perform a below-knee amputation. Acta Chir Belg. 103:238–240.
2003.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dong K, Cohen-Rosenblum A and Hartzler M:
Total knee arthroplasty after ipsilateral below-knee amputation: A
review of the literature and surgical techniques. Arthroplast
Today. 16:158–163. 2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ordaz A, Trimm C, Pedowitz J and Foran IM:
Transmetatarsal amputation results in higher frequency of revision
surgery and higher ambulation rates than below-knee amputation.
Foot Ankle Orthop. 7(24730114221112938)2022.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Li P, Li H, Ding S and Zhou J: NLR, PLR,
LMR and MWR as diagnostic and prognostic markers for laryngeal
carcinoma. Am J Transl Res. 14:3017–3027. 2022.PubMed/NCBI
|
|
13
|
Russu E, Mureșan AV, Arbănași EM, Kaller
R, Hosu I, Voidăzan S, Arbănași EM and Coșarcă CM: The predictive
role of NLR and PLR in outcome and patency of lower limb
revascularization in patients with femoropopliteal disease. J Clin
Med. 11(2620)2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Metineren H and Dülgeroğlu TC: Comparison
of the neutrophil/lymphocyte ratio and C-reactive protein levels in
patients with amputation for diabetic foot ulcers. Int J Low Extrem
Wounds. 16:23–28. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Yılmaz K and Turanlı M: A
multi-disciplinary investigation of linearization deviations in
different regression models. Asian J Probab Stat. 22:15–19.
2023.
|
|
16
|
Yilmaz K and Turanli M: A
multi-disciplinary investigation on minimizing linearization
deviations in different regression models. Change & Shaping The
Future, IV. ASC-2022/Fall Congress ISBN 978-625-8048-99-5,
2022.
|
|
17
|
Hillis SL: Equivalence of binormal
likelihood-ratio and bi-chi-squared ROC curve models. Stat Med.
35:2031–2057. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
18
|
Crane H, Boam G, Carradice D, Vanicek N,
Twiddy M and Smith GE: Through-knee versus above-knee amputation
for vascular and non-vascular major lower limb amputations.
Cochrane Database Syst Rev. 12(CD013839)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Ontario Health (Quality). Osseointegrated
prosthetic implants for people with lower-limb amputation: A health
technology assessment. Ont Health Technol Assess Ser. 19:1–126.
2019.PubMed/NCBI
|
|
20
|
Beyaz S, Güler ÜÖ and Bağır GŞ: Factors
affecting lifespan following below-knee amputation in diabetic
patients. Acta Orthop Traumatol Turc. 51:393–397. 2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Abbas ZG, Chockalingam N, Lutale JK and
Naemi R: Predicting the risk of amputation and death in patients
with diabetic foot ulcer. A long-term prospective cohort study of
patients in Tanzania. Endocrinol Diabetes Metab.
5(e00336)2022.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
Hoellwarth JS, Tetsworth K, Oomatia A,
Akhtar MA, Xu H and Al Muderis M: Association between
osseointegration of lower extremity amputation and mortality among
adults. JAMA Netw Open. 5(e2235074)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Spoden M, Nimptsch U and Mansky T:
Amputation rates of the lower limb by amputation
level-observational study using German national hospital discharge
data from 2005 to 2015. BMC Health Serv Res. 19(8)2019.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Hickson LJ, Rule AD, Thorsteinsdottir B,
Shields RC, Porter IE, Fleming MD, Ubl DS, Crowson CS, Hanson KT,
Elhassan BT, et al: Predictors of early mortality and readmissions
among dialysis patients undergoing lower extremity amputation. J
Vasc Surg. 68:1505–1516. 2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Klaphake S, de Leur K, Mulder PG, Ho GH,
de Groot HG, Veen EJ, Verhagen HJ and van der Laan L: Mortality
after major amputation in elderly patients with critical limb
ischemia. Clin Interv Aging. 12:1985–1992. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Bates B, Stineman MG, Reker DM, Kurichi JE
and Kwong PL: Risk factors associated with mortality in veteran
population following transtibial or transfemoral amputation. J
Rehabil Res Dev. 43:917–928. 2006.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Norvell DC and Czerniecki JM: Risks and
risk factors for ipsilateral re-amputation in the first year
following first major unilateral dysvascular amputation. Eur J Vasc
Endovasc Surg. 60:614–621. 2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Mustapha JA, Katzen BT, Neville RF,
Lookstein RA, Zeller T, Miller LE and Jaff MR: Determinants of
long-term outcomes and costs in the management of critical limb
ischemia: A population-based cohort study. J Am Heart Assoc.
7(e009724)2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yilmaz S, Kurt M, Dülgeroğlu TC and
Üzümcügil AO: Predictive value of systemic immune inflammation
index (SII) and prognostic nutritional index (PNI) on mortality
after below-knee amputation. Medicine (Baltimore).
102(e35703)2023.PubMed/NCBI View Article : Google Scholar
|