Introduction
Ovarian cancer is a prevalent gynecological disease.
The commonly used chemotherapeutic drugs for treating ovarian
cancer, platinum and paclitaxel, are known to have serious side
effects and often lead to drug resistance, particularly in the
advanced stages of the disease. As such, there is a need to explore
alternative, more effective treatment options for this patient
population (1,2). Gefitinib is a prevalent targeted
therapy that has been employed in the treatment of lung cancer;
however, current scientific endeavors are ongoing to investigate
the prospective use of gefitinib, alongside adjunctive
pharmacological agents, for the purpose of managing ovarian cancer
(3,4). These studies are dedicated to the
refinement and optimization of the therapeutic approach applied to
ovarian cancer, and have yielded encouraging indications of
therapeutic efficacy, particularly in patients resistant to
chemotherapy (5,6). Gefitinib acts intracellularly and
selectively inhibits epidermal growth factor receptor (EGFR) by
acting as an antagonist that binds competitively to the EGFR
ATP-binding site. In addition, gefitinib has been demonstrated to
exert anti-vascular endothelial growth factor (VEGF) activity and
to reduce tumor angiogenesis (6-8);
however, the oral absorption rate of gefitinib is relatively low,
potentially limiting its efficacy in treating cancer. Furthermore,
it has been found to upregulate the phosphorylation of
STAT3(9), which, when activated
and phosphorylated to phosphorylated (p)-STAT3, can lead to
abnormal cell proliferation and malignant transformation, promoting
tumor growth. As such, further research is required to fully
investigate the potential of gefitinib as a treatment option for
ovarian cancer.
Previous studies have revealed that the interaction
between low-intensity pulsed ultrasound and contrast agent
microbubbles in blood generates ultrasound-stimulated microbubble
cavitation (USMC), which can produce stable cavitation (10,11).
This cavitation, in turn, can produce a series of biological
effects. For example, it can enhance blood perfusion of tumor
vessels and permeability of cell membranes, thereby increasing
osmotic drug concentration and improving therapeutic efficacy.
Moreover, low-intensity ultrasound can produce biological effects
in other tissues, including bones, muscles and nerves, ultimately
leading to changes in the activation of signaling pathways.
Notably, low-intensity ultrasound has been shown to suppress
lipopolysaccharide-induced inflammatory responses by inhibiting
TLR4 signal transduction. Therefore, low-intensity ultrasound may
have biological effects in different tissues by affecting various
signaling pathways (12-15).
A previous study reported that low-intensity ultrasound may reduce
the phosphorylation of STAT3 in ovarian cancer stem cells through
the interleukin 6 (IL-6)/STAT3 pathway (16). Accordingly, the present study aimed
to elucidate the influence of USMC on the pharmacokinetics and
pharmacodynamics of oral gefitinib therapy in subcutaneously
transplanted SKOV3 ovarian cancer tumors in nude mice. Such
investigations may enhance the therapeutic outcomes associated with
oral drug administration in oncology, thereby offering prospects
for novel therapeutic advancements aimed at improving the efficacy
of oral antitumor treatment.
Materials and methods
Cells and animals
The SKOV3 ovarian cancer cell line (The Cell Bank of
Type Culture Collection of The Chinese Academy of Sciences) and
Balb/c-nude mice (n=168; Chengdu Yaokang Biotechnology Co., Ltd.,)
were used in the present study. The adult female mice were of
specific pathogen-free (SPF)-grade quality, were 6-8 weeks old and
weighed 17-21 g. The mice were housed in SPF-grade animal rooms in
individually ventilated cages (IVCs), with no more than five
animals per cage. The air cleanliness was maintained at a level of
<10,000 particles per cubic foot, noise levels were maintained
at ≤60 decibels, temperature was kept at 20-27˚C, and the humidity
level was between 40 and 70%. The mice were maintained under a 12-h
light/dark cycle, and were given free access to water and food. The
experimental animals were allowed to adapt to the animal room for 1
week before the start of the experiment. Due to the lack of an
immune system, nude mice are more susceptible to the engraftment of
exogenous cells or tissues. This model is widely employed in
oncology research, and provides crucial insights into tumor
development, growth and therapeutic response. The present study
received approval from the Scientific Research Ethics Committee of
The Second Hospital of Hebei Medical University (Shijiazhuang,
China; approval no. 2022-AE261).
Animal model
SKOV3 cells were cultured in McCoy's 5A medium (cat.
no. 16600082; Gibco; Thermo Fisher Scientific, Inc.) containing 10%
fetal bovine serum (cat. no. 10099-141; Gibco; Thermo Fisher
Scientific, Inc.) and 1% penicillin-streptomycin at 37˚C in a
40-70% humidified incubator containing 5% CO2. The
medium was changed every 2-3 days and cells were grown attached to
the flask; passaging was performed at a ratio of 1:3. The SKOV3
cells were resuspended in serum-free medium before being injected
into the nude mice, drawn into a 1-ml syringe and subcutaneously
injected into nude mice at a concentration of 150 µl/mouse
(~1.5x106 cells). The experiment started when the
diameter of the tumor reached ~0.8 cm.
B-mode ultrasound and
contrast-enhanced ultrasound (CEUS) perfusion imaging
B-mode ultrasound and CEUS imaging were performed
using an X4-12L linear scanner [VINNO70; VINNO Technology (Suzhou)
Co., Ltd.] with a frequency range of 4.0-12.0 MHz. The contrast
agent used was Sonazoid® (GE Healthcare), which is a
phospholipid-coated perfluorobutane microsphere. The microbubbles
used for ultrasound therapy were sulfur hexafluoride microbubbles,
known as SonoVue (Bracco Suisse SA). During contrast-enhanced
ultrasound imaging, 16 µl Sonazoid perfluorobutane microspheres
were diluted into 4 ml normal saline solution, and 0.01 ml was
injected through the tail vein. USMC treatment was performed after
Sonazoid contrast agent microbubbles had cleared. During treatment,
5 ml normal saline was added to the SonoVue bottle, and the mixture
was shaken well to create a microbubble suspension with a
concentration equivalent to 8 µl/ml of the microbubble content.
Next, 0.01 ml was taken from the mixture and diluted to 0.5 ml with
normal saline, and then 0.3 ml was used for treatment. Throughout
the B-mode ultrasound, CEUS and USMC procedures, the corresponding
depth, gain and other settings were consistently maintained. The
CEUS images were analyzed using CBI (VINNO70), an internal
quantitative software developed by VINNO Technology (Suzhou) Co.,
Ltd., to determine peak intensity (PI), area under the curve (AUC)
and percentage perfusion area.
Trial protocol
A total of 168 mice were used in the present study.
For ultrasound, a mechanical index (MI) of 0.25 was selected for
the present study. Before USMC treatment, the tumor-bearing mice
were anaesthetized with an intraperitoneal injection of 1.2%
tribromoethanol at a dose of 250 mg/kg. The present study initially
aimed to evaluate the effects of USMC on tumor vascular effects,
drug uptake and signaling pathway factors. The parameter estimation
method indicated that a sample size of 72 mice was required for
this particular investigation. A total of 72 tumor-bearing mice
were randomly divided into the following four groups (n=18/group)
using a random number generator: i) Control group, ii) USMC5
min group, iii) USMC10 min group and iv)
USMC20 min group. The MI was maintained at a constant
value of 0.25(17) for 5, 10 and
20 min, in the control, USMC5 min, USMC10 min
and USMC20 min groups, respectively. All nude mice in
each group were orally administered gefitinib at a dose of 112
mg/kg; after 3 h, USMC treatment was performed. Subsequently, 30
min after USMC was completed, the nude mice were sacrificed and
tumor tissues were extracted. CEUS was used to evaluate flow
perfusion, quantitative determination of gefitinib concentration in
tumor samples was performed using liquid chromatography-mass
spectrometry, western blotting (WB) was used to detect STAT3 and
phosphorylated (p)-STAT3 expression, and ELISA was used to measure
IL-6 levels.
In addition, to evaluate the antitumor effect of
combination therapy, 96 tumor-bearing mice were randomly divided
into the following four groups (n=24/group): i) Control group, ii)
USMC group; iii) gefitinib group and iv) USMC + gefitinib group.
Combination therapy was initiated on day 9 after SKOV3 cell
inoculation in nude mice. Gefitinib was orally administered once
every morning at a concentration of 112 mg/kg, after considering
the pharmacokinetics of gefitinib, 3 h before USMC treatment. USMC
treatment was performed at 3-day intervals on days 9, 12, 15, 18
and 21. After the last treatment, 18 nude mice in each group were
randomly sacrificed and tumor tissues were extracted for H&E
staining to detect the morphology of the tumor tissue, and WB was
performed to detect EGFR, p-EGFR, STAT3, p-STAT3, AKT, p-AKT, ERK,
p-ERK and Mcl-1 expression. Additionally, the expression of CD31
and VEGF in tumor tissue was evaluated by immunofluorescence, the
apoptosis of tumor tissue was detected using TUNEL staining and the
concentration of IL-6 in tumor tissue was measured using ELISA. The
drug concentration of gefitinib was determined in the gefitinib and
USMC + gefitinib groups using the liquid chromatography-mass
spectrometry method, and the remaining six animals in each group
were used to observe tumor growth and survival. The use of nude
mouse models allows for the observation of tumor growth during
combined treatment, and facilitates the evaluation of antitumor
effects of combined therapy.
When the body weight of rats decreased by >15%,
or when there were evident signs of respiratory distress or
mobility impairment, euthanasia was considered, according to the
principles of humane endpoints. Mice were euthanized in IVCs.
Briefly, CO2 was introduced at a rate of 30% cage
volume/min, and, typically within 3-4 min, the mice were observed
to cease breathing. The CO2 supply was then shut off.
After noting that the mice had ceased breathing and after waiting
an additional 2 min, secondary physical confirmations of death were
performed; these included checking for the absence of a heartbeat
using a stethoscope and the lack of a response to toe pinch.
Euthanasia was conducted in a timely and humane manner to minimize
any potential suffering for the animals.
Histology
The tumor tissues of SKOV3 tumor-bearing nude mice
were extracted 30 min after treatment. Tissues were stained with
hematoxylin for 3-5 min and with eosin for 5 min at room
temperature, and images were then captured under an optical
microscope. An experienced pathologist diagnosed all specimens, and
the pathologist remained unaware of the grouping of each specimen
throughout the diagnosis.
TUNEL staining
The tumor tissues from SKOV3 tumor-bearing nude mice
were extracted 30 min after the final combined treatment course.
The sections were fixed in 4% paraformaldehyde at room temperature
for 48 h. The slices were then incubated in two changes of xylene
(15 min each), and dehydrated in two changes of pure ethanol for 5
min followed by dehydration in 85 and 75% ethanol for 5 min each at
room temperature. Subsequently, the sections were incubated with
TUNEL reagent (TDT enzyme, dUTP and buffer in a 1:5:50 ratio) at
37˚C for 2 h and the nuclei were stained with DAPI at room
temperature for 10 min. Finally, the sections were mounted with an
anti-fade mounting medium and were observed under a fluorescence
microscope.
Multicolor immunofluorescence tyramide
signal amplification (TSA) method
The tumor tissues from SKOV3 tumor-bearing nude mice
were extracted 30 min after the final combined treatment course.
The extracted tissues were washed with PBS, followed by staining of
the nuclei with DAPI. The samples were fixed in 10%
neutral-buffered formalin at room temperature for 48 h.
Subsequently, tissue sections (2 µm) were embedded in paraffin. To
expose intracellular antigens, the sections underwent
high-temperature antigen retrieval at 98-120˚C with EDTA antigen
retrieval solution for 5 min, followed by washing with xylene and
rehydration in a descending alcohol series. To block endogenous
peroxidase activity, 3% hydrogen peroxide was incubated with the
sections at room temperature in the dark for 25 min. To reduce
background staining, 1-5% bovine serum albumin (BSA; Thermo Fisher
Scientific, Inc.) was used as the blocking reagent and the sections
were incubated with it at room temperature for 1 h. Subsequently,
anti-CD31 (1:2,000; cat. no. GB113151; Wuhan Servicebio Technology
Co., Ltd.) was incubated with the sections at 4˚C overnight. The
samples were then incubated with a HRP-labelled goat anti-rabbit
secondary antibody (1:500; cat. no. GB23303; Wuhan Servicebio
Technology Co., Ltd.) at room temperature for 30 min. After the
slices were dried, iF555-Tyramide (1:2,000; cat. no. G1233; Wuhan
Servicebio Technology Co., Ltd.) was added and incubated at room
temperature for 10 min in the dark. After incubation, the slides
were placed in PBS and washed three times (5 min/wash). Microwave
heating for 15 min removed the anti-CD31 and secondary antibodies,
after which anti-VEGF (1:200; cat. no. GB11034B; Wuhan Servicebio
Technology Co., Ltd.) was incubated with the sections at 4˚C
overnight. Subsequently, the aforementioned steps were repeated,
followed by incubation with iF488-Tyramide (1:1,000; cat. no.
G1231; Wuhan Servicebio Technology Co., Ltd.) at room temperature
for 10 min in the dark. DAPI was used to restain the nuclei at room
temperature for 10 min. The slices were slightly dried and sealed
with anti-fluorescence quenching reagents. Finally, the samples
were mounted and images were captured using a confocal laser
scanning microscope (Nikon Eclipse C1; Nikon Corporation) with
imaging software (Nikon DS-U3; Nikon Corporation).
Liquid chromatography-mass
spectrometry method
To test the concentration of gefitinib in tumors,
liquid chromatography-mass spectrometry was performed. Firstly, the
gefitinib standard (cat. no. G-231; TLC Pharmaceutical Standards
Ltd.) was accurately weighed and dissolved in methanol to prepare a
2.00 mg/ml stock solution. Subsequently, 200 mg tumor sample was
weighed and 1 ml pure methanol was added. Zirconia grinding beads
were then added and the tumor sample was ground for 10 min before
centrifuging at 14,500 x g for 10 min at 4˚C. Subsequently, the
solution was filtered using a 0.22-µm filter membrane and the
filtrate was diluted 20-fold with methanol. An UltiMate 3000 Rapid
Separation system (Thermo Fisher Scientific, Inc.) coupled with a
TSQ Quantum mass spectrometer (Thermo Fisher Scientific, Inc.) was
used. Electron spray ionization in positive mode was applied. The
operating conditions for the system were as follows: Nitrogen gas
temperature, 400˚C; electrospray voltage, (+) 3,500 V. Selected
reaction monitoring scan mode was used to detect the ion pairs of
the substances to be measured, with the precursor ion being 447.1
and the fragment ions being 100.2, 128.1 and 360.1. The most
intense fragment ion 128.1 was chosen as the quantitative ion, and
the second and third most intense ions 100.2 and 360.1 were used as
the qualitative ions. The solvent system consisted of mobile phase
A (water containing 0.1% formic acid) and phase B (acetonitrile
containing 0.1% formic acid). The gradient conditions were as
follows: 0-1.5 min, 10-95% B; 1.5-3.8 min, 95% B; 3.8-4.0 min,
95-10% B; 4.0-5.0 min, 10% B. Chromatographic separation was
performed with a Welch XB-C8 (150x4.6 mm; 5 µm). The column
temperature was 40˚C and the flow rate was 1 ml/min; the
autosampling volume was 2.00 µl. Chromatogram acquisition and
integration for gefitinib was completed using Xcalibur 3.0 software
(Thermo Fisher Scientific, Inc.).
WB detection
The tumor tissues from SKOV3 tumor-bearing nude mice
were extracted 30 min after treatment. The tissue samples were cut
into small pieces, to which 1 ml RIPA lysis buffer (Beyotime
Institute of Biotechnology) was added per 100 mg of tissue. Protein
concentration was determined using a BCA protein assay kit.
Subsequently, ~30 µg protein/lane was separated by SDS-PAGE on 10%
gels and was transferred to polyvinylidene difluoride membranes
(cat. no. IPFL00010; MilliporeSigma). After that, the membranes
were blocked for 1 h at room temperature with 5% nonfat dry milk in
TBST, washed in TBST and incubated with primary antibodies. The
membranes were incubated with anti-EGFR (1:1,000; cat. no.
ab52894), anti-p-EGFR (1:1,000; cat. no. ab40815), anti-STAT3
(1:1,000; cat. no. ab68153), anti-p-STAT3 (1:1,000; cat. no.
ab76315) (all from Abcam), anti-ERK (1:1,000; cat. no. ET1601-29;
HUABIO), anti-p-ERK (1:1,000; cat. no. 4370; Cell Signaling
Technology, Inc.), anti-AKT (1:1,000; cat. no. ET1609-51; HUABIO),
anti-p-AKT (1:1,000; cat. no. ab81283; Abcam), anti-Mcl-l (1:1,000;
cat. no. AF5311; Affinity Biosciences) and anti-β-actin (1:1,000;
cat. no. HA722023; HUABIO) antibodies at 4˚C overnight; the
antibodies were diluted in 5% BSA in TBST. Subsequently, the
membranes were washed in TBST and incubated with the corresponding
HRP-labelled goat anti-rabbit IgG (1:1,000; cat. no. A0208;
Beyotime Institute of Biotechnology), which was diluted in 5% BSA
in TBST, for 1 h at room temperature. Finally, after washing in
TBST, the blots were visualized using Western Lightning plus ECL
reagents (1:1,000; cat. no. NEL105001EAa; Shanghai Pufei
Biotechnology Co., Ltd.) and were semi-quantified using
TanonImage1.0 (Tanon Science and Technology Co., Ltd.).
ELISA
A total of 30 min after treatment, tumor tissue was
extracted from SKOV3 tumor-bearing nude mice. First, the tissue was
homogenized as follows: The tissue was rinsed with pre-cooled PBS
to remove any residual blood, and then weighed and chopped, before
being added to a glass homogenizer at a weight-to-volume ratio of
1:9 with PBS. The tissue was then ground on ice. Once the tissue
cells were sufficiently lysed, the sample was placed on ice and was
sonicated at frequency of 20-25 kHz (2 sec on, 3 sec off, repeated
for 5-10 min until the solution was clarified). The sample was then
centrifuged at 5,000 x g for 5 min at 4˚C and the supernatant was
collected for detection purposes. The concentration of the tumor
marker IL-6 was determined using a mouse anti-IL-6 ELISA kit (cat.
no. JL202688; Jianglai Biotechnology), according to the
manufacturer's instructions.
Statistical analysis
Statistical analysis was performed using SPSS 21.0
software (IBM Corporation). All data were found to conform to a
normal distribution by Shapiro-Wilk test and are presented as the
mean ± SD. Paired Student's t-test was employed to compare the data
obtained before and after treatment, whereas one-way ANOVA was used
for comparison between multiple groups and pairwise comparisons
were conducted using the Bonferroni method. Additionally, survival
analysis was conducted using the Kaplan-Meier method, with the
log-rank test being employed to compare between groups. P≤0.05 was
considered to indicate a statistically significant difference.
Results
Effects of different durations of USMC
treatment on tumor perfusion and related histology
The results showed that 10 min of ultrasound
irradiation per treatment is the optimal duration. Firstly, the
increase in tumor blood perfusion before and after USMC treatment
as demonstrated by CEUS was markedly better at 10 min than that at
5 min, and it showed no obvious change compared with that at 20 min
(Fig. 1A). This optimal effect at
10 min was further confirmed by the quantified changes in PI, AUC
and the percentage area of blood perfusion (Fig. 1B-G). The tumor vasculature in the
groups subjected to 5, 10 and 20 min of ultrasound irradiation all
exhibited an increased CEUS area compared with that in the control
group, with the duration of 10 min showing a greater widening
compared with 5 min, and no marked change compared with that in the
20 min group (Fig. 1H).
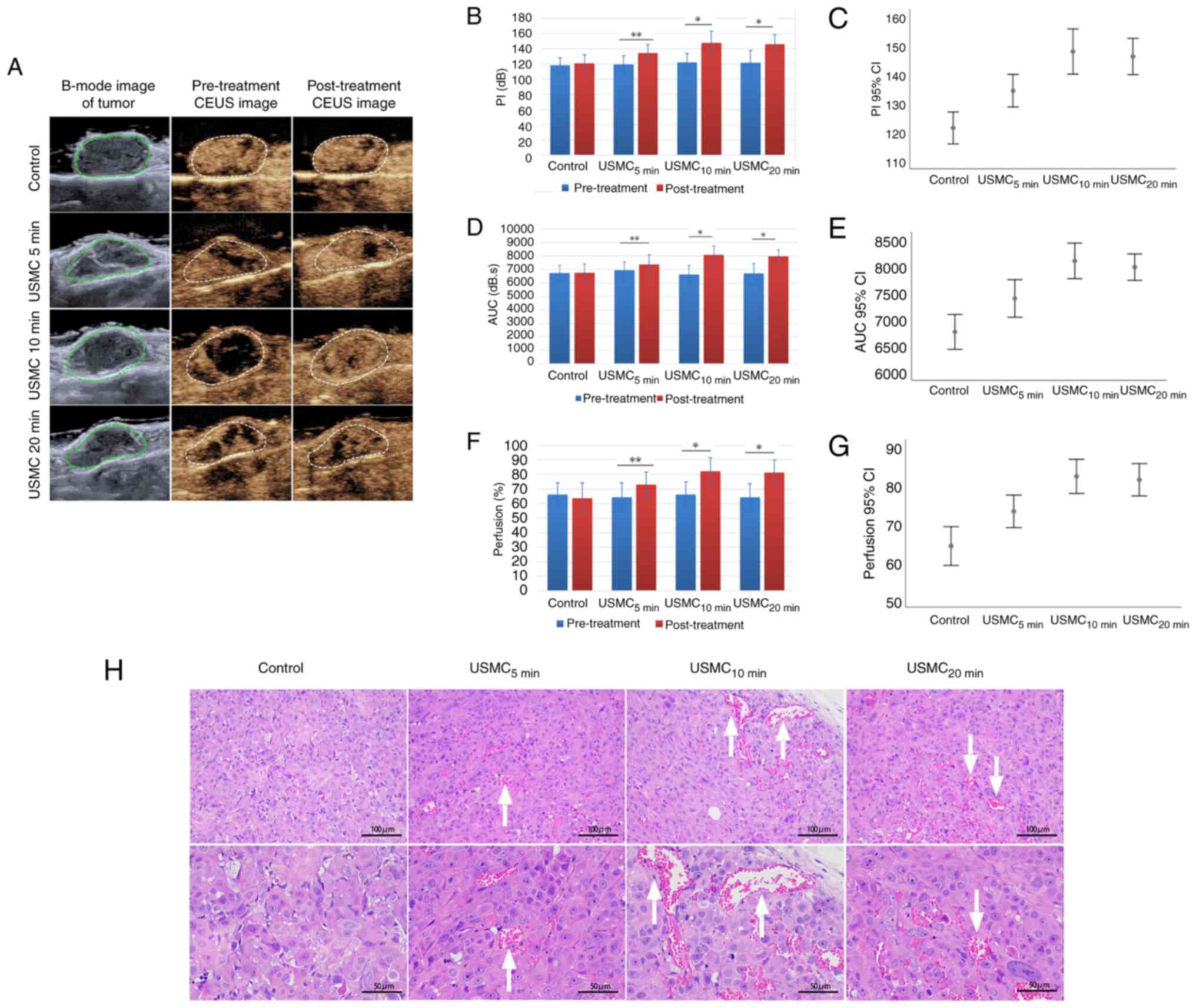 | Figure 1Effects of different durations of
USMC treatment on tumor perfusion and histology. (A) CEUS was used
to assess alterations in blood perfusion in nude mice with SKOV3
tumors before and after USMC treatment. Measurements of perfusion,
including (B) PI, (D) AUC and (F) changes in blood perfusion after
treatment compared with those before treatment, and (C) PI, (E) AUC
and (G) changes in blood perfusion among groups, with no
significant differences found between the groups prior to
treatment. (H) Histological analysis was performed to assess tumor
microvessels and demonstrated dilation of microvessels after USMC
treatment, as indicated by arrows. *P<0.05,
**P<0.01. AUC, area under the curve; CEUS,
contrast-enhanced ultrasound; PI, peak index; USMC,
ultrasound-stimulated microbubble cavitation. |
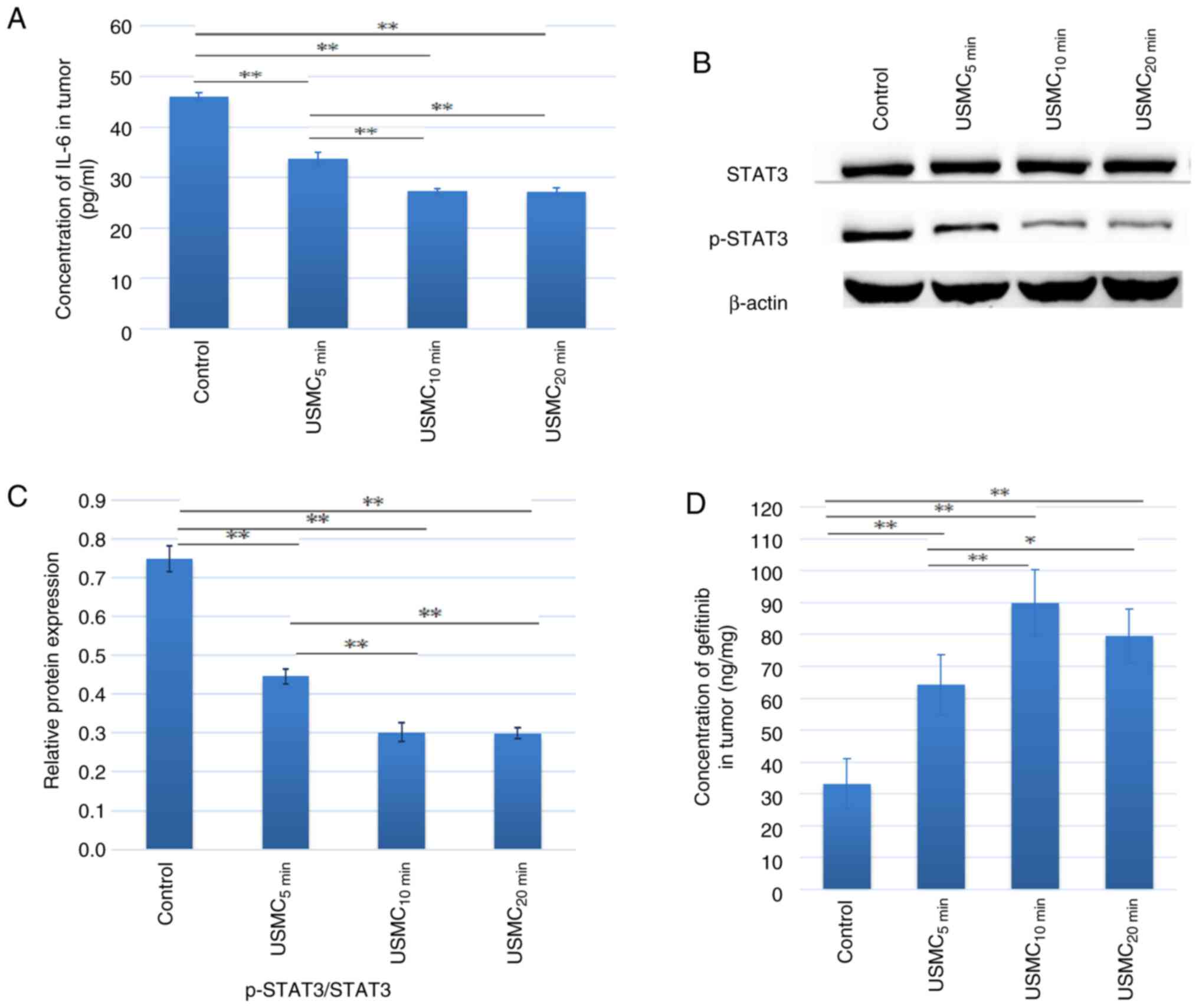
Effect of different durations of USMC
treatment on the concentration of IL-6, the expression levels of
STAT3 and p-STAT3, and gefitinib concentration in tumor tissue
In the present study, solid tumors were subjected to
the simultaneous action of USMC, and the results showed a reduction
in IL-6 levels in SKOV3 tumor tissue in nude mice. The levels of
IL-6 (Fig. 2A) and the downstream
phosphorylation of STAT3 (Fig. 2B
and C) were lower after 5, 10 and
20 min of ultrasound treatment, compared with those observed in the
control group. The decrease in IL-6 and STAT3 phosphorylation at 10
min was more notable than that at 5 min, with no significant
differences observed compared with those in the 20 min group. Nude
mice were euthanized 30 min after ultrasound treatment and tumor
tissue was extracted. Mass spectrometry was used to measure
gefitinib concentration in the tumor tissue. The results showed
that the gefitinib concentration after 5, 10 and 20 min of
ultrasound treatment was significantly higher than that of the
control group (Fig. 2D). The
gefitinib concentration after 10 min of treatment was significantly
higher than that after 5 min of treatment, whereas there was no
significant difference when compared with the 20 min treatment
group. The drug concentration in the USMC10 min group
was 2.7 times greater than that of the control group.
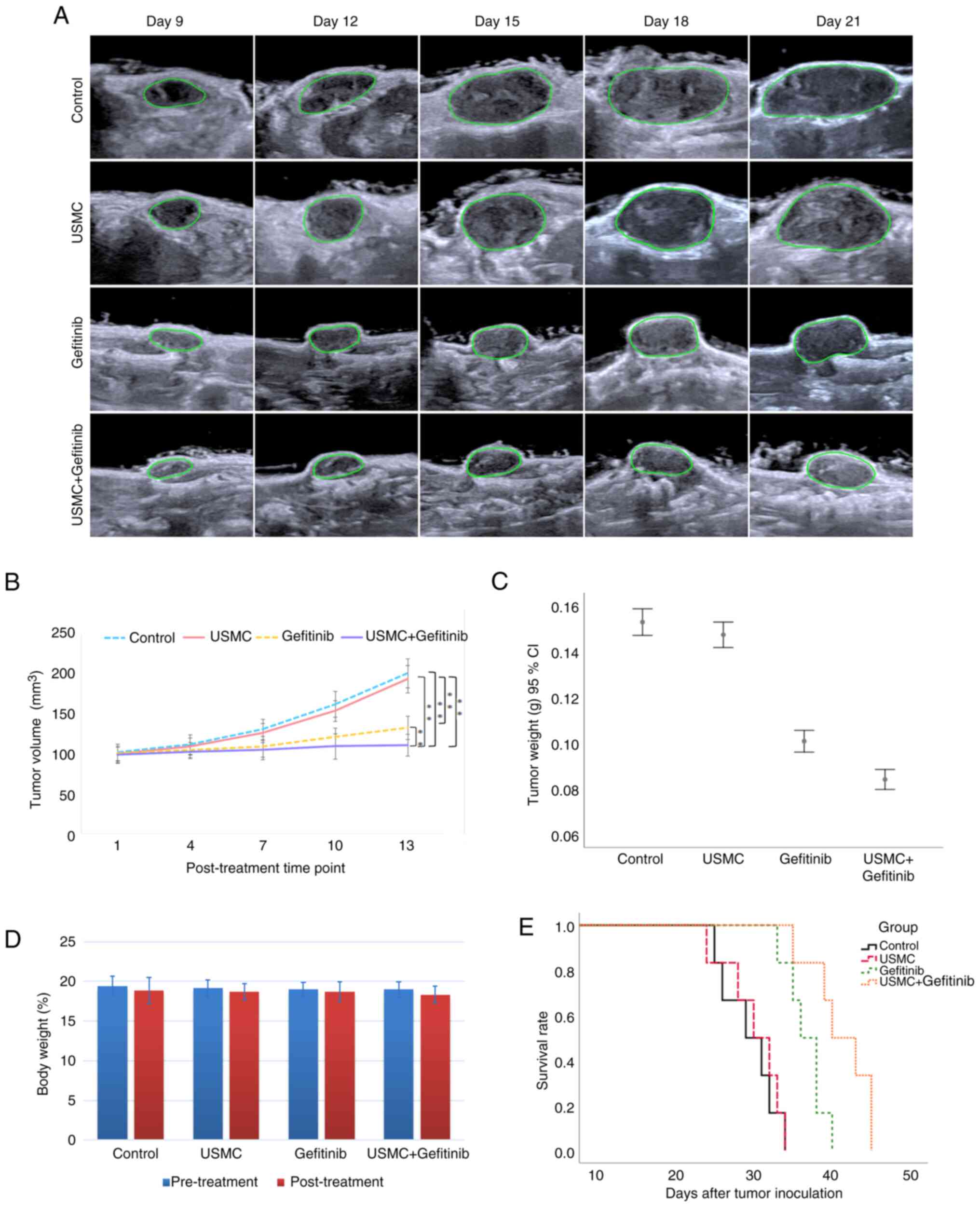
Combined effect of USMC with gefitinib
against SKOV3 tumors in nude mice
By observing the effects on PI, AUC and the
percentage area of blood perfusion, and by comparing the USMC5
min group, USMC10 min group and USMC20
min group with the control group, it was revealed that 10 min
was the optimal irradiation time for USMC; therefore, 10 min was
selected as the USMC treatment time for combined therapy. The
results of the experimental study demonstrated that USMC combined
with gefitinib was more effective in inhibiting tumor growth than
either USMC or gefitinib alone (Fig.
3A and B). Tumor tissue weight
was also analyzed and was revealed to be the smallest in the
combination group (Fig. 3C). Prior
to the final USMC treatment, the nude mice in each group were
weighed. The results showed a slight reduction in weight from the
initial treatment, but no significant difference was found between
the groups (Fig. 3D), indicating
that the combination therapy was safe. Finally, the survival period
of six nude mice per group was observed and the nude mice in the
combination group had the longest survival time (Fig. 3E).
Tumor perfusion is enhanced by
combination therapy
After the last treatment, the CEUS area of tumors
was markedly increased in the combined group and the USMC group,
whereas this effect was not detected in the control group and
gefitinib group due to the lack of USMC treatment (Fig. 4A). Additionally, PI, AUC and blood
perfusion area percentage values were significantly increased after
treatment compared with those before treatment in the ultrasound
treatment groups (Fig. 4B,
D and F). Furthermore, a comparison between the
groups indicated that the combined treatment group exhibited a more
notable increase in PI, AUC and percentage perfusion area than the
USMC group (Fig. 4C, E and G).
The results of H&E staining (Fig.
4H), in combination with the fluorescence images of tumor
apoptosis detected by TUNEL staining, demonstrated that the
combination group had the most favorable effect (Fig. 4I and K). Tumor expression of CD31 and VEGF was
also significantly lower in the combination treatment group when
compared with the other groups (Fig.
4J, L and M). Additionally, the concentration of
gefitinib was measured in the tumor tissue and was revealed to be
1.4 times higher in the combination treatment group compared with
that in the gefitinib group (Fig.
4N).
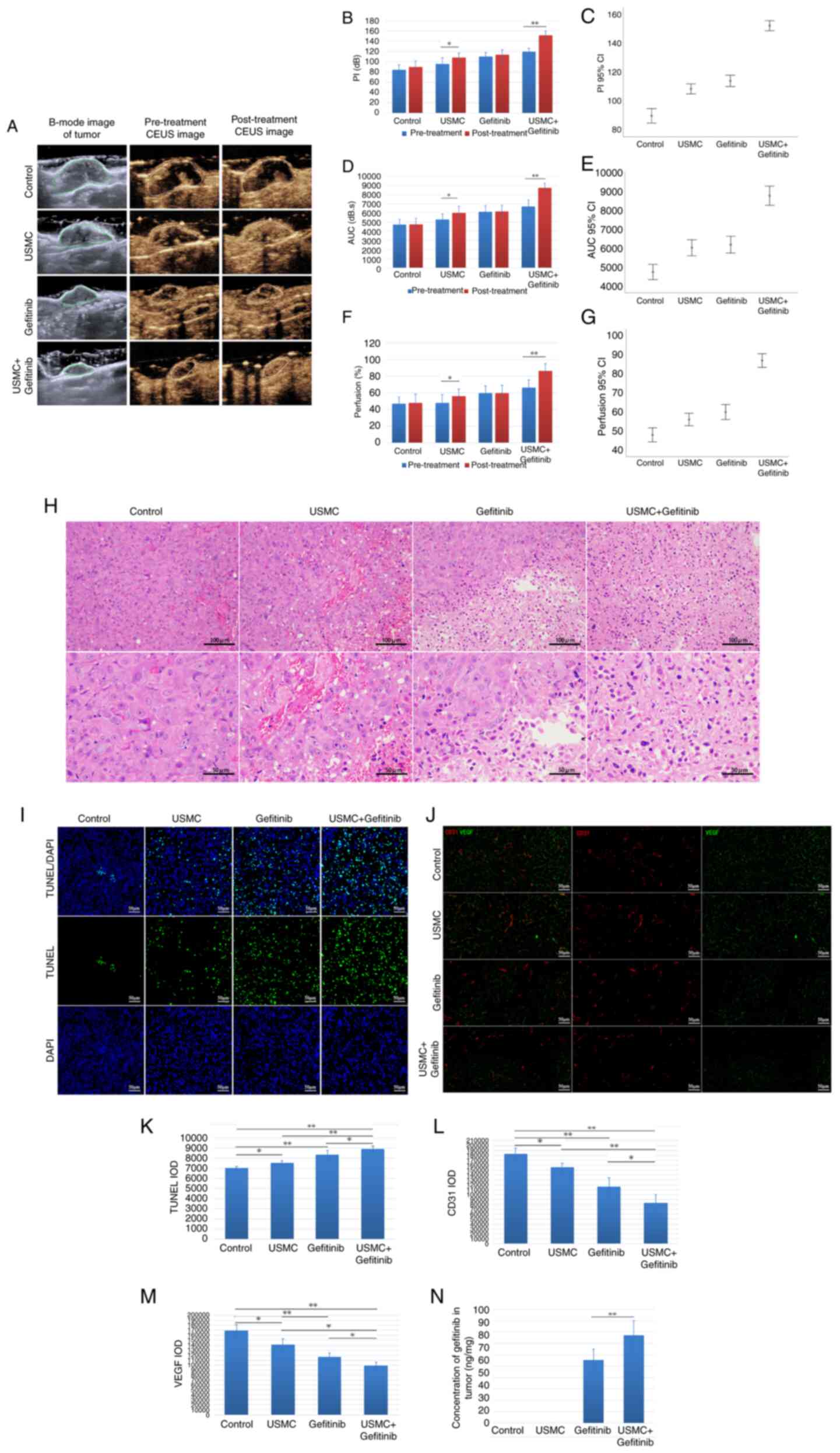 | Figure 4Tumor perfusion enhancement by
combination therapy. (A) CEUS was used to evaluate blood perfusion
before and after the last treatment. (B) PI, (D) AUC and (F)
percentage perfusion area values before and after treatment.
Comparison of (C) PI, (E) AUC and (G) perfusion values after the
last treatment among the groups. (H) Results of hematoxylin and
eosin staining after the last treatment. (I) Fluorescence staining
and (K) IOD concentration of TUNEL after the last treatment. (J)
Immunofluorescence staining, and IOD concentrations of (L) CD31 and
(M) VEGF after the last treatment. (N) Drug concentration after the
last treatment. *P<0.05, **P<0.01. AUC,
area under the curve; CEUS, contrast-enhanced ultrasound; IOD,
integrated optical density; PI, peak index; USMC,
ultrasound-stimulated microbubble cavitation; VEGF, vascular
endothelial growth factor. |
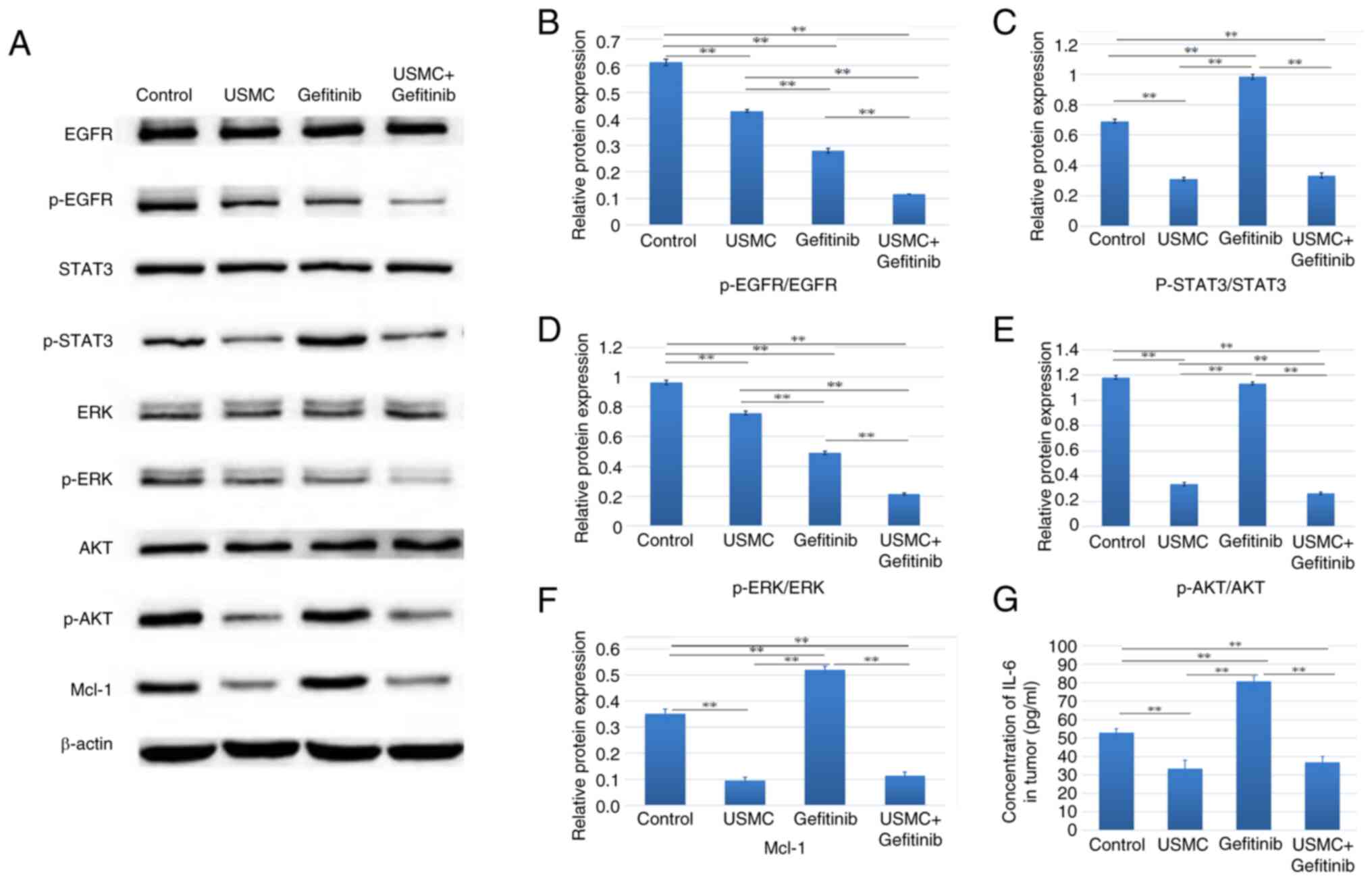
Effects of combined therapy on related
signaling pathway factors
The present study evaluated the impact of treatment
on CD31 and VEGF expression, as well as EGFR pathway molecules and
downstream signaling intermediates, including EGFR, p-EGFR, STAT3,
p-STAT3, AKT, p-AKT, ERK, p-ERK and Mcl-1. WB was used to assess
the expression levels of these proteins, as depicted in Fig. 5A. The results showed that gefitinib
upregulated STAT3 phosphorylation compared with in the control
group, whereas USMC reduced STAT3 phosphorylation. Moreover, the
combined therapy reduced the expression levels of p-AKT and p-ERK
downstream of EGFR, which are also downstream factors of VEGF, and
Mcl-1 downstream of STAT3, compared with those in the gefitinib
group (Fig. 5A-F). The ELISA
results also demonstrated a significant reduction in IL-6 levels in
both the combination therapy and USMC monotherapy groups compared
with those detected in the other two groups; however, there was no
significant difference observed between the combination therapy and
USMC monotherapy groups (Fig.
5G).
Discussion
In the past 20 years, research has been carried out
on the enhancement of drug delivery by USMC, including increased
drug delivery for tumor treatment and gene therapy (18-21).
There are typically three main routes of administration for tumor
treatment: Intravenous injection, intratumoral injection and
intraperitoneal injection (15,22).
Notably, research concerning the influence of USMC on the efficacy
of oral drug administration remains scarce (23). The present study aimed to explore
the impact of USMC on the therapeutic efficacy of orally
administered drugs, utilizing gefitinib as the primary medication.
Simultaneously, the study aimed to optimize the method of oral drug
delivery through combination with USMC. The outcomes of this study
have the potential to provide novel strategies and techniques to
improve and optimize oral drug administration. The animal model
selected in the present study involved subcutaneously inoculated
SKOV3 ovarian cancer cells in nude mice.
The results of the present study indicated that USMC
enhanced the effects of gefitinib treatment on subcutaneously
transplanted SKOV3 ovarian cancer in nude mice. The initial
experimental results revealed that the drug concentration in the
USMC10 min group was 2.7 times greater than that of the
control group, thus indicating that USMC may increase the
concentration of drugs in tumor tissues. In addition, these
findings support the results of a previous study, which
demonstrated that after the application of USMC, the concentration
of doxorubicin in the VX2 tumors of rabbits was 2.63 times that of
the control group (24). Regarding
the effect of USMC on blood perfusion, it was revealed that 10 min
of ultrasound irradiation was more effective than 5 min of
ultrasound irradiation; however, there was no marked difference
when compared with 20 min of ultrasound irradiation. Considering
the different effects resulting from varying USMC treatment
durations, the observation that USMC5 min had less of an
effect on PI, AUC and the percentage area of blood perfusion than
USMC10 min, owing to an inadequate duration of
ultrasonic irradiation, could explain the suboptimal results
obtained with a shorter treatment duration. Conversely, irradiation
durations exceeding an optimal threshold, such as the 20-min limit
identified here, may produce excessive biological effects and cause
an inflammatory reaction in tumor blood vessels, thereby narrowing
their lumen and limiting drug transport. This, in turn, may cause
irreversible damage to the surrounding normal tissue. There may be
a threshold for cavitation, indicating that a more extended
ultrasonic treatment does not give rise to better outcomes. This
threshold may be determined by factors such as MI, pulse length
(PL), probe frequency, center frequency, switching mode and
irradiation time (22), and
different combinations of these factors might produce different
thresholds; this requires further exploration. Notably, the concept
of cavitation dose has been introduced in previous studies
(22), which merges ultrasound
intensity and treatment duration into a singular parameter for
cavitation. This will further advance the research on simplifying
ultrasound therapy parameters.
The biological effects of USMC extend beyond
increasing blood perfusion to accelerate drug delivery. The present
study also showed that USMC can modulate the expression of certain
cytokines in signaling pathways. Previous studies have confirmed
that VEGF can activate PI3K/AKT and ERK signaling pathways during
angiogenesis (25), while
increased tumor blood perfusion can downregulate VEGF expression,
and downregulate AKT and ERK accordingly (26,27).
The present study demonstrated that USMC could markedly enhance
blood perfusion, and inhibit VEGF, AKT and ERK expression, thus
indicating the regulatory effects of USMC on certain cellular
factors in signaling pathways. Specifically, in the present study,
USMC was revealed to affect the levels of IL-6 and the
phosphorylation of STAT3, which are highly expressed in ovarian
cancer. IL-6 typically promotes cell proliferation and inhibits
cell apoptosis in ovarian cancer cells, and IL-6/STAT3 is an
important pathway in ovarian cancer. Notably, the inhibition of
IL-6 can reduce the phosphorylation of STAT3 (16,28).
In addition, numerous studies have employed low-intensity
ultrasound to irradiate cartilage tissue, all of which have
confirmed its ability to downregulate the expression of IL-6
(29,30). Relevant research (16) has demonstrated that low-intensity
ultrasound can suppress the expression of IL-6 in A2780 and OVCAR5
ovarian cancer stem cells, without having a significant inhibitory
impact on solid tumors. In the present study, the combined therapy
group was more effective at inhibiting the expression of IL-6 and
p-STAT3, and tumor growth, than the gefitinib group, indicating
that USMC can significantly improve the therapeutic effect of
gefitinib and thus may have an inhibitory effect on solid tumors.
Notably, the effects of USMC is reliant on various factors, such as
the treatment parameters of the ultrasound employed, microbubble
size and the individual distinctions of tumor blood vessels,
including the number and differentiation degree of tumor blood
vessels (31).
Cavitation refers to a series of biological effects
occurring when contrast agent microbubbles expand, contract or even
rupture under the action of ultrasound waves. Under specific
parameters, the interaction resulting in a mechanical jet can
temporarily increase the space between vascular endothelial cells,
lead to the formation of acoustic holes in the vascular wall,
increase endothelial vascular permeability and cause a change in
the shape of the blood vessels, as well as dilation (17,31).
The diameter of the microbubble and the inner diameter of the blood
vessel are related factors. A larger bubble diameter and smaller
blood vessel diameter result in more noticeable deformation
(32). Furthermore, the
interaction between the microbubbles, and between the microbubbles
and tube wall, rely on distance, which is closely related to
microbubble concentration (33). A
concentration of bubbles between 107-108/ml
has been reported to provide the best effect (34). In the present study, the number of
SonoVue microbubbles corresponded to the concentration suitable
(1x107/ml) for the optimal effect of cavitation.
Moreover, with the movement of the jet, cavitation helps drugs pass
through the created pores, allowing them to enter the tumor stroma
at a fast rate (35).
It has been suggested that upregulation of p-STAT3
is one of the reasons underlying the poor treatment efficacy of
gefitinib in SKOV3 tumors in nude mice (9). The present study revealed that, by
intervening with USMC, this phenomenon could be effectively
prevented. These insights have important implications for the
development of novel cancer therapies. In addition, the expression
of Mcl-1 in the combination group and the USMC group was decreased,
and Mcl-1, as a downstream factor of STAT3, exhibits a consistent
trend with STAT3 phosphorylation (9) The experimental findings revealed that
the USMC combined with gefitinib achieved the highest treatment
efficacy, with a greater tumor inhibition rate compared with
gefitinib alone, and an improved overall survival time. Xia et
al (36) used chemotherapy in
combination with USMC for the treatment of advanced prostate
cancer, and it significantly inhibited tumor growth and prolonged
the survival of tumor-bearing mice. These previous findings
indicated a synergistic effect of the combination therapy on tumor
reduction, which is consistent with the findings of the present
study and further confirms the accuracy of the results. Notably,
combination therapy of USMC and gefitinib may enhance the
accumulation and penetration of oral antitumor drugs in tumor
tissues, thereby improving the therapeutic efficacy of gefitinib
and significantly increasing its antitumor effectiveness. This
outcome implies the potential value of USMC in overcoming drug
resistance and improving targeted drug delivery. In addition, USMC
has the potential to reverse tumor resistance to gefitinib by
increasing drug concentrations and improving drug uptake. This
finding may provide a novel treatment strategy for drug-resistant
patients. The use of USMC can increase the accumulation and
penetration of gefitinib in tumor tissues, enabling more precise
drug delivery to cancer cells, and this precise drug targeting can
minimize damage to normal cells, reduce off-target side effects and
improve the therapeutic index of antitumor drugs. The strategy of
combining USMC with gefitinib can also be used in conjunction with
other treatment modalities, such as combination chemotherapy,
immunotherapy or targeted therapy. This results in new
possibilities for the treatment of ovarian cancer and other tumors,
with the potential to generate synergistic effects, improving
patient prognosis and treatment outcomes. The present findings are
of importance for enhancing the effectiveness of oral gefitinib and
improving the treatment outcomes of patients with ovarian
cancer.
The present study also revealed that the drug
concentration in the tumors of the combined therapy group was ~1.4
times that of the concentration in the gefitinib group, while the
drug concentration in the tumors of the USMC10 min group
was 2.7 times that of the concentration in the control group.
Notably, in the initial part of the experiment, the number of tumor
vessels in each group was relatively large without treatment, after
completing five cycles of USMC treatment in combined therapy
process of the experiment, the expression levels of VEGF and CD31,
which represent tumor angiogenesis, were the lowest among the four
groups. Therefore, it may be hypothesized that the degree of blood
perfusion influenced by USMC was decreased and thus the degree of
the increase of drug concentration in the combined therapy group
vs. the gefitinib group was relatively lower than that observed in
the USMC10 min group vs. the control group. Although
ultrasound can enhance the permeability of normal tissues and cell
membranes, using high microbubble concentrations and ultrasound
intensity can potentially damage healthy cells and tissues
surrounding the tumor. Moreover, the therapeutic effectiveness in
various tumors can be influenced by several factors, including
ultrasound frequency, MI, PL and pulse repetition frequency.
Therefore, identifying the optimal ultrasound microbubble
concentration and ultrasound parameters for various tumors is
crucial to ensure the effectiveness of ultrasound-enhanced
therapy.
The present study has some limitations that need
addressing. Firstly, the study predominantly focused on a single
parameter, specifically the duration of ultrasound treatment, while
keeping all other parameters at fixed values. The exploration of
multiple parameters was not extensive and this represents a
significant area for future research focus. Additionally, the
variation in tumor models, the type and concentration of
microbubbles, and various other factors can exert a considerable
influence on the study outcomes. It is precisely since there is a
multitude of factors affecting the effectiveness of USMC therapy
that more comprehensive investigations into each of these variables
should be performed in the future.
In conclusion, the present study reported that the
combination of USMC and oral gefitinib therapy for ovarian cancer
may significantly enhance the treatment efficacy, increase drug
accumulation and penetration in tumor tissue, and reduce drug
resistance. This discovery provides a novel approach to improve the
treatment outcomes for patients with ovarian cancer, potentially
reducing drug side effects and enhancing treatment
effectiveness.
Acknowledgements
The authors would like to thank Dr Jiawei Tang
(Department of Ultrasound, Xinqiao Hospital, Army Medical
University) and Dr Dan Lei (Department of Ultrasound, Xinqiao
Hospital, Army Medical University) for their assistance in this
research; their valuable insights and suggestions have been
instrumental. The present study was presented at the 5th
International Conference on Advances in Biological Science and
Technology.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YW and JC designed the study. JC and XZ confirm the
authenticity of all the raw data. XZ and JC performed the
statistical analysis. JC, ZZ, HL, XY and JW conducted the
experiments. JC wrote the manuscript. YW critically revised the
manuscript for essential intellectual content. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The authors declare compliance with the ARRIVE
guidelines. The study was approved by the Scientific Research
Ethics Committee of The Second Hospital of Hebei Medical University
(Shijiazhuang, China; approval no. 2022-AE261).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Khan MA, Vikramdeo KS, Sudan SK, Singh S,
Wilhite A, Dasgupta S, Rocconi RP and Singh AP: Platinum-resistant
ovarian cancer: From drug resistance mechanisms to liquid
biopsy-based biomarkers for disease management. Semin Cancer Biol.
77:99–109. 2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kuroki L and Guntupalli SR: Treatment of
epithelial ovarian cancer. BMJ. 371(m3773)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ohta T, Ohmichi M, Shibuya T, Takahashi T,
Tsutsumi S, Takahashi K and Kurachi H: Gefitinib (ZD1839) increases
the efficacy of cisplatin in ovarian cancer cells. Cancer Biol
Ther. 13:408–416. 2012.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Chelariu-Raicu A, Levenback CF, Slomovitz
BM, Wolf J, Bodurka DC, Kavanagh JJ, Morrison C, Gershenson DM and
Coleman RL: Phase Ib/II study of weekly topotecan and daily
gefitinib in patients with platinum resistant ovarian, peritoneal,
or fallopian tube cancer. Int J Gynecol Cancer. 30:1768–1774.
2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Thibault B and Jean-Claude B: Dasatinib +
Gefitinib, a non platinum-based combination with enhanced growth
inhibitory, anti-migratory and anti-invasive potency against human
ovarian cancer cells. J Ovarian Res. 10(31)2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chang SJ, Liao EC, Yeo HY, Kuo WH, Chen
HY, Tsai YT, Wei YS, Chen YJ, Wang YS, Li JM, et al: Proteomic
investigating the cooperative lethal effect of EGFR and MDM2
inhibitors on ovarian carcinoma. Arch Biochem Biophys. 647:10–32.
2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Karami A, Hossienpour M, Mohammadi Noori
E, Rahpyma M, Najafi K and Kiani A: Synergistic effect of gefitinib
and temozolomide on U87MG glioblastoma angiogenesis. utr Cancer.
74:1299–1307. 2022.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Odunsi A, McGray AJR, Miliotto A, Zhang Y,
Wang J, Abiola A, Eppolito C and Huang RY: Fidelity of human
ovarian cancer patient-derived xenografts in a partially humanized
mouse model for preclinical testing of immunotherapies. J
Immunother Cancer. 8(e001237)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wen W, Wu J, Liu L, Tian Y, Buettner R,
Hsieh MY, Horne D, Dellinger TH, Han ES, Jove R and Yim JH:
Synergistic anti-tumor effect of combined inhibition of EGFR and
JAK/STAT3 pathways in human ovarian cancer. Mol Cancer.
14(100)2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Ji R, Karakatsani ME, Burgess M, Smith M,
Murillo MF and Konofagou EE: Cavitation-modulated inflammatory
response following focused ultrasound blood-brain barrier opening.
J Control Release. 337:458–471. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
de Leon A, Perera R, Nittayacharn P,
Cooley M, Jung O and Exner AA: Ultrasound contrast agents and
delivery systems in cancer detection and therapy. Adv Cancer Res.
139:57–84. 2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Deng L, Liu M, Sheng D, Luo Y, Wang D, Yu
X, Wang Z, Ran H and Li P: Low-intensity focused
ultrasound-augmented Cascade chemodynamic therapy via boosting ROS
generation. Biomaterials. 271(120710)2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Luo D, Chen W, Wang W, Chen J, Xu H, Chen
J and Wang Y: Low-intensity pulsed ultrasound alleviating
myelosuppression of Sprague-Dawley rats after combined treating by
paclitaxel and carboplatin. Transl Cancer Res. 10:1183–1192.
2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Chen TT, Lan TH and Yang FY: Low-intensity
pulsed ultrasound attenuates LPS-induced neuroinflammation and
memory impairment by modulation of TLR4/NF-κB signaling and
CREB/BDNF expression. Cereb Cortex. 29:1430–1438. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li F, Park TH, Sankin G, Gilchrist C, Liao
D, Chan CU, Mao Z, Hoffman BD and Zhong P: Mechanically induced
integrin ligation mediates intracellular calcium signaling with
single pulsating cavitation bubbles. Theranostics. 11:6090–6104.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gong T, Zhang P, Jia L and Pan Y:
Suppression of ovarian cancer by low-intensity ultrasound through
depletion of IL-6/STAT3 inflammatory pathway-maintained cancer
stemness. Biochem Biophys Res Commun. 526:820–826. 2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
He J, Liu Z, Zhu X, Xia H and Gao H:
Ultrasonic microbubble cavitation enhanced tissue permeability and
drug diffusion in solid tumor therapy. Pharmaceutics.
14(1642)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li N, Tang J, Yang J, Zhu B, Wang X, Luo
Y, Yang H, Jang F, Zou J, Liu Z and Wang Z: Tumor perfusion
enhancement by ultrasound stimulated microbubbles potentiates PD-L1
blockade of MC38 colon cancer in mice. Cancer Lett. 498:121–129.
2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Dimcevski G, Kotopoulis S, Bjånes T, Hoem
D, Schjøtt J, Gjertsen BT, Biermann M, Molven A, Sorbye H,
McCormack E, et al: A human clinical trial using ultrasound and
microbubbles to enhance gemcitabine treatment of inoperable
pancreatic cancer. J Control Release. 243:172–181. 2016.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Anderson CD, Walton CB and Shohet RV: A
comparison of focused and unfocused ultrasound for
microbubble-mediated gene delivery. Ultrasound Med Biol.
47:1785–1800. 2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Matsuo M, Yamaguchi K, Feril LB Jr, Endo
H, Ogawa K, Tachibana K and Nakayama J: Synergistic inhibition of
malignant melanoma proliferation by melphalan combined with
ultrasound and microbubbles. Ultrason Sonochem. 18:1218–1224.
2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Chowdhury SM, Abou-Elkacem L, Lee T, Dahl
J and Lutz AM: Ultrasound and microbubble mediated therapeutic
delivery: Underlying mechanisms and future outlook. J Control
Release. 326:75–90. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kancheva M, Aronson L, Pattilachan T,
Sautto F, Daines B, Thommes D, Shar A and Razavi M: Bubble-based
drug delivery systems: Next-generation diagnosis to therapy. J
Funct Biomater. 14(373)2023.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang Y, Tang N, Huang L, Qiao W, Zhu Q
and Liu Z: Effect of diagnostic ultrasound and microbubble-enhanced
chemotherapy on metastasis of rabbit VX2 tumor. Med Phys.
48:3927–3935. 2021.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Arjaans M, Schröder CP, Oosting SF, Dafni
U, Kleibeuker JE and de Vries EGE: VEGF pathway targeting agents,
vessel normalization and tumor drug uptake: From bench to bedside.
Oncotarget. 7:21247–21258. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Abou Khouzam R, Brodaczewska K, Filipiak
A, Zeinelabdin NA, Buart S, Szczylik C, Kieda C and Chouaib S:
Tumor hypoxia regulates immune escape/invasion: Influence on
angiogenesis and potential impact of hypoxic biomarkers on cancer
therapies. Front Immunol. 11(613114)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang X, Huang M, Zhang Q, Chen J, Li J,
Han Q, Zhang L, Li J, Liu S, Ma Y, et al: Metformin antagonizes
ovarian cancer cells malignancy through MSLN mediated IL-6/STAT3
signaling. Cell Transplant. 30(9636897211027819)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yang T, Liang C, Chen L, Li J and Geng W:
Low-intensity pulsed ultrasound alleviates hypoxia-induced
chondrocyte damage in temporomandibular disorders by modulating the
hypoxia- inducible factor pathway. Front Pharmacol.
11(689)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sang F, Xu J, Chen Z, Liu Q and Jiang W:
Low-intensity pulsed ultrasound alleviates osteoarthritis condition
through focal adhesion kinase-mediated chondrocyte proliferation
and differentiation. Cartilage. 13 (2 Suppl):196S–203S.
2021.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ward M, Wu J and Chiu JF: Experimental
study of the effects of optison concentration on sonoporation in
vitro. Ultrasound Med Biol. 26:1169–1175. 2000.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Caskey CF, Stieger SM, Qin S, Dayton PA
and Ferrara KW: Direct observations of ultrasound microbubble
contrast agent interaction with the microvessel wall. J Acoust Soc
Am. 122:1191–1200. 2007.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Klibanov AL: Ultrasound contrast: Gas
microbubbles in the vasculature. Invest Radiol. 56:50–61.
2021.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tu J and Yu ACH: Ultrasound-mediated drug
delivery: Sonoporation mechanisms, biophysics, and critical
factors. BME Front. 2022(9807347)2022.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Xiao N, Liu J, Liao L, Sun J, Jin W and
Shu X: Improved delivery of doxorubicin by altering the tumor
microenvironment using ultrasound combined with microbubbles and
chemotherapy. J BUON. 24:844–852. 2019.PubMed/NCBI
|
|
36
|
Xia H, Yang D, He W, Zhu X, Yan Y, Liu Z,
Liu T, Yang J, Tan S, Jiang J, et al: Ultrasound-mediated
microbubbles cavitation enhanced chemotherapy of advanced prostate
cancer by increasing the permeability of blood-prostate barrier.
Transl Oncol. 14(101177)2021.PubMed/NCBI View Article : Google Scholar
|