Introduction
Acute variceal bleeding, including esophageal and
gastric variceal bleeding, is the main cause of bleeding in 50-60%
of patients with cirrhosis (1).
Acute variceal bleeding is usually complicated by ascites (34%),
infection (30%), respiratory complications (24%), intensive care
unit admission (20%), rebleeding (11%), encephalopathy (7%), acute
kidney injury (6%) and failure to control bleeding (4%) (2). In addition, acute variceal bleeding,
one of the most fatal complications of cirrhosis, is responsible
for 34-50% of cirrhosis-related deaths (3,4). In
the last two decades, mortality from acute variceal bleeding has
decreased from 40 to 15-20% due to endoscopic therapy, including
ligation, sclerotherapy and cyanoacrylate glue injection (4,5).
Endoscopy performed within 24 h is recommended for patients with
acute upper gastrointestinal bleeding (6,7).
Some studies have reported low or no risk of
infection following endoscopic therapy (8,9).
However, certain studies have reported that endoscopic therapy can
increase the risk of infection (10,11).
Therefore, certain studies have recommended the use of antibiotic
prophylaxis, leading to the on-demand use of antibiotics following
endoscopic therapy (12-15).
Hou et al (12) reported that, in addition to
preventing infection, the administration of antibiotic prophylaxis
following endoscopic therapy for acute variceal bleeding can
decrease early and late rebleeding and mortality. However, Agarwal
et al (14) and Liu et
al (13) reported conflicting
results, stating that antibiotic prophylaxis following endoscopy is
not associated with mortality and rebleeding.
Endoscopic therapy is a useful method for the
treatment of acute esophageal and gastric variceal bleeding; this
therapy can decrease the risk of mortality in patients with acute
variceal bleeding. However, it remains unknown whether antibiotic
prophylaxis is necessary to decrease infection, rebleeding and
mortality. Inconsistent results from different randomized
controlled trials (RCTs) (12-15)
have resulted in discrepancies in the reported data. Therefore, a
systematic review and meta-analysis was performed to integrate the
data and provide guidance for gastroenterologists. The aim of the
present study was to conduct a meta-analysis that could elucidate
the effects of antibiotic prophylaxis on infection, rebleeding and
mortality in patients that had undergone endoscopic therapy for
variceal hemorrhage.
Materials and methods
Eligibility of included studies
The PubMed (http://www.ncbi.nlm./pubmed/), Embase (http://www.embase.com.) and Cochrane Library
(http://www.cochranelibrary.com/www.cochranelibrary.com/)
databases were systematically searched between January 1959 and
January 2024, to ensure that all relevant literature was covered.
The search terms used were: (‘antibiotics’) AND (‘variceal
bleeding’) AND (‘endoscopy’ or ‘ligation’ or ‘sclerotherapy’ or
‘glue’).
Inclusion criteria
The comparative studies that explored the efficacy
of antibiotic prophylaxis and on-demand treatment for acute
variceal bleeding following endoscopic therapy were included. In
addition, only RCTs written in English were included. The patients
in the prophylactic group received antibiotic treatment immediately
following randomization. The antibiotics were used only when
infection was suspected or established in the on-demand
antibiotic-treatment group of patients. The main outcome was the
incidence of infection. The secondary outcomes were the incidence
of rebleeding, early rebleeding and mortality. The information on
the method of randomization, usage of antibiotics and assessment of
infection were necessary for the inclusion of the studies.
Infection was defined as fever (>38˚C), hypothermia
(<36˚C), unexpected hemodynamic instability,
tachypnea, new onset of symptoms, such as cough, dysuria,
septicemia, urinary tract infection, spontaneous peritonitis and
pneumonia, or positive blood cultures. Rebleeding was defined as
rebleeding within 2 months after initial control of bleeding. Early
bleeding was defined as rebleeding within 7 days after initial
control of bleeding.
Exclusion criteria
The following exclusion criteria were used: i)
Non-comparative studies, retrospective studies and studies that
were not RCTs; ii) patients with non-acute esophageal or gastric
variceal bleeding; iii) studies in which the patients did not
receive prophylactic antibiotics following endoscopic therapy; iv)
studies that did not include control groups with patients
administered on-demand antibiotics; and, v) studies that were not
written in English.
Data extraction
Following the initial database search, three
reviewers independently screened the titles and abstracts, and
excluded irrelevant articles according to the inclusion and
exclusion criteria. The number of studies excluded along with the
reasons for exclusion were recorded. Following the initial
examination of the titles and abstracts, the reviewers performed a
thorough evaluation of the included articles and extracted
information, including the leading author, country, publication
year, baseline characteristics, endoscopic therapeutic methods and
outcome indicators involving incidence of infection, rebleeding,
early rebleeding and mortality. In case of disagreement among the
three reviewers, the final decision was made through discussion and
a mutual consensus between the researchers was achieved.
Quality assessment of the included
studies
Two researchers were assigned to assess the risk of
bias in each trial using the Cochrane risk-of-bias assessment tool
(16). The seven domains of the
Cochrane risk-of-bias assessment tool were used to assess the bias
for each individual study as follows: Randomization, allocation,
blind involvement of participants and study personnel, outcome
assessors, incomplete outcome data, selection of reporting and
other bias (17). Following
evaluation, the included studies were graded into three levels,
including ‘unclear risk of bias’, ‘low risk of bias’, and ‘high
risk of bias’.
Statistical analysis
RevMan software version 5.4.1 (The Nordic Cochrane
Center; The Cochrane Collaboration, 2020) was used to analyze the
extracted data. The χ2 test was used to qualitatively
assess the heterogeneity of the groups (P<0.05 was considered to
indicate a statistically significant difference) and the
I2 statistic was used to quantitatively evaluate the
overall heterogeneity of the studies (18).
A random-effect model was used for all analyses as
recommended in the Cochrane Handbook for Systematic Reviews of
Interventions version 5.1.0 (https://training.cochrane.org/handbook/archive/v5.1/).
Odds ratios (ORs) and 95% confidence intervals (CIs) were used to
present the summarized estimates for the dichotomous data (17). The effect estimates and 95% CIs of
the individual and overall studies are shown in the figures using
forest plots. A funnel plot was not drawn as the total number of
studies assessed was <10(19).
Results
Study retrieval and selection
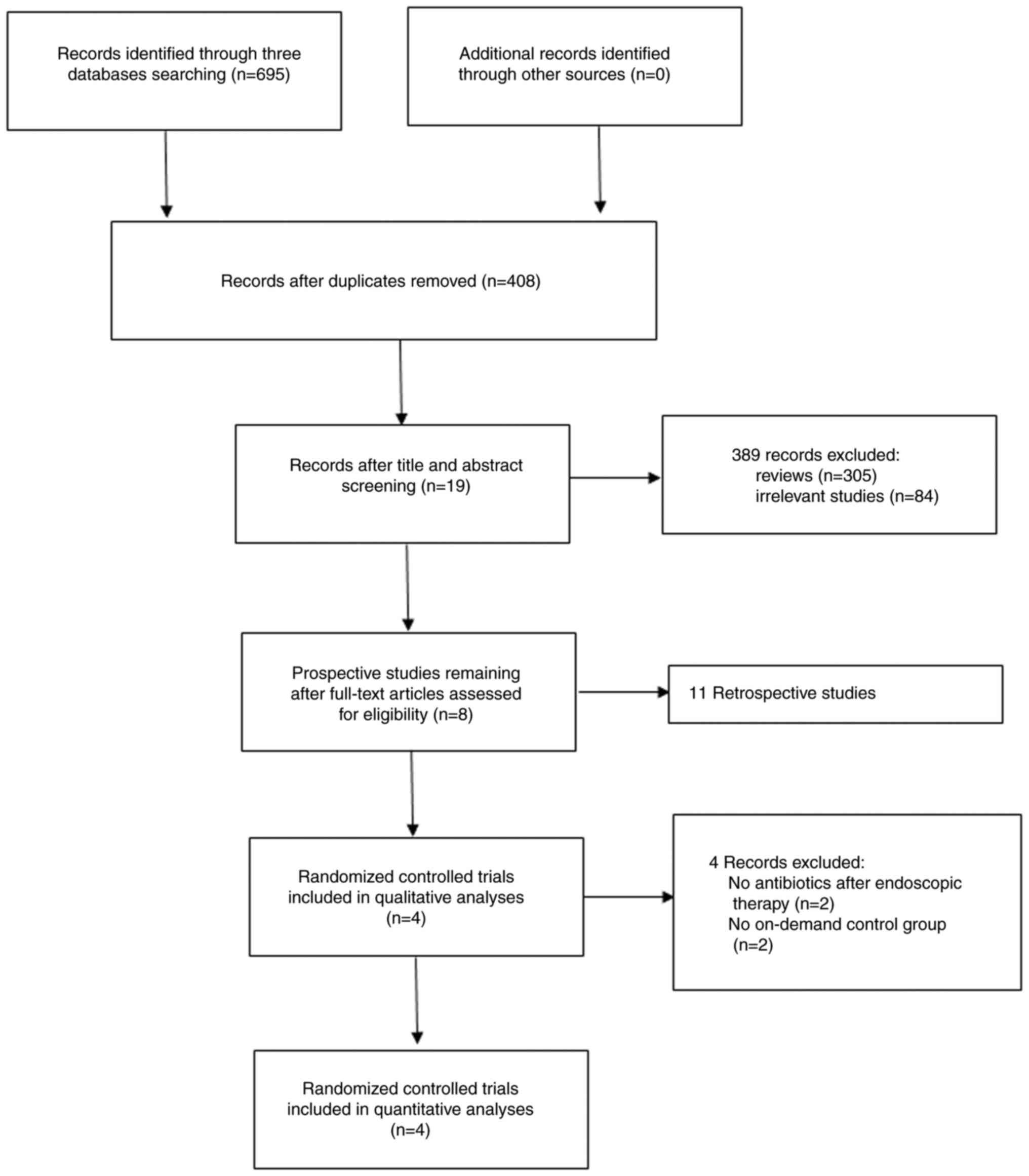
Following the database search, a total of 695
studies were selected for the initial screening. Following removal
of the duplicates, 287 studies were excluded and following
searching of the titles and abstracts of the remaining 408 studies,
389 were excluded, leaving only 19 studies. Following assessment of
the full-text articles for eligibility, 11 retrospective studies
were excluded, leaving eight prospective studies for review. The
qualitative and quantitative analyses were performed and four
prospective RCTs were finally included in the present
meta-analysis. A flow diagram of the process of study retrieval and
selection is shown in Fig. 1.
Characteristics of the eligible
studies
Table I summarizes
the basic characteristics of the four studies; they were published
from 1994 to 2019, and the study sites were distributed across the
following four areas: India, China, Taiwan and Australia. All of
the included articles were RCTs. Across the four RCTs, 326 patients
were involved. The sample size of each trial ranged from 39 to 120
patients. There were no significant differences in sex ratio, mean
age ± standard deviation and the Child-Pugh score (12-15)
or grade (A/B/C) between the intervention and control groups in
each RCT. All four studies included patients with acute variceal
bleeding and those who had undergone endoscopic treatment including
endoscopic variceal ligation (EVL), endoscopic variceal
sclerotherapy (EVS) and tissue adhesive injection. The intervention
methods of the antibiotic prophylaxis group in the included
articles were divided into two types. One intervention method was:
Intravenous ofloxacin (200 mg) q12H for 2 days, followed by oral
ofloxacin (200 mg) q12H for 5 days following endoscopic therapy.
The other intervention method was: Intravenous cefotiam (2 g) or
intravenous cefotaxime (1 g) administered one day prior to
endoscopic therapy. The intervention method for the on-demand group
in all four studies was: Antibiotics such as ofloxacin, cefotiam or
cefotaxime were used only when infection was suspected or
established. The outcomes of all four studies included the
incidence of infection and mortality, and three of the included
articles reported outcomes of the incidence of rebleeding. The
assessments of infection, rebleeding and mortality are described in
detail in the previous studies (12-15).
 | Table IBasic characteristics of all four
included studies. |
Table I
Basic characteristics of all four
included studies.
| | Sample size, n | Sex,
male/female | Mean age ± SD,
years | Child-Pugh
scorea | | Intervention
regime | Incidence of
infection, n (%) | Incidence of
rebleeding, n (%) | Incidence of
mortality, n (%) | |
|---|
| First author,
year | Country | Study type | AP | OD | AP | OD | AP | OD | AP | OD | Endoscopic
treatment | AP | OD | AP | OD | AP | OD | AP | OD | (Refs.) |
|---|
| Agarwal et
al, 2015 | India | RCT | 30 | 26 | 15/15 | 17/9 | 41.53± 2.19 | 44.15± 2.66 | 7.03± 1.24 | 7.07± 0.93 | EVL or EVS | Intravenous
ofloxacin (200 mg) q12H for 2 days or until oral fluids were
allowed, followed by oral ofloxacin (200 mg) q12H for a total of 7
days | Antibiotics used
only when infection was suspected or established | 5 (16.7) | 7 (26.9) | 7 (23.3) | 10 (38.4) | 4 (13.33) | 5 (16.67) | (14) |
| Liu et al,
2019 | China | RCT | 53 | 54 | 41/12 | 41/13 | 53.45± 12.49 | 55.76± 11.94 | 40/13/0 | 36/18/0 | Tissue adhesive
injection | Intravenous
cefotiam (2 g) | 100 ml saline
solution and antibiotics used only when infection was
suspected | 2 (3.8) | 9 (16.7) | 1 (1.9) | 5 (9.3) | 0 (0) | 0 (0) | (13) |
| Hou et al,
2004 | Taiwan | RCT | 59 | 61 | 43/16 | 48/13 | 60.02± 13.92 | 59.39± 14.85 | 8.54± 1.90 | 7.90± 2.04 | EVL, EVS, tissue
adhesive injection | Intravenous
ofloxacin (200 mg) q12H for 2 days, followed by oral ofloxacin (200
mg) q12H for 5 days | Antibiotics used
only when infection was suspected or established | 2 (3.39) | 16 (26.2) | 12 (20.3) | 27 (44.3) | 16 (27.1) | 13 (21.3) | (12) |
| Selby et al,
1994 | Australia | RCT | 19 | 20 | 15/4 | 13/7 | 58.9± 14.2 | 49.5± 10.7 | 4/8/7 | 4/10/6 | EVS | Intravenous
cefotaxime (1 g) | Antibiotics used
only when infection was suspected or established | 5 (26.3) | 7(35) | NM | NM | 2 (10.5) | 5(25) | (15) |
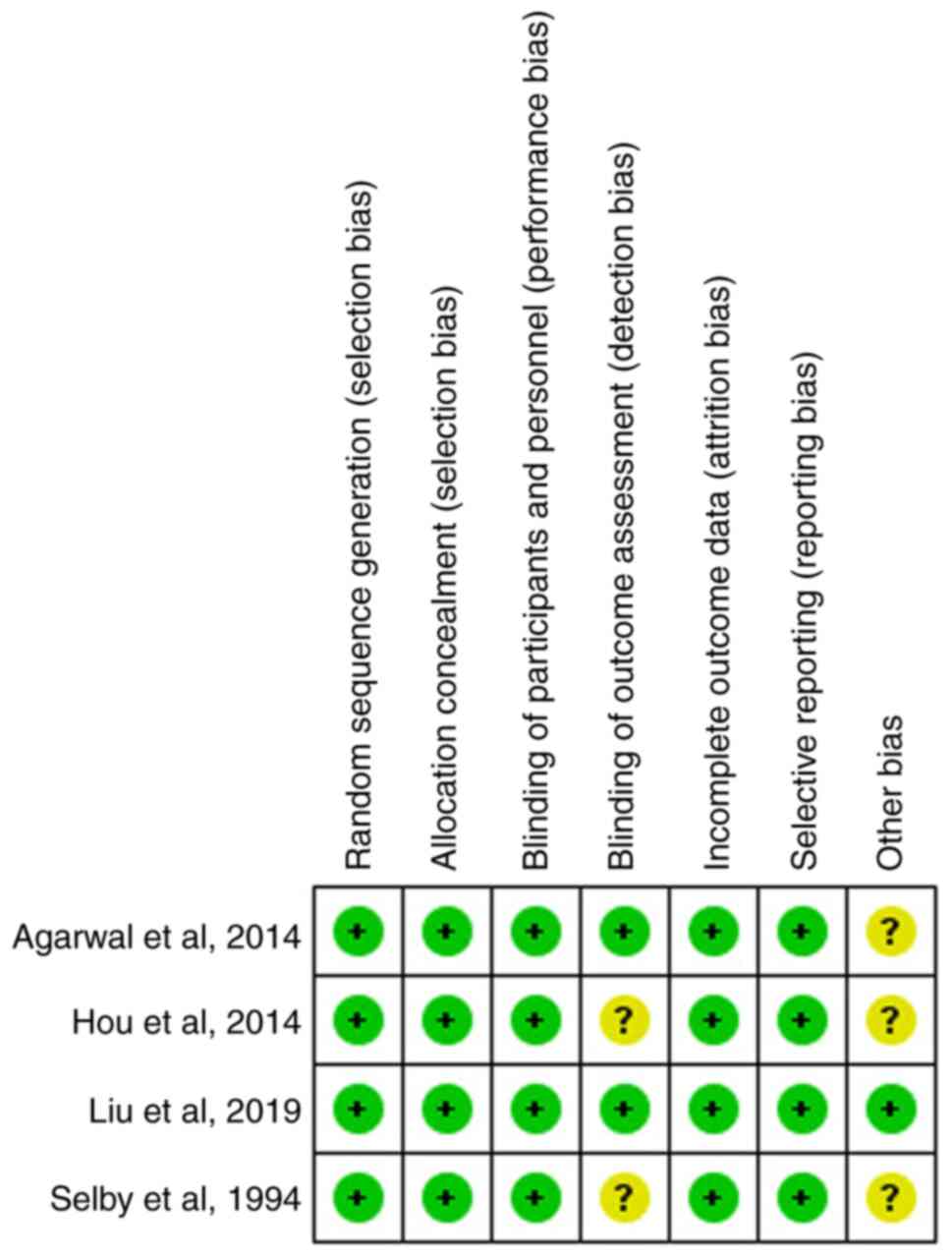
Quality assessment of the included
studies
All four studies were randomized using
computer-generated numbers and the data were reported in sealed
envelopes for allocation concealment. The patients were blinded for
intervention in only one of the included studies via the
administration of a 100 ml saline solution placebo (13). All four studies had assessed the
incidence of infection and mortality (12-15).
Three of the included studies reported the incidence of rebleeding
(12-14).
However, none of the four studies mentioned a blinded assessment of
the outcomes, while the follow-up outcomes of all the included
patients in the four studies were reported. Publication years,
countries and Endoscopic treatment methods are the unclear risks of
bias. The quality assessment of four studies is shown in Fig. 2.
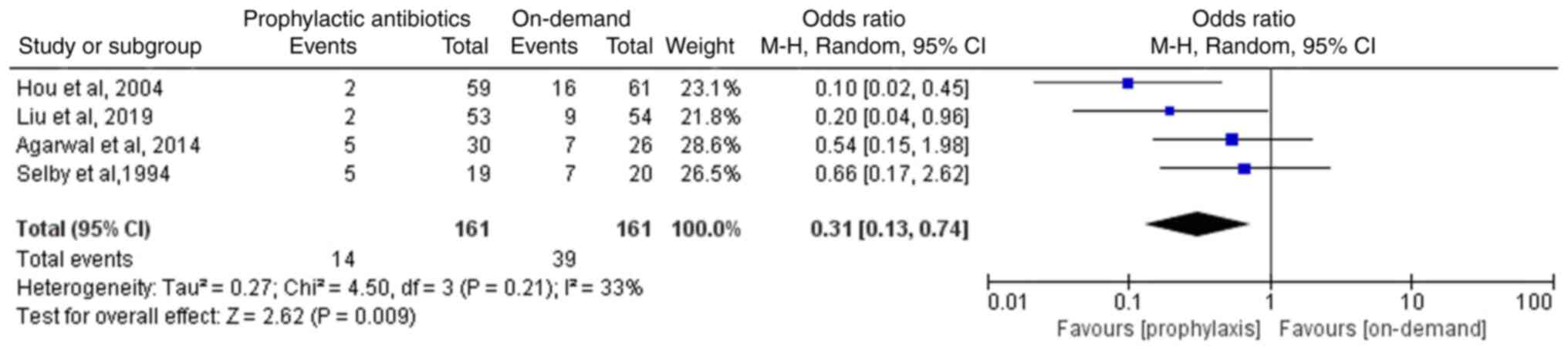
Incidence of infection
Of the four RCTs (12-15),
two studies reported that the comparisons between the on-demand and
the prophylactic antibiotics groups indicated lack of statistical
significance (14,15). The other two studies reported that
the prophylactic antibiotics group exhibited a lower incidence of
infection compared with the on-demand group (12,13).
The four eligible RCTs involving 322 patients reported the
incidence of infection following endoscopic therapy in patients
with variceal bleeding. The meta-analysis suggested that the
incidence of infection in the prophylactic antibiotics group was
significantly lower than that noted in the on-demand group (OR,
0.31; 95% CI, 0.13-0.74; P=0.009; I2, 33%; Fig. 3).
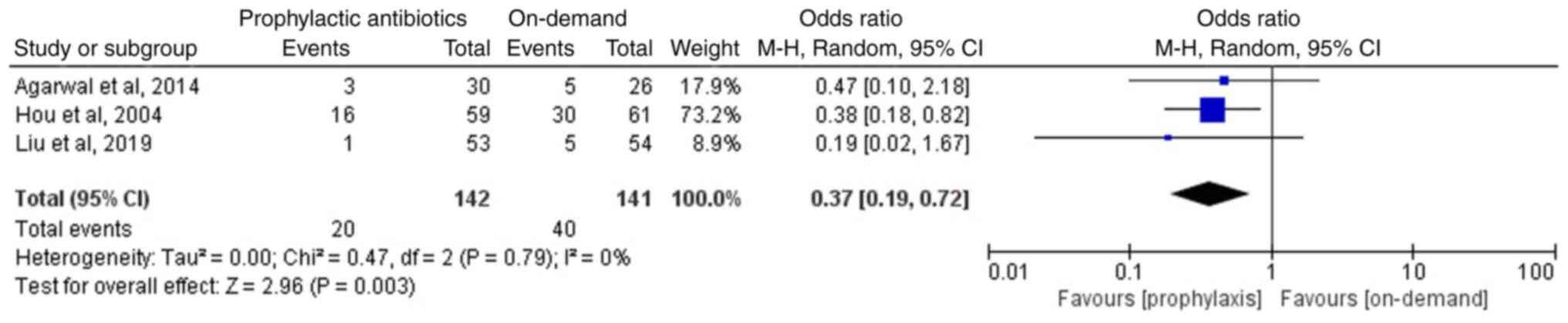
Incidence of rebleeding
Of the included articles, three studies involving
283 patients reported the incidence of rebleeding in the two groups
following endoscopic therapy (12-14);
two studies, including those by Agarwal et al (14) and Liu et al (13), reported non-significant differences
in the incidence of rebleeding between the prophylactic antibiotics
and the on-demand groups. However, Hou et al (12) reported that the prophylactic
antibiotics group exhibited a lower incidence of rebleeding than
that of the on-demand group. The meta-analysis of the three
included studies suggested that the prophylactic antibiotics group
exhibited a lower incidence of rebleeding compared with that of the
on-demand group (OR, 0.37; 95% CI, 0.19-0.72; P=0.003;
I2, 0%; Fig. 4).
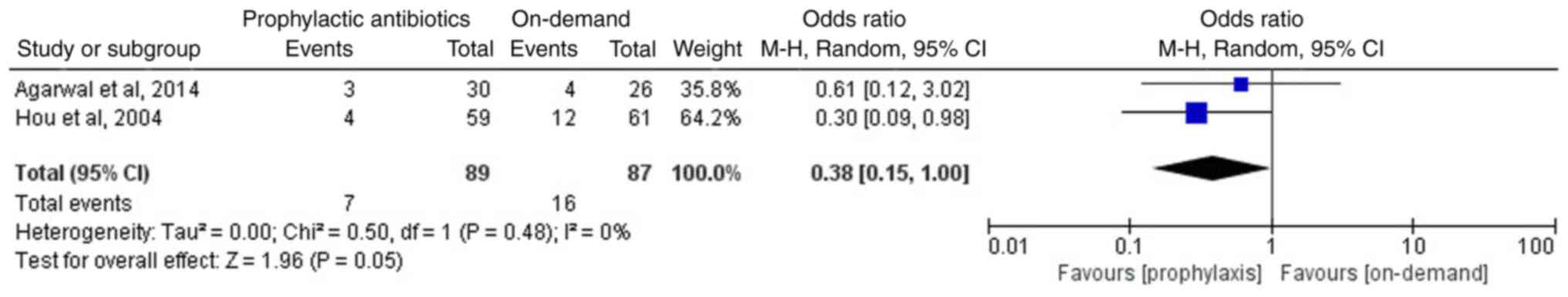
Furthermore, two studies reported the incidence of early rebleeding
(12,14). Early rebleeding was defined as
rebleeding within 7 days following the initial control of bleeding
(14). A meta-analysis of the two
studies, involving 176 patients, suggested that compared with the
on-demand group, the prophylactic antibiotics group exhibited a
lower incidence of early rebleeding (OR, 0.38; 95% CI, 0.15-1.0;
P=0.05; I2, 0%; Fig.
5).
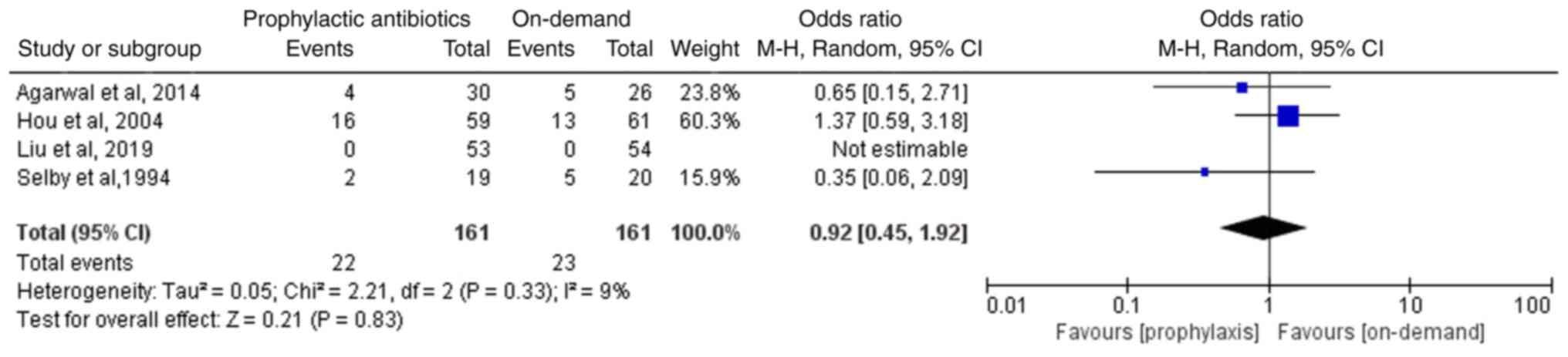
Incidence of mortality
All four included studies, involving 322 patients,
reported the incidence of mortality. Liu et al (13) reported no deaths among the included
patients, but the three other included studies reported a lack of
statistically significant differences in the incidence of mortality
between the two groups (12-15).
The meta-analysis of the four included RCTs suggested absence of a
statistically significant difference in the incidence of mortality
between the two groups (OR, 0.92; 95%CI, 0.45-1.92; P=0.83;
I2, 9%; Fig. 6).
Publication bias
In the present meta-analysis, <10 articles were
included. Therefore, a publication bias test based on a funnel plot
was not conducted.
Discussion
Acute variceal bleeding, one of the most fatal
complications of cirrhosis, is responsible for 34-50% of
cirrhosis-related deaths (3,4,20).
The management of acute variceal bleeding requires a
multidisciplinary approach, including pharmacological, endoscopic
and radiological interventions (21). Endoscopic therapy and antibiotic
prophylaxis, as independent factors, improve the survival and
decrease the mortality rate of patients with acute variceal
bleeding (22,23). Carbonell et al (22) reported that the incidence of
mortality in patients with acute variceal bleeding decreased from
42.6% in 1980 to 14.5% in 2000 due to a combination of endoscopic
therapy and antibiotic prophylaxis. In addition, for patients with
Child-Pugh class A, mortality decreased from 9% in 1980 to 0% in
2000(22). Vuachet et al
(24) reported similar results;
specifically, endoscopic therapy and antibiotic prophylaxis
decreased the 6-week mortality rate and the number of red cell unit
transfusions. For esophageal variceal bleeding, endoscopic therapy
includes EVL and EVS (25,26), and tissue adhesive injections are
recommended (27). Antibiotic use
has been reported to reduce the mortality of patients with variceal
bleeding (28); morover, it
prevented infection in patients following endoscopic therapy. The
UK guidelines and the Korean Association for the Study of the Liver
clinical practice guidelines have recommended short-term antibiotic
use (covering gram-negative antibiotic administration within 1 day)
for patients with acute variceal bleeding (7,29).
The European Society for Gastrointestinal Endoscopy guidelines have
recommended intravenous erythromycin (250 mg) 30-120 min prior to
upper gastrointestinal endoscopy in patients with suspected acute
variceal bleeding, in the absence of contraindications (30). By contrast, Lee et al
(31) reported that early
prophylactic antibiotic use increased the risk of early bacterial
infections, whereas it did not decrease the risk of infection in
cases with acute variceal bleeding and it did not prevent infection
in patients following endoscopic therapy. Bacteremia has also been
reported in patients with variceal bleeding following endoscopic
therapy (10,11). Ueno et al (32) reported that the prophylactic use of
antibiotics was not associated with the 30-day mortality rate or
the frequency of nosocomial bacterial infections. However, Jia
et al (8) reported a low
risk of bacteremia in patients with varices following endoscopic
therapy. Zuckerman et al (9) reported that bacteremia was not
associated with endoscopic therapy in patients with variceal
bleeding. Therefore, whether endoscopic therapy for patients with
varices increases the rate of infection remains to be
elucidated.
Infection is one of the main factors associated with
mortality following the cessation of initial variceal bleeding
(33). Whether antibiotic
prophylaxis is necessary to prevent bacterial infection and to
decrease the mortality of patients with variceal bleeding following
endoscopic therapy has been debated. Following a search and
screening of the databases according to the inclusion and exclusion
criteria, four RCTs were included (12-15).
All four studies reported different results involving the incidence
of infection, rebleeding and mortality; two articles reported that
antibiotic prophylaxis was not necessary for patients with variceal
bleeding following endoscopic therapy due to the lack of
significant difference in the incidence of infection between the
antibiotic prophylaxis and the on-demand groups (14,15).
By contrast, the other two studies reported opposing results, which
indicated that antibiotic prophylaxis was necessary to prevent
bacterial infection in patients with variceal bleeding following
endoscopic therapy (12,13). The present meta-analysis with 322
patients from the four included studies suggested that antibiotic
prophylaxis was necessary to prevent bacterial infection due to the
incidence of infection in the prophylactic antibiotic group being
significantly lower than that noted in the on-demand antibiotic
group. Furthermore, the meta-analysis of three included studies,
involving 283 patients, suggested that antibiotic prophylaxis
decreased the incidence of rebleeding (12-14).
Based on a meta-analysis of two included studies involving 176
patients, antibiotic prophylaxis was also beneficial in decreasing
the incidence of early rebleeding (12,14).
However, with regard to the incidence of mortality, the present
meta-analysis of the four included RCTs, involving 322 patients,
suggested that antibiotic prophylaxis did not decrease mortality in
patients with variceal bleeding following endoscopic therapy.
Inclusion and meta-analysis of the four RCTs suggested that the
interventions performed in the antibiotic prophylaxis group aided
the prevention of bacterial infection and rebleeding in patients
with variceal bleeding following endoscopic therapy compared with
those in the on-demand group; however, these interventions did not
decrease mortality.
The methods of antibiotic prophylaxis differed among
the four RCTs. The two antibiotic prophylaxis methods performed
were as follows: Intravenous ofloxacin (200 mg q12H) for 2 days,
followed by oral ofloxacin (200 mg q12H) for 5 days after
endoscopic therapy (12,14); intravenous cefotiam (2 g) or
intravenous cefotaxime (1 g) administered 1 day prior to endoscopic
therapy (13,15). The application of prophylaxis for 7
days may influence the outcomes compared with administration of
prophylaxis for 1 day. The UK guidelines recommend the 1-day
antibiotic prophylaxis program prior to endoscopic therapy
(7), while two previous studies
have used the 7-day antibiotics prophylaxis program (34,35).
The outcomes from antibiotics treatment occurring 7 days following
endoscopic therapy and those occurring during prophylaxis
antibiotics treatment on the first day prior to endoscopic therapy
have not been reported for patients with variceal bleeding; a
previous study evaluating these parameters provided certain
clarifications, including Child Pugh grade A/B/C, regarding the
optimal antibiotic prophylaxis treatment (34). An additional issue that remains to
be elucidated is the type of antibiotic; two studies involving the
prophylactic intravenous first-generation cephalosporin, cefazolin
and the third-generation cephalosporin, ceftriaxone, for patients
with variceal bleeding following endoscopic interventions reported
contrasting results that third-generation cephalosporin
ceftriaxione was not superior to the first-generation cephalosporin
cefazolin (34,35). A previous study by Wu et al
(34) that included 713 patients
reported that the third-generation cephalosporin ceftriaxone
exhibited improved efficacy compared with the first-generation
cephalosporin cefazolin in preventing infection and reducing
rebleeding in patients who underwent endoscopic therapy for acute
variceal bleeding. The UK guidelines also recommend the
third-generation cephalosporin ceftriaxione as antibiotic
prophylaxis following endoscopic therapy for acute variceal
bleeding (7). However, a previous
study by Lee et al (35)
that included 84 patients reported contradictory results, stating
that the first-generation cephalosporin cefazolin exhibited
improved efficacy than that of the third-generation cephalosporin
ceftriaxone; notably, the latter was administered to a small sample
size of patients. A clinical RCT or meta-analysis with a larger
sample size is required to determine the optimal prophylactic
antibiotic following endoscopic intervention for patients with
acute variceal bleeding.
The present meta-analysis that included four RCTs
with 322 patients suggested that the antibiotic prophylaxis group
exhibited a lower incidence of infection and rebleeding following
endoscopic therapy in patients with variceal bleeding compared with
that of the on-demand group. However, no significant difference was
noted in the mortality between the two groups. The current
meta-analysis discussed the effectiveness of prophylactic
antimicrobial therapy and included a small number of studies,
providing limited value for daily clinical practice. Notably, the
different intervention methods of the prophylaxis group in the four
studies may have affected the outcomes of the current
meta-analysis. High-quality evidence obtained from RCTs or
meta-analyses with a larger sample size are required to elucidate
the optimal prophylactic antibiotic and antibiotic prophylaxis
method for patients with variceal bleeding following endoscopic
therapy.
Acknowledgements
The authors would like to thank Professor Long Chen
from Nanchong Central Hospital (Sichuan, China) who contributed to
the statistical guidance.
Funding
Funding: The present study was funded by the Key Program of
Science & Technology Department of Sichuan Province (grant no.
2023YFS0473) and the Bureau of Science & Technology Nanchong
City (grant no. 22SXQT0401).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZT and WP conceived and designed the study. YG, YZ,
XT and YH acquired the data. DH, JC, JY, ZD, SL and SF analyzed and
interpretated the data according to the inclusion and exclusion
criteria. ZT, WP and YG confirm the authenticity of all the raw
data. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
van Leerdam ME: Epidemiology of acute
upper gastrointestinal bleeding. Best Pract Res Clin Gastroenterol.
22:209–224. 2008.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Carneiro de Moura M, Chen S, Kamath BM, Ng
VL and Ling SC: Acute variceal bleeding causes significant
morbidity. J Pediatr Gastroenterol Nutr. 67:371–376.
2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Tayyem O, Bilal M, Samuel R and Merwat SK:
Evaluation and management of variceal bleeding. Dis Mon.
64:312–320. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hsu YC, Chung CS and Wang HP: Application
of endoscopy in improving survival of cirrhotic patients with acute
variceal hemorrhage. Int J Hepatol. 2011(893973)2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
García-Pagán JC, Reverter E, Abraldes JG
and Bosch J: Acute variceal bleeding. Semin Respir Crit Care Med.
33:46–54. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lau JYW, Yu Y, Tang RSY, Chan HCH, Yip HC,
Chan SM, Luk SWY, Wong SH, Lau LHS, Lui RN, et al: Timing of
endoscopy for acute upper gastrointestinal bleeding. N Engl J Med.
382:1299–1308. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Tripathi D, Stanley AJ, Hayes PC, Patch D,
Millson C, Mehrzad H, Austin A, Ferguson JW, Olliff SP, Hudson M,
et al: U.K. guidelines on the management of variceal haemorrhage in
cirrhotic patients. Gut. 64:1680–1704. 2015.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jia Y, Dwivedi A, Elhanafi S, Ortiz A,
Othman M and Zuckerman M: Low risk of bacteremia after endoscopic
variceal therapy for esophageal varices: A systematic review and
meta-analysis. Endosc Int Open. 3:E409–E417. 2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zuckerman MJ, Jia Y, Hernandez JA, Kolli
VR, Norte A, Amin H, Casner NA, Dwivedi A and Ho H: A prospective
randomized study on the risk of bacteremia in banding versus
sclerotherapy of esophageal varices. Front Med (Lausanne).
3(16)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Galperine T, Flateau C, Venon MD, Lescure
FX, Béraud G, Said Ibrahim T, Delisle F, Durand F, Faure K, Pialoux
G and Guery B: Recurrent bacteremia, a complication of
cyanoacrylate injection for variceal bleeding: Report of two cases
and review of the literature. Case Rep Med.
2009(407053)2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Randi BA, Ninomiya DA, Nicodemo EL, Lopes
BC, Cançado ER and Levin AS: Recurrent bacteremia after injection
of N-butyl-2-cyanoacrylate for treatment of bleeding gastric
varices: A case report and review of the literature. BMC Res Notes.
8(692)2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Hou MC, Lin HC, Liu TT, Kuo BI, Lee FY,
Chang FY and Lee SD: Antibiotic prophylaxis after endoscopic
therapy prevents rebleeding in acute variceal hemorrhage: A
randomized trial. Hepatology. 39:746–753. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu C, Ma L, Wang J, Li F, Tseng Y, Luo T,
Zeng X and Chen S: Prophylactic use of antibiotics in endoscopic
injection of tissue adhesive for the elective treatment of gastric
varices: A randomized controlled study. J Gastroenterol Hepatol.
32:1486–1491. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Agarwal A, Kumar SS, Sadasivan J and Kate
V: Antibiotic prophylaxis in the prevention of rebleeding in acute
variceal hemorrhage: A randomized trial. J Pharmacol Pharmacother.
6:24–29. 2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Selby WS, Norton ID, Pokorny CS and Benn
RA: Bacteremia and bacterascites after endoscopic sclerotherapy for
bleeding esophageal varices and prevention by intravenous
cefotaxime: A randomized trial. Gastrointest Endosc. 40:680–684.
1994.PubMed/NCBI
|
|
16
|
Higgins JPT, Altman DG, Gøtzsche PC, Jüni
P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, et
al: The Cochrane Collaboration's tool for assessing risk of bias in
randomised trials. BMJ. 343(d5928)2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Higgins JP and Green S: Cochrane handbook
for systematic reviews of interventions version 5.0.0.
Naunyn-Schmiedebergs Archiv Exp Pathol Pharmackol. 5(S38)2009.
|
|
18
|
Bowden J, Tierney JF, Copas AJ and Burdett
S: Quantifying, displaying and accounting for heterogeneity in the
meta-analysis of RCTs using standard and generalised Q statistics.
BMC Med Res Methodol. 11(41)2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Palma Pérez S and Delgado Rodríguez M:
Practical considerations on detection of publication bias. Gac
Sanit. 20 (Suppl 3):S10–S16. 2006.PubMed/NCBI View
Article : Google Scholar : (In Spanish).
|
|
20
|
Zanetto A and Garcia-Tsao G: Management of
acute variceal hemorrhage. F1000Res. 8(F1000)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Alqahtani SA and Jang S: Pathophysiology
and management of variceal bleeding. Drugs. 81:647–667.
2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Carbonell N, Pauwels A, Serfaty L, Fourdan
O, Lévy VG and Poupon R: Improved survival after variceal bleeding
in patients with cirrhosis over the past two decades. Hepatology.
40:652–659. 2004.PubMed/NCBI View Article : Google Scholar
|
|
23
|
O'Brien J, Triantos C and Burroughs AK:
Management of varices in patients with cirrhosis. Nat Rev
Gastroenterol Hepatol. 10:402–412. 2013.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Vuachet D, Cervoni JP, Vuitton L, Weil D,
Dritsas S, Dussaucy A, Koch S, Di Martino V and Thevenot T:
Improved survival of cirrhotic patients with variceal bleeding over
the decade 2000-2010. Clin Res Hepatol Gastroenterol. 39:59–67.
2015.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hou MC, Lin HC, Kuo BI, Chen CH, Lee FY
and Lee SD: Comparison of endoscopic variceal injection
sclerotherapy and ligation for the treatment of esophageal variceal
hemorrhage: A prospective randomized trial. Hepatology.
21:1517–1522. 1995.PubMed/NCBI
|
|
26
|
Dai C, Liu WX, Jiang M and Sun MJ:
Endoscopic variceal ligation compared with endoscopic injection
sclerotherapy for treatment of esophageal variceal hemorrhage: A
meta-analysis. World J Gastroenterol. 21:2534–2541. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Lo GH, Lai KH, Cheng JS, Chen MH and
Chiang HT: A prospective, randomized trial of butyl cyanoacrylate
injection versus band ligation in the management of bleeding
gastric varices. Hepatology. 33:1060–1064. 2001.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Moon AM, Dominitz JA, Ioannou GN, Lowy E
and Beste LA: Use of antibiotics among patients with cirrhosis and
upper gastrointestinal bleeding is associated with reduced
mortality. Clin Gastroenterol Hepatol. 14:1629–1637.e1.
2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Korean Association for the Study of the
Liver (KASL). KASL clinical practice guidelines for liver
cirrhosis: Varices, hepatic encephalopathy, and related
complications. Clin Mol Hepatol. 26:83–127. 2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Gralnek IM, Camus Duboc M, Garcia-Pagan
JC, Fuccio L, Karstensen JG, Hucl T, Jovanovic I, Awadie H,
Hernandez-Gea V, Tantau M, et al: Endoscopic diagnosis and
management of esophagogastric variceal hemorrhage: European society
of gastrointestinal endoscopy (ESGE) guideline. Endoscopy.
54:1094–1120. 2022.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Lee S, Saxinger L, Ma M, Prado V,
Fernández J, Kumar D, Gonzalez-Abraldes J, Keough A, Bastiampillai
R, Carbonneau M, et al: Bacterial infections in acute variceal
hemorrhage despite antibiotics-a multicenter study of predictors
and clinical impact. United European Gastroenterol J. 5:1090–1099.
2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Ueno M, Fujiwara T, Tokumasu H, Mano T,
Kayahara T, Takabatake H, Morimoto Y, Matsueda K, Fukuoka T and
Mizuno M: Real-world efficacy of antibiotic prophylaxis for upper
gastrointestinal bleeding in cirrhotic patients in Japan. J
Gastroenterol. 58:766–777. 2023.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Lee SW, Lee TY, Chang CS, Ko CW, Yeh HZ
and Yang SS: Independent factors associated with early outcome in
Chinese cirrhotic patients after cessation of initial esophageal
variceal hemorrhage. J Clin Gastroenterol. 44:e123–e127.
2010.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wu CK, Wang JH, Lee CH, Wu KL, Tai WC, Lu
SN, Hu TH and Chuah SK: The outcome of prophylactic intravenous
cefazolin and ceftriaxone in cirrhotic patients at different
clinical stages of disease after endoscopic interventions for acute
variceal hemorrhage. PLoS One. 8(e61666)2013.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Lee J, Xu H, Chuah SK, et al: The effect
of intravenous cefazolin and ceftrixions as prophylactic
antibiotics in cirrhotic patients with acute variceal hemorrhage
after endoscopic interventions-A preliminary report. Hepatol Int.
6(302)2012.
|